Abstract
IMPORTANCE
The patient-centered medical home (PCMH) model of primary care is being implemented in a wide variety of socioeconomic contexts, yet there has been little research on whether its effects differ by context. Clinical preventive service use, including cancer screening, is an important outcome to assess the effectiveness of the PCMH within and across socioeconomic contexts.
OBJECTIVE
To determine whether the relationship between the PCMH and cancer screening is conditional on the socioeconomic context in which a primary care physician practice operates.
DESIGN, SETTING, AND PARTICIPANTS
A longitudinal study spanning July 1, 2009, through June 30, 2012, using data from the Blue Cross Blue Shield of Michigan Physician Group Incentive Program was conducted. Michigan nonpediatric primary care physician practices that participated in the Physician Group Incentive Program (5452 practice-years) were included. Sample size and outlier exclusion criteria were applied to each outcome. We examined the interaction between practices’ PCMH implementation scores and their socioeconomic context. The implementation of a PCMH was self-reported by the practice’s affiliated physician organizations and was measured as a continuous score ranging from 0 to 1. Socioeconomic context was calculated using a market-based approach based on zip code characteristics of the practice’s patients and by combining multiple measures using principal components analysis.
MAIN OUTCOMES AND MEASURES
Breast, cervical, and colorectal cancer screening rates for practices’ Blue Cross Blue Shield of Michigan patients.
RESULTS
The implementation of a PCMH was associated with higher breast, cervical, and colorectal cancer screening rates across most market socioeconomic contexts. In multivariable models, the PCMH was associated with a higher rate of screening for breast cancer (5.4%; 95% CI, 1.5% to 9.3%), cervical cancer (4.2%; 95% CI, 1.4% to 6.9%), and colorectal cancer (7.0%; 95% CI, 3.6% to 10.5%) in the lowest socioeconomic group but nonsignificant differences in screening for breast cancer (2.6%; 95% CI, −0.1% to 5.3%) and cervical cancer (−0.5%; 95% CI, −2.7% to 1.7%) and a higher rate of colorectal cancer (4.5%; 95% CI, 1.8% to 7.3%) screening in the highest socioeconomic group. Because PCMH implementation was associated with larger increases in screening in lower socioeconomic practice settings, models suggest reduced disparities in screening rates across these contexts. For example, the model-predicted disparity in breast cancer screening rates between the highest and lowest socioeconomic contexts was 6% (77.9% vs 72.2%) among practices with no PCMH implementation and 3% (80.3% vs. 77.0%) among practices with full PCMH implementation.
CONCLUSIONS AND RELEVANCE
In our study, the PCMH model was associated with improved cancer screening rates across contexts but may be especially relevant for practices in lower socioeconomic areas.
The patient-centered medical home (PCMH) model of primary care has become a focus for innovation in the US health care system, with endorsements by the major primary care physician (PCP) societies1 and support in the Patient Protection and Affordable Care Act of 2010.2 As a holistic, patient-centered, team-based model of care, the PCMH promotes access, coordination, comprehensiveness, quality, and safety.3 This model emphasizes the core primary care function of providing clinical preventive services4 and a comprehensive approach to care over a patient’s life course rather than focusing on episodic treatment, a specific medical issue, or a particular body system.5 Increased clinical preventive service use is considered a key indicator for evaluating the success of the PCMH,6,7 and early evaluations8-10 have shown consistent evidence of a positive association.
The PCMH model calls for practices to work within systems of care that are not restricted by organizational boundaries of the primary care practice but are coordinated across all elements of the health care system, patients’ day-to-day lives, and their communities.5 Because patient centeredness implies that patients are active partners in health care decision making,5 these contextual features may be especially relevant when evaluating the effect of the PCMH on clinical preventive services given persistent socioeconomic disparities in their use.11-22 Some23,24 have argued that the PCMH model may be especially effective in lower socioeconomic status (SES) areas by overcoming some of the challenges that drive disparities by improving access to care, care coordination, and health literacy.
Implementation of the PCMH model is occurring in a wide variety of practice settings,25-28 including areas with a predominance of low SES patients29,30; however, to our knowledge, no research to date has investigated whether the effects of PCMHs on preventive services differ by the SES contexts in which they were implemented.8 The SES context is typically defined based on residential neighborhoods or other geographic boundaries that have economic, educational, social, cultural, and political characteristics.31,32 Our study investigated (1) whether the relationship between the PCMH and cancer screening, an important type of clinical preventive service, is conditional on the socioeconomic context in which a physician practice operates and (2) whether the PCMH provides a boosting effect in lower socioeconomic contexts.
Cancer prevention services were chosen as the focus of our investigation.33,34 Several types of routine cancer screening tests are included in the Healthcare Effectiveness Data and Information (HEDIS) data set and are recommended by the US Preventive Services Task Force.35,36 This data set includes cervical cancer screening for women 21 years or older, breast cancer screening for women 50 years or older, and colorectal cancer screening for all adults 50 years or older. Results from studies that consider community socioeconomic context or area-based measures of SES are mixed, although several studies22,37,38 have reported significant positive associations between the odds of cancer screening and area SES.
Methods
Study Design
This dynamic cohort study included a total of 2218 Michigan primary care practices that participated in the Blue Cross Blue Shield of Michigan (BCBSM) Physician Group Incentive Program (PGIP). The PGIP is a voluntary incentive and payment reform program designed to support physician organizations and their affiliated practices to achieve care transformation and value-based care delivery.39 Physician organizations participating in the PGIP report their affiliated practices semiannually to BCBSM. Using these data, we included practices that participated for at least 1 full year between July 1, 2009, and June 30, 2012, measured using the June reports, for a total of 5452 observed practice-years. We excluded practices in which specialists accounted for the majority of the physicians (n = 41), pediatric practices (n = 329), and practices with missing data on PCMH capability implementation or other predictor variables (n = 127). This study was approved by the University of Michigan Institutional Review Board and determined to be exempt for the purpose of program evaluation.
PCMH Implementation
The PCMH model within the PGIP was created collaboratively with physician organization leaders based on the Joint Principles of the Patient-Centered Medical Home5 and the chronic care model40 and defined specific capabilities within 13 domains of PCMH functioning. The PGIP program supports PCMH implementation by providing physician organizations with financial incentives when their member practices initiate PCMH capabilities. Physician organizations identified PCMH capabilities begun in all affiliated practices. To validate these reports, BCBSM conducted site visits and, in 2012, confirmed that 95% of self-reported capabilities were in place at 323 randomly selected practices. Using 114 capabilities that were defined consistently from June 2009 through June 2012 (eTable 1 in the Supplement), we calculated practice-level PCMH implementation scores. Giving each domain an equal weight, we calculated continuous PCMH implementation scores ranging from 0 (no implementation) to 1 (full implementation); this calculation process is described in more detail elsewhere.41
We divided the study period into 3 study years: July 1, 2009, through June 30, 2010; July 1, 2010, through June 30, 2011; and July 1, 2011, through June 30, 2012. The PCMH implementation scores at the beginning of these study years were calculated using capabilities reported in the preceding June. In addition, the change in PCMH implementation scores between consecutive June reporting periods was used to measure the incremental implementation during the study year.
Socioeconomic Context
We defined a medical practice’s socioeconomic context as the geographic environments in which its patients reside. We operationalized this market-based approach by calculating zip code characteristics for each practice, weighted by the proportion of the practice’s professional services provided to BCBSM members residing within that zip code. We identified 8 standard measures of socioeconomic position that were available at a zip code level and are relevant to population health31: (1) percentage of individuals with income below the poverty level, (2) median household income, (3) percentage of adults 25 years or older with less than a high school education, (4) percentage of individuals unemployed, (5) percentage of households with affordable housing (paying <30% of their income to housing), (6) percentage of families with single parents, (7) percentage of households with public assistance income, and (8) a foreclosure risk score. Measures 1 through 7 were identified using the 2011 American Community Survey.42 The foreclosure risk score was obtained from the Local Initiatives Support Corporation43 and combined measures of subprime lending, foreclosures, delinquency, and vacancy rates to assign a score relative to the neediest jurisdiction in the state, with a score of 100 representing the neediest jurisdiction and 0 the least needy. We calculated a weighted mean of these zip code characteristics for each practice using the locations of their patients and then used principal components analysis to combine these 8 measures into a single practice-level index of SES context. We assumed stability in zip code characteristics during the study period, but we calculated the practice’s scores separately for each study year to account for shifts in their patient populations over time. Thus, a practice could change SES categories during the study period. The SES index was standardized to have a mean (SD) of O (1).
Outcomes
The outcomes for this study were practice-level, age-appropriate breast, cervical, and colorectal cancer screening rates, defined using HEDIS.44-46 Breast cancer screening was measured as the proportion of women aged 52 to 64 years who received a mammogram during the study year or the previous year. The lower age limit was modified from the HEDIS specifications to reflect the updated US Preventive Services Task Force recommendations.47 All upper age limits were 64 years because the BCBSM cohort of commercial members 65 years or older is small and the findings are likely not generalizable to the older population. Cervical cancer screening was based on the proportion of women aged 24 to 64 years who were evaluated in the previous 3 years. Colorectal cancer screening was measured as the proportion of patients aged 51 to 64 years who received a fecal occult blood test during the study year or a flexible sigmoidoscopy or colonoscopy in the previous 4 years and 9 months. The period used to identify colorectal screening was shortened from HEDIS specifications owing to limited historical claims data, but exclusion and continuous enrollment criteria were consistent with those of HEDIS.
Screening rates were calculated using the practice panels of adult BCBSM members, identified through a retrospective claims-based algorithm that assigns members to a single PCP based on administrative claims from the previous 24 months (eMethods in the Supplement). Outcomes were measured separately for each study year using administrative claims.
Covariates
We controlled for characteristics of the practice, its patient population, its physician organization, and other geographic characteristics. Practice characteristics included (1) the number of physicians, (2) whether the practice contained nonprimary care specialists, (3) BCBSM patient volume, (4) mean number of years during which the practice’s PCPs participated in the PGIP, (5) physician turnover in the practice over time, and (6) whether the practice moved between physician organizations over time. Patient characteristics included (1) the proportion of adults who were female and (2) the mean prospective risk score (determined with OptumInsight Symmetry, version 8; OptumInsight). We operationalized physician organization size as the number of practices in the physician organization with PCPs. We also controlled for the following geographic characteristics, calculated using the market-based approach of weighting zip code characteristics based on the residences of the practices’ patients: (1) BCBSM market share, (2) percentage of residents living in a rural area, (3) number of PCPs per 1000 residents, and (4) percentage of nonwhite or Hispanic residents. Sensitivity analyses demonstrated that models excluding the race/ethnicity variable did not change the results.
Statistical Analysis
The physician practice was the unit of analysis. To test the conditional effects of SES context, we analyzed the interaction between the level of PCMH implementation at the beginning of each study year and the market-based SES index for each practice in predicting the 3 practice-level cancer screening rates. We used multivariable, cross-classified linear models, with a random effect for the practice and a cumulative random effect for the physician organization. These random effects accounted for the longitudinal design, clustering of practices within physician organizations, and movement of practices between physician organizations over time.48
The market-based SES context index was stratified into 4 categories based on the number of SDs from the mean to account for potential nonlinearity in relationships. This approach captured the tail of the distribution while retaining sufficient sample sizes in each category. The groups were (1) greater than 1 SD above the mean (the highest SES category), (2) greater than the mean to 1 SD above, (3) the mean to 1 SD below, and (4) greater than 1 SD below the mean (the lowest SES category). We report estimates and 95% CIs of the effect of PCMH implementation scores on cancer screening for each of these SES index categories and the P value of the interaction term comparing each SES category with the highest SES category as the reference group. We also calculated marginal estimates for the mean of each cancer screening rate at different combinations of the PCMH score and SES index category. These marginal means are predictions from the model after controlling for all other covariates.
Both sample size and statistical outlier exclusion criteria were applied to each cancer screening outcome before constructing multivariable models. To be included in each model, practice-years had to have a minimum of 30 patients eligible for cancer screening (ie, meeting the HEDIS denominator criteria) and could not have a score that exceeded 2 interquartile range units from the median. Using residual diagnostics and models with categorized PCMH implementation scores, we found no departures from linear regression model assumptions including normality, homoscedasticity, and linearity. All analyses were performed using SAS, version 9.2 (SAS Institute Inc).
Results
Across the 3-year study period, the median practice-level cancer screening rates were 75.0% for breast cancer, 75.0% for cervical cancer, and 50.0% for colorectal cancer. Screening rates for breast and cervical cancer decreased slightly during the study period from 76.1% in July 2009 to June 2010 to 74.6% in July 2011 to June 2012 for breast cancer and from 76.9% to 73.7% for cervical cancer; colorectal cancer screening rates remained constant from 50.3% in July 2009 to June 2010 to 50.0% in July 2011 to June 2012. Practices in higher market-based SES index categories had higher cancer screening rates (Table 1). The PCMH implementation scores increased from a median of 0.17 in June 2009 to 0.45 in June 2012 for a median increase of 0.07 per year. Table 2 reports the distribution of variables included in the SES index for each of the 4 SES index categories.
Table 1. Characteristics of Physician Group Incentive Program Primary Care Practices by Socioeconomic Environment, July 2009-June 2012.
| Characteristic | SES Index (Practice-years), Median (IQR)a | |||
|---|---|---|---|---|
| Category 1 (n = 602) |
Category 2 (n = 2752) |
Category 3 (n = 1448) |
Category 4 (n = 650) |
|
| Outcomes | ||||
| Cancer screening rate, % | ||||
| Breast | 78.2 (71.4-83.4) | 75.9 (67.4-82.5) | 73.5 (62.1-82.1) | 68.8 (54.2-78.4) |
| Cervical | 80.6 (75.0-85.2) | 76.5 (70.3-81.8) | 71.8 (63.8-79.1) | 67.9 (58.1-76.5) |
| Colorectal | 55.7 (48.2-62.3) | 51.4 (44.0-58.2) | 47.2 (38.9-56.1) | 46.2 (33.8-58.8) |
| Continuous Variables | ||||
| PCMH score at beginning of study year | 0.31 (0.13-0.56) | 0.32 (0.15-0.54) | 0.27 (0.12-0.52) | 0.22 (0.08-0.43) |
| Change in PCMH score during study year | 0.09 (0.01-0.21) | 0.08 (0-0.20) | 0.05 (0-0.17) | 0.05 (0-0.19) |
| Professional services per PCP in practice | 1931 (1199-3286) | 1714 (1046-2666) | 1286 (754-2072) | 670 (335-1195) |
| Mean No. of years in PGIP for PCPs | 3.00 (2.50-3.50) | 2.75 (2.25-3.50) | 2.50 (2.00-3.50) | 2.75 (2.50-3.50) |
| Turnover of physicians during study year | 0 | 0 | 0 | 0 |
| Total practices in PO with a PCP | 111 (58-177) | 90 (56-499) | 124 (59-535) | 99 (59-140) |
| BCBSM market share, % | 38.2 (35.5-39.9) | 33.5 (30.2-36.4) | 29.5 (26.0-32.9) | 21.7 (18.3-25.0) |
| Nonwhite or Hispanic residents, % | 16.1 (10.4-20.0) | 15.7 (9.0-21.7) | 20.8 (10.6-28.2) | 56.8 (40.8-73.6) |
| % Rural | 8.1 (4.1-24.3) | 23.6 (4.9-48.0) | 24.1 (2.9-57.8) | 0.8 (0.2-4.5) |
| No. of PCPs per 1000 residents | 0.89 (0.60-1.09) | 0.76 (0.60-0.95) | 0.74 (0.59-0.92) | 0.83 (0.63-1.09) |
| Female-attributed members, % | 49.8 (44.1-58.1) | 50.7 (45.9-57.2) | 50.9 (46.3-57.0) | 53.1 (47.1-61.5) |
| Mean prospective risk score (adult) | 1.62 (1.44-1.83) | 1.70 (1.51-1.92) | 1.81 (1.59-2.12) | 1.97 (1.68-2.38) |
| Categorical Variables | ||||
| Study year, No. (%) | ||||
| July 2009-June 2010 | 167 (27.7) | 833 (30.3) | 433 (29.9) | 202 (31.1) |
| July 2010-June 2011 | 211 (35.0) | 930 (33.8) | 486 (33.6) | 215 (33.1) |
| July 2011-June 2012 | 224 (37.2) | 989 (35.9) | 529 (36.5) | 233 (35.8) |
| Practice size, No. (%) | ||||
| Solo physician practice | 302 (50.2) | 1520 (55.2) | 897 (61.9) | 440 (67.7) |
| 2-3 Physicians | 179 (29.7) | 666 (24.2) | 332 (22.9) | 140 (21.5) |
| 4-5 Physicians | 63 (10.5) | 278 (10.1) | 117 (8.1) | 20 (3.1) |
| ≥6 Physicians | 58 (9.6) | 288 (10.5) | 102 (7.0) | 50 (7.7) |
| Practice specialty | ||||
| Primary care | 586 (97.3) | 2642 (96.0) | 1390 (96.0) | 622 (95.7) |
| Multispecialty | 16 (2.7) | 110 (4.0) | 58 (4.0) | 28 (4.3) |
| Practice changed POs during time period, No. (%) |
||||
| No | 562 (93.4) | 2500 (90.8) | 1345 (92.9) | 586 (90.2) |
| Yes | 40 (6.6) | 252 (9.2) | 103 (7.1) | 64 (9.8) |
Abbreviations: BCBSM, Blue Cross Blue Shield of Michigan; IQR, interquartile range; PCMH, patient-centered medical home; PCP, primary care physician; PGIP, physician group incentive program; PO, physician organization; SES, socioeconomic status.
The SES index was calculated using principal components analysis and was standardized to have a mean (SD) of O (1). Category 1 was the highest level; category 4, the lowest level.
Table 2. Categorization of Eligible Primary Care Practices by Socioeconomic Environment.
| Characteristic | SES Index (Practice-years), Median (Range) | |||
|---|---|---|---|---|
| Category 1 (n = 602) |
Category 2 (n = 2752) |
Category 3 (n = 1448) |
Category 4 (n = 650) |
|
| SES indexa | 1.21 (1.00 to 1.83) | 0.42 (0 to 1.00) | −0.34 (−1.00 to 0) | −1.93 (−4.23 to −1.00) |
| Variables in SES index | ||||
| Below poverty, % | 7.7 (5.2 to 12.6) | 11.9 (7.5 to 22.0) | 16.4 (8.9 to 32.1) | 24.8 (17.6 to 44.8) |
| Median income, $ | 73 379 (56 903 to 96 484) | 54 208 (33 148 to 84 032) | 46 098 (34 616 to 66 941) | 39 831 (23 070 to 54 958) |
| Less than HS education, % | 6.9 (4.2 to 9.7) | 10.0 (5.4 to 16.7) | 13.0 (8.2 to 20.8) | 16.9 (11.0 to 49.0) |
| Unemployed, % | 9.2 (6.2 to 11.5) | 11.0 (6.4 to 16.5) | 13.8 (8.2 to 18.7) | 18.9 (10.4 to 29.2) |
| Affordable housing, % | 62.7 (59.0 to 67.2) | 60.6 (53.1 to 72.4) | 57.2 (47.9 to 65.1) | 47.7 (36.2 to 56.5) |
| Foreclosure risk score | 1.36 (0.3 to 5.7) | 2.34 (0.1 to 16.9) | 4.94 (0.1 to 25.2) | 24.09 (0.5 to 73.3) |
| Public assistance, % | 1.9 (1.1 to 3.1) | 2.8 (1.0 to 5.6) | 3.8 (1.8 to 8.0) | 6.3 (3.8 to 11.8) |
| Single parents, % | 21.6 (14.9 to 28.9) | 28.7 (16.8 to 43.2) | 35.7 (22.4 to 48.8) | 50.0 (18.9 to 79.8) |
Abbreviations: HS, high school; SES, socioeconomic status.
The SES index was calculated using principal components analysis and was standardized to have a mean (SD) of O (1). Category 1 was the highest level; category 4, the lowest level.
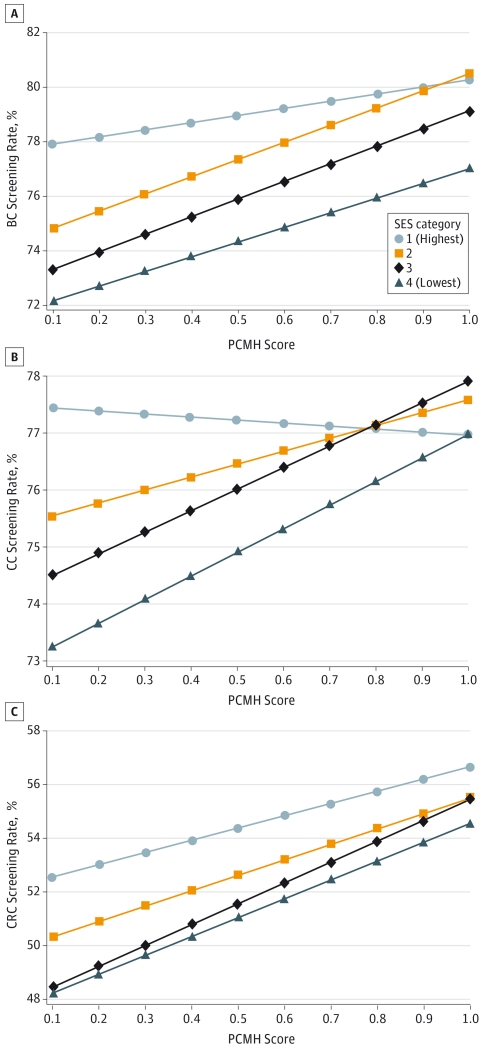
In multivariable analysis, practices in the highest market-based SES index category demonstrated a nonsignificant increase of 2.6% in breast cancer screening rates for an increase in the PCMH implementation score at the beginning of each study year from 0 (no implementation) to 1 (full implementation). The effect estimate in the lowest SES category (5.4%) was larger but not statistically significantly different from the effect in the highest SES category (P = .21), and the effect estimates in the middle categories were significantly larger than the effect in the highest SES category (P = .005 and P = .01), assessed using interaction terms (Table 3). A graphical representation of this relationship is demonstrated (Figure, A) using marginal estimates of the mean breast cancer screening rate at different combinations of PCMH score and SES category. For all SES categories, the model predicted an increase in breast cancer screening as PCMH scores increased, but the slope of the line was less for the highest SES category, illustrating the smaller predicted effect. In the Figure, the difference in the mean breast cancer screening rates between the highest and lowest SES categories is 6 percentage points at the lowest PCMH scores (77.9% vs 72.2%) and 3 percentage points at the highest PCMH scores (80.3% vs 77.0%). For cervical cancer screening, practices in the highest SES category revealed a small, nonsignificant (P = .64) decrease in screening rates (−0.5%) with an increase in the PCMH score from 0 to 1. All other SES categories had a statistically significant positive effect in conjunction with a monotonic relationship between lower SES categories and larger effect estimates (Table 3). At full PCMH implementation, the highest and lowest SES categories are predicted to have the same cervical cancer screening rates (77.0%) despite having a 4% absolute disparity in screening rates at the lowest PCMH score (77.4% vs 73.3%) (Figure, B).
Table 3. Adjusted Effects of PCMH Implementation on Cancer Screening by Practice Socioeconomic Environmenta.
| SES Index Categoryb | Cancer Screening (Practice-years) |
|||||
|---|---|---|---|---|---|---|
| Breast (n = 367) |
Cervical (n = 4406) |
Colorectal (n = 4630) |
||||
| Effect of PCMH Score, β Estimate (95% CI)c |
P Valued | Effect of PCMH Score, β Estimate (95% CI)c |
P Valued | Effect of PCMH Score, β Estimate (95% CI)c |
P Valued | |
| 1 (Highest) | 2.6 (−0.1 to 5.3) | 1 [Reference] | −0.5 (−2.7 to 1.7) | 1 [Reference] | 4.5 (1.8 to 7.3) | 1 [Reference] |
|
| ||||||
| 2 | 6.3 (4.5 to 8.1) | .005 | 2.3 (0.9 to 3.6) | .01 | 5.7 (4.0 to 7.5) | .38 |
|
| ||||||
| 3 | 6.5 (4.2 to 8.7) | .01 | 3.8 (2.1 to 5.5) | <.001 | 7.7 (5.6 to 9.9) | .04 |
|
| ||||||
| 4 (Lowest) | 5.4 (1.5 to 9.3) | .21 | 4.2 (1.4 to 6.9) | .004 | 7.0 (3.6 to 10.5) | .23 |
Abbreviations: PCMH, patient-centered medical home; SES, socioeconomic status.
Covariates included the following: (1) practice characteristics (mean prospective risk score for attributed pediatric patients, percentage of attributed female patients, paid services per primary care provider [PCP], mean number of years that the physicians in the practice have participated in the Physician Group Incentive Program, the percentage of PCPs in the practice who left during the time period, practice size based on total physicians in the practice, practice as primary care only or mixed primary and specialty care, whether the practice was pediatric, and whether the practice changed physician organizations (POs) during the time period); (2) practice environment characteristics (total primary care practices in the PO, PCPs per 1000 population, Blue Cross Blue Shield of Michigan market share, percentage of nonwhite residents, percentage of residents who lived in a rural area, and SES index); and (3) the study year.
The SES index was calculated using principal components analysis using the following variables: (1) percentage below poverty level, (2) median income, (3) percentage with less than a high school education, (4) percentage unemployed, (5) percentage with affordable housing, (6) foreclosure risk score, (7) percentage receiving public assistance, and (8) percentage of families with single parents.
Estimates of the main effect of the PCMH score at the beginning of each study year on cancer screening outcomes specific to each SES index category.
Interaction effect of the PCMH score and SES index for each outcome, using the highest category as the reference group.
Figure. Mean Marginal Estimates of Mean Cancer Screening Rates From Multivariable Mixed Models.
The interaction between practice socioeconomic environment and patient-centered medical home (PCMH) implementation. A, Breast cancer (BC) screening. B, Cervical cancer (CC) screening. C, Colorectal cancer (CRC) screening. SES indicates socioeconomic status.
The effect of the PCMH score on colorectal cancer screening was positive and statistically significant for all SES categories. Similar to breast cancer screening, lower SES categories generally had larger effect estimates, but the effect was not monotonic. Only the effect estimate for the third SES category was statistically significantly different from the highest SES category (P = .04). A similar larger disparity in screening rates was demonstrated at the lowest PCMH scores (52.6% vs 48.2%; 4 percentage points between the highest and lowest SES categories) than at the highest PCMH scores (56.6% vs 54.6%; 2 percentage points between the highest and lowest SES categories) (Figure, C). Full multivariable model results for each screening type are reported in eTables 2 through 4 in the Supplement.
Discussion
These research results suggest that increased implementation of a PCMH model has a greater potential to increase cancer screening in physician practices operating within lower SES contexts. Our multivariable model spredicted that disparities across SES contexts in medical practice screening rates could be halved or, in the case of cervical cancer, eliminated as a result of full implementation of the PCMH model. The observed greater increases in cancer screening rates in lower SES practice contexts may indicate that individuals in these environments benefit more from the PCMH model for this type of preventive service. The greater changes in the lower SES contexts could also occur because there is less room for improvement in cancer screening rates in higher SES environments. Although the observed reduction in disparities attributable to the PCMH implementation score (2%-4%) was modest, this reduction could translate into important gains in early detection at the population level. In addition, this modest decrease in cancer screening disparities should be considered along with the other potential benefits of implementing the PCMH model.
In almost all SES context categories, we found a significant effect of PCMH implementation on improved cancer screening outcomes. This finding is consistent with literature8-10 describing evidence of a positive association between the PCMH model and preventive service use, including cancer screening rates. In contrast to many previous studies,8-10 we quantified progression toward the PCMH model of care through implementation of relevant capabilities as opposed to measuring the dichotomous effect of a PCMH intervention. In addition, many of the previous studies were cross-sectional while ours was strengthened by 3 years of longitudinal data.
We did not observe significant associations between PCMH implementation and improved screening rates for breast and cervical cancer in practices in the highest SES contexts. Breast and cervical cancer screening rates in the United States are relatively high, but colorectal cancer screening rates have lagged.49,50 It is possible that in higher SES contexts, physicians have reached a ceiling effect whereby it is much more difficult to improve breast and cervical cancer screening rates. It is also possible that patients and physicians in higher SES practice contexts are more aware of changes in guidelines that recommend less frequent screening for breast and cervical cancer. However, the US Preventive Services Task Force guidelines36 for cervical cancer screening were not released until March 2012, which was past the data collection period for the present study. In addition, mammography guideline changes and controversy have only addressed the 40- to 49-year-old age group.51 Cancer screening rates in any practice will never reach 100% owing to contraindications not observable in available data.
The observed significant effects of the PCMH on cancer screening rates could be explained by the general shift in focus of physicians implementing the PCMH model toward more preventive and comprehensive care. This shift is especially important considering that a physician’s recommendation is one of the greatest predictors of screening.52,53 The observed impact could also reflect specific capabilities included in the BCBSM PCMH implementation approach, such as the focus on coordination of care across the health system including community resources, increased access to the practice, reminder systems for needed services, and performance reports tracking preventive service use, all of which may be relevant for increasing preventive service use.33,54-62 This model of care may be especially relevant in lower SES contexts where there are greater patient- and system-level barriers to screening. The idea that health care interventions can have a greater effect on lower SES populations is consistent with findings from Rothman et al,63 who demonstrated that patients with low literacy derived more benefit from a diabetes mellitus disease management program than did patients with higher literacy. Health care interventions in the racial disparities literature64-66 have been mixed, with some interventions increasing and others reducing disparities, suggesting that the specific characteristics of the interventions, including the amount of cultural competency, may be important for disparity reduction. Our study did not try to determine the specific components of the model that are related to improving cancer screening rates since we considered that many of these processes are interrelated within the PCMH model.
There are several limitations in our research approach. Our study was set in the context of the PGIP, which provided financial incentives related to PCMH implementation and for breast and cervical cancer screening. Incentive structures did not differ by the context of the practice. This study also used a self-selected group of practices by virtue of participating in the PGIP that may not be generalizable. The PGIP does, however, comprise almost two-thirds of the PCP practices in Michigan, including a mixture of practice sizes, organizational structures, and rural and urban geographies. In addition, patients included in the practice-level cancer screening rates were all commercially insured, so their experiences may differ from those of uninsured or publically insured patients. The use of administrative claims data, combined with a limited data collection period of 4 years and 9 months, may have caused some misclassification of patients in terms of their cancer screening behavior and needs. However, this misclassification is likely nondifferential with respect to PCMH score and SES category. In addition, few practices (2%) were excluded from the analysis because of missing covariate data. These practices were mostly lacking PCMH capability data (92%). Covariate data were more likely to be missing in earlier periods of the study and for smaller practices as well as those that had recently moved to a different physician organization. It is unlikely that missing data were systematically related to cancer screening rates, so the exclusion is unlikely to have caused substantial bias.
The Institute of Medicine’s 2001 report, Crossing the Quality Chasm: A New Health System for the 21st Century,67 considers equity to be a core dimension of a high-quality health care system; nevertheless, interventions to reduce socioeconomic disparities in health and health care have been difficult to implement.68,69 Previous studies23,70-72 examining the role of the PCMH in reducing health disparities have focused on whether the patient had a regular source of primary care rather than looking at the level of practice transformation by a patient’s PCP. Our study provides additional support for the idea that the PCMH model can help close socioeconomic gaps in the quality of preventive care. Many practices in lower socioeconomic areas face resource constraints, including lower payments received for Medicaid and uninsured patients and difficulty recruiting highly qualified physicians and staff,73-75 that lead to reduced PCMH implementation.65 Thus, it is important that reimbursement models provide sufficient support for practices with fewer resources to maximize the potential benefit of the PCMH.
Conclusions
The results of this study suggest that the PCMH model has potential to increase cancer screening rates. However, our findings also suggest that the effects of the PCMH model on cancer screening rates vary depending on the socioeconomic context of the practice, with greater effects occurring in lower socioeconomic contexts. Thus, the PCMH model could also contribute to reductions in disparities in cancer screening rates across socioeconomic contexts.
Supplementary Material
Acknowledgments
Funding/Support: Funding was received from the Agency for Healthcare Research and Quality (grant R18 RFA-HS-10-002) for our foundational work on this topic. Ms Markovitz was supported by training grant T32HD060454 in Reproductive, Perinatal, and Pediatric Epidemiology from the National Institute of Child Health and Human Development, National Institutes of Health.
Role of the Funder/Sponsor: The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions: Dr Paustian had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Markovitz, Alexander, Paustian.
Acquisition, analysis, or interpretation of data: Markovitz, Lantz, Paustian.
Drafting of the manuscript: All authors.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Markovitz, Alexander, Paustian.
Administrative, technical, or material support: Paustian.
Study supervision: Alexander, Paustian.
Conflict of Interest Disclosures: Ms Markovitz and Dr Paustian were employed by Blue Cross Blue Shield of Michigan during the time of this study. No other disclosures were reported.
REFERENCES
- 1.American Academy of Family Physicians. American Academy of Pediatrics. American College of Physicians. American Osteopathic Association [Accessed July 23, 2014];Guidelines for patient-centered medical home (PCMH) recognition and accreditation programs. 2011 Feb; http://www.acponline.org/running_practice/delivery_and_payment_models/pcmh/understanding/guidelines_pcmh.pdf. Published.
- 2.Patient Protection and Affordable Care Act, 2 USC §2703, 3 USC §3021. 2010. [Google Scholar]
- 3.Scholle SH, Torda P, Peikes D, Han E, Genevro J. Engaging Patients and Families in the Medical Home. Agency for Healthcare Research and Quality; Rockville, MD: 2010. Prepared by Mathematica Policy Research under contract No. HHSA290200900019I TO2. AHRQ publication No. 10-0083-EF. [Google Scholar]
- 4.Ferrer RL, Hambidge SJ, Maly RC. The essential role of generalists in health care systems. Ann Intern Med. 2005;142(8):691–699. doi: 10.7326/0003-4819-142-8-200504190-00037. [DOI] [PubMed] [Google Scholar]
- 5.American Academy of Family Physicians. American Academy of Pediatrics. American College of Physicians. American Osteopathic Association [Accessed July 23, 2014];Joint principles of the patient-centered medical home. Patient-Centered Primary Care Collaborative. 2007 Feb; http://www.aafp.org/dam/AAFP/documents/practice_management/pcmh/initiatives/PCMHJoint.pdf Published.
- 6.Jaén CR, Crabtree BF, Palmer RF, et al. Methods for evaluating practice change toward a patient-centered medical home. Ann Fam Med. 2010;8(suppl 1):S9–S20. S92. doi: 10.1370/afm.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenthal M, Abrams M, Bitton A, Patient-Centered Medical Home Evaluators’ Collaborative . Recommended core measures for evaluating the patient-centered medical home: cost, utilization, and clinical quality. Commonwealth Fund; [Accessed July 23, 2014]. May, 2012. http://www.commonwealthfund.org/~/media/files/publications/data-brief/2012/1601_rosenthal_recommended_core_measures_pcmh_v2.pdf. Published. [Google Scholar]
- 8.Alexander JA, Bae D. Does the patient-centred medical home work? a critical synthesis of research on patient-centred medical homes and patient-related outcomes. Health Serv Manage Res. 2012;25(2):51–59. doi: 10.1258/hsmr.2012.012001. [DOI] [PubMed] [Google Scholar]
- 9.Hoff T, Weller W, DePuccio M. The patient-centered medical home: a review of recent research. Med Care Res Rev. 2012;69(6):619–644. doi: 10.1177/1077558712447688. [DOI] [PubMed] [Google Scholar]
- 10.Jackson GL, Powers BJ, Chatterjee R, et al. Improving patient care: the patient centered medical home: a systematic review. Ann Intern Med. 2013;158(3):169–178. doi: 10.7326/0003-4819-158-3-201302050-00579. [DOI] [PubMed] [Google Scholar]
- 11.Franks P, Fiscella K, Beckett L, Zwanziger J, Mooney C, Gorthy S. Effects of patient and physician practice socioeconomic status on the health care of privately insured managed care patients. Med Care. 2003;41(7):842–852. doi: 10.1097/00005650-200307000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Sambamoorthi U, McAlpine DD. Racial, ethnic, socioeconomic, and access disparities in the use of preventive services among women. Prev Med. 2003;37(5):475–484. doi: 10.1016/s0091-7435(03)00172-5. [DOI] [PubMed] [Google Scholar]
- 13.Kirby JB, Kaneda T. Neighborhood socioeconomic disadvantage and access to health care. J Health Soc Behav. 2005;46(1):15–31. doi: 10.1177/002214650504600103. [DOI] [PubMed] [Google Scholar]
- 14.Jain N, Euler GL, Shefer A, Lu P, Yankey D, Markowitz L. Human papillomavirus (HPV) awareness and vaccination initiation among women in the United States, National Immunization Survey-Adult 2007. Prev Med. 2009;48(5):426–431. doi: 10.1016/j.ypmed.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 15.Linn ST, Guralnik JM, Patel KV. Disparities in influenza vaccine coverage in the United States, 2008. J Am Geriatr Soc. 2010;58(7):1333–1340. doi: 10.1111/j.1532-5415.2010.02904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agency for Healthcare Research and Quality . 2013 National healthcare disparities report. Agency for Healthcare Research and Quality; Rockville, MD: [Accessed July 23, 2014]. May, 2014. http://www.ahrq.gov/research/findings/nhqrdr/nhdr13/2013nhdr.pdf. Published. [Google Scholar]
- 17.Hughes MC, Hannon PA, Harris JR, Patrick DL. Health behaviors of employed and insured adults in the United States, 2004-2005. Am J Health Promot. 2010;24(5):315–323. doi: 10.4278/ajhp.080603-QUAN-77. [DOI] [PubMed] [Google Scholar]
- 18.Doubeni C, Robinson S, Fouayzi H, Roblin D, Field T, Fletcher R. C-C3-04: neighborhood socioeconomic conditions and use of preventive health care services in insured populations. Clin Med Res. 2010;8(3-4):194. [Google Scholar]
- 19.Lian M, Schootman M, Yun S. Geographic variation and effect of area-level poverty rate on colorectal cancer screening. BMC Public Health. 2008;8:358. doi: 10.1186/1471-2458-8-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katz SJ, Hofer TP. Socioeconomic disparities in preventive care persist despite universal coverage: breast and cervical cancer screening in Ontario and the United States. JAMA. 1994;272(7):530–534. [PubMed] [Google Scholar]
- 21.McMorrow S, Kenney GM, Goin D. Determinants of receipt of recommended preventive services: implications for the Affordable Care Act. Am J Public Health. 2014;104(12):2392–2399. doi: 10.2105/AJPH.2013.301569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doubeni CA, Jambaulikar GD, Fouayzi H, et al. Neighborhood socioeconomic status and use of colonoscopy in an insured population—a retrospective cohort study. PLoS One. 2012;7(5):e36392. doi: 10.1371/journal.pone.0036392. doi:10.1371/journal.pone.0036392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berenson J, Michelle D, Abrams M, Shih A. Achieving better quality of care for low-income populations: the roles of health insurance and the medical home in reducing health inequities. Commonwealth Fund; [Accessed August 8, 2014]. May, 2012. http://www.commonwealthfund.org/~/media/files/publications/issue-brief/2012/may/1600_berenson_achieving_better_quality_care_low_income_v2.pdf. Published. [PubMed] [Google Scholar]
- 24.Wong W, Anderson K, Dankwa-Mullan I, Simon M, Vega W. The Patient-Centered Medical Home: A Path Toward Health Equity? Institute of Medicine; Washington, DC: [Accessed August 7, 2014]. 2012. http://www.iom.edu/~/media/Files/Perspectives-Files/2012/Discussion-Papers/PatientCenteredMedicalHome.pdf. [Google Scholar]
- 25.Fields D, Leshen E, Patel K. Driving quality gains and cost savings through adoption of medical homes. Health Aff (Millwood) 2010;29(5):819–826. doi: 10.1377/hlthaff.2010.0009. [DOI] [PubMed] [Google Scholar]
- 26.Commonwealth Fund [Accessed July 23, 2014];Patient centered coordinated care. http://www.commonwealthfund.org/grants-and-fellowships/programs/archived-programs/patient-centered-coordinated-care.
- 27.Nielsen M, Olayiwola JN, Grundy P, Grumbach K. [Accessed July 23, 2014];The medical home’s impact on cost and quality: an annual update of the evidence, 2012-2013. 2014 Jan; http://www.pcpcc.org/resource/medical-homes-impact-cost-quality. Published.
- 28.Patient-Centered Primary Care Collaborative [Accessed July 23, 2014];Proof in practice: a compilation of patient centered medical home pilot and demonstration projects. 2009 http://www.pcpcc.org/sites/default/files/media/PilotGuidePip.pdf. Published.
- 29.Berry CA, Mijanovich T, Albert S, et al. Patient-centered medical home among small urban practices serving low-income and disadvantaged patients. Ann Fam Med. 2013;11(suppl 1):S82–S89. doi: 10.1370/afm.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quinn MT, Gunter KE, Nocon RS, et al. Undergoing transformation to the patient centered medical home in safety net health centers: perspectives from the front lines. Ethn Dis. 2013;23(3):356–362. [PMC free article] [PubMed] [Google Scholar]
- 31.Lantz PM, Pritchard A. Socioeconomic indicators that matter for population health. Prev Chronic Dis. 2010;7(4):A74. [PMC free article] [PubMed] [Google Scholar]
- 32.Krieger N, Chen JT, Waterman PD, Soobader M-J, Subramanian SV, Carson R. Geocoding and monitoring of US socioeconomic inequalities in mortality and cancer incidence: does the choice of area-based measure and geographic level matter? the Public Health Disparities Geocoding Project. Am J Epidemiol. 2002;156(5):471–482. doi: 10.1093/aje/kwf068. [DOI] [PubMed] [Google Scholar]
- 33.Sarfaty M, Wender R, Smith R. Promoting cancer screening within the patient centered medical home. CA Cancer J Clin. 2011;61(6):397–408. doi: 10.3322/caac.20125. [DOI] [PubMed] [Google Scholar]
- 34.Coffield AB, Maciosek MV, McGinnis JM, et al. Priorities among recommended clinical preventive services. Am J Prev Med. 2001;21(1):1–9. doi: 10.1016/s0749-3797(01)00308-7. [DOI] [PubMed] [Google Scholar]
- 35.Smith RA, Manassaram-Baptiste D, Brooks D, et al. Cancer screening in the United States, 2014: a review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin. 2014;64(1):30–51. doi: 10.3322/caac.21212. [DOI] [PubMed] [Google Scholar]
- 36.US Preventive Services Task Force [Accessed January 16, 2015];Recommendations for adults. http://www.uspreventiveservicestaskforce.org/BrowseRec/Index/browse-recommendations.
- 37.Pruitt SL, Shim MJ, Mullen PD, Vernon SW, Amick BC., III Association of area socioeconomic status and breast, cervical, and colorectal cancer screening: a systematic review. Cancer Epidemiol Biomarkers Prev. 2009;18(10):2579–2599. doi: 10.1158/1055-9965.EPI-09-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baron RC, Rimer BK, Coates RJ, et al. Task Force on Community Preventive Services Client-directed interventions to increase community access to breast, cervical, and colorectal cancer screening: a systematic review. Am J Prev Med. 2008;35((1)(suppl)):S56–S66. doi: 10.1016/j.amepre.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 39.Share DA, Mason MH. Michigan’s Physician Group Incentive Program offers a regional model for incremental ‘fee for value’ payment reform. Health Aff (Millwood) 2012;31(9):1993–2001. doi: 10.1377/hlthaff.2012.0328. [DOI] [PubMed] [Google Scholar]
- 40.Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness: the chronic care model, part 2. JAMA. 2002;288(15):1909–1914. doi: 10.1001/jama.288.15.1909. [DOI] [PubMed] [Google Scholar]
- 41.Alexander JA, Paustian M, Wise CG, et al. Assessment and measurement of patient-centered medical home implementation: the BCBSM experience. Ann Fam Med. 2013;11(suppl 1):S74–S81. doi: 10.1370/afm.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.US Census Bureau . Design and Methodology: American Community Survey. US Government Printing Office; Washington, DC: 2011. [Google Scholar]
- 43.Local Initiatives Support Corporation [Accessed July 24, 2014];Zip code foreclosure risk score methodology appendix. 2014 http://www.foreclosure-response.org/assets/maps&data/ZIPCodeForeclosureRiskScore_Methodology_IntraState_2014.pdf. Published.
- 44.National Council for Quality Assurance . HEDIS 2011: Healthcare Effectiveness Data and Information Set. National Council for Quality Assurance; Washington, DC: 2010. [Google Scholar]
- 45.National Council for Quality Assurance . HEDIS 2012: Healthcare Effectiveness Data and Information Set. National Council for Quality Assurance; Washington, DC: 2011. [Google Scholar]
- 46.National Council for Quality Assurance . HEDIS 2013: Healthcare Effectiveness Data and Information Set. National Council for Quality Assurance; Washington, DC: 2012. [Google Scholar]
- 47.US Preventive Services Task Force [Accessed August 6, 2014];Screening for breast cancer. http://www.uspreventiveservicestaskforce.org/uspstf/uspsbrca.htm.
- 48.Raudenbush S, Bryk A. Hierarchical Linear Models: Applications and Data Analysis Methods. 2nd ed. Sage Publications; Newbury Park, CA: 2002. [Google Scholar]
- 49.Centers for Disease Control and Prevention (CDC) Cancer screening—United States, 2010. MMWR Morb Mortal Wkly Rep. 2012;61(3):41–45. [PubMed] [Google Scholar]
- 50.Centers for Disease Control and Prevention (CDC) Vital signs: colorectal cancer screening test use—United States, 2012. MMWR Morb Mortal Wkly Rep. 2013;62(44):881–888. [PMC free article] [PubMed] [Google Scholar]
- 51.Agency for Health Care Quality and Research . Guide to clinical preventive services, 2014: recommendations of the US Preventive Services Task Force. Department of Health and Human Services; Washington, DC: [Accessed November 6, 2014]. 2014. http://www.ahrq.gov/professionals/clinicians-providers/guidelines-recommendations/guide/cpsguide.pdf. [Google Scholar]
- 52.Sarfaty M. [Accessed August 8, 2014];How to increase colorectal cancer screening rates in practice: a primary care clinician’s evidence-based toolbox and guide 2008. 2008 http://www.cancer.org/acs/groups/content/documents/document/acspc-024588.pdf. Published.
- 53.Roman L, Meghea C, Ford S, et al. Individual, provider, and system risk factors for breast and cervical cancer screening among underserved black, Latina, and Arab women. J Womens Health. 2014;23(1):57–64. doi: 10.1089/jwh.2013.4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pandhi N, DeVoe JE, Schumacher JR, et al. Preventive service gains from first contact access in the primary care home. J Am Board Fam Med. 2011;24(4):351–359. doi: 10.3122/jabfm.2011.04.100254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pandhi N, DeVoe JE, Schumacher JR, et al. Number of first-contact access components required to improve preventive service receipt in primary care homes. J Gen Intern Med. 2012;27(6):677–684. doi: 10.1007/s11606-011-1955-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bindman AB, Grumbach K, Osmond D, Vranizan K, Stewart AL. Primary care and receipt of preventive services. J Gen Intern Med. 1996;11(5):269–276. doi: 10.1007/BF02598266. [DOI] [PubMed] [Google Scholar]
- 57.DeVoe JE, Fryer GE, Phillips R, Green L. Receipt of preventive care among adults: insurance status and usual source of care. Am J Public Health. 2003;93(5):786–791. doi: 10.2105/ajph.93.5.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Parkerton PH, Smith DG, Straley HL. Primary care practice coordination versus physician continuity. Fam Med. 2004;36(1):15–21. [PubMed] [Google Scholar]
- 59.Flocke SA, Stange KC, Zyzanski SJ. The association of attributes of primary care with the delivery of clinical preventive services. Med Care. 1998;36((8)(suppl)):AS21–AS30. doi: 10.1097/00005650-199808001-00004. [DOI] [PubMed] [Google Scholar]
- 60.O’Malley AS, Beaton E, Yabroff KR, Abramson R, Mandelblatt J. Patient and provider barriers to colorectal cancer screening in the primary care safety-net. Prev Med. 2004;39(1):56–63. doi: 10.1016/j.ypmed.2004.02.022. [DOI] [PubMed] [Google Scholar]
- 61.Meissner HI, Klabunde CN, Breen N, Zapka JM. Breast and colorectal cancer screening: US primary care physicians’ reports of barriers. Am J Prev Med. 2012;43(6):584–589. doi: 10.1016/j.amepre.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 62.Sabatino SA, Habarta N, Baron RC, et al. Task Force on Community Preventive Services Interventions to increase recommendation and delivery of screening for breast, cervical, and colorectal cancers by healthcare providers: systematic reviews of provider assessment and feedback and provider incentives. Am J Prev Med. 2008;35((1)(suppl)):S67–S74. doi: 10.1016/j.amepre.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 63.Rothman RL, DeWalt DA, Malone R, et al. Influence of patient literacy on the effectiveness of a primary care–based diabetes disease management program. JAMA. 2004;292(14):1711–1716. doi: 10.1001/jama.292.14.1711. doi:10.1001/jama.292.14.1711. [DOI] [PubMed] [Google Scholar]
- 64.Chien AT, Chin MH, Davis AM, Casalino LP. Pay for performance, public reporting, and racial disparities in health care: how are programs being designed? Med Care Res Rev. 2007;64((5)(suppl)):283S–304S. doi: 10.1177/1077558707305426. [DOI] [PubMed] [Google Scholar]
- 65.Chin MH, Walters AE, Cook SC, Huang ES. Interventions to reduce racial and ethnic disparities in health care. Med Care Res Rev. 2007;64((5) (suppl)):7S–28S. doi: 10.1177/1077558707305413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chin MH, Clarke AR, Nocon RS, et al. A roadmap and best practices for organizations to reduce racial and ethnic disparities in health care. J Gen Intern Med. 2012;27(8):992–1000. doi: 10.1007/s11606-012-2082-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Committee on Quality of Health Care in America, Institute of Medicine . Crossing the Quality Chasm: A New Health System for the 21st Century. National Academies Press; Washington, DC: 2001. [Google Scholar]
- 68.Adler NE, Newman K. Socioeconomic disparities in health: pathways and policies. Health Aff (Millwood) 2002;21(2):60–76. doi: 10.1377/hlthaff.21.2.60. [DOI] [PubMed] [Google Scholar]
- 69.Gallo LC, de Los Monteros KE, Shivpuri S. Socioeconomic status and health: what is the role of reserve capacity? Curr Dir Psychol Sci. 2009;18(5):269–274. doi: 10.1111/j.1467-8721.2009.01650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Aysola J, Bitton A, Zaslavsky AM, Ayanian JZ. Quality and equity of primary care with patient-centered medical homes: results from a national survey. Med Care. 2013;51(1):68–77. doi: 10.1097/MLR.0b013e318270bb0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Beal A, Doty M, Hernandez S, Shea K, Davis K. Closing the divide: how medical homes promote equity in health care: results from the Commonwealth Fund 2006 Health Care Quality Survey. Commonwealth Fund; New York, NY: [Accessed August 8, 2014]. 2007. http://www.commonwealthfund.org/usr_doc/1035_Beal_closing_divide_medical_homes.pdf. [Google Scholar]
- 72.Beal A, Hernandez S, Doty M. Latino access to the patient-centered medical home. J Gen Intern Med. 2009;24(suppl 3):514–520. doi: 10.1007/s11606-009-1119-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rosenblatt RA, Andrilla CHA, Curtin T, Hart LG. Shortages of medical personnel at community health centers: implications for planned expansion. JAMA. 2006;295(9):1042–1049. doi: 10.1001/jama.295.9.1042. [DOI] [PubMed] [Google Scholar]
- 74.Fairbrother G, DuMont KA, Friedman S, Lobach KS. New York City physicians serving high volumes of Medicaid children: who are they and how do they practice? Inquiry. 1995;32(3):345–352. [PubMed] [Google Scholar]
- 75.Coleman K, Phillips K. Providing underserved patients with medical homes: assessing the readiness of safety-net health centers. Issue Brief (Commonw Fund) 2010;85:1–14. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.