Abstract
Objective: The aim of this study was to analyze the effects of low-level laser therapy (LLLT) when associated with treadmill training on the recovery of skeletal muscle, during two periods of rest after muscle injury in rats. Background data: Because of photostimulation, LLLT has been presented as an alternative for accelerating the tissue healing process. Materials and methods: Forty rats were divided into two groups (A and B) containing four subgroups each: GC (Control Group)–cryolesion untreated; EG (Exercise Group)–cryolesion treated with physical exercise; LG (Laser Group)–cryolesion treated with laser; ELG (Exercise and Laser Group)–cryolesion treated with laser and physical exercise. The right tibialis anterior (TA) of the middle belly was injured by a cooling iron bar (cryoinjury). Group A remained at rest for 3 days, whereas Group B remained at rest for 7 days. The laser parameters utilized were 780 nm with 15 mW average optical power and spot size of 0.04 cm2 applied during 10 sec, leading to 0.152 J and 3.8 J/cm2. Treadmill training with and without laser application was performed during 5 days, with each session lasting for 12 min at a velocity of 17 m/min. Subsequently, the TA muscle was removed for a histological and morphometric analysis. Results: The damaged area was significantly smaller for the ELG at both periods of rest, 3 and 7 days, respectively (4.4 ± 0.42% and 3.5 ± 0.14%, p < 0.05), when compared with the LG (18.6 ± 0.64% and 7.5 ± 0.13%), the EG (21 ± 0.26% and 8.7 ± 0.32%), and the CG (23.9 ± 0.37% and 21.4 ± 0.38%). In addition, the number of blood vessels were significantly higher for the ELG at both periods of rest, 3 and 7 days, respectively (71.2 ± 13.51 and 104.5 ± 11.78, p < 0.05), when compared with the LG (60.6 ± 11.25 and 93.5 ± 16.87), the EG (51.6 ± 7.3 and 93.8 ± 15.1) and the CG (34.4 ± 2.54 and 65.7 ± 14.1). Conclusions: The LLLT applied before the physical exercise on the treadmill stimulated the angiogenesis and accelerated the process of muscle recovery.
Introduction
Muscle injury may be traumatic (including crush, contusion, laceration and freezing)1 or nontraumatic (caused by acute exhaustive exercise which promotes oxidative stress and muscle damage) both may conduct to a neuromuscular dysfunction.2
The muscle regeneration process depends, for example, on the skeletal muscle type (anaerobic or aerobic metabolism), injury type (strain or stress), localization, severity, and grade injury, as well as age, gender, species, or body size. The metabolism of smaller or larger adults from different or even from the same species can be compared, as the metabolic rate per body mass is inversely related to the body size, what indicates that the cells and tissues of smaller animals use energy at a higher rate when compared with larger animals or even human beings.3
In addition, the muscle wound healing process occurs in three overlapping phases: degeneration and inflammation (1–3 days), regeneration and fibrosis (3–4 weeks), and the remodeling process (3–6 months). Satellite cells are activated by a variety of cytokines and growth factors within 18 h after the injury, as the result of a response to a chemical stimulus, in order to facilitate the vascular and muscle fiber repair. The complete injury recovery can be compromised by excessive fibrosis. The constituted scar tissue always is mechanically inferior; therefore, the muscle can be more susceptible to a re-injury.4 Effective therapeutic strategies to improve regeneration/repair of muscle after injury are related to the regulation of the inflammation, the enhancement of the regeneration process, and the blocking of excessive fibrosis.1
Early rest accelerates the capillary ingrowth, promoting the muscle fiber regeneration and the gaining of strength. However, rest also may lead to larger scars and higher incidence of re-ruptures. Therefore, it is advised to rest only during the first 3–7 days to allow the scar tissue to gain strength. For this acute phase of pain and inflammation modulation, rest, ice, compression, elevation (RICE) and nonsteroidal anti-inflammatory drugs (NSAIDs) are recommended, but continuous rest may lead to muscle atrophy, excessive deposition of connective tissue in muscles, and a retarded recovery of the injured muscle strength.4,5 Therefore, therapeutic exercises should be started gradually, within the limits of pain. In addition, low intensity treadmill training does not produce a risk of re-ruptures,6,7 and the phototherapeutic procedure may modulate the inflammatory process, provide pain relief,8 and stimulate tissue regeneration.9
It is a well-known fact that physical training promotes muscle adaptation such as molecular, biochemical, and structural changes in the mitochondria and in the muscle fiber, as well as an increased capillarity and blood flux, improving metabolic activity.10,11 In addition, physical exercises are also required to provide muscle recovery between the training sessions of athletes, increasing muscle performance. Other recovery modalities are stretching, massage, cryotherapy, contrast temperature water immersion therapy, hyperbaric oxygen therapy, NSAIDs, compression garments, electrostimulation,12 and phototherapy.13
Photons penetrate through the skin and the underlying tissues, varying the rates of absorption, scattering, transmission, and reflection, to stimulate the body's natural repair processes. Many in vitro14 and in vivo9,15–17 studies have shown positive effects of low-level laser therapy (LLLT) on the muscle repair process. In an in vitro study, LLLT stimulated the cell cycle entry and the accumulation of satellite cells around isolated single fibers. The authors of this study also showed the survival of the fibers as well as of their adjacent cells and cultured myogenic cells under serum-free conditions that would, normally, lead them to apoptosis.14 The study of Rizzi et al.15 showed the potential of LLLT on the optimization of the gastrocnemius muscle healing process in rats, as it reduces the inflammatory response induced by trauma and is able to block the effects of reactive oxygen species (ROS) released, and provides the activation of nuclear factor kappa B (NF-kB) proteins. In addition, Avni et al.16 showed the ability of the LLLT to prevent the degeneration that follows ischemia/reperfusion injury in gastrocnemius muscles in rats by the induction of antioxidant synthesis and other cytoprotective proteins. In another study, infrared laser applied in four sessions was started 24 h after the surgically induced muscle injury, promoting anti-inflammatory effects, reducing the number of leukocytes at the injury site, and accelerating the regeneration of connective tissue during the acute inflammatory phase.17 Silveira et al.18 also found an accelerated muscle healing process, evidenced by the reduction of superoxide anion production, thiobarbituric acid reactive substance (TBARS) levels, superoxide dismutase (SOD) activity, and hydroxyproline content, also demonstrating an increased collagen synthesis.
A review of the literature has shown that only one study has investigated the effects of LLLT after downhill running on a muscle strain injury in rats.19 However, in this study, the eccentric exercise was used to induce muscle injury by strain in rats. The results indicated that red laser enhances muscle antioxidative capacity and reduces the inflammatory response in injured muscle tissue, which was evidenced by an enhanced muscle SOD activity and the reduction of both serum creatine kinase (CK) activity and muscle malondialdehyde (MDA) level.
Moreover, several experimental studies from our group have observed the potential effects of both LLLT on muscle damage20–22 and LLLT associated with physical training on muscle performance23,24 in Wistar rats. However, to our knowledge, the associated effects of LLLT and a physical exercise program on muscle repair have not yet been investigated. Therefore, the aim of this study was to evaluate the effect of LLLT applied before treadmill training on the muscle injury recovery process in Wistar rats. Our hypothesis is that LLLT associated with treadmill training may accelerate the muscle healing process in animals.
Materials and Methods
Animal care and experimental groups
This study was approved by the Ethics Committee of the Federal University of São Carlos (UFSCar) in São Carlos, Brazil (number 035/2012). All animal procedures were performed according to the principles in the Guide for the Care and Use of Laboratory Animals.
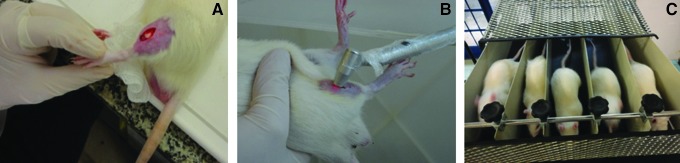
Forty male Wistar rats (body weight 400–500 g) were kept in a cage with food and water ad libitum, on a 12:12 h light–dark cycle at 23 ± 1°C. The animals were randomized into two groups containing 20 rats per group: Group A, 3 days of rest post-injury (n = 20); and Group B, 7 days of rest post-injury (n = 20). Each group was subdivided into four groups containing five rats each: Control Group (CG), cryolesion untreated (n = 5); Laser Group (LG), cryolesion treated with laser (n = 5); Exercise Group (EG), cryolesion treated with physical exercise (n = 5); and Exercise and Laser Group (ELG), cryolesion treated with laser and physical exercise (n = 5). The experimental procedure can be seen in Fig. 1.
FIG. 1.
Experimental procedure: cryoinjury (A), LLLT (B), and treadmill training (C).
Muscle damage induced by cryolesion
The animals were anaesthetized using an intraperitoneal injection of xylazine (0.03 mg/kg) and ketamine (0.05 mg/kg). To induce the muscle injury to the middle belly of both left and right tibialis anterior (TA), the skin around the muscle was trichotomized and cleaned. A transversal cut of the skin over the middle muscle belly was performed, exposing the TA muscle. Afterwards, the TA of the middle belly was injured by a cooling iron bar (cryoinjury). An iron bar of 0.5 cm2 immersed in liquid nitrogen and pressed transversely against on the muscle belly for 10 sec was used. This procedure was performed twice.22 Immediately after the induced muscle injury by cryoinjury, the skin was sutured and cleaned with iodine alcohol. After 3 days of rest for Group A and after 7 days of rest for Group B, the treatments were started.
Laser therapy and treadmill training
An aluminum gallium arsenide (AlGaAs) diode laser device (Twin laser, MMOptics, São Carlos, SP, Brazil) was used. The irradiation parameters were: infrared wavelength (780 nm), a spot area of 0.04 cm2, and an average optical power of 15 mW operating in a continuous mode for 10 sec, leading to an irradiance of 37.5 mW/cm2, energy of 0.15 J, and fluence of 3.8 J/cm2.23,24 LLLT was applied at one point for 5 consecutive days in contact mode at a 90 degree angle with the skin, using slight pressure. The animals were irradiated directly on the skin of the middle region of the left and right TA muscle (location of the muscle damage) and immediately before physical exercise. Treadmill training with and without LLLT was performed for 5 days, with each session lasting for 12 min at a treadmill velocity of 17 m/min.24
Histological and morphometric analysis
After the completion of the protocol, with the animals anesthetized, the right TA muscle was carefully dissected and removed for histological and morphometric analysis. Then, the animals were submitted to a process of euthanasia consisting of an intracardiac injection of potassium chloride (3M KCl). The muscles were immediately removed and washed with saline solution, and then fixed in 10% buffered neutral formalin solution. After the fixation, the muscle was processed by paraffin-embedded tissues. Posteriorly, the tissue was sectioned and stained with toluidine blue and Hematoxylin and Eosin, for the analysis of the damaged area and blood capillaries. A light microscope (Carl Zeiss, Jena, Germany) with magnifications of 10× and 40× was usedto measure the damaged area and to count the blood capillaries.22 The capillary blood images were analyzed using ImageJ software (Rasband, WS, ImageJ, National Institutes of Health, Bethesda, MD; <http://imagej.nih.gov/ij/>).
Statistical analysis
Measurements were expressed by mean and standard deviations. The Shapiro–Wilk and Levene's tests were used to analyze the normality and homogeneity of the variance. Comparisons between the groups were made using the two way analysis of variance (ANOVA) with the Tukey's post-hoc test. To investigate the relationship between key variables, we used the Pearson product-moment correlation coefficient. The Statistica for Windows Release 7 software (Statsoft Inc., Tulsa, OK) was used to produce the statistical analysis, and the significance level was set at 5% (p < 0.05).
Results
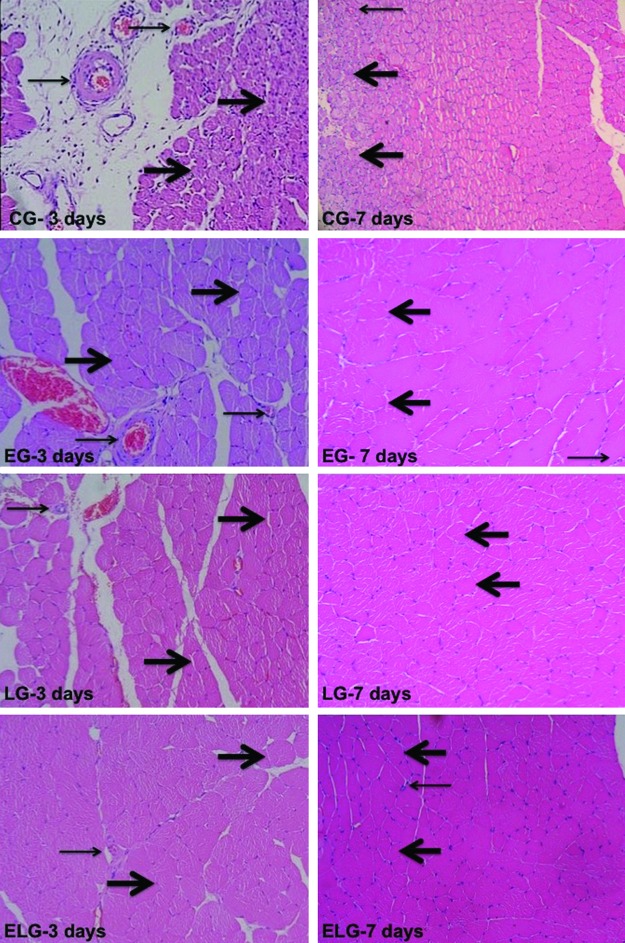
The histological findings showed the injured muscle area of all groups with regard to days of rest (3 and 7 days). The CG with 7 days of rest (Group B) presented a smaller necrosis area than the CG that had 3 days of rest (Group A). For the EG, there was better muscle tissue organization for the group with 7 days of rest than for the group that had 3 days of rest. The findings of the LG with 7 days of rest had similar patterns of tissue repair when compared to the EG that had 7 days of rest; however, the muscle fibers were better organized than in the EG with 3 days of rest. The histological findings for the ELG with 7 days of rest showed a better structural tissue than the ELG with 3 days of rest, as well as when compared with the other groups (CG, EG, and LG with 3 and 7 days of rest) (Fig. 2).
FIG. 2.
Injured muscle in Group A (with 3 days of rest) and in Group B (with 7 days of rest): (CG) Control Group; (LG) Laser Group; (EG) Exercise Group; and (ELG) Exercise and Laser Group. Thick arrows indicating formed muscle fibers and thin arrow indicating blood capillaries (40×).
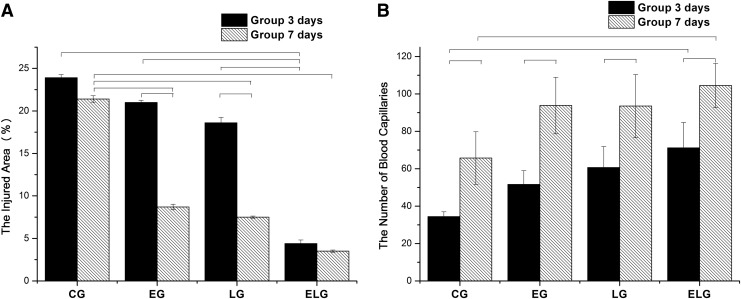
The damaged area (%) and the number of blood capillaries can be seen in the Fig. 3A and B, respectively. The damaged area was significantly smaller for the ELG at both periods of rest, 3 and 7 days, respectively (4.4 ± 0.42% and 3.5 ± 0.14%, p < 0.05), when compared with the LG (18.6 ± 0.64% and 7.5 ± 0.13%), the EG (21 ± 0.26% and 8.7 ± 0.32%) and the CG (23.9 ± 0.37% and 21.4 ± 0.38%). The group that remained at rest for 7 days had a significant reduction of the damaged area when compared with the group that had 3 days of rest, in both the LG and the EG. In addition, the number of blood vessels was significantly higher for the ELG at both periods of rest, 3 and 7 days, respectively (71.2 ± 13.51 and 104.5 ± 11,78, p < 0.05), when compared with the LG (60.6 ± 11.25 and 93.5 ± 16.87), the EG (51.6 ± 7.3 and 93.8 ± 15.1), and the CG (34.4 ± 2.54 and 65.7 ± 14.1). The group that remained at rest for 7 days had a significant improvement of blood vessel quantity in all subgroups evaluated (CG, EG, LG, and ELG) when compared with the group that had 3 days of rest. However, the number of blood vessels in the ELG was significantly higher than in the CG for the group that remained at rest for 7 days. There were significant inverse correlations between the damaged area and the number of blood capillaries for both groups, 3 days of rest (r = −0.71; p < 0.001) and 7 days of rest (r = −0.76; p < 0.001).
FIG. 3.
Mean and standard deviation of injured area (A) and blood vessels (B). CG, Control Group; EG, Exercise Group; LG, Laser Group; ELG, Exercise and Laser Group. Black bars represent significant difference between groups, with p < 0.05.
Discussion
This is the first study that presents the effects of LLLT when associated with treadmill training on the recovery of skeletal muscle during two periods of rest (3 and 7 days) after skeletal muscle injury in rats. There was an increase in the number of blood capillaries and a reduction of the damaged area for all groups. However, the number of blood vessels was significantly higher and the damaged area was significantly smaller when LLLT was applied before physical exercise on the treadmill.
Many in vitro14,25 and in vivo20–22 studies showed the positive effects of LLLT on the process of muscle regeneration. An in vitro study showed that red laser (632.8 nm, 1.8 mm beam diameter, and 4.5 mW at 3 sec) stimulated the cell cycle entry and accumulated satellite cells around isolated single fibers. The authors also showed the survival of myogenic cells and fibers under serum-free conditions that could lead them to apoptosis.14 Another in vitro25 study showed that red laser (632.8 nm, 1.8 mm beam diameter, and 4.5 mW at 3 sec) increased the gene expression of satellite cells and stimulated the myogenic cell proliferation.
In the current study, we did not use parameters of irradiation similar to those applied for in vitro studies, because in vivo studies show some complexities regarding the variety of biological tissues and immunological response.26 We choose an infrared wavelength, because the penetration is deeper if compared with the red wavelength used in in vitro studies. In addition, we used a greater power and fluence, if compared with in vitro studies, but smaller values compared with clinical trials,27,28 because human skin levels, skin color, and body composition (fat, muscle, and bone) may create a biological barrier for the phototherapy, suggesting the use of a greater power and fluence in treatment protocols, in contrast to what is used in animals.
The principle of the Arndt–Schultz law states that very low doses of radiation can cause a strong physiological response. Therefore, a small amount of energy is biostimulatory and a high dose is inhibitory.29 In this way, we chose to apply a low dose of the infrared radiation (780 nm, 15 mW, 0.25 J, 10 sec, 37.5 mW/cm2, and 3.8 J/cm2) in order to accelerate the tissue healing process in Wistar rats. Moreover, these parameters were applied in previous studies with LLLT associated with physical training in rats, which showed positive effects on muscle function.23,24
There are several models to induce muscle injury by strain19,30 or stress18; however, we used the cryoinjury because this model ensures a homogeneous injured area. In this context, several animal studies22,31,32 have induced muscle injury by cryoinjury to evaluate treatment with LLLT. Recent studies9,22,32 have investigated the effects of infrared laser on TA muscle regeneration after cryoinjury. In the study by Freitas et al.,32 it was found that LLLT promotes a higher number of regenerating fibers and fewer degenerating fibers, as well as a reduction in the expression of tumor necrosis factor (TNF)-α and myogenin, contributing to muscle recovery. Vatansever et al.22 showed that LLLT on TA, after cryoinjury, can increase the myogenic regulatory factor (MyoD) and the vascular endothelial growth factor (VEGF) gene expression as well as eliciting higher capillary blood count in aged rats. Alves et al.9 demonstrated that LLLT on TA after cryoinjury presented a reduction in the inflammatory infiltrate and myonecrosis as well as an increase in the number of both blood vessels and immature muscle fibers. In addition, photomodulation of inflammatory process and higher matrix metalloproteinase-2 (MMP-2) activity can lead to improvements in both the organization and the distribution of collagen fibers during repair process of rat skeletal muscle.9 Likewise, Souza et al.31 promoted the photostimulation of angiogenesis and collagen synthesis (types I and III) as well as the reduction of myonecrosis after cryoinjury. Stimulated angiogenesis have an essential role for muscle regeneration, because an increased local blood flow generates greater supplies of oxygen and nutrients to accelerate the injured muscle healing process with greater tissue quality.21 Moreover, increased cytochrome oxidase activity leads to an improvement of the oxidative capacity of muscle fibers for the muscle injury healing process and the muscle function.33
The TA muscle has the highest proportions of glycolytic fast-twitch muscles (type II). These fibers are classically white and with little myoglobin, being characterized by a low oxidative capacity and high activity of myosin adenosine triphosphatase (ATPase) and phosphorylase.3,34 In anaerobic metabolism, there are greater amounts of depleted phosphocreatine as well as storages of glycogen that are broken down and metabolized to lactate and adenosine triphosphate (ATP), resulting in a quick decrease of the maximal work production in the muscles, fatigability, and higher risk of injury recurrence.35 In this context, we chose the TA muscle with the predominance of the anaerobic metabolism for rehabilitation using aerobic training. The aerobic metabolism increases the endurance performance by improving the ability of the muscles to sustain ATP production through the metabolism of carbohydrates (muscle glycogen and blood glucose) and lipid (fatty acids generated by the lipolysis from intramuscular and adipocyte triglyceride). It reduces the fatigue and the risk of fiber re-rupture or injury recurrence.
Moreover, we applied the LLLT before the aerobic training to stimulate the microcirculation, oxidative capacity, analgesia, reabsorption of edema, and anti-inflammatory action.30,36,37 Therefore, LLLT may promote the best physical conditions for performing exercises and, consequently, may contribute to muscle recovery.
The current study showed that the injured area was similar between the groups that had 3 and 7 days of rest for the cryolesioned rats treated with LLLT and treadmill training, indicating accelerated muscle regeneration.
In this context, LLLT applied before aerobic training reduced inflammatory markers [interleukin (IL)-6 and TNF-α],36 increased fatigue resistance, and decreased post-exercise blood lactate and creatine kinase,37 also improving the functional performance of the rats.30 Other studies also showed the potential benefits of phototherapy applied after exercise programs in animals, including the biomodulation of cytokines, stimulation of insulin-like growth factor 1 (IGF-1) production, increasing muscle volume,38 as well as increasing MMP-2 gene expression and decreasing lactate levels, indicating a skeletal muscle remodeling and an enhanced functionality.39 In addition, the phototherapy applied during high intensity exercise was able to promote an improvement in both power muscle and fatigue resistance without leading to muscle damage.39 Therefore, phototherapy has a protective effect against muscle damage. Ribeiro et al.40 demonstrated that LLLT previously applied to muscle injury reduces myonecrosis and inflammatory cells, increases blood vessels and immature muscle fibers as well as increasing MMP-2 activity, decreases collagen deposition, and provides a better collagen organization and distribution.
Moreover, a recent study41 has demonstrated that 3–6 h could be an optimum time to apply phototherapy in order to improve muscle metabolism before exercise. This study has also suggested possible cumulative effects if the illumination is applied at intervals of <24 h for muscle recovery after exercise. This is an important finding, as the photostimulation of muscle cells (myotubes) is related to time-response in order to increase ATP and the mitochondrial membrane potential.
These findings corroborate with our study, which demonstrates that LLLT applied in combination with physical exercise on the treadmill provides better results in muscle recovery when compared with groups receiving other treatments. The suppositions42 that may elucidate these benefits are: (1) formation of a giant mitochondria with a larger amount of cytochrome c oxidase and greater ATP synthesis; (2) changed gene expression, leading to greater protein synthesis, increased angiogenesis, reduced protein degradation and less inflammation; and (3) reduced fatigue and delayed-onset muscle soreness caused by fiber type shifts (aerobic predominance), greater phosphocreatine and ATP resynthesis, reduced blood lactate concentration, and increased production of ATP, aerobically.
Several clinical trials have been investigating the effects of phototherapy before,43 during,27,39 and after physical exercise,42 demonstrating improvements in muscle function as well as a protective effect on muscle. Therefore, phototherapy and physical exercise may lead to the development of new treatment strategies for skeletal muscle injuries, and future studies should be performed.
Conclusions
LLLT applied before treadmill training accelerated the recovery process of the injured skeletal muscle. In addition, the combined techniques showed greater quality of skeletal muscle tissue. These findings may prove an effective treatment of the skeletal muscle injury for athletes, children, young adults, and and the elderly, who may sustain traumatic or nontraumatic injuries at work, sport, or during day-to-day activities.
Acknowledgments
We thank the São Paulo Research Foundation (FAPESP)–grant no. 2013/07276-1 and 2013/14001-9, and the National Council for Scientific and Technological Development (CNPq)–grant no. 573587/2008. We also acknowledge scientific contributions and helpful advice from Vitória H. Maciel.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Filippin LI, Moreira AJ, Marroni NP, Xavier RM. Nitric oxide and repair of skeletal muscle injury. Nitric Oxide 2009;21:157–163 [DOI] [PubMed] [Google Scholar]
- 2.Malaguti M, Angeloni C, Garatachea N, et al. Sulforaphane treatment protects skeletal muscle against damage induced by exhaustive exercise in rats. J Appl Physiol 2009;107:1028–1036 [DOI] [PubMed] [Google Scholar]
- 3.Hirofuji C, Nakatani T, Ishihara A, et al. Cell size and succinate dehydrogenase activity of different types of fibers in different regions of the tibialis anterior muscle in mice and rats. Acta Histochem Cytochem 2000;33:295–303 [Google Scholar]
- 4.Baoge L, Van Den Steen E, Rimbaut S, et al. Treatment of skeletal muscle injury: a review. ISRN Orthop 2012;26:689012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Järvinen TA, Järvinen M, Kalimo H. Regeneration of injured skeletal muscle after the injury. Muscles Ligaments Tendons J 2013;3:337–345 [PMC free article] [PubMed] [Google Scholar]
- 6.Baltgalvis KA, Call JA, Cochrane GD, Laker RC, Yan Z, Lowe DA. Exercise training improves plantarflexor muscle function in mdx mice. Med Sci Sports Exerc 2012;44:1671–1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Järvinen TA, Järvinen TL, Kääriäinen M, et al. Muscle injuries: optimising recovery. Best Pract Res Clin Rheumatol 2007;21:317–331 [DOI] [PubMed] [Google Scholar]
- 8.Jang H, Lee H. Meta-analysis of pain relief effects by laser irradiation on joint areas. Photomed Laser Surg 2012;30:405–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alves AN, Fernandes KP, Melo CA, et al. Modulating effect of low level-laser therapy on fibrosis in the repair process of the tibialis anterior muscle in rats. Lasers Med Sci 2014;29:813–821 [DOI] [PubMed] [Google Scholar]
- 10.Scribbans TD, Edgett BA, Vorobej K, et al. Fibre-specific responses to endurance and low volume high intensity interval training: striking similarities in acute and chronic adaptation. PLoS One 2014;9:e98119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Irrcher I, Adhihetty PJ, Joseph AM, Ljubicic V, Hood DA. Regulation of mitochondrial biogenesis in muscle by endurance exercise. Sports Med 2003;33:783–793 [DOI] [PubMed] [Google Scholar]
- 12.Barnett A. Using recovery modalities between training sessions in elite athletes: does it help? Sports Med 2006;36:781–796 [DOI] [PubMed] [Google Scholar]
- 13.Santos VBC, de Paula Ramos S, Milanez VF, et al. LED therapy or cryotherapy between exercise intervals in Wistar rats: anti-inflammatory and ergogenic effects. Lasers Med Sci 2014;29:599–605 [DOI] [PubMed] [Google Scholar]
- 14.Shefer G, Partridge TA, Heslop L, Gross JG, Oron U, Halevy O. Low-energy laser irradiation promotes the survival and cell cycle entry of skeletal muscle satellite cells. J Cell Sci 2002;115:1461–1469 [DOI] [PubMed] [Google Scholar]
- 15.Rizzi CF, Mauriz JL, Corrêia DSF. Effects of low-level laser therapy (LLLT) on the nuclear factor (NF)-kB signaling pathway in traumatized muscle. Lasers Surg Med 2006;38:704–713 [DOI] [PubMed] [Google Scholar]
- 16.Avni D, Levkovitz S, Maltz L, Oron U. Protection of skeletal muscles from ischemic injury: low-level laser therapy increases antioxidant activity. Photomed Laser Surg 2005;23:273–277 [DOI] [PubMed] [Google Scholar]
- 17.Cressoni MD, Dib Giusti HH, Casarotto RA, Anaruma CA. The effects of a 785-nm AlGaInP laser on the regeneration of rat anterior tibialis muscle after surgically-induced injury. Photomed Laser Surg 2008;26:461–466 [DOI] [PubMed] [Google Scholar]
- 18.Silveira PC, da Silva LA, Pinho CA, et al. Effects of low-level laser therapy (GaAs) in an animal model of muscular damage induced by trauma. Lasers Med Sci 2013;28:431–436 [DOI] [PubMed] [Google Scholar]
- 19.Liu XG, Zhou YJ, Liu TC, Yuan JQ. Effects of low-level laser irradiation on rat skeletal muscle injury after eccentric exercise. Photomed Laser Surg 2009;27:863–869 [DOI] [PubMed] [Google Scholar]
- 20.Brunelli RM, Rodrigues NC, Ribeiro DA, et al. The effects of 780-nm low-level laser therapy on muscle healing process after cryolesion. Lasers Med Sci 2014;29:91–96 [DOI] [PubMed] [Google Scholar]
- 21.Assis L, Moretti AI, Abrahão TB, de Souza HP, Hamblin MR, Parizotto NA. Low-level laser therapy (808 nm) contributes to muscle regeneration and prevents fibrosis in rat tibialis anterior muscle after cryolesion. Lasers Med Sci 2013;28:947–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vatansever F, Rodrigues NC, Assis LL, et al. Low intensity laser therapy accelerates muscle regeneration in aged rats. Photonics Lasers Med 2012;1:287–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paolillo FR, Ross A, Dutra DB, et al. Low-level laser therapy associated with high intensity resistance training on cardiac autonomic control of heart rate and skeletal muscle remodeling in Wistar rats. Lasers Surg Med 2014;46:796–803 [DOI] [PubMed] [Google Scholar]
- 24.Vieira WHB, Goes R, Costa FC, et al. Adaptation of LDH enzyme in rats undergoing aerobic treadmill training and low intensity laser therapy. Rev Bras Fisioter 2006;10:205–211 [Google Scholar]
- 25.Ben-Dov N, Shefer G, Irintchev A, Wernig A, Oron U, Halevy O. Low-energy laser irradiation affects satellite cell proliferation and differentiation in vitro. Biochim Biophys Acta 1999;1448:372–380 [DOI] [PubMed] [Google Scholar]
- 26.Leite DP, Paolillo FR, Parmesano TN, Fontana CR, Bagnato VS. Effects of photodynamic therapy with blue light and curcumin as mouth rinse for oral disinfection: a randomized controlled trial. Photomed Laser Surg 2014;32:627–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paolillo FR, Corazza AV, Borghi–Silva A, Parizotto NA, Kurachi C, Bagnato VS. Infrared-LED applied during high-intensity treadmill training improved maximal exercise tolerance in postmenopausal women: a 6-month longitudinal study. Lasers Med Sci 2013;28:415–422 [DOI] [PubMed] [Google Scholar]
- 28.Paolillo AR, Paolillo FR, João JP, João HA, Bagnato VS. Synergic effects of ultrasound and laser on the pain relief in women with hand osteoarthritis. Lasers Med Sci 2015;30:279–286 [DOI] [PubMed] [Google Scholar]
- 29.Chow RT. Dose dilemmas in low level laser therapy–the effects of different paradigms and historical perspectives. Laser Ther 2000;13:102–109 [Google Scholar]
- 30.Ramos L, Leal Junior EC, Pallotta RC, et al. Infrared (810 nm) low-level laser therapy in experimental model of strain-induced skeletal muscle injury in rats: effects on functional outcomes. Photochem Photobiol 2012;88:154–160 [DOI] [PubMed] [Google Scholar]
- 31.Souza TO, Mesquita DA, Ferrari RA, et al. Phototherapy with low-level laser affects the remodeling of types I and III collagen in skeletal muscle repair. Lasers Med Sci 2011;26:803–814 [DOI] [PubMed] [Google Scholar]
- 32.Freitas CE, Bertaglia RS, Vechetti Júnior IJ, et al. High final energy of low-level gallium arsenide laser therapy enhances skeletal muscle recovery without a positive effect on collagen remodeling. Photochem Photobiol 2015;91:957–965 [DOI] [PubMed] [Google Scholar]
- 33.Hayworth CR, Rojas JC, Padilla E, Holmes GM, Sheridan EC, Gonzalez–Lima F. In vivo low-level light therapy increases cytochrome oxidase in skeletal muscle. Photochem Photobiol (2010);86:673–680 [DOI] [PubMed] [Google Scholar]
- 34.Nakatani T, Nakashima T, Kita T, et al. Cell size and oxidative enzyme activity of different types of fibers in different regions of the rat plantaris and tibialis anterior muscles. Jpn J Physiol 2000;50:413–418 [DOI] [PubMed] [Google Scholar]
- 35.Baldwin KM, Tipton CM. Work and metabolic patterns of fast and slow twitch skeletal muscle contracting in situ. Pflugers Arch 1972;334:345–356 [DOI] [PubMed] [Google Scholar]
- 36.Amadio EM, Serra AJ, Guaraldo SA, et al. The action of pre-exercise low-level laser therapy (LLLT) on the expression of IL-6 and TNF-α proteins and on the functional fitness of elderly rats subjected to aerobic training. Lasers Med Sci 2015;30:1127–1134 [DOI] [PubMed] [Google Scholar]
- 37.Leal Junior EC, Lopes–Martins RA, de Almeida P, Ramos L, Iversen VV, Bjordal JM. Effect of low-level laser therapy (GaAs 904 nm) in skeletal muscle fatigue and biochemical markers of muscle damage in rats. Eur J Appl Physiol 2010;108:1083–1088 [DOI] [PubMed] [Google Scholar]
- 38.Corazza AV, Paolillo FR, Groppo FC, Bagnato VS, Caria PH. Phototherapy and resistance training prevent sarcopenia in ovariectomized rats. Lasers Med Sci 2013;28:1467–1474 [DOI] [PubMed] [Google Scholar]
- 39.Paolillo FR, Corazza AV, Paolillo AR, et al. Phototherapy during treadmill training improves quadriceps performance in postmenopausal women. Climacteric 2014;16:1–9 [DOI] [PubMed] [Google Scholar]
- 40.Ribeiro BG, Alves AN, Santos LA, et al. The effect of low-level laser therapy (LLLT) applied prior to muscle injury. Lasers Surg Med 2015. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 41.Ferraresi C, Kaippert B, Avci P, et al. Low-level laser (light) therapy increases mitochondrial membrane potential and ATP synthesis in C2C12 myotubes with a peak response at 3-6 h. Photochem Photobiol 2015;91:411–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ferraresi C, Oliveira TB, Zafalon LO, et al. Effects of low level laser therapy (808 nm) on physical strength training in humans. Lasers Med Sci 2011;26:349–358 [DOI] [PubMed] [Google Scholar]
- 43.Leal Junior EC, Lopes–Martins RA, Baroni BM, et al. Effect of 830nm low-level laser therapy applied before high-intensity exercises on skeletal muscle recovery in athletes. Lasers Med. Sci 2009;24:857–863 [DOI] [PubMed] [Google Scholar]