Abstract Abstract
The diagnosis of pulmonary vascular disease (PVD) is usually based on hemodynamic and/or clinical criteria. Noninvasive imaging of the heart and proximal vasculature can also provide useful information. An alternate approach to such criteria in the diagnosis of PVD is to image the vascular abnormalities in the lungs themselves. Hyperpolarized (HP) 129Xe magnetic resonance imaging (MRI) is a novel technique for assessing abnormalities in ventilation and gas exchange in the lungs. We applied this technique to two patients for whom there was clinical suspicion of PVD. Two patients who had significant hypoxemia and dyspnea with no significant abnormalities on computed tomography imaging or ventilation-perfusion scan and only mild or borderline pulmonary arterial hypertension at catheterization were evaluated. They underwent HP 129Xe imaging and subsequently had tissue diagnosis obtained from lung pathology. In both patients, HP 129Xe imaging demonstrated normal ventilation but markedly decreased gas transfer to red blood cells with focal defects on imaging, a pattern distinct from those previously described for idiopathic pulmonary fibrosis or obstructive lung disease. Pathology on both patients later demonstrated severe PVD. These findings suggest that HP 129Xe MRI may be useful in the diagnosis of PVD and monitoring response to therapy. Further studies are required to determine its sensitivity and specificity in these settings.
Keywords: pulmonary vascular disease, pulmonary hypertension, magnetic resonance imaging, xenon
Pulmonary vascular diseases (PVDs), such as pulmonary arterial hypertension (PAH), are diseases of the pulmonary vasculature that are typically associated with endothelial cell apoptosis, smooth muscle proliferation, and formation of obliterative vascular lesions.1 This results in the development of higher pulmonary arterial pressure, as the right ventricle must maintain cardiac output in the setting of high pulmonary vascular resistance. PVD is heterogeneous and, at times, can be difficult to diagnose. For example, while the diagnosis of PAH is based on hemodynamic criteria of a mean pulmonary arterial pressure of ≥25 mmHg at rest with a pulmonary arterial occlusion pressure of ≤15 mmHg, some patients do not meet these strict criteria because of the coexistence of left heart disease or a lack of significant elevation of their mean pulmonary arterial pressure measurement at rest.
A potential alternative approach to diagnosing PVD is to image the vascular abnormalities in the lungs themselves. These abnormalities are commonly associated with decreased pulmonary gas transfer, which manifests in pulmonary function tests as a reduction in the diffusion capacity of the lungs for carbon monoxide (DLCO).2,3 However, the DLCO is a global measure of gas exchange whose reduction is not specific to PVD and cannot address spatial variations or heterogeneity of disease.4 An alternative technology, hyperpolarized (HP) 129Xe magnetic resonance imaging (MRI), can be safely used in human subjects4 to generate high-quality images of pulmonary ventilation and gas exchange (Fig. 1). HP 129Xe ventilation MRI has been used to characterize specific patterns of disease in asthma5 and chronic obstructive pulmonary disease.6 129Xe has notable qualities, including its solubility in pulmonary barrier tissues and its unique frequency shift in red blood cells,7 by which it can be distinguished from 129Xe in barrier tissue or gas, as demonstrated in idiopathic pulmonary fibrosis (IPF).8,9 To our knowledge, there are no reports of its use in patients with PVD. Here we describe two patients with severe abnormalities noted on HP 129Xe MRI research scans who were later confirmed to have PVD on pathology. Institutional review board approval was obtained to undergo these research studies, and informed consent was given by each patient.
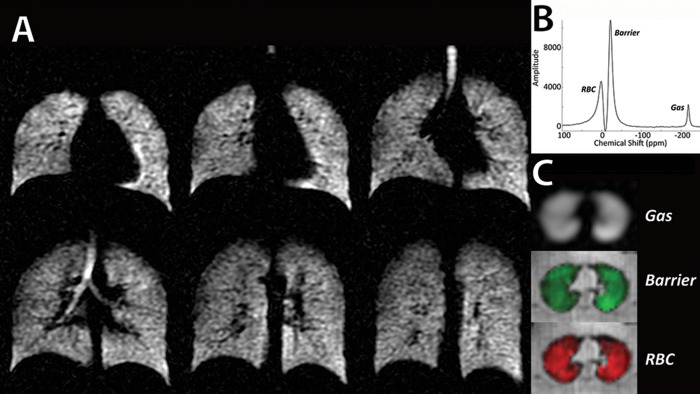
Figure 1.
129Xe hyperpolarized magnetic resonance imaging (MRI) and spectroscopy in a healthy individual. A, High-resolution 129Xe ventilation MRI with normal homogeneous distribution. B, Gas spectroscopy demonstrating xenon signals in red blood cells (RBCs), barrier tissue, and gas-phase xenon in the airspaces. C, Representative slices from single-breath 3-D images of Xe in gas, barrier, and RBCs, depicting homogeneous distributions.
Case description
Case 1
Ms. KS is a 61-year-old nonsmoking woman and former ballet dancer. In December 2012, she presented to the pulmonary hypertension (PH) clinic at Duke University with dyspnea with minimal exertion and two recent episodes of syncope. She did not have dyspnea at rest, chest pain, palpitation, or persistent cough. Her vital signs were notable for blood pressure of 104/60 mmHg, respiratory rate of 18 breaths/min, heart rate of 72 beats/min, and oxygen saturation (Spo2) of 91% on room air. She had an otherwise normal systemic examination except for a holosystolic murmur. Hemoglobin was 11.3 g/dL, with normal white blood cells and platelets. She had normal electrolytes and kidney and liver function tests. Her pulmonary function testing was unremarkable except for a reduced DLCO to 5.4 mL/mmHg/min (28% predicted). Arterial blood gas oxygen tension measured while breathing ambient air was 61 mmHg. Echocardiography revealed normal left and right ventricular size and function, with an estimated right ventricular systolic pressure of 40 mmHg and a negative microcavitation study. Her initial 6-minute walk distance was 451 m on 6 L/min oxygen, with Spo2 of 100% at rest and a nadir of 85%.
Her chest radiograph was normal. Computed tomography of the chest, abdomen, and pelvis revealed no evidence of parenchymal disease (Fig. 2A). Ventilation-perfusion scan showed a very low probability of pulmonary embolism. No evidence of arteriovenous malformations or shunting that could explain her hypoxemia was noted on contrasted computed tomography scans of her chest, abdomen, and pelvis. She underwent right heart catheterization that revealed only mild PAH: right atrial pressure of 13 mmHg, right ventricular pressure of 48/13 mmHg, mean pulmonary arterial pressure of 32 mmHg, wedge pressure of 14 mmHg, cardiac index of 2.76 L/min/m2, and pulmonary vascular resistance of 2.9 Wood units. She was started earlier on hydroxychloroquine and azathioprine by her local rheumatologist for possible lupus in the setting of weakly positive antinuclear antibody (1∶40) and double-stranded DNA antibody test results but without any symptoms. She did not improve clinically, and both were discontinued by a rheumatologist at our institution who did not feel that she had lupus. She had increasing dyspnea and an increase in her oxygen requirement from 6 to 10 L/min with exertion. She was felt to be a poor candidate for further pulmonary vasodilators, as there was concern for pulmonary venoocclusive disease (PVOD); therefore, she was referred to lung transplantation.
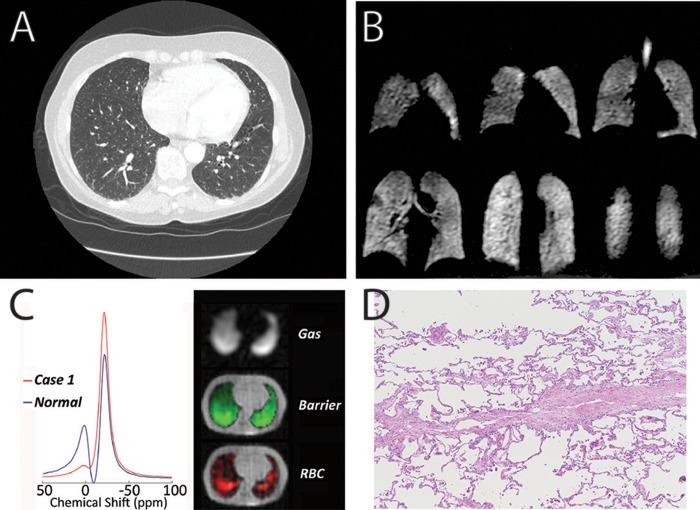
Figure 2.
Imaging and pathology from case 1. A, Representative axial computed tomography image demonstrating no significant abnormalities. B, 129Xe magnetic resonance imaging of ventilation demonstrating no significant abnormalities. C, 129Xe spectroscopy showing a severe decrease in xenon signal in red blood cells (RBCs) relative to barrier tissue (left) and representative single-breath gas exchange images showing homogeneous gas and barrier signal while 129Xe transfer to RBCs contains severe focal abnormalities (right). D, Pathology demonstrating pulmonary venoocclusive disease.
During her lung transplantation evaluation, she underwent further studies to rule out shunt in the setting of her severe hypoxemia and mild PAH. Her high oxygen requirement precluded lung biopsy. During this evaluation, she had a research HP 129Xe MRI study performed that demonstrated normal ventilation images (Fig. 2B). However, 129Xe gas exchange spectroscopy and imaging (Fig. 2C) indicated severely diminished gas transfer to the red blood cells (13% of that seen in healthy volunteers). With her worsening clinical status, she was listed for lung transplantation and subsequently underwent transplantation. The pathology of her explanted lungs demonstrated PVOD (Fig. 2D).
Case 2
Mr. GC is a 70-year-old male with systemic hypertension, hyperlipidemia, and allergic rhinitis. Five years ago, he noted exertional dyspnea that increased over months. He had no cough, sputum production, chest pain, or syncope. His dyspnea increased over time, and he sought medical evaluation at several specialty clinics. Controlling his allergic rhinitis did not improve his dyspnea. Evaluation revealed blood pressure of 149/85 mmHg, heart rate of 66 beats/min, respiratory rate of 20 breaths/min, and Spo2 of 93% on ambient air. His clinical examination was unremarkable. Arterial blood gas oxygen tension measured while breathing ambient air was 74 mmHg. Laboratory testing was unremarkable except for a serum creatinine of 1.6 mg/dL. Pulmonary function tests revealed normal spirometry and lung volumes with an isolated reduction of the DLCO to 5.4 mL/mmHg/min (44% predicted). Echocardiography was normal; RVSP was estimated at 30 mmHg. Chest radiograph and chest computed tomography scan were unremarkable (Fig. 3A). Right heart catheterization demonstrated right atrial pressure of 4 mmHg, right ventricular pressure of 32/6 mmHg, mean pulmonary arterial pressure of 21 mmHg, wedge pressure of 6 mmHg, cardiac output of 6 L/min, cardiac index of 2.82 L/min/m2, and pulmonary vascular resistance of 2.4 Wood units. He underwent cardiopulmonary exercise testing that revealed normal oxygen consumption, maximal heart rate of 150 beats/min with no changes in spirometry parameters, and Spo2 of 88% at the end of the test. Cardiac MRI revealed a right ventricle with normal size and function but with delayed enhancement at its insertion sites, a finding seen in PH. Clinically, he was thought to have borderline PAH.
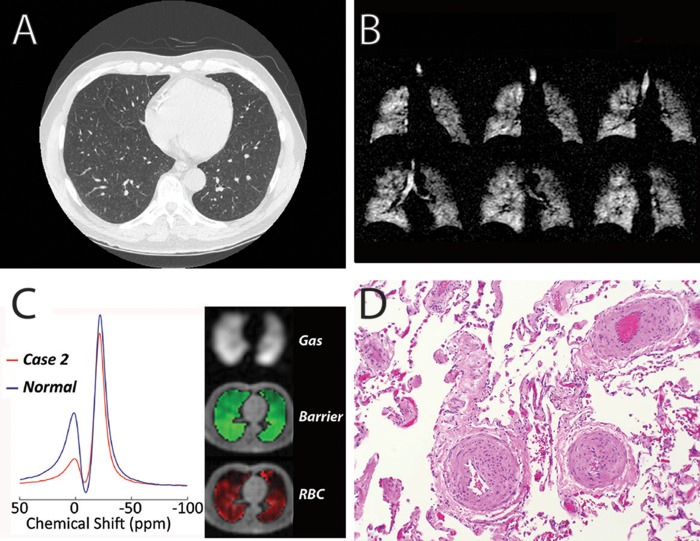
Figure 3.
Imaging and pathology from case 2. A, Representative axial computed tomography image of the lungs demonstrating no significant abnormalities near the bases. Mild upper lobe emphysema was noted on other images (not shown). B, 129Xe magnetic resonance imaging of ventilation demonstrating mild peripheral ventilation defects and mild upper lobe emphysema. C, 129Xe spectroscopy exhibiting a moderate decrease in xenon signal in red blood cells (RBCs) relative to barrier tissue (left) and single-breath gas exchange images demonstrating homogeneous gas and barrier signal while xenon uptake in RBCs contained severe focal abnormalities (right). D, Pathology demonstrating pulmonary hypertensive arteriopathy.
During this workup, Mr. GC also underwent a research HP 129Xe MRI study that demonstrated moderate ventilation defects with mild emphysema in the apical lobes (Fig. 3B). This subject also exhibited significantly diminished 129Xe gas transfer (42% of that seen in healthy volunteers) and focal defects on imaging (Fig. 3C). As he had worsening of his dyspnea and hypoxemia, he was referred for videoscopic-assisted thoracoscopic lung biopsy. Pathology revealed severe and focal arteriopathy with minimal interstitial fibrotic changes (Fig. 3D). He has since been treated with tadalafil, with subjective improvement in his symptoms.
Discussion
The diagnosis of PVD can be difficult in many cases, such as in PAH with borderline elevations of mean pulmonary arterial pressure (21–24 mmHg),10 exercise-induced PH,11 significant precapillary PH in the setting of elevated wedge pressure,12 or PVOD, which is most commonly a diagnosis of exclusion. Here we presented two cases in which there was a significant discordance between the severity of symptoms and hemodynamics at right heart catheterization. While both patients continued to have appropriate care with presumed clinical diagnoses (PVOD and borderline PAH), the degree of their disease was unclear on the basis of their diagnostic studies. In both of these cases, HP 129Xe MRI demonstrated abnormalities that were consistent with findings later noted on pathology. This suggests that HP 129Xe imaging may have potential utility in the diagnosis of PVD.
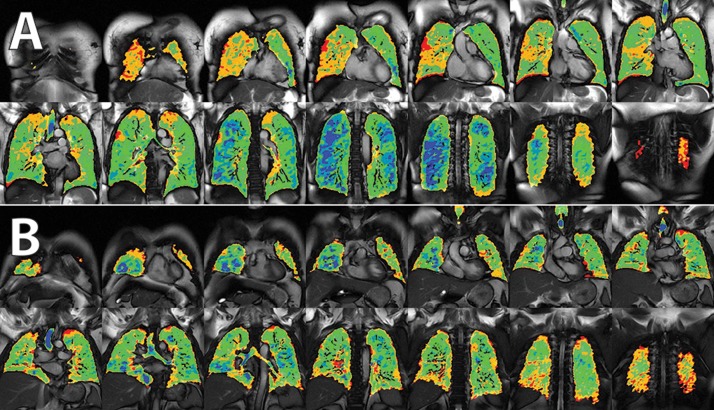
HP 129Xe imaging enables 3-D regional assessment of lung ventilation and gas exchange in a single breath hold. This technique has demonstrated specific patterns of airway and parenchymal lung disease.4 In asthma, it was superior to 3He MRI5 and revealed ventilation abnormalities before bronchodilation. In IPF, it clearly identifies diffusion limitation along with regional defects and pattern of disease that may be unique compared with other causes of pulmonary fibrosis.8 The gas exchange patterns seen in these two PVD patients have some similarities to those seen in IPF, although with notable differences in 129Xe ventilation patterns, which show a more compliant lung in PVD (Fig. 4).
Figure 4.
Differences in ventilation imaging between subjects with pulmonary vascular disease (PVD; A) and idiopathic pulmonary fibrosis (IPF; B). Subjects with PVD appear to have fairly normal ventilation with relatively high lung compliance (blue), while those with IPF appear to lose high-intensity signal (loss of blue signal), consistent with a loss of lung compliance.
This is the first report of this technology demonstrating its potential utility in the diagnosis of PVD. Further studies of patients with known PVD are needed to fully characterize their ventilation and gas transfer patterns in HP 129Xe MRI and to determine the utility of this technology in its diagnosis and management.
Source of Support: BD is supported by National Institutes of Health (NIH) grants R01HL105643 and P41EB015897. SR is supported by NIH grant K08HL114643, Gilead Research Scholars in Pulmonary Arterial Hypertension, and a Burroughs Wellcome Career Award for Medical Scientists.
Conflict of Interest: BD is a shareholder in Polarean, which is commercializing hyperpolarized 129Xe magnetic resonance imaging technology. All other authors: none declared.
References
- 1.Tuder RM, Archer SL, Dorfmüller P, et al. Relevant issues in the pathology and pathobiology of pulmonary hypertension. J Am Coll Cardiol 2013;62(suppl. 25):D4–D12. [DOI] [PMC free article] [PubMed]
- 2.Souza R, Fernandes CJ, Hoeper MM. Carbon monoxide diffusing capacity and the complexity of diagnosis in pulmonary arterial hypertension. Eur Respir J 2014;43(4):963–965. [DOI] [PubMed]
- 3.Elliott CG, Colby TV, Hill T, Crapo RO. Pulmonary veno-occlusive disease associated with severe reduction of single-breath carbon monoxide diffusing capacity. Respiration 1988;53(4):262–266. [DOI] [PubMed]
- 4.Cleveland ZI, Cofer GP, Metz G, Beaver D, Nouls J, Kaushik SS, Kraft M, et al. Hyperpolarized Xe MR imaging of alveolar gas uptake in humans. PLoS ONE 2010;5(8):e12192. [DOI] [PMC free article] [PubMed]
- 5.Svenningsen S, Kirby M, Starr D, Leary D, Wheatley A, Maksym GN, McCormack DG, Parraga G. Hyperpolarized 3He and 129Xe MRI: differences in asthma before bronchodilation. J Magn Reson Imaging 2013;38(6):1521–1530. [DOI] [PubMed]
- 6.Kaushik SS, Cleveland ZI, Cofer GP, Metz G, Beaver D, Nouls J, Kraft M, et al. Diffusion-weighted hyperpolarized 129Xe MRI in healthy volunteers and subjects with chronic obstructive pulmonary disease. Magn Reson Med 2011;65(4):1154–1165. [DOI] [PMC free article] [PubMed]
- 7.Weathersby PK, Homer LD. Solubility of inert gases in biological fluids and tissues: a review. Undersea Biomed Res 1980;7(4):277–296. [PubMed]
- 8.Kaushik SS, Freeman MS, Yoon SW, Liljeroth MG, Stiles JV, Roos JE, Foster W, Rackley CR, McAdams HP, Driehuys B. Measuring diffusion limitation with a perfusion-limited gas–hyperpolarized 129Xe gas-transfer spectroscopy in patients with idiopathic pulmonary fibrosis. J Appl Physiol 2014;117(6):577–585. [DOI] [PMC free article] [PubMed]
- 9.Kaushik SS, Robertson SH, Freeman MS, He M, Kelly KT, Roos JE, Rackley CR, Foster WM, McAdams HP, Driehuys B. Single-breath imaging of hyperpolarized 129Xe in the airspaces, barrier and red blood cells using an interleaved 3D radial 1-point Dixon acquisition. Magn Reson Med. Electronically published May 18, 2015. doi:10.1002/mrm.25675. [DOI] [PMC free article] [PubMed]
- 10.Hoeper MM, Bogaard HJ, Condliffe R, Frantz R, Khanna D, Kurzyna M, Langleben D, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol 2013;62(suppl. 25):D42–D50. [DOI] [PubMed]
- 11.Naeije R, Vanderpool R, Dhakal BP, Saggar R, Saggar R, Vachiéry JL, Lewis GD, et al. Exercise-induced pulmonary hypertension: physiological basis and methodological concerns. Am J Respir Crit Care Med 2013;187(6):576–583. [DOI] [PMC free article] [PubMed]
- 12.Vachiéry JL, Adir Y, Barberà JA, Champion H, Coghlan JG, Cottin V, De Marco T, et al. Pulmonary hypertension due to left heart diseases. J Am Coll Cardiol 2013;62(suppl. 25):D100–D108. [DOI] [PubMed]