Abstract
Background
We previously reported the safety of a self‐administered subcutaneous immunotherapy (SCIT) protocol. Here we report the results of the retrospective efficacy trial of the United Allergy Service (UAS) self‐administered SCIT protocol. We hypothesized that by utilizing a slow SCIT buildup phase, designed to attain recommended allergen concentrations on a cumulative basis, efficacious outcomes and clinical relevance would be achieved.
Methods
We enrolled 60 SCIT patients and 56 control patients. The study contrasted baseline and treatment period combined symptom plus medication scores (CSMS) as the primary outcome measure and rhinoconjunctivitis quality of life questionnaire (RQLQ) scores as the secondary study outcome measure. Changes in pollen counts were also examined with regard to effects on these efficacy parameters.
Results
The treatment group showed significantly improved CSMS (standardized mean difference [SMD]: −1.57; 95% confidence interval [CI], −1.97 to −1.18; p < 0.001) and RQLQ (SMD: −0.91; 95% CI, −1.23 to −0.59; p < 0.001). These treatment group outcome measures were respectively improved by 33% and 29% compared to baseline and greater than 40% in comparison to the control group (p < 0.0001). Significant results were also shown when examining these outcome measures with regards to either monotherapy or poly‐allergen SCIT. Furthermore, a comparison to recent meta‐analyses of SCIT studies showed equivalent efficacy and clinical relevance. Assessment of pollen counts during the baseline and treatment periods further corroborated the efficacy of the UAS SCIT protocol.
Conclusion
These efficacy results, and our previous safety results, show that a carefully designed and implemented self‐administered SCIT protocol is efficacious and safe.
Keywords: allergic rhinitis, allergy immunotherapy, allergy injections, subcutaneous immunotherapy, aeroallergens
In contrast to pharmacotherapy, allergen immunotherapy (IT) is the only therapeutic modality that specifically addresses the central pathophysiology of allergic rhinitis and possibly diminishes the development of allergic asthma and severity of other preexisting allergic comorbidities.1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11
Several studies, including a recent study by this group, have showed safe outcomes for carefully designed self‐administered subcutaneous immunotherapy (SCIT) protocols.12, 13, 14, 15, 16, 17 A recent criticism hypothesized that 1 set of these safety outcomes was achieved by utilizing subtherapeutic allergen concentrations and thus, the attainment of clinical efficacy was questioned.18 Therefore, the demonstration of clinical efficacy is a salient issue, particularly for self‐administered SCIT protocols.
The majority of SCIT prescribed in the United States uses several allergen extracts to compliment the patient with multiple sensitivities.19, 20, 21 Consistent with this commonly prescribed therapeutic approach in the United States, we report the findings of a SCIT protocol designed for patients with multiallergen sensitivities. Specifically, we present and discuss the results of a self‐administered SCIT retrospective efficacy study using the United Allergy Services (UAS) immunotherapy protocol.
Patients and methods
Patients
All subjects were diagnosed with seasonal or seasonal plus perennial allergic rhinitis (AR) as described.22 Patients with contraindications consistent with the current recommendations for allergy skin prick testing (SPT) and SCIT utilization were excluded.2, 22 All patients underwent SPT utilizing relevant extracts consistent with standard practices.2, 22 Both male and female patients aged 18 to 65 years with diagnosed AR were openly enrolled in the study. Patients who were enrolled and opted for SCIT were deemed treatment patients and those that declined SCIT were deemed control patients.
Study design
To control for geographic differences, patients were primarily recruited from clinics located in Dallas, TX, and San Antonio, TX. Research personnel were blinded to patient identification, age, gender, and primary care clinic affiliation. The study design, informed consent, and execution were approved by the Salus Independent Review Board (IRB) (Austin, TX).
All study subjects were allowed to continue oral and topical antihistamines and nasal steroids prescribed by their physician. Study subjects using systemic steroids were excluded. Individuals enrolled in the study completed validated questionnaires as discussed below (see Assessment).
UAS immunotherapy protocol
Immunotherapy formulation guidelines were in accordance with published recommendations.2, 22, 23, 24, 25, 26, 27 Due to the documented increased risk of systemic reactions (SR) during the immunotherapy buildup phase (ie, escalation phase),25, 26, 27, 28 the UAS protocol was designed to complete the buildup phase over a 6‐month period. Also, based upon the administration frequency and allergen concentrations used, cumulative allergen dosing is equivalent to current recommendations.2, 12 A more detailed description of the UAS SCIT protocol has been recently published.12
Assessment
Enrolled subjects completed validated questionnaires for rhinoconjunctivitis symptom scores (SS) in accord with both the U.S. Food and Drug Administration (FDA) and World Allergy Organization (WAO) recommendations.29, 30, 31 The medication scores (MS) paralleled the methods of Stelmach et al.32 The aggregate of the SS and the MS (CSMS) followed previously utilized methods.32, 33, 34, 35, 36 The Rhinoconjunctivitis Quality of Life Questionnaire (RQLQ) used consists of 28 queries divided into 7 domains.37
These questionnaires were initially completed at the end of the baseline year of therapy (2010 to 2011). During this period, all study participants were undergoing pharmacotherapy and avoidance therapy only. After undergoing a further 12 or more months of immunotherapy (2011 to late 2012), the second set of surveys were completed. This survey methodology is consistent with the format used in several previous trials.38, 39, 40, 41 (See the online Supporting Information for the questionnaires.)
Statistical methods
For each study subject, their SS, MS, CSMS, and RQLQ were averaged for both the baseline and the follow‐up assessments. The difference in mean scores between baseline and follow‐up was obtained and within group differences were assessed by use of a paired Student t test. Group differences between mean scores at baseline and at follow‐up were assessed by use of the Student t test. Standardized mean differences (SMDs) were computed as the mean difference divided by the standard deviation of the mean difference. The SMD inclusion was so that the results from this project could be compared to other published reports which may have used modified assessments of symptom and medication scores.
Adverse events, safety, and compliance determination
Patients were required to report all suspected adverse events to their primary care clinics as soon as possible and to cease continued treatment until evaluated and provided guidance by their physician.
To promote safety and compliance, all patients were only given a single unit‐dose vial set and a preprinted IT injection log book that enumerated all required doses to utilize. Patients were instructed to maintain the injection log and to record any pertinent events related to the injections. All patients were interviewed and their logs were reviewed when they returned to the clinic to acquire their next unit‐dose vials.
Pollen count analyses
Atmospheric sampling for aeroallergens was performed and reported by certified staff through the American Academy of Allergy, Asthma and Immunology Aeroallergen Network. Aeroallergen samplers used were approved by the National Allergy Bureau (NAB) and identification/measurements met NAB requirements.
The grass pollen seasons were determined by examining pollen counts of greater than 15 grains/m3 for 3 consecutive days, which defined the season starting date. Whereas, grass pollen counts that were less than 15 grains/m3 for 3 consecutive days defined the season ending date.42, 43, 44 Weed‐based and tree‐based pollen seasons were determined by examining the raw data for respective pollen counts of greater than 20 grains/m3 for 3 consecutive days, which defined the season starting date, whereas respective pollen counts that were <20 grains/m3 for 3 consecutive days defined the season ending date. The delineation of pollen seasons by these levels are consistent with NAB‐defined “moderate” pollen exposure.42, 43, 44
Results
Protocol compliance
One of the 60 individuals in the SCIT treatment group did not comply with the specified treatment protocol and that individual was excluded from the study. Another treatment group participant was compliant for 75% of scheduled injections and he was not excluded from study participation. All other treatment and control group study participants were fully compliant.
Adverse events and systemic disorders
One study subject in the SCIT treatment group reported a WAO grade II SR.45 After medical assessment and advisement, this patient's SCIT protocol was appropriately addressed.2 His SCIT regimen subsequently continued without further clinical incident. Three other participants in the SCIT treatment group reported local reactions without sequelae. There were no reported adverse events by subjects in the control group.
Patient demographics
The patient demographics are shown in Table 1.
Table 1.
Characteristics of treatment and control patients
| Treatment | Control | |
|---|---|---|
| Sex (n) | ||
| Male | 24 | 5 |
| Female | 36 | 51 |
| Race (n) | ||
| White | 37 | 34 |
| African American | 12 | 7 |
| Hispanic | 2 | 7 |
| Other | 9 | 8 |
| Age (years), mean ± SD | 37.09 ± 13.92 | |
| Duration of therapy (years), mean ± SD | 1.22 ± 0.27 | |
| Administered SCIT‐based aeroallergen extracts (n), mean ± SD | 9.08 ± 1.84 | N/A |
SCIT = subcutaneous immunotherapy; SD = standard deviation.
Identified allergies
The most common allergies manifested by both the treatment and control groups are: cedar and oak tree pollens (>60% of treatment group and 40% of controls); Bermuda and Timothy grass pollen (>62% of treatment group and 58% of controls); ragweed pollen (57% of treatment group and 45% of controls); and dust mites (>67% of treatment group and 57% of controls). Also, allergies to oak tree pollen, Timothy grass pollen, and dust mites are shared by 35% of the treatment group and 27% of the control group.
Rhinoconjunctivitis SS
Assessment showed that individuals in the treatment group (ie, SCIT therapy) experienced a 35% SS improvement over their baseline scores. The treatment group's SS improvement in comparison to that of the control group is consistent with a >45% improvement (p < 0.0001; data not shown). Also, the control group experienced a significant increase in SS in year 2 vs year 1 of this study (data not shown; p = 0.05).
MS
MS analyses showed that the treatment group had a 30% decrease in medication use (data not shown, p < 0.001). Also, there is a significant decrease in the MS of the treatment group in comparison to the control group's scores (p < 0.001). In contrast, the control group experienced a significant increased use of medications in the second vs the initial study year (paired SMD of −1.57; p < 0.0001, Table 2). Furthermore, there was a 33% improvement for the treatment group with respect to baseline results and a >40% improvement in comparison to the control group (p < 0.001).
Table 2.
Differences in CSMS and RQLQ for the treatment and control groups
| n | Diff: T2‐T1 | Paired SMD | CI | p a | |
|---|---|---|---|---|---|
| Medication use in the second vs the initial study year | |||||
| CSMS | |||||
| Control | 56 | 0.56 | 0.34 | −0.06 to 0.73 | 0.01 |
| Treatment | 60 | −1.84 | −1.57 | −1.97 to −1.18 | <0.0001 |
| RQLQ | |||||
| Control | 56 | 0.07 | 0.05 | −0.32 to 0.41 | 0.7 |
| Treatment | 60 | −1.24 | −0.91 | −1.23 to −0.59 | <0.0001 |
| Common allergies to oak, dust mite, and Timothy grass | |||||
| CSMS | |||||
| Control | 48 | 0.62 | 0.39 | −0.02 to 0.81 | 0.009 |
| Treatment | 56 | −1.83 | −1.54 | −1.95 to −1.14 | <0.0001 |
| RQLQ | |||||
| Control | 48 | 0.19 | 0.13 | −0.27 to 0.52 | 0.39 |
| Treatment | 56 | −1.22 | −0.87 | −1.20 to −0.54 | <0.0001 |
| Common allergies to ragweed | |||||
| CSMS | |||||
| Control | 22 | 0.73 | 0.49 | 0.08 to 1.06 | 0.03 |
| Treatment | 34 | −1.86 | −1.46 | −2.02 to −0.91 | <0.0001 |
| RQLQ | |||||
| Control | 22 | 0.11 | 0.07 | −0.54 to 0.69 | 0.7 |
| Treatment | 34 | −1.20 | −0.83 | −1.27 to −0.39 | <0.0001 |
| Common allergies to dust mites | |||||
| CSMS | |||||
| Control | 47 | 0.59 | 0.37 | −0.05 to 0.79 | 0.01 |
| Treatment | 40 | −1.86 | −1.70 | −2.21 to −1.20 | <0.0001 |
| RQLQ | |||||
| Control | 47 | 0.18 | 0.12 | −0.29 to 0.52 | 0.4 |
| Treatment | 40 | −1.16 | −0.79 | −1.16 to −0.41 | <0.0001 |
Bold values are significant.
CI = 95% confidence interval; CSMS = combined symptom plus medication scores; Diff: T2‐T1 = difference in average CSMS and RQLQ scores from final patient surveys in comparison to initial or baseline surveys; Paired SMD = standard mean difference calculated per patient from baseline survey to follow‐up survey; RQLQ = rhinoconjunctivitis quality of life questionnaire.
The treatment group's RQLQ showed a 29% improvement in comparison to baseline results and a 42% improvement in comparison to the control group (p < 0.0001). In contrast, the control group results did not show a significant RQLQ SMD score change during the intervals under assessment (p = 0.7, Table 2). A comparison of RQLQ scores for each of the 7 domains further showed that the treatment group attained significant improvement in 6 of the 7 domains (data not shown).
From a multiallergen perspective, Table 2 depicts the CSMS and RQLQ SMD differences for patient groups with common allergies to oak trees, dust mites, and Timothy grass. In a similar manner, Table 2 shows the results for sizeable patient populations manifesting respective common allergies to ragweed and dust mites, respectively. The CSMS results for the treatment groups with common allergies to oak tree, dust mites, and Timothy grass, or the individual allergens of ragweed or dust mites (all in Table 2) show significant improvement. In contrast, each respective control group showed significant increases (or worsening) of their CSMS scores. With regard to other combinations of manifested allergies, either shared dual‐pollen or single‐pollen allergies, similar results were evident (data not shown).
The RQLQ SMD results (Table 2) show significant improvement for subjects in the treatment group (mean improvement of 28% over baseline and a >40% improvement with respect to the control group). The control group results showed no significant change in RQLQ scores during the study period. With regard to other allergy combinations, similar results were evident (data not shown).
Pollen count assessment
Figure 1A and B show respective total seasonal pollen counts for both metropolitan locations in which the participants reside. These figures display pollen counts for the periods encompassing the baseline year (2010 to 2011) and the second year of study (2011 through 2012 inclusive).
Figure 1.
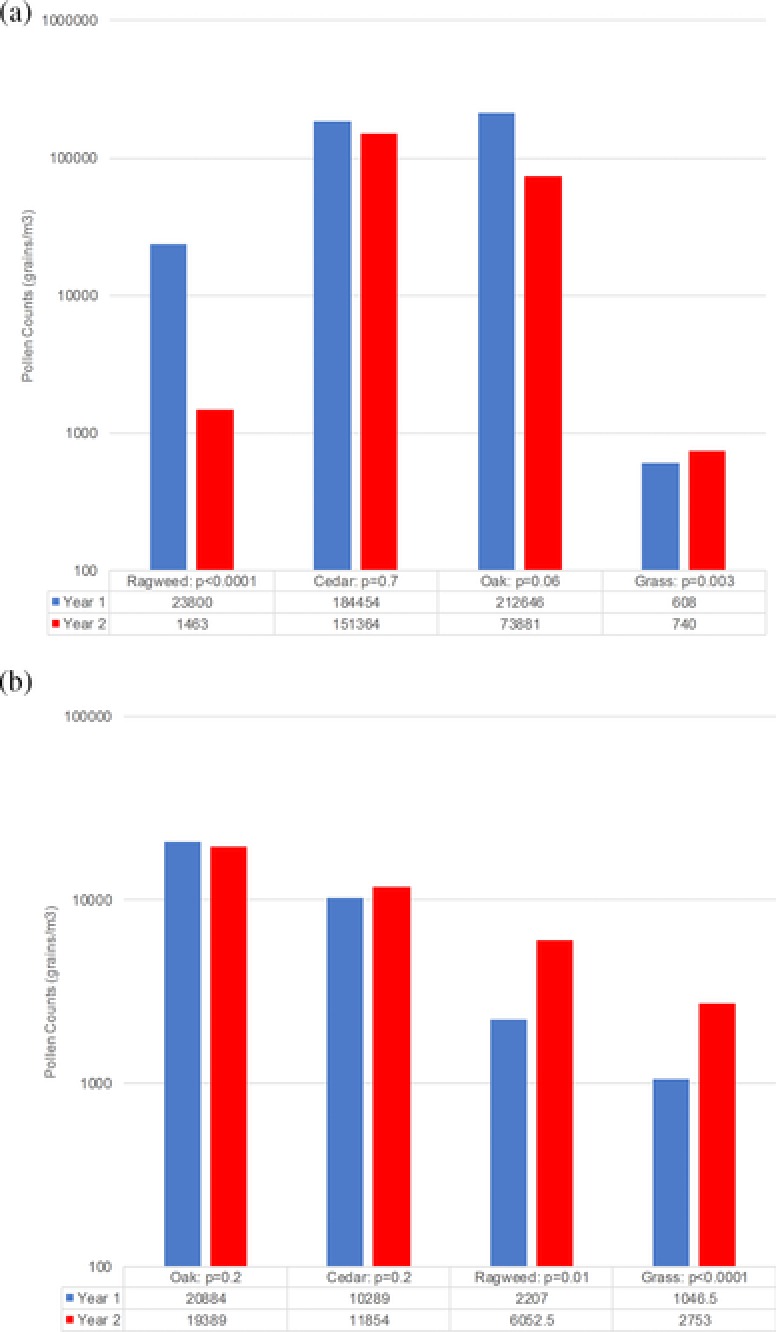
(A) Pollen counts in San Antonio for 2010 through 2012. (B) Pollen counts in Dallas for 2010 through 2012. Bars represent total pollen count for the specified pollen season. Total season pollen count is on a log10 scale.
An assessment of the pollen results available for the years of 2011 through 2012 in comparison to 2010 to 2011 in San Antonio show that total seasonal pollen counts were relatively unchanged for mountain cedar tree and oak tree (Fig. 1A), while in contrast, a significant decrease in total pollen counts for ragweed occurred. Furthermore, there was increased grass pollen counts documented (Fig. 1A). Analysis of the Dallas pollen seasons reveals that during this same time period, unchanged pollen counts for oak tree and mountain cedar tree pollen were evident (Fig. 1B). In contrast, significant increased total grass pollen and ragweed pollen counts were recorded.
Discussion
In this study we assayed the efficacy of the UAS poly‐allergen SCIT protocol by examining changes in the study outcome measures of CSMS as the primary measure and RQLQ results as the secondary outcome measure.
The total SS, MS, CSMS, and RQLQ scores were significantly improved for those undergoing SCIT (ie, treatment group) when examined at a mean of 1.22 years of therapy in comparison to baseline data and the control group scores (Table 2). When analyses focused on the 6 most common individual allergen sensitivities (cedar tree, oak tree, ragweed mix, Bermuda grass, Timothy grass, and dust mites, in part shown in Table 2) or combinations shared by both groups (in part shown in Table 2), similar significant improvement in the respective treatment group CSMS and RQLQ results were shown in comparison to baseline and control group results.
Because statistical significance may not equate to clinical relevance, several means of evaluating clinical significance or relevancy have historically been used.46, 47, 48 For non‐placebo controlled studies (eg, current study), Erekosima et al.46 determined that a difference of ≥15% in the efficacy outcome measures in comparison to the control group were considered clinically significant. Furthermore, the WAO taskforce recommended that efficacy study outcomes of 20% or more than placebo are consistent with clinical significance.47 Thus, the current study changes in the primary efficacy outcome measures are both statistically significant and clinically relevant.
The RQLQ results showed a significant treatment group improvement compared to its baseline results and in comparison to the control group results. In order to address clinical significance, a change in RQLQ of greater than ± 0.5 surpasses the threshold of clinical relevance and is evident in the current study treatment group (with an RQLQ SMD of −0.91).29, 49 It is understood that differences in study design, methodology used, and scoring methods warrant careful consideration in contrasting study results. In a review by Nelson,19 the majority of published SCIT efficacy studies to date include only monotherapy trials. Thus, in our comparisons several of the trials are monotherapy studies. A comparison of the current study CSMS‐SMD to the majority of studies (ie, those with study populations >20 subjects) referenced by Dretzke et al.10 and DiBona et al.50 is illustrated in Figure 2A.51, 52, 53, 54, 55, 56, 57, 58 Figure 2A shows (study population) weighted CSMS‐SMD with 95% confidence intervals (CIs) for the 8 studies reported by Dretzke et al.10 and DiBona et al.50 (where the mean of all studies is depicted in red), and the CSMS‐SMD for the current UAS protocol study (depicted in blue).51, 52, 53, 54, 55, 56, 57, 58 Evident in Figure 2A is the statistical equivalency of our results with those reviewed by Dretzke et al.10 and DiBona et al.50, 51, 52, 53, 54, 55, 56, 57, 58 When assessing the CSMS‐SMD for the grouped or individual allergens reviewed in this study, the same statistical equivalency of our results is evident (data not shown).
Figure 2.
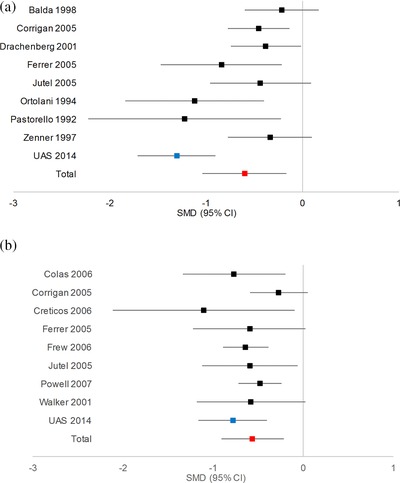
(A) CSMS results compared to previous published results.10, 50, 51, 52, 53, 54, 55, 56, 57, 58 (B) RQLQ results compared to previous published results.10, 56, 57, 58, 59, 60, 61, 62, 63 Blue data point is the SMD for the UAS SCIT trial. The red data point is the mean SMD for all studies enumerated in the graph. CI = confidence interval; CSMS = combined symptom plus medication scores; RQLQ = rhinoconjunctivitis quality of life questionnaire; SCIT = subcutaneous immunotherapy; SMD = standardized mean difference; UAS = United Allergy Service.
For analysis of RQLQ, Figure 2B shows (study population) weighted RQLQ‐SMD with 95% CIs for the 8 studies reported by Dretzke et al.10 and the UAS RQLQ‐SMD results.56, 57, 58, 59, 60, 61, 62, 63 Evident in this figure is the statistical equivalency of our results with those reviewed by Dretzke et al.10, 56, 57, 58, 59, 60, 61, 62, 63 When assessing the RQLQ‐SMD for the grouped or individual allergens reviewed in this study, the same statistical equivalency of our results is also noted (data not shown).
Therefore, the results of our primary and secondary outcome measures show that the utilization of the UAS protocol has statistically equivalent efficacy and comparable clinical relevancy to recently conducted meta‐analyses of both American and European based SCIT studies.
A direct correlation has been reported between the level of pollen counts and the corresponding SS and MS (the components of the CSMS) among affected individuals.64 Because total pollen counts were either unchanged or increased during the second year of the study in Dallas (Fig. 1B), depending on the individual's allergen sensitivities, their SS and CSMS scores would be expected to be similar or worse during the second year in comparison to the first year of the study. Specifically, each control group's (eg, Dallas and San Antonio) CSMS worsened from year 1 to year 2 of the study while the treatment groups’ outcomes (in both Dallas and San Antonio) significantly improved (as shown for all study subjects; see Table 2). Thus, for the subjects enrolled in Dallas only, the treatment group's improvement (eg, SS and CSMS), in contrast to the geographically matched control group, further corroborates the efficacy of the UAS SCIT protocol employed in this study. With regard to the study participants in San Antonio, the findings show that in an environment of increasing grass and unchanged tree pollen counts, the improved study parameters (eg, SS and CSMS) also corroborate the efficacy of the UAS SCIT protocol. The lower total ragweed pollen count in year 2 could have contributed to the improved SS and CSMS exhibited by the San Antonio treatment group. However, without a parallel efficacy measurement improvement showed by the control group, the treatment group's efficacy measurement improvement is more likely a consequence of their clinical response to SCIT therapy. Furthermore, unlike the respective control group, the San Antonio and Dallas treatment groups’ medication utilization (ie, MS) significantly decreased in conjunction with specific pollen‐related improved SS, which further corroborates the efficacy of the immunotherapy protocol used.43
This corroboration of the efficacy of the UAS SCIT protocol holds under the assumption that exposure to perennial allergens are not significantly changed throughout the study period. Similar assumptions were considered in the analyses of monotherapy grass and ragweed studies in which 78% to 90% of the study subjects were polysensitized to other allergens.20, 21 As noted by those authors, the majority of U.S. patients manifest poly‐allergen sensitivities and are treated with poly‐allergen SCIT (as exemplified in the present study).
In our recently published study of the largest population of self‐administered SCIT patients studied to date, safety results were derived from the medical review of approximately 24,000 patients who had self‐administered over 2 million SCIT injections. The systemic reaction rate was only 0.16% per individual and 0.002% per injection in comparison to significantly higher office‐based rates of approximately 4% to 14%.12 Consistent with these results, several other reports have previously documented the safety of self‐administered or home‐based SCIT.13, 14, 15, 16, 17 The authors concluded that the low systemic reaction rates reported showed that home‐based immunotherapy was safe.13, 14, 15, 16, 17
The efficacy and safety of the UAS SCIT protocol are hypothesized to be due to: a slower than traditional SCIT buildup (ie, escalation) phase that attains recommended allergen concentrations on a cumulative dosing basis, a preselection of low‐risk patients, and emphasis on patient education.12 The rate of allergen dose increase during the buildup phase is considered a prominent factor in immunotherapy‐based systemic reactions.9, 65, 66, 67 Consistent with these principles, the UAS protocol was designed to incorporate a slow buildup phase in order to diminish the associated high frequency of systemic reactions.27
The cumulative administered allergen dose has been postulated to be the significant factor in SCIT efficacy.3, 4 Although we hypothesize that the efficacy of the UAS SCIT protocol is dependent upon cumulative allergen dosing as exemplified by the results of previous IT reports, further studies are warranted to substantiate this thesis.68, 69, 70, 71, 72
A weakness in this study, as evident in other retrospective studies, is the potential occurrence of recall bias.73 In order to minimize the effects of this potential bias, it has been recommended to use standardized well‐structured surveys.73 Consistent with those recommendations, we have employed well‐structured questionnaires for assessment of the SS, MS, CSMS, and the validated RQLQ questionnaire.37 Previously, several immunotherapy studies have encompassed similar retrospective periods of analysis, and other studies have used significantly longer periods with valid results despite possible recall bias.38, 39, 40, 41
Conclusion
In conclusion, our results show significant improvement in SS, MS, CSMS, RQLQ, and clinical relevancy associated with the use of the UAS SCIT protocol. These efficacy results, and our previously reported safety results, show that a carefully designed and implemented self‐administered SCIT protocol is both efficacious and safe.
Supporting information
Supporting Information
Acknowledgments
We thank Sylvana Research in San Antonio, TX, for supplying the pollen counts for San Antonio, and the Allergy Testing and Treatment Center in Dallas, TX, for supplying the pollen counts for the Dallas/Fort Worth area.
How to Cite this Article: Schaffer FM, Garner LM, Ebeling M, Adelglass JM, Hulsey TC, Naples AR. The efficacy assessment of a self‐administered immunotherapy protocol. Int Forum Allergy Rhinol. 2016;6:148–155.
Potential conflict of interest: F.M.S., A.R.N., and L.M.G. are employees of UAS. T.C.H. and M.E. are paid by UAS for their statistical services.
References
- 1. Soyer OU, Akdis M, Akdis CA. Mechanisms of subcutaneous allergen immunotherapy. Immunol Allergy Clin North Am. 2011;31:175–190. [DOI] [PubMed] [Google Scholar]
- 2. Cox L, Nelson H, Lockey R. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol. 2011;127(1 Suppl):S1‐S55. [DOI] [PubMed] [Google Scholar]
- 3. Eng PA, Borer‐Reinhold M, Heijnen IAFM, et al. Twelve‐year follow‐up after discontinuation of preseasonal grass pollen immunotherapy in childhood. Allergy. 2006;61:198–201. [DOI] [PubMed] [Google Scholar]
- 4. Jacobsen L, Niggemann B, Dreborg S, et al. Specific immunotherapy has long‐term preventive effect of seasonal and perennial asthma: 10‐year follow‐up on the PAT study. Allergy. 2007;62: 943–948. [DOI] [PubMed] [Google Scholar]
- 5. Durham SR, Walker SM, Varga EM, et al. Long‐term clinical efficacy of grass‐pollen immunotherapy. N Engl J Med. 1999;341:468–475. [DOI] [PubMed] [Google Scholar]
- 6. Schneider L, Tilles S, Lio P, et al. Atopic dermatitis: a practice parameter update 2012. J Allergy Clin Immunol. 2013;131:295–299. [DOI] [PubMed] [Google Scholar]
- 7. Caraballo JMS, Villa RC. Clinical and immunological changes of immunotherapy in patients with atopic dermatitis: randomized controlled trial. ISRN Allergy. 2012;2012:Article ID 183983. doi:10.5402/2012/183983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Panjo GB, Caminiti L, Vita D, et al. Sublingual immunotherapy in mite‐sensitized children with atopic dermatitis: a randomized, double‐blind, placebo‐controlled study. J Allergy Clin Immunol. 2007;120:164–170. [DOI] [PubMed] [Google Scholar]
- 9. Lin MS, Tanner E, Lynn J, et al. Nonfatal systemic allergic reactions induced by skin testing and immunotherapy. Ann Allergy. 1993;71:557–562. [PubMed] [Google Scholar]
- 10. Dretzke J, Meadows A, Novielli N, et al. Subcutaneous and sublingual immunotherapy for seasonal allergic rhinitis: a systemic review and indirect comparison. J Allergy Clin Immunol. 2013;131:1361–1366. [DOI] [PubMed] [Google Scholar]
- 11. White P, Smith H, Baker N, et al. A symptom control in patients with hay fever in UK general practice: how well are we doing and is there a need for allergen immunotherapy? Clin Exp Allergy. 1998;28:266–270. [DOI] [PubMed] [Google Scholar]
- 12. Schaffer FM, Naples AR, Ebeling M, Hulsey TC, Garner LM. The safety of self‐administered allergen immunotherapy during the buildup and maintenance phases. Int Forum Allergy Rhinol. 2015;5:149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cook PR, Bryant JL, Davis WE, et al. Systemic reactions to immunotherapy: the AAOA Morbidity and Mortality Survey. Otolaryngol Head Neck Surg. 1994;110:487–493. [DOI] [PubMed] [Google Scholar]
- 14. Wells JH. Home allergenic extract administration. J Allergy Clin Immunol. 1995;95:1061–1063. [DOI] [PubMed] [Google Scholar]
- 15. Falliers CJ. At‐home administration of allergenic extracts. J Allergy Clin Immunol. 1995;95:1061–1063. [DOI] [PubMed] [Google Scholar]
- 16. Filley WV. Safety of home immunotherapy. J Allergy Clin Immunol. 2006;117:S159. [Google Scholar]
- 17. Hurst DS, Gordon BR, Fornadley JA, et al. Safety of home‐based and office allergy immunotherapy: a multicenter prospective study. Otolaryngol Head Neck Surg. 1999;121:553–561. [DOI] [PubMed] [Google Scholar]
- 18. Cox L, Aaronson D, Casale TB, et al. Allergy immunotherapy safety: location matters! J Allergy Clin Immunol Pract. 2013;1:455–457. [DOI] [PubMed] [Google Scholar]
- 19. Nelson HS. Multiallergen immunotherapy for allergic rhinitis and asthma. J Allergy Clin Immunol. 2009;123:763–769. [DOI] [PubMed] [Google Scholar]
- 20. Cox LS, Casale TB, Nayak AS, et al. Clinical efficacy of 300IR 5‐grass pollen sublingual tablet in a US study: the importance of allergen‐specific serum IgE. J Allergy Clin Immunol. 2012;130:1327–1334. [DOI] [PubMed] [Google Scholar]
- 21. Skoner D, Gentile D, Bush R, et al. Sublingual immunotherapy in patients with allergic rhinoconjunctivitis caused by ragweed pollen. J Allergy Clin Immunol. 2010;125:660–666. [DOI] [PubMed] [Google Scholar]
- 22. Bernstein LI, Li JT, Bernstein DI, et al. Allergy diagnostic testing: an updated practice parameter. Ann Allergy Asthma Immunol. 2008;100:S1‐S148. [DOI] [PubMed] [Google Scholar]
- 23. Grier TJ, LeFevre DM, Duncan EA, et al. Stability of standardized grass, dust mite, cat, and short ragweed allergens after mixing with mold or cockroach extracts. Ann Allergy Asthma Immunol. 2007;99:151–160. [DOI] [PubMed] [Google Scholar]
- 24. Esch RE, Grier TJ. Allergen compatibilities in extract mixtures. Immunol Clin North Am. 2011;31:227–239. [DOI] [PubMed] [Google Scholar]
- 25. Bernstein DI, Wanner M, Borish L, et al. Twelve‐year survey of fatal reactions to allergen injections and skin testing: 1990–2001. J Allergy Clin Immunol. 2004;113:1129–1136. [DOI] [PubMed] [Google Scholar]
- 26. Reid MJ, Lockey RF, Turkeltaub PC, et al. Survey of fatalities from skin testing and immunotherapy 1985–1989. J Allergy Clin Immunol. 1993;91:6–15. [DOI] [PubMed] [Google Scholar]
- 27. Epstein TG, Liss GM, Murphy‐Berendts K, et al. Immediate and delayed‐onset systemic reactions after subcutaneous immunotherapy injections: ACAAI/AAAAI surveillance study of subcutaneous immunotherapy–year 2. Ann Allergy Asthma Immunol. 2011;107:426–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cox L, Li JT, Nelson HS, et al. Allergen immunotherapy: a practice parameter second update. J Allergy Clin Immunol. 2007;120:S25‐S85. [DOI] [PubMed] [Google Scholar]
- 29. Pfaar O, Demoly P, Gerth van Wijk R, et al. Recommendations for the standardization of clinical outcomes used in allergen immunotherapy trials for allergic rhinoconjunctivitis: EAACI position paper. Allergy. 2014;69:854–867. [DOI] [PubMed] [Google Scholar]
- 30. U.S. Department of Health and Human Services, Food and Drug Administration Center for Drug Evaluation and Research (CDER) . Guidance for Industry Allergic Rhinitis: Clinical Development Programs for Drug Products. Draft Guidance, 2000. Rockville, MD: U.S. Food and Drug Administration; 2000. http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm071293.pdf. Accessed September 15, 2015. [Google Scholar]
- 31. European Medicine Agency. Committee for Medicinal Products for Human Use (CHMP) . Guideline on the Clinical Development of Products for specific Immunotherapy for the Treatment of Allergic Diseases. London, UK: European Medicine Agency; 2008. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003605.pdf. Accessed September 15, 2015. [Google Scholar]
- 32. Stelmach I, Kaluzinska‐Parzyszek I, Jerzynska J, et al. Comparative effect of pre‐co‐seasonal and continuous grass sublingual immunotherapy in children. Allergy. 2012;67:312–320. [DOI] [PubMed] [Google Scholar]
- 33. Nelson HS, Nolte H, Creticos P, et al. Efficacy and safety of timothy grass allergy immunotherapy tablet treatment in North America adults. J Allergy Clin Immunol. 2011;127:72–80. [DOI] [PubMed] [Google Scholar]
- 34. Blaiss M, Maloney J, Nolte H, et al. Efficacy and safety of timothy grass allergy immunotherapy tablet treatment in North American children and adolescents. J Allergy Clin Immunol. 2011;127:64–71. [DOI] [PubMed] [Google Scholar]
- 35. Murphy K, Gawchik S, Bernstein D, et al. A phase 3 trial assessing the efficacy and safety of grass allergy immunotherapy tablet in subjects with grass pollen‐induced allergic rhinitis with or without conjunctivitis, with or without asthma. J Negat Results Biomed. 2013;12:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bufe A, Eberle P, Franke‐Beckmann E, et al. Safety and efficacy in children of an SQ‐standardized grass allergen tablet for sublingual immunotherapy. J Allergy Clin Immunol. 2009;123:167–173.e7. [DOI] [PubMed] [Google Scholar]
- 37. Juniper EF, Thompson AK, Ferrie PJ, Roberts JN. Validation of the standardized version of the Rhinoconjunctivitis Quality of Life Questionnaire. J Allergy Clin Immunol. 1999;104:364–369. [DOI] [PubMed] [Google Scholar]
- 38. Petersen K, Gyrd‐Hansen D, Kjaergarrd S, et al. Clinical and patient based evaluation of immunotherapy for grass pollen and mite allergy. Allergol Immunopathol (Madr). 2005;33:264–269. [DOI] [PubMed] [Google Scholar]
- 39. Petersen KD, Kronborg C, Larsen JN, et al. Patient related outcomes in a real life prospective follow up study: allergen immunotherapy increases quality of life and reduces sick days. World Allergy Organ J. 2013;6:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Han DH, Choi YS, Lee JE, et al. Clinical efficacy of sublingual immunotherapy in pediatric patients with allergic rhinitis sensitized to house dust mites: comparison to adult patients. Acta Otolaryngol. 2012;132:S88‐S93. [DOI] [PubMed] [Google Scholar]
- 41. Lee JE, Choi YS, Kim MS, et al. Efficacy of sublingual immunotherapy with house dust mite extract in polyallergen sensitized patients with allergic rhinitis. Ann Allergy Asthma Immunol. 2011;107:79–84. [DOI] [PubMed] [Google Scholar]
- 42. Kiotseridis H, Cilio CM, Bjermer L, et al. Grass pollen allergy in children and adolescents‐symptoms, health related quality of life and the value of pollen prognosis. Clin Transl Allergy. 2013;3:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pfaar O, Kleine‐Tebbe J, Hörmann K, Klimek L. Allergen‐specific immunotherapy: which outcome measures are useful in monitoring clinical trials? Immunol Allergy Clin North Am. 2011;31:289–309, x. [DOI] [PubMed] [Google Scholar]
- 44. Esch RE, Bush RK. Aerobiology of outdoor allergens In: Adkinson NF, Bochner BS, Busse WW, et al., eds. Middleton's Allergy: Principles and Practice. 7th ed. Philadelphia: Mosby Elsevier; 2003:510–512. [Google Scholar]
- 45. Cox L, Larenas‐Linnemann D, Lockey RF, et al. Speaking the same language: the World Allergy Organization subcutaneous immunotherapy systemic reaction grading system. J Allergy Clin Immunol. 2010;125:569–574. [DOI] [PubMed] [Google Scholar]
- 46. Erekosima N, Suarez‐Cuervo C, Ramanthan M, et al. Effectiveness of subcutaneous immunotherapy for allergic rhinoconjunctivitis and asthma: a systemic review. Laryngoscope. 2014;124:616–627. [DOI] [PubMed] [Google Scholar]
- 47. Canonica GW, Baena‐Cagnani CE, Bousquet J, et al. Recommendations for standardization of clinical trials with allergen specific immunotherapy for respiratory allergy. A statement of a World Allergy Organization (WAO) taskforce. Allergy. 2007;62:317–324. [DOI] [PubMed] [Google Scholar]
- 48. Malling HJ. Immunotherapy as an effective tool in allergy treatment. Allergy. 1998;53:461–472. [DOI] [PubMed] [Google Scholar]
- 49. Calderon MA, Alves B, Jacobsen M, et al. Allergen injection immunotherapy for seasonal allergic rhinitis. Cochrane Database Syst Rev. 2007;1:CD001936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Di Bona D, Plaia A, Leto‐Barone SF, et al. Efficacy of subcutaneous and sublingual immunotherapy with grass allergens for seasonal allergic rhinitis: a meta‐analysis‐based comparison. J Allergy Clin Immunol. 2012;130:1097–1107. [DOI] [PubMed] [Google Scholar]
- 51. Balda BR, Wolf H, Baumgarten C, et al. Tree‐pollen allergy is efficiently treated by short‐term immunotherapy (STI) with seven preseasonal injections of molecular standardized allergens. Allergy. 1998;53:740–748. [DOI] [PubMed] [Google Scholar]
- 52. Zenner HP, Baumgarten C, Rasp G, et al. Short term immunotherapy: a prospective, randomized, double‐blind, placebo‐controlled multicenter study of molecular standardized grass and rye allergens in patients with grass pollen‐induced allergic rhinitis. J Allergy Clin Immunol. 1997;100:23–29. [DOI] [PubMed] [Google Scholar]
- 53. Drachenberg KJ, Wheeler AW, Stuebner P, Horak F. A well‐tolerated grass pollen specific allergy vaccine containing a novel adjuvant, monophosphoryl lipid A, reduces allergic symptoms after only four preseasonal injections. Allergy. 2001;56:498–505. [DOI] [PubMed] [Google Scholar]
- 54. Pastorello EA, Pravettoni V, Incorvaia C, et al. Clinical and immunological effects of immunotherapy with alum‐absorbed grass allergoid in grass‐pollen‐induced hay fever. Allergy. 1992;47:281–290. [DOI] [PubMed] [Google Scholar]
- 55. Ortolani C, Pastorello EA, Incorvaia C, et al. A double‐blind, placebo‐controlled study of immunotherapy with an alginate‐conjugated extract of Parietaria judaica in patients with Parietaria hay fever. Allergy. 1994;49:13–21. [DOI] [PubMed] [Google Scholar]
- 56. Jutel M, Jaeger L, Meyer H, et al. Allergen‐specific immunotherapy with recombinant grass pollen allergens. J Allergy Clin Immunol. 2005;116:608–613. [DOI] [PubMed] [Google Scholar]
- 57. Ferrer M, Burches E, Peláez A, et al. Double‐blind, placebo‐controlled study of immunotherapy with Parietaria judaica: clinical efficacy and tolerance. J Investig Allergol Clin Immunol. 2005;15:283–292. [PubMed] [Google Scholar]
- 58. Corrigan CJ, Kettner J, Doemer C, et al. Efficacy and safety of preseasonal‐specific immunotherapy with an aluminium‐adsorbed six‐grass pollen allergoid. Allergy. 2005;60:801–807. [DOI] [PubMed] [Google Scholar]
- 59. Colás C, Monzón S, Venturini M, Lezaun A. Double‐blind, placebo‐controlled study with a modified therapeutic vaccine of Salsola kali (Russian thistle) administered through use of a cluster schedule. J Allergy Clin Immunol. 2006;117:810–816. [DOI] [PubMed] [Google Scholar]
- 60. Creticos PS, Schroeder JT, Hamilton RG, et al. Immunotherapy with a ragweed‐toll‐like receptor 9 agonist vaccine for allergic rhinitis. N Engl J Med. 2006;355:1445–1455. [DOI] [PubMed] [Google Scholar]
- 61. Frew AJ, Powell RJ, Corrigan CJ, et al. Efficacy and safety of specific immunotherapy with SQ allergen extract in treatment‐resistant seasonal allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2006;117:319–325. [DOI] [PubMed] [Google Scholar]
- 62. Powell RJ, Frew AJ, Corrigan CJ, Durham SR. Effect of grass pollen immunotherapy with Alutard SQ on quality of life in seasonal allergic rhinoconjunctivitis. Allergy. 2007;62:1335–1338. [DOI] [PubMed] [Google Scholar]
- 63. Walker SM, Pajno GB, Lima MT, et al. Grass pollen immunotherapy for seasonal rhinitis and asthma: a randomized, controlled trial. J Allergy Clin Immunol. 2001;107:87–93. [DOI] [PubMed] [Google Scholar]
- 64. Pfaar O, Robinson DS, Sager A, Emuzyte R. Immunotherapy with depigmented‐polymerized mixed tree pollen extract: a clinical trial and responder analysis. Allergy. 2010;65:1614–1621. [DOI] [PubMed] [Google Scholar]
- 65. Davis WE, Cook PR, Mckinsey JP, et al. Anaphylaxis in immunotherapy. Otolaryng Head Neck Surg. 1992;107:78–83. [DOI] [PubMed] [Google Scholar]
- 66. Tinkelman DG, Cole WQ 3rd, Tunno J. Immunotherapy: a one‐year prospective study to evaluate risk factors of systemic reactions. J Allergy Clin Immunol. 1995;95:8–14. [DOI] [PubMed] [Google Scholar]
- 67. Iliopoulos O, Proud D, Atkinson F, et al. Effects of immunotherapy on the early, late, and rechallenge nasal reaction to provocation with allergen: changes in inflammatory mediators and cells. J Allergy Clin Immunol. 1991;87:855–866. [DOI] [PubMed] [Google Scholar]
- 68. Tworek D, Bochenska‐Marciniak M, Kuprys‐Lipinska I, et al. Perennial is more effective than preseasonal subcutaneous immunotherapy in the treatment of seasonal allergic rhinoconjunctivitis. Am J Rhinol Allergy. 2013;27:304–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Creticos PS, Marsh DG, Proud D, et al. Responses to ragweed‐pollen nasal challenge before and after immunotherapy. J Allergy Clin Immunol. 1989;84:197–205. [DOI] [PubMed] [Google Scholar]
- 70. Haugaard L, Dahl R, Jacobsen L. A controlled dose‐response study of immunotherapy with standardized, partially purified extract of house dust mite: clinical efficacy and side effects. J Allergy Clin Immunol. 1993;91:556–566. [DOI] [PubMed] [Google Scholar]
- 71. Ewbank PA, Murray J, Sanders K, et al. A double‐blind, placebo‐controlled immunotherapy does‐response study with standardized cat extract. J Allergy Clin Immunol. 2003;111:155–161. [DOI] [PubMed] [Google Scholar]
- 72. Lent A, Harbeck R, Strand M, et al. Immunological response to administration of standardized dog allergen extract at differing doses. J Allergy Clin Immunol. 2006;118:1249–1256. [DOI] [PubMed] [Google Scholar]
- 73. Hassan E. Recall bias can be a threat to retrospective and prospective research designs. Internet J Epidemiol. 2005;3(2):1–6. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


