Abstract
Background
We evaluated thrombotic and bleeding outcomes in patients with continuous flow left ventricular assist devices (CF-LVADs), stratified by anticoagulation intensity. Previous studies of outpatients with CF-LVADs have suggested that target international normalized ratio (INR) values less than 2.5 (range 2-3) may be used. However, recent studies reported an increase in pump thrombosis among CF-LVADs, especially within the first 6 months of implant.
Methods and Results
We retrospectively reviewed 249 outpatients at our center who received a CF-LVAD between 1/2005 and 8/2013. Using Poisson models we analyzed their 10,927 INRs to determine INR-specific rates of thrombotic (ischemic stroke and suspected pump thrombosis) and hemorrhagic (gastrointestinal bleeding and hemorrhagic stroke) events occurring outside of the hospital. In multivariate analyses, we adjusted for age, sex, atrial fibrillation, coronary disease, and LVAD type as time-dependent Cox proportional hazard models. During a mean follow-up of 17.6 ± 13.6 months, thrombotic events occurred in 46 outpatients. The highest event rate (0.40 thrombotic events per patient-year) was in the INR range of < 1.5, but INR values of 1.5-1.99 also had high rates (0.16 thrombotic events per patient-year). INR was inversely associated with thrombotic events (HR 0.40, 95% 0.22-0.72; P = 0.002). The optimal INR based on weighted mortality of thrombotic and bleeding events was 2.6.
Conclusions
INR is inversely related to thrombotic events occurring outside of the hospital among patients supported with CF-LVADs. INR values less than 2.0 increase the rate of thrombotic events occurring outside of the hospital among patients supported with CF-LVADs.
Keywords: left ventricular assist device, anticoagulation, stroke, bleeding, pump thrombosis
Continuous-flow left ventricular assist devices (CF-LVAD) have become the standard of care for medically refractory, end stage heart failure (HF) as either bridge to transplant (BTT) or destination therapy (DT).1-3 In spite of their success in improving mortality and quality of life, thrombotic and bleeding events remain significant complications.1 In a secondary analysis of the HeartMate II Bridge-to-Transplant (BTT) trial, the incidence of thrombotic events (confirmed pump thrombosis and ischemic stroke) and hemorrhagic events within 6 months of discharge were found to be 2.7% and 12.9% respectively.4 Whereas the trial by the HeartMate II Investigators specified an international normalized ratio (INR) goal of 2.0-3.0,2 the relatively greater burden of hemorrhagic events in this trial and the HeartMate II BTT trial4 fostered the conclusion that a target INR of 1.5 to 2.5 (in addition to aspirin therapy) might be safer. Single center studies also found low rates of thrombotic events and suggested that INRs less than 2.0 were acceptable as was withholding warfarin for those at high risk of bleeding.5 Practice patterns therefore have tended towards lower INR goals.
Over time, the question of anticoagulation in LVAD support has become complicated. In the HeartMate II DT Pivotal Study the incidence of thrombotic events (defined as confirmed pump thrombosis or ischemic stroke) was higher than the BTT trial study - 16% at 1.7 years of followup.2 The Pivotal Study used warfarin with a goal INR between 2.0 and 3.0, in addition to aspirin. Subsequently, analysis of the HeartWare ADVANCE trial noted INR < 2.0 as a risk factor for pump thrombosis.6 Finally, more recently others have reported an increase in pump thrombosis among CF-LVADs, especially within the first 6 months of implant.7, 8
The reason for increased thrombosis is not clear, but is likely multifactorial, with gastrointestinal bleeding, infection, varying anticoagulation bridging strategies, and suboptimal INRs potentially contributing. Additionally, patient selection has changed since FDA approval for Destination Therapy in January 2010, allowing more implants in patients potentially at higher risk of thrombosis. The objective of this study was to examine the association between INR and both thrombotic and hemorrhagic events amongst outpatients supported with CF-LVADs.
Methods
Study Population
We retrospectively identified patients that underwent implantation of a HeartMate II ® (Thoratec Corp., Pleasanton, CA) or HVAD® (HeartWare Corp., Framingham, MA) between January 2005 and August 2013 at our center. Inclusion criteria for the present study were: CF-LVAD placement, age 18 years or older, survived to discharge, and followed up at our institution. Exclusion criteria included patients who underwent repeat LVAD implantation (i.e., pump exchange), or patients who had a hemorrhagic or thrombotic event prior to discharge from their implant hospitalization.
Patients were censored at the time of transplantation, LVAD explant or exchange, death, or last known follow up. To avoid confounding by indication, patients were censored at their first hemorrhagic event (intracranial hemorrhage (ICH) or gastrointestinal bleeding (GIB)), or thrombotic event (suspected pump thrombosis or ischemic stroke)-i.e. a patient could only contribute a maximum of one thrombotic event and/or one hemorrhagic event. Only events occurring outside of the hospital were included; if a patient had a thrombotic or hemorrhagic event while an inpatient they were censored.
Data Collection
Clinical data, including baseline characteristics, medications, blood products, and outcomes, were abstracted from the electronic medical record. The data were managed in the Research Electronic Data Capture (REDCap) database.9 The Institutional Review Board at Washington University in St. Louis approved the study.
Institutional protocol dictates outpatient INRs are checked weekly when not in therapeutic range, and every other week when within therapeutic range. The target therapeutic range for our patients supported with HeartMate II evolved: from 2005-2008 it was 2.0-2.5, from 2008-2010 it was 1.5-2.0, and from 2010-curent it was 2.0-3.0. The target INR range for patients supported with HeartWare VAD was 2.0-3.0 throughout.
We used the INR at time of the adverse event. In patients treated with vitamin K, fresh frozen plasma, and/or blood transfusion prior to measuring the INR, we used the INR before treatment. In patients admitted with a pump thrombosis, we used the last available outpatient INR (because pump thrombosis often starts with a subclinical phase and can elevate the INR).
Definitions and outcomes
Suspected pump thrombosis was defined as observation of obstructive thrombus in the pump or conduit post pump exchange or severe hemolysis. Severe hemolysis was defined as lactate dehydrogenase (LDH) level greater than 1,000 mg/dL (4 times the upper limit of normal for our laboratory) or plasma free hemoglobin level greater than 40 mg/dL with symptoms of decompensated HF in the absence of a kinked inflow or outflow cannula. An LDH value >3.5 times the upper limit of normal has excellent specificity and sensitivity for the diagnosis of pump thrombosis.10
GI bleeding was defined as clinically evident or occult GI bleeding prompting hospital admission and endoscopic evaluation. Severe anemia requiring blood transfusion in the absence of hemolysis and a bleeding source was classified as occult bleeding.
Stroke was identified as an acute neurological deficits persisting for greater than 24 hours. A stroke was classified as hemorrhagic or ischemic based on head CT. If the head CT was read as ischemic stroke with hemorrhagic conversion, or both ischemic and hemorrhagic stroke, then the stroke was classified as ischemic.
Weights of Thrombotic and Bleeding Events
To balance the benefits and risks of more intensive anticoagulation, events were weighted based on their expected 30-day mortality rates. Mortality rates were calculated from the onset of adverse event by averaging internal data based on 455 CF-LVAD patients (Appendix) with published mortality data of ischemic stroke,11, 12 intracranial hemorrhage,12, 13 GI bleeding14-16 and pump thrombosis.7, 17 The baseline mortality rate in our LVAD population (N = 455) was 0.036 deaths per month. To this baseline mortality, we added the incremental mortality rate (per month) from an adverse event (Appendix): Pump thrombosis 0.16, GIB 0.01, ischemic stroke 0.11, and ICH 0.49. Of note the mortality rate after LVAD related GI bleeding varied from 0 to 0.02 deaths per patient-month.
Statistical analysis
When calculating event rates, the numerator was the number of adverse events. The denominator was days in each INR range, as calculated according to Rosendaal and colleagues’ method for linear interpolation.18 The rate for each INR category was calculated using Poisson regression via generalized estimating equations to account for correlated data (Supplementary Appendix).
The optimal INR was determined by combining exponential models created for thrombosis and for bleeding rates. Rates for each INR category were log-transformed and the mean INR value within each range was used as the independent variable to develop the two exponential models. To prevent taking the logarithm of 0, the constant 0.1 was added to each thrombosis rate. (An advantage of this approach is that it avoids relying on the Yates correction for continuity, which is conservative for two-sided tests with rare events).19 When developing the exponential models, INR ranges were weighted by the frequency of observations.
The sum of the exponential bleeding and thrombosis models was obtained by weighting each based on mortality-associated event rates obtained independently (as detailed above). This weighted U-shaped curve was then used to determine the optimal INR value that minimized mortality.
The association between INR and clinical events was further quantified in a multivariable analysis that used Cox regression. Time to event was left-truncated to account for time 0 being implant date but hazard-time not beginning until discharge. Individuals were part of the at-risk group once their first INR was measured. INR was allowed to vary over time to account for repeated measurements taken during follow up. Separate Cox models were created for both event types (bleeding, thrombosis). Cox models adjusted for age, gender, LVAD type (HeartMate II or HeartWare), history of atrial fibrillation and history of coronary artery disease as these covariates influence bleeding or thrombotic events.8, 15, 20 Additionally, the interaction of INR and time was investigated to determine if the association between INR and clinical event changed over time. Time was categorized into discharge-3 months, 3-6 months, and greater than 6 months based on when INR was obtained from time of implant.
Significance was identified as a two-sided alpha < 0.05. Analyses were conducted in SAS v9.4 (SAS Institute Inc., Cary, NC).
Results
Patient population
A total of 305 patients received a CF-LVAD from 1/2005 until 8/2013 and were discharged alive. Fifty-six patients were excluded from analysis: 20 were LVAD exchanges, 11 had thrombotic events during their index stay, 4 were followed at other institutions, and 21 had incomplete data due to censoring before 2 or more consecutive outpatient INRs were obtained. The analysis cohort consisted of 249 patients.
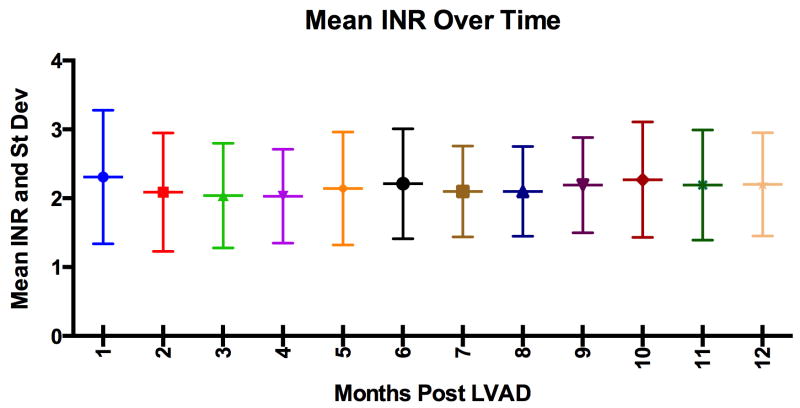
The 249 patients analyzed had a median outpatient follow-up of 17.6 ± 13.6 months with 10,927 INR measurements available, an average of 1 INR every 11.8 days. Most patients were male (81%), classified as INTERMACS profile 2, and bridge-to-transplant approach was used for 69% of the patients (Table 1). Nearly all patients were discharged on warfarin and aspirin (96%), with 45% of patients being on 325 mg of aspirin and 51% on 81 mg of aspirin. The mean INR at discharge after LVAD implant was 2.18 ± 0.62, and during outpatient follow-up the mean INR was 2.16 ± 0.77 (Figure 1).
Table 1.
Baseline characteristics of entire cohort:
| N = 249 | Value |
|---|---|
|
| |
| Age mean, range (years) | 55.5 (18.12- 78.8) |
| Male No. (%) | 195 (78) |
| Caucasian No. (%) | 190 (76) |
| BMI (kg/m2) mean, range | 28.5 (17.3-49) |
| LOS implant to discharge (days) | 21.5 ± 16.5 |
| Medical history | |
| Atrial fibrillation No. (%) | 105 (42) |
| Current smoker No. (%) | 27 (11) |
| CAD No. (%) | 123 (49) |
| DM No. (%) | 107 (43) |
| INTERMACS profile median (IQ range) | 2.0 (1.0, 2.0) |
| Intracardiac thrombus No. (%) | 28 (11) |
| Bridge to transplant No. (%) | 173 (69) |
| Labs on discharge | |
| AST | 51.3 ± 41.6 |
| Hgb (g/dL) | 9.58 ± 1.34 |
| INR | 2.18 ± 0.62 |
| PLT | 331 ± 131 |
| Aspirin on discharge No. (%) | 244 (98) |
| Aspirin dose | |
| 325 mg daily No. (%) | 112 (45) |
| 81 mg daily No. (%) | 137 (55) |
| Warfarin on discharge No. (%) | 240 (96) |
Values are shown as absolute numbers (percentages), mean ± SD, or median (IQR). BMI = body mass index; CAD = coronary artery disease; DM = diabetes mellitus; Hgb = hemoglobin; LOS = length of stay.
Figure 1.
International normalized ratio (INR) measurements after hospital discharge with a left ventricular assist device (LVAD). The horizontal line in the middle of each box indicates the mean INR for the month; and the whiskers mark the standard deviation.
Thrombotic Events
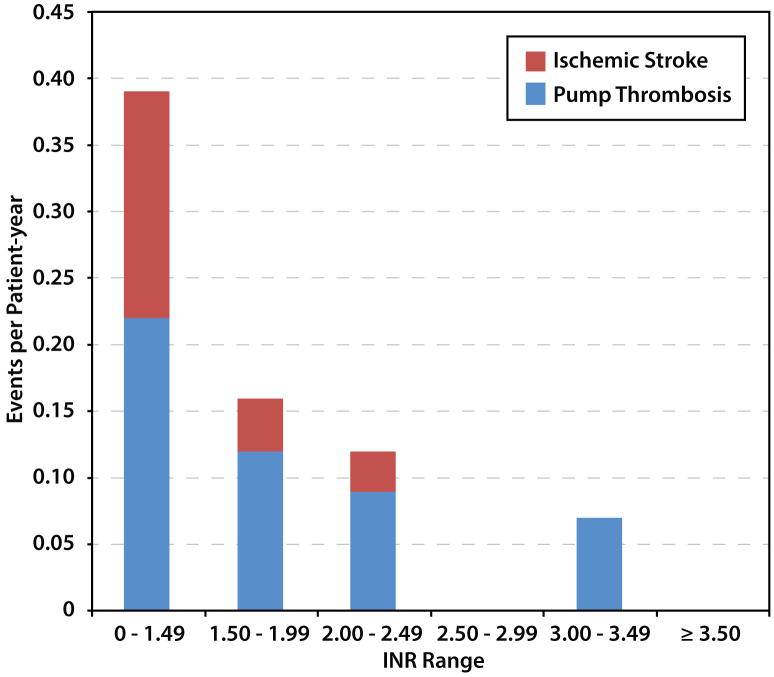
A total of 46 thrombotic events occurred: 32 suspected pump thrombosis and 14 ischemic strokes. The highest event rate (0.40 events per patient-year) occurred in the INR range of <1.5, but INR values of 1.5-1.99 also had high rates (0.16 thrombotic events per patient-year). Among the INRs > 2.5 categories, there was no episode of ischemic stroke, and only 1 episode of suspected pump thrombosis (Figure 2a). Twenty-three of the 32 patients who developed the suspected pump thrombosis expired, had pump exchange, or were transplanted within 90 days of hemolysis. Only 3 of the 32 survived for greater than 1 year with their index LVAD with all 3 having resolution of hemolysis with stronger anticoagulants.
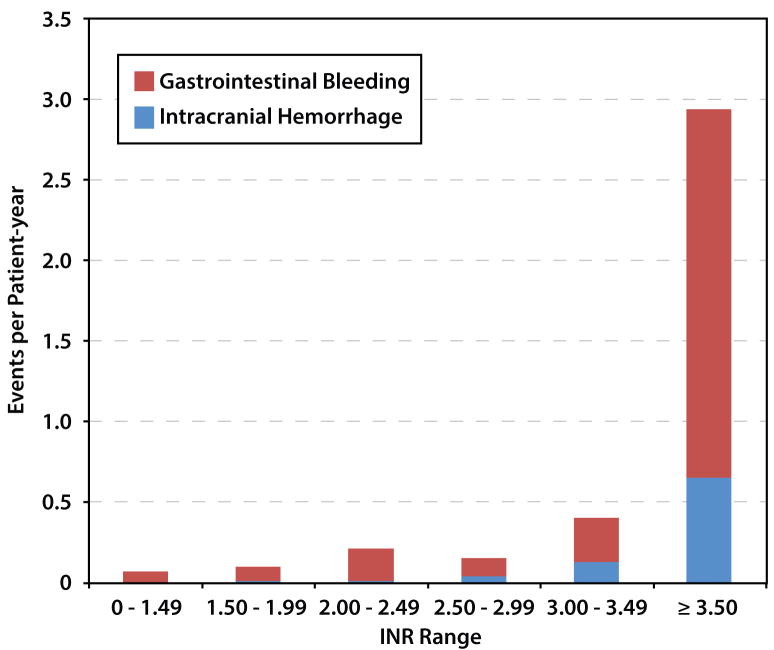
Figure 2.
(A) Thrombotic and (B) hemorrhagic event rates as a function of international normalized ratio (INR) at time of event per total patient years in a given INR range.
In univariate analysis, INR was inversely associated (P = 0.002) with thrombotic events (HR 0.39, 95% CI 0.22-0.70 per 1-unit increase in INR) (Table 2). In Cox proportional hazard models adjusting for age, sex, atrial fibrillation, coronary disease, and LVAD type, INR remained inversely associated with thrombotic events (HR 0.40, 95% CI 0.22-0.72; P = 0.002) (Table 3). The inverse association between INR and thrombotic events was statistically significant for the 3-6 month and > 6 month post-implant timeframe, but was not significant prior to 3 months (HR 0.75, 95% CI 0.34-1.65; P = 0.47) (Table 2). The lack of significance between INR and thrombotic events before 3 months was in spite of this period being the time of highest incidence rate of pump thrombosis (0.37 events per patient-year). Comparing the HR of thrombotic event by INR before 3 months to after 3 months bordered on statistical significance (0.75 vs. 0.25, p = 0.05).
Table 2.
Hazard ratio for thrombotic events as a function of INR
| HR | 95% CI | p-value | Events PPY | |
|---|---|---|---|---|
| INR overall | 0.39 | (0.22, 0.70) | 0.002 | 0.14 |
| INR Discharge- 3 months* | 0.75 | (0.34, 1.65) | 0.47 | 0.37 |
| INR >3-6 months | 0.201 | (0.04, 0.94) | 0.041 | 0.18 |
| INR > 6 months | 0.269 | (0.11, 0.67) | 0.005 | 0.09 |
Hazard ratio shown per 1 unit increase in INR
p = 0.05 when compared to INR > 3 months
Table 3.
Hazard ratio for thrombotic events based on multivariate Cox model
| HR | 95% CI | p-value | |
|---|---|---|---|
| INR (per 1 unit increase) | 0.40 | (0.22, 0.72) | 0.002 |
| Age (per 1 year increase) | 0.98 | (0.96, 1.000) | 0.06 |
| Gender (Male vs. female) | 0.91 | (0.45, 1.89) | 0.80 |
| LVAD type (HMII vs. HW) | 0.90 | (0.31, 2.59) | 0.84 |
| AFIB hx (yes vs. no) | 0.71 | (0.37, 1.34) | 0.29 |
| CAD hx (yes vs. no) | 1.05 | (0.55, 2.02) | 0.88 |
Hemorrhagic Events
A total of 62 hemorrhagic endpoints occurred: 53 GI bleeds and 9 ICHs. The highest bleeding event rate (1.4 events per patient year) occurred in the INR range >3.5, and the lowest event rates (≤0.1 events per patient year) were in with the lowest INR categories (Figure 2b). There were 7 episodes of ICH in those with INR > 2.5, and only 2 episodes of ICH with INR < 2.5.
In univariate analysis, INR was associated with hemorrhage (HR 1.63, 95% CI 1.41-1.88; P < .001) (Table 4). In a Cox proportional hazard models that adjusted for age, sex, atrial fibrillation, coronary disease, and LVAD type, INR remained associated with hemorrhage (HR 1.66, 95% CI 1.43.-1.93; P < 0.001) (Table 5). When evaluated with a time interaction to determine whether the relationship of INR and hemorrhagic events changed over time, increased INR remained significant across all time periods (Table 4). INR was also found to have a greater association with hemorrhagic events before 3 months vs. >3-6 months (0-3 months HR = 2.33 vs. >3-6 months HR = 1.44, p = 0.021) and trended towards significance when compared to >6 months (HR = 1.60, p = 0.06).
Table 4.
Hazard ratio for bleeding events as a function of INR
| HR | 95% CI | p-value | |
|---|---|---|---|
| INR | 1.63 | (1.41, 1.88) | <.001 |
| INR Discharge - 3 months interaction | 2.33 | (1.70, 3.20) | <.001 |
| INR 3-6 months interaction | 1.43* | (1.10, 1.87) | 0.007 |
| INR >6 months interaction | 1.60** | (1.26, 2.02) | <.001 |
Hazard ratio shown per 1 unit increase in INR
p = 0.021 when compared to INR ≤ 3 months
p < 0.06 when compared to INR ≤ 3 months
Table 5.
Hazard ratio for bleeding events based on multivariate Cox model
| HR | 95% CI | p-value | |
|---|---|---|---|
| INR (per 1 unit increase) | 1.66 | (1.43, 1.93) | <.001 |
| Age (per 1 year increase) | 1.026 | (1.00, 1.05) | 0.05 |
| Gender (Male vs. female) | 0.99 | (0.51, 1.90) | 0.97 |
| LVAD type (HMII vs. HW) | 0.70 | (0.34, 1.45) | 0.33 |
| AFIB hx (yes vs. no) | 1.30 | (0.77, 2.20) | 0.33 |
| CAD hx (yes vs. no) | 0.85 | (0.49, 1.48) | 0.57 |
Optimal INR range
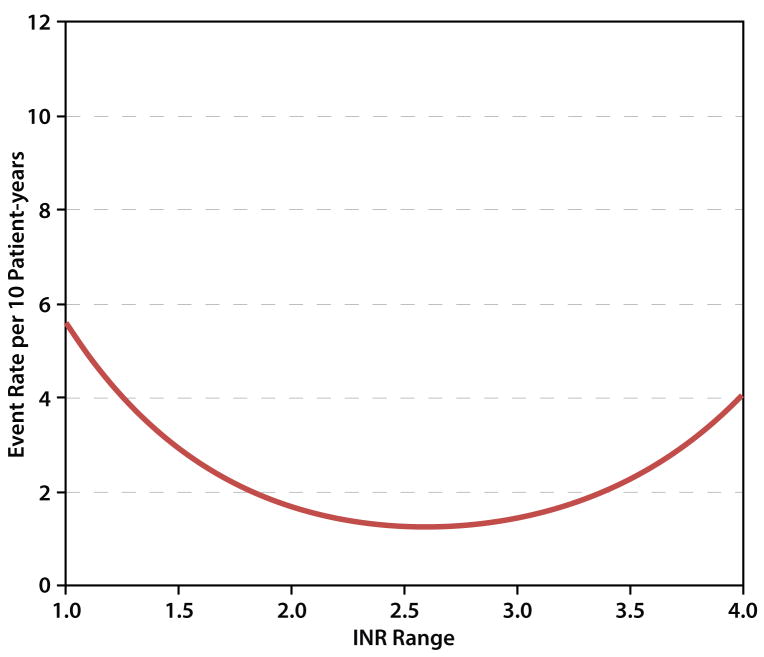
The optimal INR was determined to be 2.6, with low rates of adverse events falling between INR values of 2.0-3.2. A model with no weighting (equal mortality weights for all events) favored an INR of 2.4. (Figure 3). Sensitivity analyses were performed by varying the weights of events over their plausible ranges, with minor effect on the optimal INR of 2.6 (Supplementary Appendix Fig S3).
Figure 3.
Thrombotic and hemorrhagic event rates as a function of international normalized ratio (INR) at time of event per total patient years.
Discussion
Anticoagulation management of outpatients with CF-LVADs remains a delicate balance between avoiding hemorrhagic and thrombotic complications. Previous studies suggested that low rates of thrombotic events may allow for lower INRs for those at high risk of bleeding,4, 5 but reports of higher rates of thrombosis have cast doubt upon this assertion.7,8 Here we characterized the relationship between outpatient management of anticoagulation and both thrombotic and hemorrhagic events occurring outside of the hospital.
By analyzing nearly 11,000 outpatient INRs among 249 outpatients, we demonstrated that thrombotic outcomes (suspected pump thrombosis and ischemic stroke) were highest amongst the lowest INR range (<1.5), but INR values of 1.5-1.99 also had high rates (0.16 thrombotic events per patient-year). Although the finding that lower INR was associated with thrombosis was hypothesized, the observed time-sensitive relationship between INR and thrombosis was unexpected. This lack of a statistically significant association between INR and thrombotic events in the 0-3 month timeframe suggests that early pump thrombosis may be affected by INR-independent events during the index hospitalization, such as operative or device characteristics and intensity of post-operative bridging anticoagulation. Conversely, after the early post-surgical period, anticoagulation intensity predicts pump thrombosis.
An optimal INR based on weighted mortality of bleeding and thrombotic events was 2.6. In the future it might be interesting to develop patient-specific anticoagulation strategies based on risk models that stratify a patient’s propensity to have hemorrhagic and thrombotic complications.
Our conclusions differ from an analysis of from the HeartMate II Pivotal trial. That study did not find a statistical association between lower INR and thrombotic events, but there were no ischemic strokes when the INR exceeded 2.0.4 The different conclusions between studies may have been driven by the greater rate of suspected pump thrombosis that we observed (12.9% of patients over a mean follow-up of 17.6-months). Our rate was higher because we included persistent hemolysis in our definition of suspected pump thrombosis. In contrast, the HeartMate II Pivotal trial captured only a thrombus in the device or its conduits. Our event rate is commensurate to what Starling and others reported (e.g. 12.3% at one year of follow up).7, 21 Our study had high precision because we included 11,000 INR values over a mean follow up of 17.6 months, whereas the HeartMate II Pivotal trial evaluated 1294 INR values over 6months of follow-up.
When interpreting the results of our study, several limitations must be considered. The study is retrospective and non-randomized. To reduce confounding by indication, we censored patients at the time of their first event. However, patients at higher risk of thrombotic events may have been assigned higher INRs goals from the outset. Censoring patients after their first event and excluding events occurring in the hospital both lead to underestimation of overall event rates. There are inherent limitations to the timing of INR measures and clinical events. For example, it is possible that our associations with INR and hemorrhagic events are overestimated due to the presence of a consumptive coagulopathy. In terms of secular trends in anticoagulation management, the rate of pump thrombosis has been increasing over time, and anticoagulation and antiplatelet regimens have varied over time.7, 8, 22 Although we did not quantify von-Willebrand factor or platelet function, other LVAD studies have found that acquired von-Willebrand s contributes to GI bleeding.23 Furthermore, screening for thrombosis has intensified, potentially resulting in more frequent detection of suspected pump thrombosis. Similarly, given our modest sample size, some putative risks for thrombosis, such as non type O blood type, were not analyzed.24
Finally, our event weighting may not be optimal because it was based on local mortality rates. There is almost no literature describing provider or patient weighting of LVAD-associated adverse events. Given that pump thrombosis is associated with >50% one year mortality or major surgery with high morbidity, we chose to weigh it slightly greater than stroke. Literature in non-VAD supported patients has weighted GI bleeding anywhere between 0 and 0.6. 25 Modeling of optimal INR with a priori patient- or provider-derived weights for different events may a reasonable framework for prospective analyses of anticoagulation management.
In conclusion, INR and hemorrhagic events were highly correlated and INR and thrombotic events were inversely correlated. By considering both bleeding and thrombotic events, an optimal INR was determined to be 2.6, with low rates of adverse events falling between INR values of 2.0-3.2.
Supplementary Material
Clinical Perspective.
The approach to anticoagulation after continuous-flow LVAD implantation has varied over time. Prior studies have suggested lower INR targets may have more acceptable risk profiles. In light of a subsequently noted increase in thrombotic complications, more aggressive anticoagulation strategies have again been adopted, though evidence to support this approach is limited. We conducted a retrospective study to examine the association between INR and both thrombotic and hemorrhagic events amongst outpatients supported with CF-LVADs. In reviewing event rates by INR range for 249 patients, we demonstrate increased rates of thrombotic complications in ranges with an INR below 2 and increased rates of hemorrhagic complications with an INR above 3, providing support for the current INR goal range of 2 to 3. Additionally, weighting each complication by its associated mortality provided a similar goal range, with an ‘ideal’ INR of 2.6. This study is the first to provide substantive evidence supporting the current outpatient INR goal of 2 to 3 in continuous-flow LVAD supported patients.
Acknowledgments
Sources of Funding
This study was supported in part by research funds from the National Institutes of Health (NIH grant U10 HL110309, Heart Failure Network), (NIH Grant T32HL110837) and Grant # UL1 TR000448 R01 HL097036.
Footnotes
Disclosures
GAE receives consulting fees from Thoratec. No other relevant conflicts. Content is solely the responsibility of the authors.
References
- 1.Kirklin JK, Naftel DC, Kormos RL, Stevenson LW, Pagani FD, Miller MA, Timothy Baldwin J, Young JB. Fifth INTERMACS annual report: risk factor analysis from more than 6,000 mechanical circulatory support patients. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2013;32:141–56. doi: 10.1016/j.healun.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 2.Slaughter MS, Rogers JG, Milano CA, Russell SD, Conte JV, Feldman D, Sun B, Tatooles AJ, Delgado RM, 3rd, Long JW, Wozniak TC, Ghumman W, Farrar DJ, Frazier OH HeartMate III. Advanced heart failure treated with continuous-flow left ventricular assist device. The New England journal of medicine. 2009;361:2241–51. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 3.Griffith BP, Kormos RL, Borovetz HS, Litwak K, Antaki JF, Poirier VL, Butler KC. HeartMate II left ventricular assist system: from concept to first clinical use. The Annals of thoracic surgery. 2001;71:S116–20. doi: 10.1016/s0003-4975(00)02639-4. discussion S114-6. [DOI] [PubMed] [Google Scholar]
- 4.Boyle AJ, Russell SD, Teuteberg JJ, Slaughter MS, Moazami N, Pagani FD, Frazier OH, Heatley G, Farrar DJ, John R. Low thromboembolism and pump thrombosis with the HeartMate II left ventricular assist device: analysis of outpatient anti-coagulation. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2009;28:881–7. doi: 10.1016/j.healun.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 5.John R, Kamdar F, Liao K, Colvin-Adams M, Miller L, Joyce L, Boyle A. Low thromboembolic risk for patients with the Heartmate II left ventricular assist device. The Journal of thoracic and cardiovascular surgery. 2008;136:1318–23. doi: 10.1016/j.jtcvs.2007.12.077. [DOI] [PubMed] [Google Scholar]
- 6.Najjar SS, Slaughter MS, Pagani FD, Starling RC, McGee EC, Eckman P, Tatooles AJ, Moazami N, Kormos RL, Hathaway DR, Najarian KB, Bhat G, Aaronson KD. Boyce SW and Investigators HBtTAT. An analysis of pump thrombus events in patients in the HeartWare ADVANCE bridge to transplant and continued access protocol trial. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2014;33:23–34. doi: 10.1016/j.healun.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Starling RC, Moazami N, Silvestry SC, Ewald G, Rogers JG, Milano CA, Rame JE, Acker MA, Blackstone EH, Ehrlinger J, Thuita L, Mountis MM, Soltesz EG, Lytle BW, Smedira NG. Unexpected abrupt increase in left ventricular assist device thrombosis. The New England journal of medicine. 2014;370:33–40. doi: 10.1056/NEJMoa1313385. [DOI] [PubMed] [Google Scholar]
- 8.Kirklin JK, Naftel DC, Kormos RL, Pagani FD, Myers SL, Stevenson LW, Acker MA, Goldstein DL, Silvestry SC, Milano CA, Timothy Baldwin J, Pinney S, Eduardo Rame J, Miller MA. Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) analysis of pump thrombosis in the HeartMate II left ventricular assist device. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2014;33:12–22. doi: 10.1016/j.healun.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics. 2009;42:377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shah P, Mehta VM, Cowger JA, Aaronson KD, Pagani FD. Diagnosis of hemolysis and device thrombosis with lactate dehydrogenase during left ventricular assist device support. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2014;33:102–4. doi: 10.1016/j.healun.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 11.Willey JZ, Demmer RT, Takayama H, Colombo PC, Lazar RM. Cerebrovascular disease in the era of left ventricular assist devices with continuous flow: risk factors, diagnosis, and treatment. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2014;33:878–87. doi: 10.1016/j.healun.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 12.Morgan JA, Brewer RJ, Nemeh HW, Gerlach B, Lanfear DE, Williams CT, Paone G. Stroke while on long-term left ventricular assist device support: incidence, outcome, and predictors. ASAIO journal. 2014;60:284–9. doi: 10.1097/MAT.0000000000000074. [DOI] [PubMed] [Google Scholar]
- 13.van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. The Lancet Neurology. 2010;9:167–76. doi: 10.1016/S1474-4422(09)70340-0. [DOI] [PubMed] [Google Scholar]
- 14.Morgan JA, Paone G, Nemeh HW, Henry SE, Patel R, Vavra J, Williams CT, Lanfear DE, Tita C, Brewer RJ. Gastrointestinal bleeding with the HeartMate II left ventricular assist device. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2012;31:715–8. doi: 10.1016/j.healun.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 15.Kushnir VM, Sharma S, Ewald GA, Seccombe J, Novak E, Wang IW, Joseph SM, Gyawali CP. Evaluation of GI bleeding after implantation of left ventricular assist device. Gastrointestinal endoscopy. 2012;75:973–9. doi: 10.1016/j.gie.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aggarwal A, Pant R, Kumar S, Sharma P, Gallagher C, Tatooles AJ, Pappas PS, Bhat G. Incidence and management of gastrointestinal bleeding with continuous flow assist devices. The Annals of thoracic surgery. 2012;93:1534–40. doi: 10.1016/j.athoracsur.2012.02.035. [DOI] [PubMed] [Google Scholar]
- 17.Nassif ME, Patel JS, Shuster JE, Raymer DS, Jackups R, Jr, Novak E, Gage BF, Prasad S, Silvestry SC, Ewald GA, LaRue SJ. Clinical outcomes with use of erythropoiesis stimulating agents in patients with the HeartMate II left ventricular assist device. JACC Heart failure. 2015;3:146–53. doi: 10.1016/j.jchf.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosendaal FR, Cannegieter SC, van der Meer FJ, Briet E. A method to determine the optimal intensity of oral anticoagulant therapy. Thrombosis and haemostasis. 1993;69:236–9. [PubMed] [Google Scholar]
- 19.Maxwell EA. Analysis of Contingency Tables and Further Reasons for not Using Yates Correction in 2×2 Tables. The Canadian Journal of Statistics / La Revue Canadienne de Statistique. 1976;4:277–290. [Google Scholar]
- 20.Stulak JM, Deo S, Schirger J, Aaronson KD, Park SJ, Joyce LD, Daly RC, Pagani FD. Preoperative atrial fibrillation increases risk of thromboembolic events after left ventricular assist device implantation. The Annals of thoracic surgery. 2013;96:2161–7. doi: 10.1016/j.athoracsur.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 21.Whitson BA, Eckman P, Kamdar F, Lacey A, Shumway SJ, Liao KK, John R. Hemolysis, pump thrombus, and neurologic events in continuous-flow left ventricular assist device recipients. The Annals of thoracic surgery. 2014;97:2097–103. doi: 10.1016/j.athoracsur.2014.02.041. [DOI] [PubMed] [Google Scholar]
- 22.Rame JE, Atluri P, Acker MA. Unexpected abrupt increase in left ventricular assist device thrombosis. The New England journal of medicine. 2014;370:1466–7. doi: 10.1056/NEJMc1402425. [DOI] [PubMed] [Google Scholar]
- 23.Uriel N, Pak SW, Jorde UP, Jude B, Susen S, Vincentelli A, Ennezat PV, Cappleman S, Naka Y, Mancini D. Acquired von Willebrand syndrome after continuous-flow mechanical device support contributes to a high prevalence of bleeding during long-term support and at the time of transplantation. Journal of the American College of Cardiology. 2010;56:1207–13. doi: 10.1016/j.jacc.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 24.Kirklin JK, Naftel DC, Pagani FD, Kormos RL, Myers S, Acker MA, Rogers J, Slaughter MS, Stevenson LW. Pump thrombosis in the Thoratec HeartMate II device: An update analysis of the INTERMACS Registry. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2015;34:1515–26. doi: 10.1016/j.healun.2015.10.024. [DOI] [PubMed] [Google Scholar]
- 25.Singer DE, Chang Y, Fang MC, Borowsky LH, Pomernacki NK, Udaltsova N, Go AS. The net clinical benefit of warfarin anticoagulation in atrial fibrillation. Annals of internal medicine. 2009;151:297–305. doi: 10.7326/0003-4819-151-5-200909010-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.