Abstract
Spider silk is regarded as one of the best natural polymer fibers especially in terms of low density, high tensile strength and high elongation until breaking. Since only a few bio-engineering studies have been focused on spider silk ageing, we conducted nano-tensile tests on the vertical naturally spun silk fibers of the bridge spider Larinioides cornutus (Clerck, 1757) (Arachnida, Araneae) to evaluate changes in the mechanical properties of the silk (ultimate stress and strain, Young’s modulus, toughness) over time. We studied the natural process of silk ageing at different time intervals from spinning (20 seconds up to one month), comparing silk fibers spun from adult spiders collected in the field. Data were analyzed using Linear Mixed Models. We detected a positive trend versus time for the Young’s modulus, indicating that aged silks are stiffer and possibly less effective in catching prey. Moreover, we observed a negative trend for the ultimate strain versus time, attesting a general decrement of the resistance force. These trends are interpreted as being due to the drying of the silk protein chains and the reorientation among the fibers.
One of the most supreme examples of animal architecture are spiders’ orb webs, which assume a crucial importance for survival, growth and reproduction of a huge number of spiders. One of the main functions of the orb web is to withstand the high stresses needed to absorb the kinetic energy of flying insect prey1,2,3. Since silk is so important for spiders, it has most probably been subjected to strong selective pressures over the 400 million years of spider evolution and can be regarded as one of the keys to the evolutionary success of spiders4,5. Spider silk is regarded as one of the best natural polymer fibers especially in terms of low density, high tensile strength and high elongation until breaking giving it great toughness. It also compares well compared to the majority of the best performing artificial fibers3,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26.
Environmental factors such as temperature2,3,7, humidity10,27,28,29, diet30,31,32 and UV radiation33 have been proved to affect spider silk mechanical properties in different ways. In particular, ageing causes the cleavage of hydrogen bonds linking silk proteins, the decay of amino acids via emission of ammonia gas from the silk fiber and even oxidation. These are well-documented degradation processes for silkworm silk and, as they also occur in many polymers33 they are likely to be present in spider silk10 too. This could lead to a transient rearrangement of the silk polymer chains and in general to less compliant silk fibers determining a reduction in the mechanical properties of the silk as recorded during our experiments10,34.
Moreover, other factors such as reeling methods29,35,36,37, environmental conditions during silk spinning7,10,27,29,38,39 and types of silk (e.g. dragline, viscid or egg sac silk)14,22,40 affect silk diameter whereas the strain rate of tensile testing affects the results of the mechanical silk characterization. Therefore, mechanical performances of silk may vary at the inter-individual as well as the intra-individual level2,13,20,37,41,42.
Although spider silk has been intensively studied, few scientific papers have focused on the effects of ageing on mechanical performance10,34,43. In particular, six- and 720-hours ageing of Argiope trifasciata spider MA silk fibers10, as well as four- and 1-year ageing of different silks and spiders33 and 21-days ageing43 were analyzed in literature. The bridge spider Larinioides cornutus (Clerck, 1757) has been studied looking at its silk biomechanics44,45,46 and at the contribution of diet to its lifetime reproductive success, growth and lifespan30,31,32. So far, no bio-engineering studies have focused on the effect of time (both silk ageing and spider senescence) on the silk of the bridge spider L. cornutus.
In this study, we focused on the silk threads of the bridge orb web spider L. cornutus. Our data are referred to mixed types of silk, consisting of at least 2 major ampullate (MA), 2 minor ampullate (mA) and abundant aciniform (AF) silk threads. Spiders use MA silk as radial structural silk in orb webs together with capture spiral threads which are able to dissipate the kinetic energy of prey impacts into viscoelastic deformation1,7. MA silk is also used as a lifeline in case of danger to rapidly descend or escape from predators thanks to its unique properties even under torsion20,34,45,47. In general, MA silk shows extremely high strength and toughness in araneomorph spiders. mA silk threads are used for temporary scaffolding during web construction while AF threads are used to wrap and secure freshly captured prey. Orb weaving spiders such as L. cornutus generally renew their webs daily by repairing the old broken silk threads or spinning a new one. As a consequence, no orb web silks are normally required to function for longer than ~1 day33 even if the bridge line and frames of orbs can be very long lasting. Most technological and future applications of silk, such as super strong cables or clothing or biomedical scaffolds, need to maintain their mechanical performance over time and the degradation mechanism caused by ageing should be avoided10,30,33,48. Thus, it is important to understand how the mechanical properties of spider silk are affected by natural ageing.
In this study, we hypothesize that the mechanical properties of the silk of an orb weaver spider varies as function of time (silk ageing). In particular, we conducted nano-tensile tests on vertical naturally spun silk fibers. We measured stress, strain, Young’s modulus and toughness at different time intervals from spinning (20 seconds) up to one month later, coherently with the most exhaustive study in literature10.
In order to provide reliable regression coefficients and highlight significant statistical trends relating the dependent variables (mechanical properties) to silk ageing, we specifically designed the study in order to obtain a suitable dataset (both from the quantitative and qualitative point of view) for the application of Linear Mixed Modelling techniques. Such methods allow us to deal with the potentially confusing of individual variability and sampling period, both of which were appropriately taken into account in the design of the statistical methods.
Results
Nano-tensile testing
We performed 240 nano-tensile tests of naturally spun silk threads of six adult females of Larinioides cornutus over a period of 1 month of natural ageing. Females were collected in two different sampling periods (Summer and Autumn).
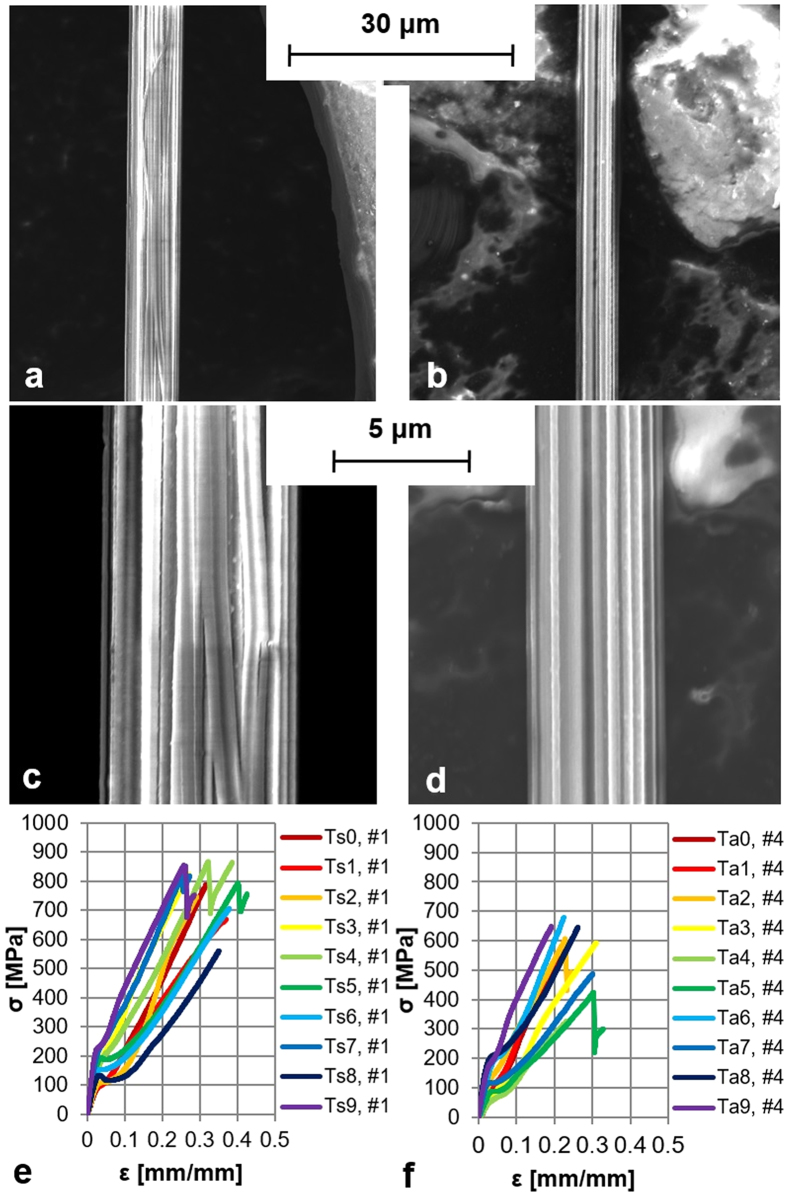
From the various tensile tests (Fig. 1a–f), we obtained rough data of ultimate stress and strain, Young’s modulus and toughness. The maximum failure stress and strain were 1.3 GPa and 117% for Summer silk or 1.1 GPa and 56% for Autumn silk, showing an Autumn strain value higher than the Summer one against the main trend evaluated with LMMs. The maximum value of toughness was 660 MJ/m3 for Summer silk and 351 MJ/m3 for Autumn silk. Young’s modulus is calculated as the initial slope of the stress-strain curve and the maximum of the Young’s modulus is equal to 14 GPa for Summer silk or 36 GPa for Autumn silk. Figure 2 shows the stress-strain curves corresponding to the maximum values of stress reported above.
Figure 1. FESEM characterization of silk samples and nano-tensile testing.
FESEM micrographies of silk of spider number #2 (a) and at high magnification (c) and spider number #5 (b) and at high magnification (d), as representatives of the silk multi-fiber thread diameter of Summer spiders (a,c) and Autumn spiders (b,d) samples. Stress-strain curves, one at each time interval from spinning, of silk of spider number #1 (e) and spider number #4 (f), as representatives of stress-strain curves of samples of Summer (e) and Autumn (f).
Figure 2. The best performing stress-strain curves of silk threads.
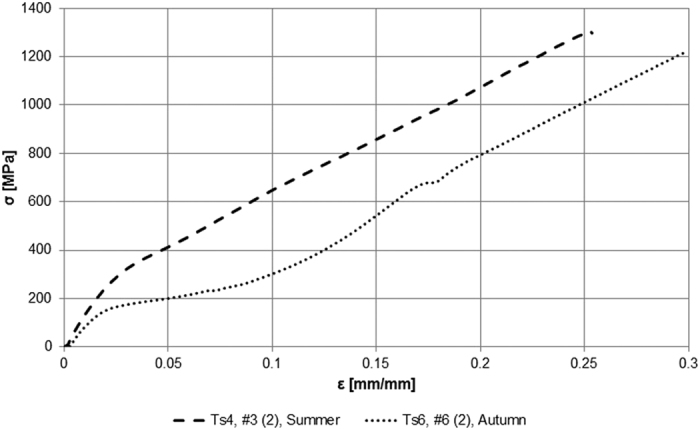
Results of the stress-strain curves corresponding to the maximum stress value observed during experiments of Summer (second sample (2) of spider #3) and Autumn silks (second sample (2) of spider #6).
Linear Mixed Models (LMMs)
In order to evaluate the behavior of the mechanical properties of the silk versus ageing (up to 1 month), raw data of ultimate stress and strain, Young’s modulus and toughness were analyzed with Linear Mixed Models (LMMs).
There was a significant increase in the Young’s modulus over time (log Time2 estimates: 0.2557; std. err.: 0.082; p: 0.002; Fig. 3), but no significant variation in respect to sampling period (Autumn estimates: − 0.2859; std. err.: 0.7806; p: 0.7327). The latter variable was therefore dropped during model selection.
Figure 3. Linear Mixed Models applied to values of Young’s modulus.
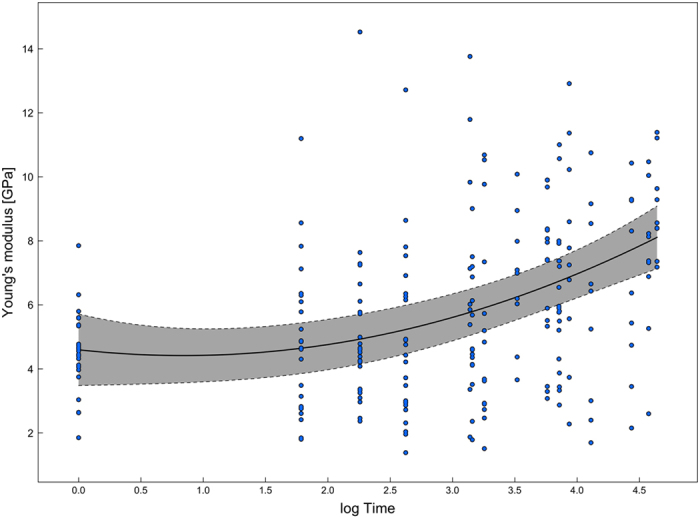
Observed values of Young’s modulus with a fitted polynomial LMM curve (solid line) and 95% confidence band (grey surface) (details in the Supplementary information).
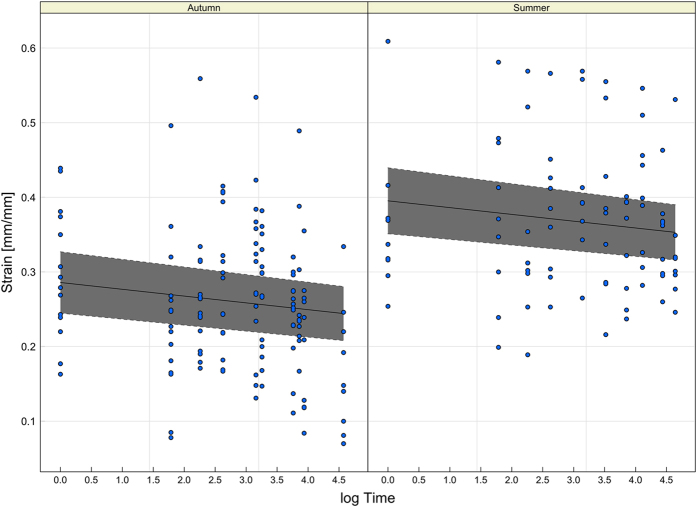
There was a significant decrease in ultimate strain values over time (log Time estimates: − 0.009; std. err.: 0.0048; p: 0.051), attesting a general decrease in silk properties with ageing (Fig. 4). We also observed a significant effect of the sampling period, with lower values of strain in Autumn in respect to the reference category Summer (Autumn estimates: − 0.1094; std. err.: 0.023; p: 0.009).
Figure 4. Linear Mixed Models applied to strain values.
Observed values of strain with a fitted LMM curve (solid line) and 95% confidence bands (grey surface) in the two sampling periods. The relation versus time is significant for both sampling periods.
Stress showed no significant decrease over time (log Time estimates: − 12.3709; std. err.: 9.2726; p: 0.183) nor significant difference with the sampling period (Autumn estimates: − 99.6723 ; std err: 44.2764; p: 0.08).
Toughness showed lower values in Autumn in respect to the reference category Summer (Autumn estimates: − 73.2082; std err: 19.0551; p: 0.018), whereas no significant trend over time was observed (log Time estimates: − 0.5919; std. err.: 2.6597; p: 0.824).
Results of model validation are reported in Supplementary Information.
Discussion
Despite of the fact that our results could be affected by the number and arrangement of fibers composing the tested silk bundle, in general, we obtained stress-strain curves which are coherent with previously tensile test results reported in literature for naturally spun silk threads2,10,45,49 and drops of stress values during testing45,50.
Indeed, we collected multi-stranded silk threads composed of at least 2 major ampullate, 2 minor ampullate and abundant aciniform threads while the spider was descending. As a consequence of the presence of parallel multi-fibers, sudden slight drops of stress values are sometimes exhibited during tensile test before failure (see Fig. 1e,f), but the stress-strain curves suddenly recover to their previous stiffness after these drops45. Note that the mechanical properties of a multi-fiber thread exhibiting this behavior were not noticeably different from tests where these drops did not appear and all the silk multi-fibers broke together.
Concerning the variation in the silk properties with ageing, we observed a non-linear increase in the Young’ Modulus values (Fig. 3), as well as a linear decrease of ultimate strain values over time (Fig. 4). On the contrary, ultimate stress and toughness did not vary with time, at least up to 1 month.
More specifically, silk ageing induces an increase of about + 88% in the Young’s Modulus, which is ~6 times higher than the increment of the dragline silk of Argiope trifasciata10 and ~1.6 or 2.6 times higher than that measured for 1- or 4-year-aged dragline silks of different species of Araneomorphae33. In biological terms, aged silks are then stiffer and possibly less effective in catching prey. We also recorded a slight percentage decrement of − 13% in the ultimate stress. These results are not in line with values reported in literature33, where values are stable for 1-year-aged dragline silks but − 35% for 4-year-aged dragline silks. Toughness, on the other hand, showed no significant trend in respect to silk ageing, which is in line with results for 1-year-aged dragline silks reported in literature33.
Overall, despite the individual variability and other ecological factors such as environment and food intake that may influence silk properties30,31,32, we underline a general decay of the silk over time. The degradation process during the monitored period of 1 month results probably from the loss of water as the result of establishing the thermodynamic equilibrium between the water content in the fiber and that of the atmosphere reducing the elastic mobility of silk proteins33,51,52. In addition, the highly mobile and extensible “nanosprings” of the silk protein chains could be degraded by processes other than mere drying, such as an increased cross-linking and reorientation among them, which indeed determines an increased stiffening of the silk structure with a simultaneous decrease of ultimate strain values33.
The potential effects due to individual variability and sampling season were appropriately taken into account by the statistical methods here adopted. In particular, the use of the mixed regression procedure allowed us to include the possible variability at the individual level and provide a more realistic representation of the observed trends. Moreover, in order to avoid bias in the ageing process, all silk samples were stored under controlled laboratory conditions of temperature and humidity and protected from UV light.
An additional source of bias may arise from the effect of the sampling period (“Summer” and “Autumn” populations). Accordingly, the categorical variable Season was introduced in the regression structure as a fixed term (categorical factors made up of less than five levels can not be introduced as random factors53), in order to take into account potential variation induced by the sampling period.
The sampling period explained variation in the data for both ultimate strain and toughness. Although differences between the two sampling periods are statistically significant, such variation could possibly be entirely driven by random variation due to the low number of individuals tested for each period. Tentatively, this trend can also be related to the spider senescence (i.e. its age), owing to the effect of the lower intake of food or, more generally, in relation to the natural decline of metabolic processes at the end of the season. The influence of the spider diet on growth, reproduction, survival and silk production is documented in literature30,31,32,45, showing that when resources are scarce, spiders spin thinner silk threads, being able to sustain lower applied loads although the maximum tensile stress remains unaffected. Given the small population size for the two sampling periods (n = 3), however, we are unable to fully support this hypothesis from a statistical standpoint. Inferences on spider senescence would need additional observations from different reproductive stages, and a higher population size for the two seasons.
Nano-tensile tests on vertical naturally spun silk fibers of the bridge spider Larinioides cornutus revealed changes with silk ageing in some of the mechanical properties here tested (ultimate strain and Young’s modulus).
The potential technological applications of silk threads with properties that change with ageing could be of huge interest in particular for medical applications as such as reabsorbable scaffolds for muscles, nerves, ligaments tissue repairs, as well as absorbable suture materials.
Methods
We collected three adult females of Larinioides cornutus (Araneae, Araneidae) of comparable size in September 2013 (Summer spiders) and in November 2013 (Autumn spiders). Since larger spiders spin proportionately thicker draglines in order to sustain almost two or three times the body weight of the spider itself45, we standardized the thread diameter selecting spiders similar in body size. The dry weight of the autumn spiders ranged from 123.0 to 137.6 mg and from 124.7 to 140.1 mg for the summer ones. Table 1 reports the SEM measurements of diameters of the distal part of tibia and metatarsus of the individuals considered in this study. Spiders were collected at the same site, a small canal on the edge of apple orchards in Costigliole Saluzzo, Province of Cuneo, Italy; Geographical coordinates: 44.566 East, 7.493 North). The sampling site was populated by a very abundant population (approximately 20–30 individuals/m2), weaving their webs almost in contact and in some cases attached to each other.
Table 1. SEM measurements of diameters of the distal part of tibia and metatarsus of the individuals considered in this study.
| Spider ID: | #1 | #2 | #3 | #4 | #5 | #6 |
|---|---|---|---|---|---|---|
| Distal diameter of tibia [mm] | 0.85 | 0.82 | 0.74 | 0.80 | 0.73 | 0.84 |
| Distal diameter of metatarsus [mm] | 0.33 | 0.35 | 0.32 | 0.36 | 0.36 | 0.37 |
Our model species, the bridge spider L. cornutus, is a nocturnal synanthropic species that is very abundant near water and lives in aggregations. Under laboratory conditions, females produce up to 15 cocoons over a lifespan of 1.5 years. All developmental stages are present throughout the year, with adult males abundant mainly in September31.
According to54, L. cornutus is one of very few Central European spiders known to be found in “colonies” in which the orb-webs are in direct contact. Individuals of different generations cooperate at least in web-building (i.e. they share the same framework or irregular “web carpet”).
Spiders were immediately put in plastic boxes and transferred in our lab after collection. All samples were collected from freely climbing spiders, following the purpose of previously published papers in order to obtain stronger silk threads and more reproducible results with less variability2,7,9,47,49,50.
Spider silk samples were collected within 1 hour of the spiders being brought into the laboratory. In order to reduce the possible sources of noise in the analysis2,7,9,47,49,50, the silk was collected using a simple standard method as follows: under controlled laboratory conditions, we forced spiders to fall down vertically from a wooden stick, coherently with previously published papers10,33,45,50 providing the first source of highly reproducibility of experimental results47,55. This collecting method allows spiders to produce stronger silk threads and reflects more closely the natural condition34,45,47,56. Another viable alternative is collecting silk spun horizontally, which was not used in this paper. This is advantageous compared to artificial forced spinning methods, which are influenced by different parameters such as the spinning speed and the anaesthetization of the spider. It is evident that the silk flow is under the active control of the spider, which can calibrate the tensile behavior of the silk by varying the fiber diameter and microstructure10,45. The natural vertically spun silk method adopted here standardizes the spinning of the spider (where the spider falls at a constant speed under its own weight) and it avoids anaesthetizing the spider itself, which has been shown to influence the tensile properties of the spider silk37,39,57. Thus, it can be considered a less invasive method as shown by the more reproducible results, of the vertical naturally spun silk (reported for the spider Argiope trifasciata10,47 or the spider Nephila maculata45) compared to other forced horizontal spinning methods where the spider must be immobilized and forced to spin silk on a collector10,39,55 or to harvesting from webs9. No spider were damaged during the manipulation required for this silking method. The height of spider falls was standardized to 20 cm and the collecting region of the thread was fixed at the mid point of the fall (10 cm) in order to decrease the likelihood that the intrinsic variation of spider speed during each descent could interfere with the reproducibility of results. One fall per collected sample, so each spider fell 3 different times to spin 3 different samples. We successfully collected 3 samples for each Summer spider and 5 for each Autumn spider for each nano-tensile test (3 × 3 + 3 × 5 = 24 samples for each of the ten nanotensile tests). Altogether, we collected 90 samples of Summer silk and 150 samples of Autumn silk, coherently with previously published papers10,38,45 (see Supplementary Information). The analysis was carried out up to 1 month after the spinning. After one month a rapid and dramatic so-called “degradation” occurs, as documented in33. The first tests were performed at Ts0 namely within 20 seconds of spinning. The rest of the samples were left in the laboratory in a closed black box to avoid the exposure to UV radiation under the same controlled ambient conditions adopted for the collecting procedure and nano-tensile tests. Since the ambient conditions such as temperature and humidity during testing may affect the mechanical characteristics of the silk thread10,27, we monitored the variation of the experimental ambient conditions with a portable datalogger (EL-USB-2, Lascar Electronics) (see Supplementary Information).
Nano-tensile testing
Nano-tensile tests were planned at 0.16 (Ts0), 60 (Ts1), 180 (Ts2), 420 (Ts3), 1380 (Ts4), 3300 (Ts5), 7140 (Ts6), 12900 (Ts7), 27300 (Ts8), 43740 (Ts9) minutes after spinning for Summer spiders and at 0.16 (Ta0), 60 (Ta1), 180 (Ta2), 420 (Ta3), 1440 (Ta4), 1800 (Ta5), 5760 (Ta6), 7200 (Ta7), 8600 (Ta8), 37440 (Ta9) for Autumn spiders. Samples were pulled at a constant rate of 1%/s until the silk fibers failed. This strain rate was selected because it is coherent with the parameter setting of many previous studies on spider silk mechanics2,3,8,12,20,33,44,50,56,58, so maximizing the comparability of results.
With the extreme accuracy needed for nano-tensile tests it was necessary to correctly evaluate the elongation of each sample, the computer program (Nanosuite®, Agilent, Santa Clara, USA) automatically recalculated the exact initial gage length (l0, previously fixed at approx. 20 mm) at the beginning of each test when any pre-load is recorded by the software. This avoids any over- or under-estimation which might lead to a uncorrected determination of elongation. The length of the initial collected fiber was ~30 cm. Thus, load-extension data were transformed into stress-strain curves to normalize data across silk samples of different lengths. This procedure guarantees an extreme accuracy of the initial gage length compared to the less precise “in web” length44 or fixed original initial length2,3,33,34,45 method for calculating stress σ , strain ε , the Young’s modulus E and the toughness (see Supplementary Information).
Linear Mixed Models (LMMs)
The data exploration was carried out following59 (see Supplementary Information). Rather than calculating the mean values of the mechanical parameters and their percentage variation as previously reported in literature33, we adopted the LMMs analysis to estimate the significance of the trends versus the two variables, namely to understand if and how the mechanical properties of the silk vary with silk ageing. In order to capture potential non-linear trends in the response of dependent variables, we allowed up to second order polynomial for the independent variable.
Prior to model fitting, the independent variable Time was log transformed to achieve homogeneity. The statistic mixed procedure allowed us to deal with violation of independence derived from the fact that silk samples were collected from the same individual and showed high variability. Rather than to test for its direct effect on the dependent variable (i.e. the different mechanical properties), the “individual” level was included in the analysis as random factor in order to account for the variation it introduced in our samples and thus to correctly estimate the regression coefficient. Moreover, we included in the analysis the sampling season, in order to take into account its possible effect on the silk mechanical properties. Being made up of only two levels, it was not possible to include the latter variable as a random component53.
The resulting structure of our models was:
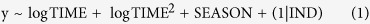 |
where y = one of ultimate stress or strain, Young’s modulus and toughness; log TIME = log transformed time interval from the spinning) (continuous variable, fixed effect), SEASON = Summer or Autumn (categorical variable, fixed effect). The random part of the model (1|IND) includes the effect of the “individual” variable.
Whenever the effect of a variable was not significant, we simplified the model by dropping it from the initial structure. The process was reiterated until a minimum adequate model of significant fixed effects remained. Model validation was carried out following60 (see Supplementary information).
FESEM characterization of the Larinioides cornutus spider silk fibers
The diameter was determined using the FESEM (FEI-InspectTM F50, at 5 kV) micrographs of the silk threads of individual #2 and #5 for Summer and Autumn spiders. Silk threads appears to be composed of parallel multi-fibers of circular cross-sections (at least 2 major ampullate, 2 minor ampullate and abundant aciniform threads), as shown in Fig. 1a–d and confirmed in previously published papers42. We made no attempts to isolate single fibers in order to preserve the silk structure the spider naturally spins. For mechanical characterization, the average diameter of silk samples was measured at five points along the length of the fiber using the SEM. Thus, the cross-sectional area was calculated assuming a circular cross-section so treating the multi-stranded structure as a solid cylinder neglecting the air gaps visible in Fig. 1a–d. This hypothesis was made on the basis of evidence used in two previously published papers with the same scope (for details see3) and due to the experimental limitations in isolating single fibers even if this adds uncertainty to the correct assessment of the bundle cross-sectional areas which then leads to a high standard deviation on stress values derived from them. The mean diameter is of ~7.15 ± 0.07 μm (or 5.21 ± 0.05 μm) for Summer (or Autumn) silks.
Additional Information
How to cite this article: Lepore, E. et al. The effect of ageing on the mechanical properties of the silk of the bridge spider Larinioides cornutus (Clerck, 1757). Sci. Rep. 6, 24699; doi: 10.1038/srep24699 (2016).
Supplementary Material
Acknowledgments
We are grateful to “Nanofacility Piemonte”, INRIM Institute, for the FESEM microscope facility. N.M.P. is supported by the European Research Council (ERC StG Ideas 2011 BIHSNAM n. 279985, ERC PoC 2013 KNOTOUGH n. 632277, ERC PoC 2015 SILKENE nr. 693670), by the European Commission under the Graphene Flagship (WP10 “Nanocomposites”, n. 604391).
Footnotes
Author Contributions N.M.P. conceived the idea and supervised the entire research, E.L. performed silk nano-tensile testing, SEM analyses and analyzed the experimental data, M.I. and S.M. collected the spiders in the field and performed the statistical analysis. All the authors contributed to the writing of the paper.
References
- Vollrath F. Biology of spider silk. Int. J. Biol. Macrmol. 24, 81–88 (1999). [DOI] [PubMed] [Google Scholar]
- Vollrath F., Madsen B. & Shao Z. The effect of spinning conditions on the mechanics of a spider’s dragline silk. Proc. R. Soc. Lond. B 268, 2339–2346 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore A. M. F. & Tran K. Material properties of cobweb silk from the black widow spider Latrodectus hesperus. Int. J. Biol. Macromol. 24, 277–282 (1999). [DOI] [PubMed] [Google Scholar]
- Craig C. L. Spiderwebs and silk: Tracing evolution from molecules to genes to phenotypes (Oxford University Press, New York, 2003). [Google Scholar]
- Sensenig A., Agnarsson I. & Blackledge T. A. Behavioral and biomaterial coevolution in spider orb webs. J. Evolution. Biol. 23, 1839–1856 (2010). [DOI] [PubMed] [Google Scholar]
- Lepore E., Marchioro A., Isaia M., Buehler M. & Pugno N. Evidence of the most stretchable egg sac silk stalk, of the European spider of the year Meta Menardi. Plos one 7, 1–12 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foelix R. F. Biology of Spiders (Oxford University Press, 1996). [Google Scholar]
- Blackledge T. A. & Hayashi C. Y. Silken toolkits: biomechanics of silk fibers spun by the orb web spider Argiope argentata (Fabricius 1775). J. Exp. Biol. 209, 2452–2461 (2006). [DOI] [PubMed] [Google Scholar]
- Perez-Rigueiro J., Elices M., Llorca J. & Viney C. Tensile properties of Argiope trifasciata drag line silk obtained from the spider’s web. J. Appl. Polym. Sci. 82, 2245–2251 (2001). [Google Scholar]
- Elices M., Pérez-Rigueiro J., Plaza G. R. & Guinea G. V. Finding inspiration in Argiope trifasciata spider silk fibers. J. Mineral. Metals. Materials Soc. 57, 60–66 (2005). [Google Scholar]
- Poza P., Perez-Rigueiro J., Elices M. & Llorca J. Fractographic analysis of silkworm and spider silk. Eng. Fract. Mech. 69, 1035–1048 (2002). [Google Scholar]
- Hayashi C. Y., Blackledge T. A. & Lewis R. V. Molecular and mechanical characterization of aciniform silk: uniformity of iterated sequence modules in a novel member of the spider silk fibroin gene family. Mol. Biol. Evol. 21, 1950–1959 (2004). [DOI] [PubMed] [Google Scholar]
- Madsen B., Shao Z. Z. & Vollrath F. Variability in the mechanical properties of spider silks on three levels: interspecific, intraspecific and intraindividual. Int. J. Biol. Macromol. 24, 301–306 (1999). [DOI] [PubMed] [Google Scholar]
- Van Nimmen E., Gellynck K., Gheysens T., Van Langenhove L. & Mertens J. Modelling of the Stress-Strain behaviour of egg sac silk of the spider Araneus diadematus. J. Arachnol. 33, 629–639 (2005). [Google Scholar]
- Gosline J. M., Guerette P. A., Ortlepp C. S. & Savage K. N. The mechanical design of spider silks: from fibroin sequence to mechanical function. J. Exp. Biol. 202, 3295–3303 (1999). [DOI] [PubMed] [Google Scholar]
- Köhler T. & Vollrath F. Thread biomechanics in the two orb weaving spiders Araneus diadematus (Araneae, Araneidae) and Uloborus walckenaerius (Araneae, Uloboridae). J. Exp. Zool. 271, 1–17 (1995). [Google Scholar]
- Römer L. & Scheibel T. The elaborate structure of spider silk. Prion 4, 154–161 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosline J. M., Demont E. M. & Denny M. W. The structure and properties of spider silk. Endeavor 10, 37–44 (1986). [Google Scholar]
- Ortlepp C. & Gosline J. M. The scaling of safety factor in spider draglines. J. Exp. Biol. 211, 2832–2840 (2008). [DOI] [PubMed] [Google Scholar]
- Swanson B. O., Blackledge T. A., Beltran J. & Hayashi C. Y. Variation in the material properties of spider dragline silk across species. Appl. Phys. A-Mater. 82, 213–218 (2006). [Google Scholar]
- Zhao A. C. et al. Novel molecular and mechanical properties of egg case silk from wasp spider, Argiope bruennichi. Biochemistry 45, 3348–3356 (2006). [DOI] [PubMed] [Google Scholar]
- Stauffer S. L., Coguill S. L. & Lewis R. V. Comparison of physical properties of three silks from Nephila clavipes and Araneus gemmoides. J. Arachnol. 22, 5–11 (1994). [Google Scholar]
- Denny M. The physical properties of spider’s silk and their role in the design of orb-webs. J. Exp. Biol. 65, 483–506 (1976). [Google Scholar]
- Dunaway D. L., Thiel B. L. & Viney C. Tensile mechanical property evaluation of natural and epoxide-treated silk fibers. J. Appl. Polym. Sci. 58, 675–683 (1995). [Google Scholar]
- Cunniff P. M. et al. Mechanical and thermal properties of dragline silk from the spider Nephila clavipes. Polym. Advan. Technol. 5, 401–410 (1994). [Google Scholar]
- Agnarsson I., Kuntner M. & Blackledge T. A. Bioprospecting finds the toughest biological material: extraordinary silk from a giant riverine orb spider. Plos one 5, 1–8 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agnarsson I., Dhinojwala A., Sahni V. & Blackledge T. A. Spider silk as a novel high performance biomimetic muscle driven by humidity. J. Exp. Biol. 212, 1990–1994 (2009). [DOI] [PubMed] [Google Scholar]
- Brown C. P. et al. The critical role of water in spider silk and its consequence for protein mechanics. Nanoscale 3, 3805 (2011). [DOI] [PubMed] [Google Scholar]
- Liu Y., Shao Z. & Vollrath F. Relationships between supercontraction and mechanical properties of spider silk. Nat. mater. 12, 901–905 (2005). [DOI] [PubMed] [Google Scholar]
- Kleinteich A., Wilder S. M. & Schneider J. M. Contributions of juvenile and adult diet to the lifetime reproductive success and lifespan of a spider. Oikos 124, 130–138 (2015). [Google Scholar]
- Kleinteich A. & Schneider J. M. Developmental strategies in an invasive spider: constraints and plasticity. Ecol. Entomol. 36, 82–93 (2011). [Google Scholar]
- Kleinteich A. & Schneider J. M. Evidence for Rensch’s rule in an orb-web spider with moderate sexual size dimorphism. Evol. Ecol. Res. 12, 667–683 (2010). [Google Scholar]
- Agnarsson I., Boutry C. & Blackledge T. A. Spider silk aging: initial improvement in a high performance material followed by slow degradation. J. Exp. Zool. 309, 494–504 (2008). [DOI] [PubMed] [Google Scholar]
- Blackledge T. A. et al. How super is supercontraction? Persistent versus cyclic responses to humidity in spider dragline silk. J. Exp. Biol. 212, 1981–1989 (2009). [DOI] [PubMed] [Google Scholar]
- Boutry C., Řezáč M. & Blackledge T. A. Plasticity in major ampullate silk production in relation to spider phylogeny and ecology. Plos one 6, 1–8 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim M., Keerl D. & Scheibel T. Spider silk: from soluble protein to extraordinary fiber. Angew. Chem. Int. Ed. 48, 3584–3596 (2009). [DOI] [PubMed] [Google Scholar]
- Pérez-Rigueiro J., Elices M., Plaza G., Real J. I. & Guinea G. V. The effect of spinning forces on spider silk properties. J. Exp. Biol. 208, 2633–2639 (2005). [DOI] [PubMed] [Google Scholar]
- Guinea G. V., Elices M., Perez-Rigueiro J. & Plaza G. Self-tightening of spider silk fibers induced by moisture. Polymer 44, 5785–5788 (2003). [Google Scholar]
- Work R. W. A comparative study of the supercontraction of major ampullate silk fibers of orb-web-building spiders (Araneae). J. Arachnol. 9, 299–308 (1981). [Google Scholar]
- Van Nimmen E., Gellynck K. & Van Langenhove L. The tensile behaviour of spider silk. Autex Research Journal 5, 120–126 (2005). [Google Scholar]
- Madsen B., Shao Z. Z. & Vollrath F. Variability in the mechanical properties of spider silks on three levels: interspecific, intraspecific and intraindividual. Int. J. Biol. Macromol. 24, 301–306 (1999). [DOI] [PubMed] [Google Scholar]
- Silva L. P. & Rech E. L. Unravelling the biodiversity of nanoscale signatures of spider silk fibres. Nat. commun. 4, 3014 (2013). [DOI] [PubMed] [Google Scholar]
- Griffiths J. R. & Salanitri V. R. The strength of spider silk. J. Mater. Sci. 15, 491–496 (1980). [Google Scholar]
- Swanson B. O., Blackledge T. A. & Hayashi C. Y. Spider capture silk: performance implications of variation in an exceptional biomaterial. J. Exp. Zool. 307, 654–666 (2007). [DOI] [PubMed] [Google Scholar]
- Heiling A. M. & Herberstein M. E. Activity patterns in different developmental stages and sexes of Larinioides sclopetarius (Clerck) (Araneae, Araneidae). Selden P. A. (ed.). Proceedings of the 17th European Colloquium of Arachnology, Edinburgh (1998). [Google Scholar]
- Wolff J. O., Schneider J. M. & Gorb S. N. How to pass the gap – functional morphology and biomechanics of spider bridging threads. Biotechnology of Silk 5, 165–177 (2014). [Google Scholar]
- Garrido M. A., Elices M., Viney C. & Perez-Rigueiro J. Active control of spider silk strength: comparison of drag line spun on vertical and horizontal surfaces. Polymer 43, 1537–1540 (2002). [Google Scholar]
- Lu Q. et al. Degradation mechanism and control of silk fibroin. Biomacromolecules 4, 1080–1086 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemlin L. C. A study of the mechanical behaviour of spider silk. Cloting and organic materials laboratory, US Army Natick Laboratories (1968). [Google Scholar]
- Blackledge T. A., Swindeman J. E. & Hayashi C. Y. Quasistatic and continuous dynamic characterization of the mechanical properties of silk from the cobweb of the black widow spider Latrodectus Hesperus. J. Exp. Biol. 208, 1937–1949 (2005). [DOI] [PubMed] [Google Scholar]
- Hayashi C. Y. & Lewis R. V. Evidence from flagelliform silk cDNA for the structural basis of elasticity and modular nature of spider silks. J. Mol. Biol. 275, 773–784 (1998). [DOI] [PubMed] [Google Scholar]
- Becker N. et al. Molecular nanosprings in spider capture-silk threads. Nat. Mater. 2, 278–283 (2003). [DOI] [PubMed] [Google Scholar]
- Gelman A. & Hill J. Data analysis using regression and multi level/hierarchical models. New York: Cambridge University Press (2007). [Google Scholar]
- Marcus S. Larinioides sclopetarius, eine parasoziale Spinne Mitteleuropas? Arachnologische Mitteilungen 27/28, 5 (2004). [Google Scholar]
- Guinea G. V., Elices M., Real J. I., Gutierrez S. & Perez-Rigueiro J. Reproducibility of the tensile properties of spider (Argiope trifasciata) silk obtained by forced silking. J. Exp. Zool. 303, 37–44 (2005). [DOI] [PubMed] [Google Scholar]
- Boutry C. & Blackledge T. A. Biomechanical variation of silk links spinning plasticity to spider web function. Zoology 112, 451–460 (2009). [DOI] [PubMed] [Google Scholar]
- Madsen B. & Vollrath F. Mechanics and morphology of silk drawn from anesthetized spiders. Naturwissenschaften 87, 148–153 (2000). [DOI] [PubMed] [Google Scholar]
- Lawrence B. A., Vierra C. A. & Moore A. M. F. Molecular and mechanical properties of major ampullate silk of the black widow spider, Latrodectus hesperus. Biomacromolecules 5, 689–695 (2004). [DOI] [PubMed] [Google Scholar]
- Zuur A. F., Ieno E. N. & Elphick S. C. A protocol for data exploration to avoid common statistical problem. Methods in Ecology and Evolution 1, 3–14 (2010). [Google Scholar]
- Zuur A. F., Ieno E. N., Walker N. J., Saveliev A. A. & Smith G. M. Mixed effect models and extensions in ecology with R (Springer, New York, 2009). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.