Abstract
To date, clinical trials have failed to find an effective therapy for victims of traumatic brain injury (TBI) who live with motor, cognitive, and psychiatric complaints. Pre-clinical investigators are now encouraged to include male and female subjects in all translational research, which is of particular interest in the field of neurotrauma given that circulating female hormones (progesterone and estrogen) have been demonstrated to exert neuroprotective effects. To determine whether behavior of male and female C57BL6/J mice is differentially impaired by TBI, male and cycling female mice were injured by controlled cortical impact and tested for several weeks with functional assessments commonly employed in pre-clinical research. We found that cognitive and motor impairments post-TBI, as measured by the Morris water maze (MWM) and rotarod, respectively, were largely equivalent in male and female animals. However, spatial working memory, assessed by the y-maze, was poorer in female mice. Female mice were generally more active, as evidenced by greater distance traveled in the first exposure to the open field, greater distance in the y-maze, and faster swimming speeds in the MWM. Statistical analysis showed that variability in all behavioral data was no greater in cycling female mice than it was in male mice. These data all suggest that with careful selection of tests, procedures, and measurements, both sexes can be included in translational TBI research without concern for effect of hormones on functional impairments or behavioral variability.
Key words: : learning and memory, motor, mouse, sex differences, traumatic brain injury
Introduction
Traumatic brain injury (TBI) affects millions worldwide and can result in profound, long-term functional deficits, including personality changes, motor incoordination, and neuropsychiatric and cognitive issues. Subsequent to the initial insult causing the injury, a series of secondary pathophysiological cascades persist for days to months,1–3 providing multiple targets and a relatively large window for potential therapeutic intervention. Animal models of TBI provide a controlled environment in which the biological and functional effects of injury can be studied and manipulated, and a number of pharmaceutical agents have demonstrated efficacy in mitigating the effects of brain injury in a laboratory setting.4 However, despite the number of potential treatments that have shown promise in pre-clinical TBI studies, more than 30 agents have failed in phase III prospective clinical trials5; at present, there is no U.S. Food and Drug Administration–approved drug for brain injury, and treatment continues to be based on supportive therapies and symptom management.
Methodological challenges to pre-clinical TBI investigations and questions of relevance to the clinical setting are numerous, including choice of experimental details, such as injury method, species, strain, and sex of animals. A new challenge to pre-clinical studies in all disciplines of biomedical research is the recent National Institutes of Health (NIH) requirement that female animals or cells be employed in all laboratory translational studies.6–8 Efforts continue to urge researchers to incorporate female animals in translational studies,9 but TBI research, and biomedical research in general, has continued to focus largely on males. It was recently estimated that male bias is most pronounced in the neuroscience discipline, with male animals in experiments outnumbering female animals 5.5 to 1.10 One reason for the exclusion of females from animal research is the belief that the fluctuating hormones resulting from the estrous cycle in females introduces variability, complicating interpretation of the effects of experimental manipulations. However, a recent meta-analysis of 293 studies reporting behavioral, molecular, physiological, and morphological measures in male and female mice concluded that the data obtained from female mice in random stages of estrous do not have greater variability than data collected from male mice.11
Inclusion of female animals in pre-clinical research is of particular concern in the context of TBI, given that circulating sex hormones (progesterone and estrogen) have been demonstrated to have neuroprotective effects by acting through multiple pathways in the secondary injury process.12–15 However, potential advantages incurred by females after brain injury owing to higher levels of endogenous progesterone and estrogen are uncertain. Although some clinical studies have concluded that females fare better after moderate to severe TBI than men,16,17 others have shown that gender-dependent differences are dependent on the functional tests employed18 or that memory performance of male TBI patients is superior to that of female patients.19 A recent systematic review concluded that a poorer long-term outcome after mild or concussive brain injury is associated with female gender.20
Differences in outcome between males and females post-TBI are also unclear in the laboratory setting. There is substantial evidence to suggest that female mice and rats fare better on many biological measures after brain injury, for example, reduced cerebral edema and increased release of anti-inflammatory cytokines, and these differences can be attributed to levels of estrogen or progesterone. However, changes in these measures do not translate to reductions in cortical and hippocampal injury or neurodegeneration.21–24
Although administration of progesterone has been demonstrated to improve behavioral performance in male rats post-TBI,25,26 direct comparisons of postinjury functional differences between male and female rodents are sparse. O'Connor and colleagues reported that after diffuse brain injury, female rats performed better than males on the rotarod test of motor coordination and also incurred a slight advantage on the Barnes maze test of learning and memory.27 Male rats subjected to controlled cortical impact (CCI) also showed inferior motor performance to females assessed using beam balance and walking tasks, but cognitive performance on the Morris water maze (MWM) was equally impaired in both sexes.28,29 In female rats, estrous cycle stage (and resulting levels of circulating hormones) at the time of TBI had a slight effect on motor performance shortly after the injury, but hormonal level had no effect on MWM performance 2 weeks postinjury.28 Further, a recent study also demonstrated that certain motor and cognitive tasks were unaffected by estrous cycle stage at the time of CCI in female rats housed in standard cages or enriched environments.30 To date, there is only one study directly comparing male and female functional performance post-TBI in mice: Females outperform males after brain injury on a grid-walking foot-fault test, but have equivalent cognitive deficits in a modified version of the MWM.31
The aim of this work was to gain a more comprehensive understanding of sex differences on several behavioral tasks commonly selected to assess post-TBI function, recovery, and potential therapies. We employed male and cycling female C57BL/6J mice, a strain commonly used as a background for transgenic and knockout manipulations, and used CCI, a popular brain injury model, to deliver the injury. We hope to provide pre-clinical investigators with preliminary understanding and expectations of the practical implications of including female animals in translational TBI research. Exploratory, cognitive, and motor performance after mild or severe brain injury induced by CCI was assessed long term to determine sex-specific effects of the injury on function. Our findings from repeated open field (OF), rotarod, y-maze, and MWM testing demonstrate that the behavioral effects of TBI are largely comparable in male and female mice. Further, female mice, at random stages of the estrous cycle at time of injury, did not have greater variability in performance than male mice. These findings suggest that including female mice that sustained brain injury at various or undetermined stages of the estrous cycle will not contribute to behavioral variability and obscure the effects of injury, and that labor-intensive monitoring of female estrous cycles at times of the injury and postinjury testing may be unnecessary. However, female mice had greater levels of activity in many tests, and this must be considered in the context of experimental design and data interpretation.
Methods
Animals and housing
Male and female C57BL/6J mice seven weeks of age were obtained from The Jackson Laboratory (Bar Harbor, ME) and group-housed (3–5 mice in same-sex cages for study duration) in the same room of Association for Assessment and Accreditation of Laboratory Animal Care–accredited facilities with a standard 12-h light-dark cycle. Standard rodent chow and water was available ad libitum, and mice were allowed to acclimate after arrival for at least 1 week before sham or CCI procedures. Surgical procedures and behavioral testing were performed by female investigators,32 and all behavioral testing took place during the light phase of the cycle. Procedures were approved by the institutional animal care and use committee at the Uniformed Services University of the Health Sciences (Bethesda, MD).
Controlled cortical impact and sham procedures
Cages of mice were randomly assigned to sham (male, n=18; female, n=18), mild CCI (male, n=18; female, n=19), or severe CCI (male, n=18; female, n=17) conditions, and each mouse was weighed before surgical procedures. Male and female mice weighed 20–28 and 15–20 g at the time of procedures, respectively, and female mice were at random (undetermined) stages of the estrous cycle. All CCI or sham procedures were performed in an aseptic manner, as previously described.33 Induction of anesthesia was achieved in a rodent volatile anesthesia box with 3% isoflurane in 100% oxygen. Once corneal and paw-withdrawal reflexes were absent, protective eye ointment (Lacri-Lube®) was applied, head hair clipped, and mice were placed into a standard rodent stereotaxic device and secured into position with an incisor bar and ear bars (Stoelting, Wood Dale, IL). Maintenance of anesthesia was achieved with 1.5% isoflurane/oxygen administered by a flow-through nose cone, and body temperature was maintained at 37°C throughout the procedure using an isothermal heating pad with feedback controller (Stoelting). A unilateral 5-mm craniectomy over the parietal cortex was performed with a high-speed drill. The CCI device (Impact One™; Leica Microsystems, Buffalo Grove, IL) is an electronically controlled, electromagnetically driven impactor, fitted with a 3.0-mm-diameter steel tip and mounted on a stereotax micromanipulator. The tip of the impactor was centered over the injury site (2.5 mm posterior to bregma and 1.5 mm left of midline) at a 15-degree angle relative to the sagittal plane, and the injury was produced with the following parameters: 5.0 m/sec velocity with a dwell time of 0.1 sec, and a depth of 1.0 mm (mild CCI) or 2.0 mm (severe CCI). Postinjury, the skin incision was closed with a silk suture and mice were placed into a warm, clean housing cage and monitored until ambulatory. Sham-operated mice underwent all described procedures except the cortical impact.
Open field
All mice were tested in an OF environment for 20 minutes on days 1, 7, 14, and 21 post-CCI or sham procedures. The OF arena was 40×40 cm with opaque black walls (Stoelting) and a light level of approximately 175 lux. Mice were individually placed into the center of the OF, and a camera above the apparatus recorded activity in the apparatus and was connected to a computer with Any-Maze tracking software (Stoelting). The software detected and tracked the mouse and reported total distance traveled, average speed, number of mobile episodes (with mobility defined as at least 35% of the animal moving for at least 2 sec), and distance traveled in a software-defined 20×20 cm center region of the arena (expressed as a percent of the total distance traveled). Additionally, the arena was equipped with photobeam arrays positioned on either side of the apparatus, allowing the measurement of vertical rearing.
Rotarod
Motor coordination was assessed on days 1, 2, 3, 7, 14, and 21 post-CCI or sham procedures on an accelerating rotarod (Ugo-Basile, Collegeville, PA). Before the first trial on day 1 post-TBI or sham surgery, mice were placed on the slowly rotating rod (four rotations per minute; RPM) and allowed to acclimate to the apparatus for 1 min. After the 1-min acclimation, the rod accelerated from 4 to 60 RPM over a period of 3 min. Latency to fall from the rod (or cling to and rotate with the rod for three consecutive rotations) was recorded for three trials on each testing day, allowing 5–10 min of rest with access to food and water between each trial. A mouse that remained on the rod for the entire 3 min was assigned a score of 180 sec for that trial. Scores from the three trials on each testing day were averaged to give a single score for each mouse on each testing day.
Y-maze spontaneous alternation
The y-maze spontaneous alternation behavior (SAB) test, to assess hippocampal-dependent working (short-term) memory, was performed on postinjury day 10, as previously described.34,35 The apparatus consisted of three arms at a 120-degree angle to one another with a triangular central zone. Each arm was 36 cm in length, enclosed by walls 16 cm high, and a small visual cue was placed at the distal wall of each arm. Other objects in the room provided further spatial cues to mice during testing. Each mouse was placed at the end of a randomly chosen arm and allowed to freely explore the maze for 5 min. An animal with nonimpaired working memory is expected to exhibit SAB, visiting all three arms in alternation, not immediately returning to either of the arms that had been most recently explored. An overhead camera linked to Any-Maze computer software (Stoelting) recorded movements of the mouse and reported the distance traveled by each mouse during the test. Visits to arms were manually scored; a visit to an arm of the maze was counted when all four paws of the mouse entered the arm. An alternation was counted when the mouse visited three different arms consecutively, and the percent correct alternation was calculated as 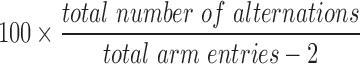 .
.
Morris water maze
Spatial learning and memory was assessed by standard MWM36 training trials on postinjury days 23–26 followed by a probe trial approximately 1 h after the final training trial. The apparatus was a circular white tank 122 cm in diameter, filled with water (23±1°C) to a depth of approximately 30 cm. A transparent acrylic platform (11 cm diameter) was submerged approximately 1.0 cm below the surface of the water and was located approximately 15 cm from the edge of the maze. A ceiling camera directly over the water maze was connected to a computer with Any-Maze tracking software (Stoelting). Within the software, the water maze was divided into four equal quadrants, with the platform located in the northwest (NW) quadrant. Large visual cues (black and white geometric shapes) were positioned around the apparatus in locations that could be viewed by mice during testing. Each mouse received four training trials (with an intertrial interval of 4–5 min) for 4 days. Four start positions were designated along the perimeter of the tank, and the order in which these positions were used was changed each day. For each training trial, the mouse was gently placed in the designated start position, facing the wall. Mice were allowed 60 sec to locate the platform using the spatial cues in the room, after which the animal remained on the platform for 15 sec. If the mouse did not find the platform in the allotted 60 sec, it was gently guided to the platform and allowed to remain there for 15 sec and received the maximum score of 60 sec for that trial. After each trial, mice were towel dried and placed into a heated cage. Latency to platform, distance swam during the test trials, and swimming speed were recorded by the software and averaged over the four trials each training day.
Approximately 1 h after the final training trial on postinjury day 26, a single probe trial was conducted in which the platform was removed from the tank and the mouse was placed against the wall, opposite the former location of the platform. The mouse was allowed to swim for 60 sec, during which time the software recorded the amount of time spent in the NW quadrant (that previously housed the platform), number of times the mouse crossed the previous exact location of the platform, and amount of time spent in the precise platform location.
Beginning on day 27 postinjury, reversal training trials were conducted to assess behavioral flexibility. These trials were conducted identically to the original training trials on postinjury days 22–26, except the platform was moved to the opposite quadrant (southeast; SE) of the apparatus. A reversal probe trial in which the platform was removed from the tank was conducted approximately 1 h after the final reversal training trial on postinjury day 30.
Histological assessment of injury
Immediately after the conclusion of behavioral testing on day 30 postinjury, mice were deeply anesthetized (60 mg/kg of ketamine with 60 mg/kg of xyalzine, intraperitoneally) and transcardially perfused with 0.1 M of phosphate buffer followed by 4% paraformaldehyde (PFA) in 0.1 M of phosphate buffer. Brains were postfixed for 24 hours in 4% PFA, followed by 24 hours of cryoprotection in 20% sucrose solution in 0.1 M of phosphate buffer. After cryoprotection, each brain was blocked identically, weighed, frozen in powdered dry ice, and stored at −80°C.
Eight of the brains from each sex and injury group were randomly chosen for lesion volume analysis. A Leica cryostat was used to cut serial sections through the lesion site 30 μm thick; sections were stained with cresyl violet. Slides were scanned with an Epson scanner, and the area of serial sections at 540-μm intervals (approximately 10 sections for each brain) was measured with ImageJ software (NIH, Bethesda, MD).37 Lesion area for each section was calculated as the area of the contralateral hemisphere minus the area of the ipsilateral hemisphere. The sum of the area values for each mouse brain was multiplied by 0.54 mm (the distance between each measured section) to estimate lesion volume.38
Statistical analyses
Effects of injury and sex on behavioral measures were evaluated using SPSS software (version 20; IBM SPSS Statistics, Armonk, NY). A mixed-design three-way analysis of variance (ANOVA) was performed on measures from tests on which mice were tested repeatedly over time (rotarod, OF, and MWM training and reversal training trials), with injury (sham, mild CCI, and severe CCI) and sex (male, female) as between-subjects variables and test day as a within-subject variable. Wilks' lambda F-statistics resulting from multivariate tests with measures from each testing day as separate dependent variables are reported. When interaction effects were significant, subsequent univariate two-way ANOVAs were performed for each day, and Bonferroni-adjusted t-test values were used to determine significant differences between groups.
For lesion volume data and for tests that were only performed at one time point (MWM probe trial measures, MWM reversal probe trial measures, and Y-maze test), two-way ANOVAs with sex and injury as between-subjects variables were performed and Bonferroni-adjusted t-test values were used to determine specific group differences. For data that failed to meet the homogeneity of variance assumption as assessed by Levene's test of equality of error variance, variables were transformed to square root or reciprocal values. Transformed variables passed homogeneity of variance tests. Where main statistical effects of sex were found, effect size (Cohen's d) was calculated as 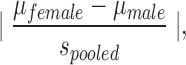 where
where 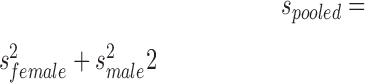 .
.
Finally, variability of behavioral data was compared between injury and sex groups. To control for potential differences in scale across the behavioral measures, the coefficient of variation (CV; standard deviation/mean) for each of the six treatment groups was calculated separately for each behavioral variable for which an ANOVA was performed. Repeated measures (e.g., distance traveled in OF) were collapsed across days to yield a single CV for each treatment group for that variable. A two-way (injury×sex) ANOVA was performed to compare mean CVs for each group. Additionally, Levene's test for equality of variances was conducted to compare the distributions of CVs for each group11,39: The median CV for each treatment group was determined, and absolute values of the differences between the CVs for each behavioral measure and the median were calculated. A two-way ANOVA with sex and injury as between-subjects variables was performed on these values.
Data summarized in all figures represent the mean±standard error of the mean (SEM), and results were considered significant when the p value was less than or equal to 0.05. For ease of viewing, results from behavioral tests measured over multiple days (i.e., OF, rotarod, and MWM) are divided into two panels on a single figure, with comparisons between mild CCI and sham controls on one panel, and comparisons between severe CCI and sham controls on a separate panel. The same group of sham controls are represented on both panels of these figures.
Results
Extent of injury
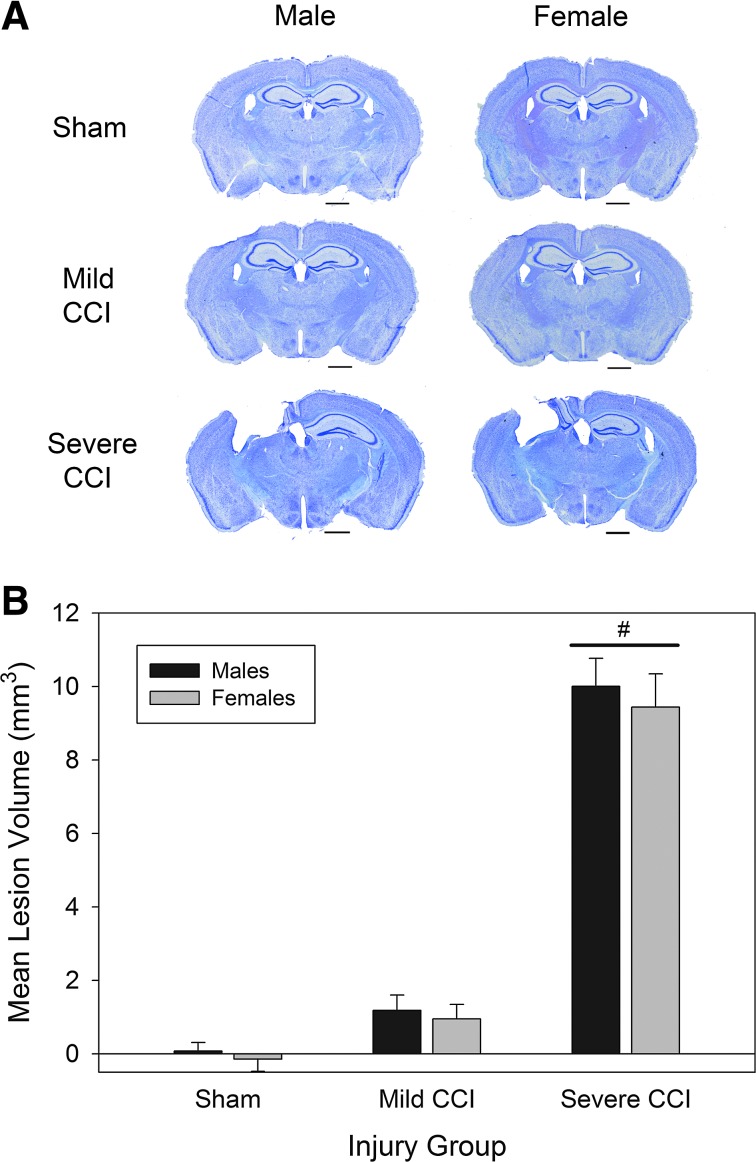
Figure 1A shows representative cresyl violet–stained brain sections from each injury group for both males and females. The extent of injury was comparable in male and female mice. Mild CCI, defined as impact delivered at a velocity of 5.0 m/s and a depth of 1.0 mm, resulted in very little gross, observable damage to the cortex and underlying hippocampal formation. The severe level of CCI (velocity, 5.0 m/s; depth, 2.0 m/s) delivered substantial damage to the left cortex and hippocampal formation. Lesion volume analysis (Fig. 1B) confirmed that there was no main effect of sex (F1,42=0.12; p=0.731) or interaction between sex and injury (F2,42=0.03; p=0.971). However, mice that had sustained severe CCI had significantly larger lesions than mice that had undergone sham procedures or mild CCI (F2,42=218.04; p<0.0001). The difference in lesion volume between mice with mild CCI and sham-operated mice was not significant (p=0.104).
FIG. 1.
Extent of injury post-CCI. Representative brain sections (stained with cresyl violet) at 30 days after traumatic brain injury (A). The severe level of CCI (velocity, 5 m/s; depth, 2.0 mm) resulted in substantial injury to the ipsilateral cortex and underlying hippocampus. Mild CCI (velocity, 5 m/s; depth, 1.0 mm) resulted in little macroscopic damage. Lesion volume analysis (B) showed that mice that sustained severe CCI had significantly greater tissue loss than mice that sustained mild CCI or sham procedures. Horizontal bars in (A) represent 1 mm. Pound sign (#) in (B) represents a main effect of injury on lesion volume, Severe CCI>Sham. CCI, controlled cortical impact.
Open field test
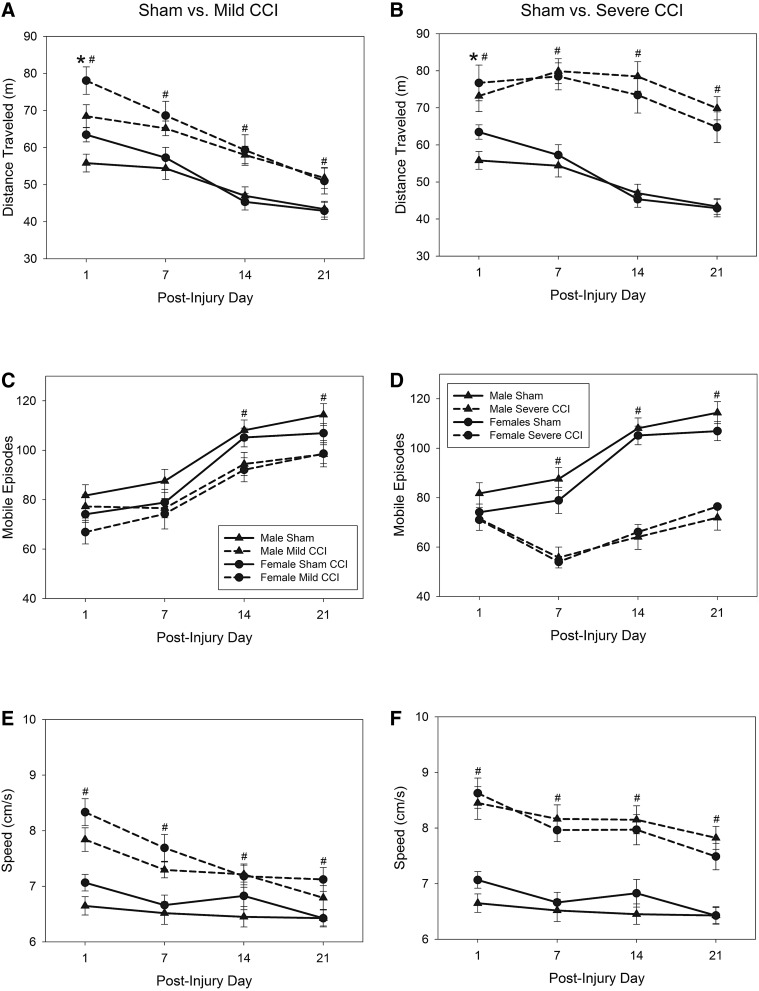
Analysis of distance traveled during a 20-min test session on days 1, 7, 14, and 21 after sham or TBI surgery revealed significant day×sex (F3,100=3.22; p=0.026) and day×injury (F6,200=4.05; p=0.001) interactions (Fig. 2A [mild CCI vs. sham controls] and 2B [severe CCI vs. sham controls]). Subsequent univariate analyses of each day revealed that female mice were more active than male mice in the OF, as measured by distance traveled on the first day postinjury (F1,102=6.70; p=0.011), but not on any other days tested. Univariate analyses for each day also showed that injury had an effect on distance traveled on all days tested (F2,102=10.68, F2,102=26.62, F2,102=37.91, and F2,102=31.54 for postinjury days [PIDs] 1, 7, 14, and 21, respectively; p≤0.0001 for all days), mice with both mild and severe CCI traveled a greater distance in the arena than sham-operated mice (Bonferroni's t-test comparisons sham<mild CCI and severe CCI; p<0.025).
FIG. 2.
Motor behavior in an open field after traumatic brain injury (TBI). Mice that sustained mild or severe CCI were significantly hyperactive on all days tested postinjury in the open field, as measured by total distance traveled (mild CCI [A] and severe CCI [B]). Additionally, female mice were more active than male mice in the open field on the day postinjury (day 1, A and B). The increase in activity in the injured mice was the result of fewer episodes of mobility (injured mice stopped and started moving less often, C and D) and of greater speed when mobile (E and F). Injured mice and sham-treated mice are represented by broken lines and solid lines, respectively; pound sign (#) denotes a significant effect of the represented injury level on the given test day. Asterisks (*) in (A) and (B) represents a main effect of sex (female>male) on distance traveled on day 1. CCI, controlled cortical impact.
The number of movement bouts for mice with mild or severe CCI, compared to sham controls, is shown in Figure 2C,D, respectively. Male and female mice engaged in the same number of mobile episodes (F1,102=1.05; p=0.309), and sex did not interact with either injury status (F2,102=0.55; p=0.578) nor day of testing (F3,100=0.61; p=0.610). There was a significant day×injury interaction for the number of mobile episodes exhibited by animals in OF testing (F6,200=8.03; p<0.0001; Fig. 2B). On the first day postinjury, all animals engaged in the same number of mobile episodes. Injury status had a significant effect on mobility on days 7, 14, and 21 postinjury (F2,102=17.28, 39.05, and 31.65, respectively; p<0.0001). Mice with mild CCI were indistinguishable from sham controls in mobility on day 7, but engaged in fewer bouts of movement on days 14 and 21 (Bonferroni's t-test comparison; p<0.029), indicating greater continuity of movement with fewer stops. Mice with severe CCI had fewer mobile episodes than sham-treated mice on days 7, 14, and 21 (Bonferroni's t-test comparison; p≤0.0002).
Analysis of movement speed during mobile episodes (Fig. 2E [mild CCI vs. sham controls] and 2F [severe CCI vs. sham controls]) showed that male and female mice moved at similar speeds (F1,102=0.65; p=0.423) regardless of injury condition (F2,102=1.31; p=0.273) or test day (F3,100=1.51; p=0.217). A day×injury interaction was found for the average speed of animals in the OF arena (F6,200=2.32; p=0.035; Fig. 2E,F); injury status affected speed on all days tested (F2,102=28.92 [day 1], F2,102=25.23 [day 7], F2,102=24.10 [day 14], and F2,102=18.93 [day 21]; p<0.0001 for all days). Sham-operated controls moved slower than mice with either mild or severe CCI on each test (Bonferroni's t-test comparison; p<0.042 and p<0.0001, respectively).
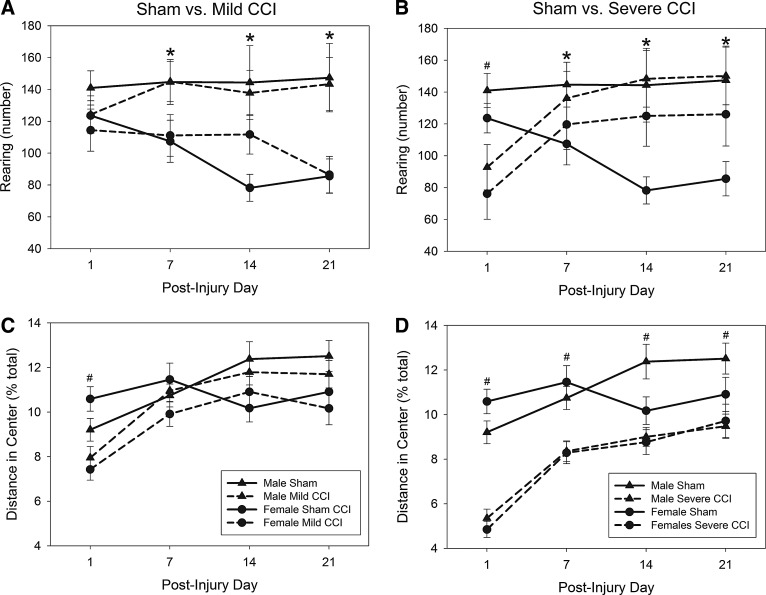
A day×sex (F3,100=3.42; p=0.02) interaction was found for vertical movement as assessed by rearing activity (number of rearing events for mice with mild or severe CCI compared to sham controls; Fig. 3A,B, respectively). Male and female mice were equal in rearing on the day postinjury, but on all other days tested, female mice reared less than male mice (F1,102=6.23, p=0.014; F1,102=8.05, p=0.005; and F1,102=12.09, p=0.001 for PIDs 7, 14, and 21, respectively). A significant day×injury (F6,200=5.97; p<0.0001) interaction was also reported; TBI reduced the number of rearing episodes only on the day after the procedure (F2,102=7.46; p=0.001), and only mice with the severe level of CCI were different from sham-operated controls (Bonferroni's t-test comparison; p=0.001).
FIG. 3.
Rearing and center activity in the open field after traumatic brain injury (TBI). Vertical movements (rearing) were unaffected by mild CCI (A), but were reduced on day 1 postinjury in mice that sustained severe CCI (B). On all other days tested (7, 14, and 21), female mice had reduced vertical movements, compared to male mice. Assessment of activity in the center of the apparatus, expressed as a function of total activity, suggested that mice with mild TBI were anxious on the first day postinjury (C), and severe TBI resulted in anxiety on all days tested postinjury (D). Injured mice and sham-treated mice are represented by broken lines and solid lines, respectively; pound sign (#) denotes a significant effect of the represented injury level on the given test day. Asterisks (*) in (A) and (B) represents a main effect of sex (male>female) on rearing on days 7, 14, and 21. CCI, controlled cortical impact.
A three-way ANOVA performed on distance traveled in the center zone of the OF (expressed as a percent of the total distance traveled) revealed no main sex differences (F1,102=2.79; p=0.098) or interactions between sex and injury (F2,102=0.89; p=0.414) or test day (F3,100=1.84; p=0.145), but we found a significant day×injury interaction (F6,200=2.57; p=0.020). Injury (mild CCI, Fig. 3C; severe CCI, Fig. 3D) significantly reduced distance traveled by mice in the center of the apparatus (expressed as a percent of the total distance traveled) on all days tested (injury factor main effect: F2,102=50.40, p<0.0001; F2,102=13.51, p<0.0001; F2,102=10.49, p<0.0001; and F2,102=5.15, p=0.007 for PIDs 1, 7, 14, and 21, respectively). Mice with severe brain injury traveled less distance in the center than sham-operated controls on all days tested (Bonferroni's t-test comparison; p≤0.0001 for PIDs 1, 7, and 14 and p=0.006 for PID 21), but mice that had sustained a mild CCI were less active in the center of the apparatus than sham-treated animals only on the day postinjury (Bonferroni's t-test comparison; p<0.0001).
Rotarod
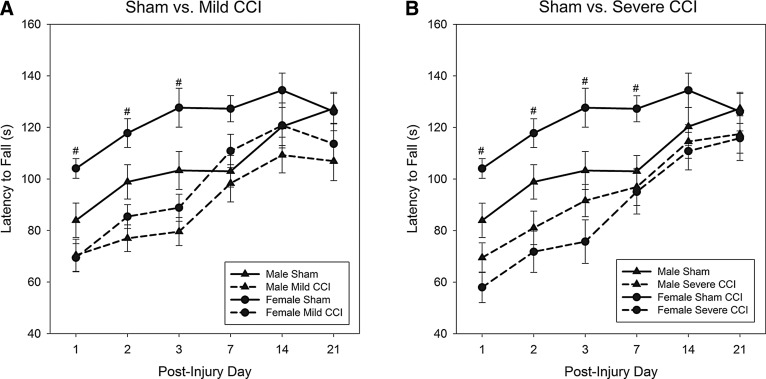
Analysis of motor coordination as assessed by the latency to fall from an accelerating rotarod found a sex×injury interaction that neared significance (F2,102=2.84; p=0.063); Figure 4A (mild CCI vs. sham controls) and 4B (severe CCI vs. sham controls) shows that for the sham-treated groups, females may have outperformed males. Performance was affected by brain injury for 1 week postinjury (F2,102=15.72, p<0.0001; F2,102=16.10, p<0.0001; F2,102=14.92, p<0.0001; and F2,102=3.90, p=0.023 for PIDs 1, 2, 3, and 7, respectively), but injured animals performed equivalent to controls on PIDs 14 and 21 (day×injury interaction; F10,196=1.90; p=0.048). Bonferroni-adjusted t-tests after univariate analyses revealed that mice that had sustained a severe CCI fell from the rotating rod faster than sham-operated mice on all days for which a significant effect of injury was found (p<0.0001 for PIDs 1, 2, and 3; p=0.019 for PID 7; Fig. 4B). Mice with a mild brain injury were impaired compared to sham-operated mice on the 3 days after CCI (p<0.0001 for PIDs 1, 2, and 3), but their performance was equal to uninjured animals on all subsequent rotarod tests of motor coordination (Fig. 4A).
FIG. 4.
Motor coordination assessed on an accelerating rotarod. Both mild and severe levels of CCI significantly impaired motor coordination on the 3 days immediately after traumatic brain injury (TBI; A and B, respectively). Mice with severe CCI continued to show impairments 1 week postinjury, but all mice had equivalent performance to sham controls by 14 days post-CCI. Injured mice and sham-treated mice are represented by broken lines and solid lines, respectively; pound sign (#) denotes a significant effect of the represented injury level on the given test day. CCI, controlled cortical impact.
Y-maze spontaneous alternation behavior
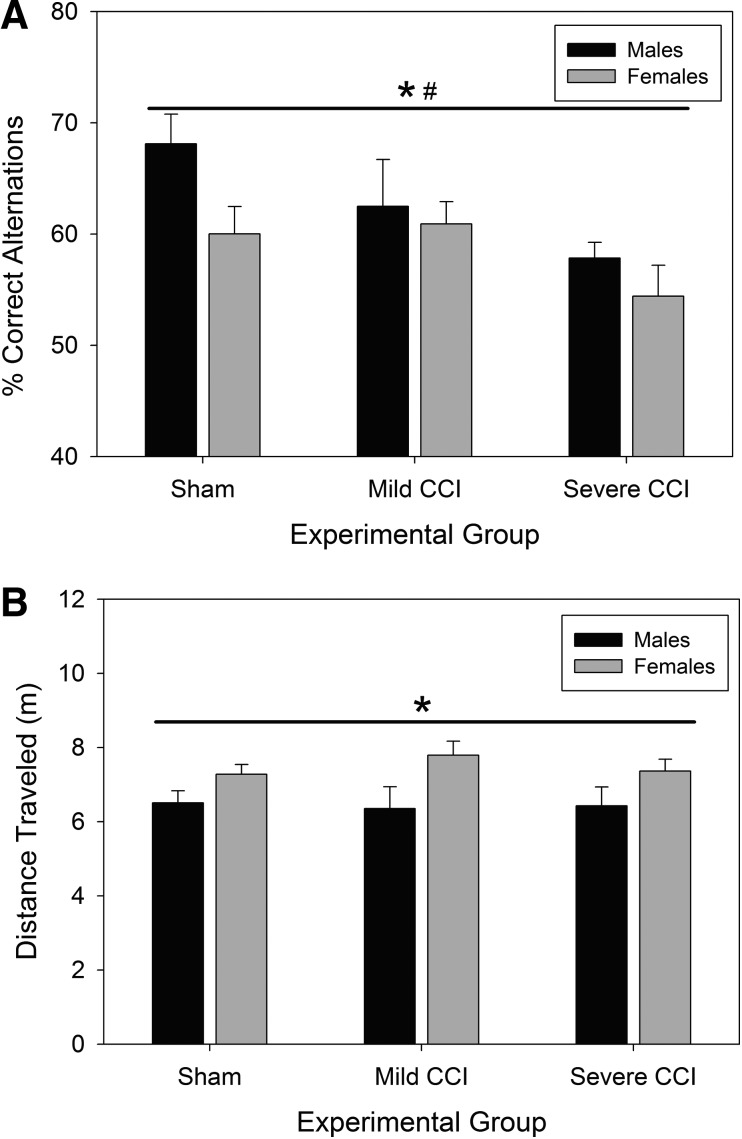
Figure 5A shows working memory performance in the y-maze SAB test of male and female mice post-TBI. There were significant main effects of both sex (F1,102=4.75; p=0.032) and injury (F2,102=5.46; p=0.006) on memory performance on this test. Female mice showed inferior performance to male mice in this working memory task. Additionally, female mice were more active in the y-maze apparatus than male mice, as evidenced by greater distance traveled (Fig. 5B; F1,102=11.97; p=0.001). Although mild CCI did not affect SAB in male and female mice, severely injured mice had significantly impaired working memory compared to sham-controls, as evidenced by a lower SAB score (Bonferroni's t-test comparison; p=0.005).
FIG. 5.
Activity in a y-maze. Working memory was impaired 10 days postinjury in mice with severe, but not mild, CCI as assessed by spontaneous alternation behavior (A). Female mice were also more active in the y-maze, having a greater distance traveled during the test session (B). Asterisks (*) represent a main effect of sex on percent correct alternations (female <male; A) and on distance traveled in the y-maze (females >male; B); pound sign (#) denotes inferior performance of all mice with severe CCI, compared to sham-treated animals (A). CCI, controlled cortical impact.
Morris water maze
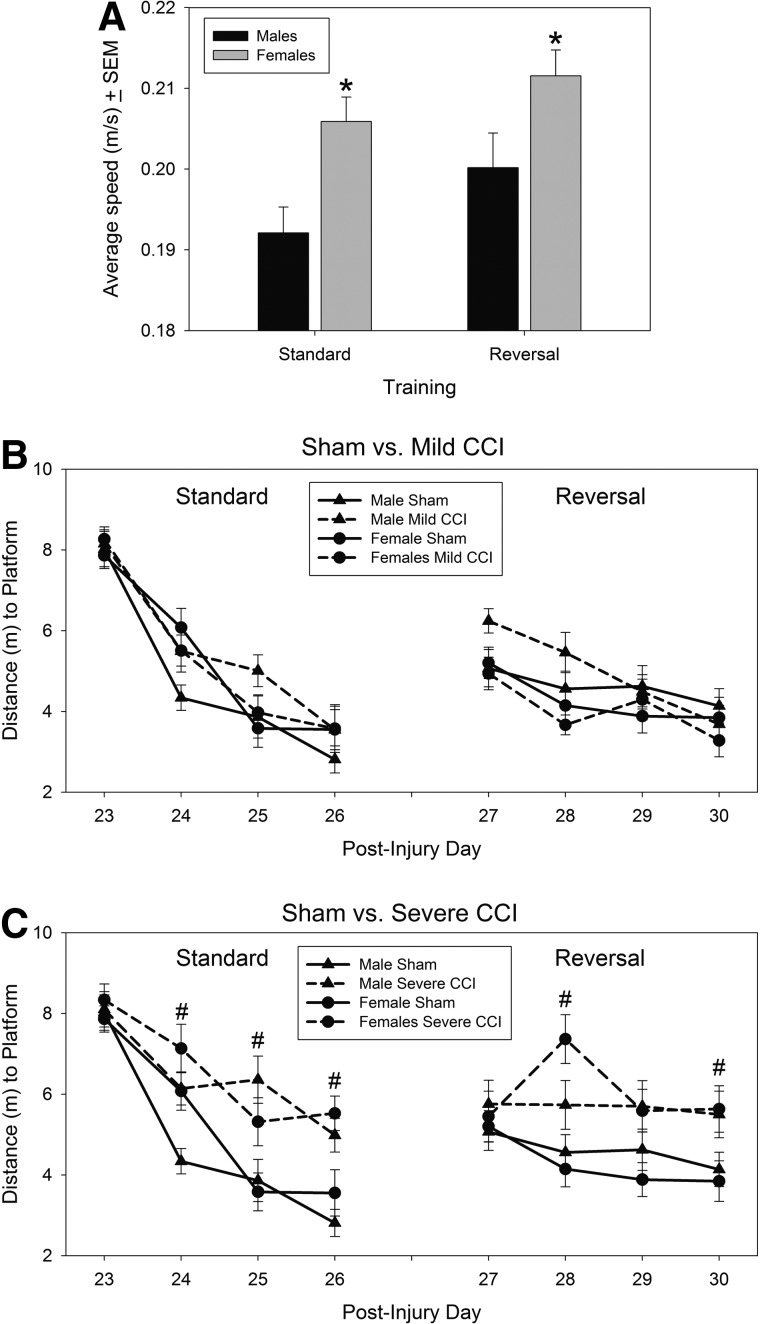
Analysis of swimming speed in the MWM showed that female mice swam significantly faster than male mice during standard training trials (F1,102=9.08; p=0.003) and reversal training trials (F1,102=4.23; p=0.04; Figure 6A). Injury did not impact swimming speed during standard or reversal training trials (F2,102=0.78, p=0.459 and F2,102=0.44, p=0.664, respectively; data not shown).
FIG. 6.
Spatial learning in the Morris water maze. Female mice swam faster than male mice during both standard and reversal training trials (A). Mice with mild CCI had equivalent learning abilities to sham controls (B), but all mice with severe CCI were impaired in their ability to learn the location of a hidden platform in the Morris water maze, during both standard training and reversal training trials (C). Brain-injured mice and sham-treated mice are represented by broken lines and solid lines, respectively. Asterisks (*; A) denote faster swimming speeds of female mice; pound sign (#; C) represents poorer performance of mice with severe CCI, compared to sham controls, on the denoted training days. CCI, controlled cortical impact.
Distance swam before reaching the platform was employed as the measurement of animals' cognitive performance during MWM training trials (Fig. 5B,C [left plots, labeled “standard”] for mild or severe CCI vs. sham controls, respectively). Analysis of spatial learning, as assessed by the distance swam before locating a submerged platform in a pool of water, revealed a significant day×injury×sex interaction during standard training days 23–26 postinjury (F6,200=2.22; p=0.043). Subsequent two-way (injury×sex) ANOVAs for each day showed that the three-way interaction from the repeated-measures ANOVA resulted from an injury×sex interaction on the third training day that almost reached statistical significance (F2,102=2.98; p=0.055). Injury status did not affect cognitive performance on the first day of standard training, but there was a significant effect of injury status on the subsequent 3 days (F2,102=6.96, p=0.001; F2,102=7.97, p=0.001; and F2,102=10.26, p<0.0001, respectively). Mice with mild CCI were not impaired on this cognitive task (Fig. 6B), but severe CCI resulted in significantly longer distances swam, when compared to sham-operated controls, on those 3 days (Bonferroni's t-test comparison; p=0.001, 0.001, and 0.0002, respectively; Fig. 6C).
During reversal training trials on PIDs 27–30 (Figure 6B,C; right plots, labeled “reversal”), the three-way ANOVA on distance to platform revealed no main sex differences (F1,102=0.04; p=0.551), nor sex×injury (F2,102=1.93; p=0.151) or sex×training day (F3,100=1.13; p=0.342) interactions. There was a significant day×injury interaction (F6,200=4.90; p=0.0001); injury status had a significant effect on learning on PIDs 28–30 (F2,102=13.06, p<0001; F2,102=4.55, p=0.017; and F2,102=8.92, p=0.0003, respectively), but not on PID 27. Severe CCI, compared to sham procedures, resulted in longer distances swam before the platform was found on PIDs 28 and 30 (Bonferroni's t-test comparison; p<0.0001 and p=0.001, respectively).
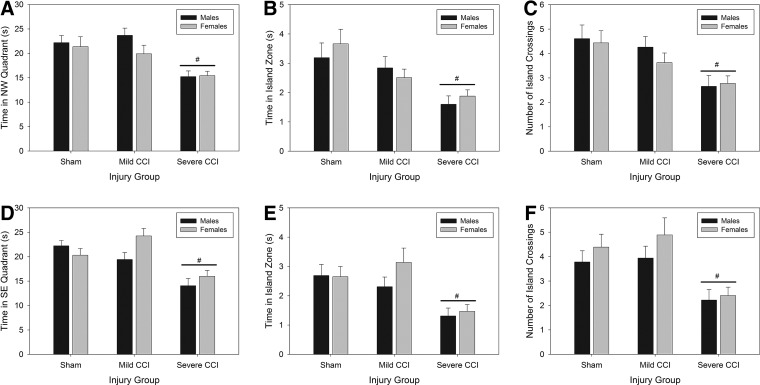
Analysis of the time spent in the NW quadrant during the probe trial after standard training on PID 26 (Fig. 7A) showed no significant main effect of sex (F1,102=1.30; p=0.256), nor a sex×injury interaction (F2,102=0.36; p=0.696). There was a significant effect of injury (F2,102=10.10; p<0.0001); the severe level of CCI, but not mild CCI, reduced the amount of time spent in the NW zone (Bonferroni's t-test; p=0.001). Time spent in the exact platform location (Fig. 7B) was also unaffected by sex (F1,102=0.520; p=0.473), and there was no sex×injury interaction on this measure (F2,102=0.54; p=0.582); there were also no main effects of sex (F1,102=0.75; p=0.390) or sex×injury interaction (F2,102=0.51; p=0.600) for the number of times the platform location was crossed (Fig. 7C).
FIG. 7.
Results from probe trials, designed to assess memory for the location of the platform, are shown in (A) through (C) and (D) through (F) for the standard probe trial and the reversal probe trial, respectively. Time in the quadrant formerly housing the platform (northwest quadrant for the standard probe trial [A], southeast quadrant for the reversal probe trial [D]) was significantly reduced in mice with severe CCI, indicating memory impairments. Specific platform measurements—time in platform zone (B and E) and number of platform crossings (C and F)—distinguished all mice with severe CCI from sham-operated controls. #Severe CCI versus sham. CCI, controlled cortical impact.
There were significant main effects of injury (F2,102=8.33, p=0.0004; F2,102=8.21, p=0.0005) for time in platform location (Fig. 7B) and platform crossings (Fig. 7C). Mice with the severe level of brain injury had poorer memory of the platform than sham-control mice did (p=0.0002 for time in platform location; p=0.0004 for number of crossings), but mildly injured mice performed equally to sham-operated mice.
Similar results were found after the reversal probe trial on PID 30. There were no main effects of sex on any of the probe trial variables (F1,102=2.07, p=0.154; F1,102=1.28, p=0.260; and F1,102=1.95, p=0.166 for time in SE quadrant [Fig. 7D], time in island zone [Fig. 7E], or number of island crossings [Fig. 7F], respectively). An interaction between sex and injury for time in the SE quadrant neared significance (F2,102=3.05; p=0.052); there were no sex×injury interactions found for time in the platform zone or number of platform location crossings (F2,102=0.20, p=0.818 and F2,102=0.28, p=0.759, respectively). However, injury significantly impacted the ability of mice to remember the location of the platform, as assessed by the amount of time spent in the SE quadrant (Fig. 7D; F2,102=14.91; p<0.0001), amount of time spent in the specific former location of the platform (Fig. 7E; F2,102=7.79; p=0.001), and number of times mice entered/crossed the former platform location (Fig. 7F; F2,102=9.61; p=0.0002). Mice that had sustained severe TBI were impaired, compared to sham-operated controls, on all measures (Bonferroni's t-tests; p<0.002), but mice with mild CCI had similar memory performance to uninjured animals.
Variability in behavioral results
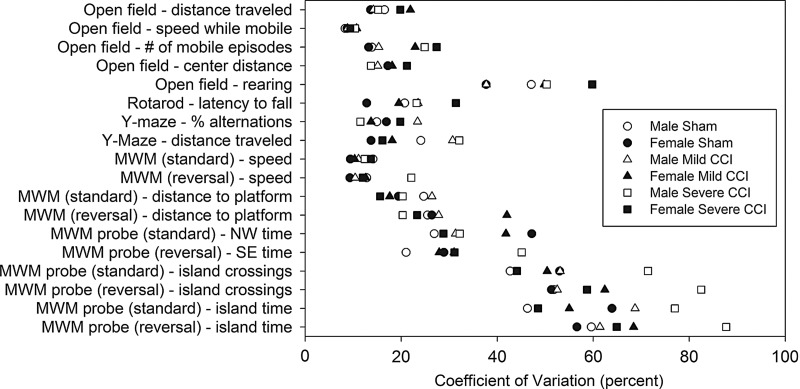
Table 1 lists the CVs for each group, on each reported behavioral measure. Mean CVs for all reported behavioral measures (n=18) were not significantly affected by injury (F2,102=0.81; p=0.448) or sex (F1,102=0.14; p=0.712). Figure 8 provides a graphical depiction of the CV values as an illustration of the range of values for each behavioral test. Distribution of variability in behavioral results (Fig. 8), compared with Levene's test of equality of variances, was also not affected by injury (F2,102=0.17; p=0.842) or sex (F1,102=0.30; p=0.582).
Table 1.
Mean Coefficients of Variation on Each Behavioral Measure for all Treatment Groupsa
| Sham (%) | Mild CCI (%) | Severe CCI (%) | Summary (%) | ||||
|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | Mean±SEM | |
| Open field—distance traveled | 16.5 | 13.6 | 14.2 | 21.9 | 15.2 | 19.8 | 16.9±1.3 |
| Open field—speed while mobile | 8.3 | 8.7 | 8.8 | 10.7 | 10.4 | 9.4 | 9.4±0.4 |
| Open field—no. of mobile episodes | 13.9 | 13.2 | 15.3 | 22.9 | 24.9 | 27.4 | 19.6±2.5 |
| Open field—center distance | 17.2 | 17.2 | 15.1 | 18.1 | 13.7 | 21.2 | 17.1±1.1 |
| Open field—rearing | 47.1 | 37.7 | 37.7 | 49.8 | 50.3 | 59.8 | 47.0±3.4 |
| Rotarod—latency to fall | 20.7 | 12.8 | 23.5 | 19.5 | 23.2 | 31.4 | 21.8±2.5 |
| Y-maze—% alternations | 14.9 | 16.9 | 23.4 | 13.7 | 11.5 | 19.8 | 16.7±1.8 |
| Y-maze—distance traveled | 24.1 | 13.7 | 30.7 | 18.1 | 32.1 | 16.1 | 22.5±3.2 |
| MWM (standard)—speed | 14.1 | 9.4 | 11.1 | 10.3 | 12.4 | 13.7 | 11.8±0.8 |
| MWM (reversal)—speed | 12.8 | 9.3 | 10.4 | 12.6 | 22.1 | 12.0 | 13.2±1.9 |
| MWM (standard)—distance to platform | 24.7 | 19.4 | 26.4 | 17.6 | 20.3 | 15.6 | 20.7±1.7 |
| MWM (reversal)—distance to platform | 25.5 | 26.4 | 27.8 | 42.0 | 20.3 | 23.3 | 27.6±3.1 |
| MWM probe (standard)—NW time | 26.9 | 47.2 | 31.3 | 41.8 | 32.2 | 28.8 | 34.7±3.3 |
| MWM probe (reversal)—SE time | 21.0 | 28.9 | 31.0 | 27.9 | 45.1 | 31.1 | 30.8±3.2 |
| MWM probe (standard)—island crossings | 42.7 | 53.0 | 53.1 | 50.4 | 71.4 | 44.1 | 52.5±4.2 |
| MWM probe (reversal)—island crossings | 51.8 | 51.3 | 52.5 | 62.4 | 82.5 | 58.7 | 59.9±4.9 |
| MWM probe (standard)—island time | 46.3 | 63.9 | 68.7 | 55.0 | 77.0 | 48.5 | 59.9±4.9 |
| MWM probe (reversal)—island time | 59.6 | 56.6 | 61.4 | 68.4 | 87.7 | 64.9 | 66.4±4.6 |
| Mean | 27.1 | 27.7 | 30.1 | 31.3 | 36.2 | 30.3 | |
| SEM | 3.6 | 4.4 | 4.3 | 4.5 | 6.2 | 4.1 | |
No statistical differences in mean variation were observed between levels of injury or sex.
MWM, Morris water maze; CCI, controlled cortical impact; NW, northwest; SE, southeast; SEM, standard error of the mean.
FIG. 8.
Variability in behavioral data. Coefficients of variation are shown for 18 behavioral measures for each of the six groups. There was no effect of sex or injury on the mean coefficients of variation (data not shown), and the distribution of variability was also unaffected by sex or injury as assessed by Levene's test of equality of error variance. Open and closed symbols represent males and females, respectively, and circles, triangles, and squares represent sham, mild and severe injury, respectively. MWM, Morris water maze; CCI, controlled cortical impact.
Discussion
Summary of sex differences in behavioral measures
The data presented in this article confirm numerous previous studies describing behavioral changes in mice post-CCI. Of the 18 behavioral measures reported in Table 1, there were no sex×injury significant interactions, suggesting that the effects of CCI are equivalent in male and female mice. However, there were five measures for which significant main effects of sex were found (Table 2), and power and effect size suggest that these variables manifest important sex differences. Although one of the measures (percent alternation in the y-maze) suggests inferior memory performance of female mice, the remaining measures relate to activity levels, with female mice moving a greater distance or at a greater speed than male mice. These differences are considered below in the interpretation of the effects of brain injury on behavior.
Table 2.
Summary of Significant Differences Between Male (n=54) and Female (n=54) Micea
| Main effect of Sex | p value | Power | Effect size (Cohen's d) | |
|---|---|---|---|---|
| OF distance traveled, day 1 | female>male | 0.027 | 0.604 | 0.4317 |
| Y-maze, distance traveled | female>male | 0.001 | 0.938 | 0.6800 |
| Y-maze, % alternation (memory) | male>female | 0.044 | 0.523 | 0.3921 |
| MWM swim speed, standard trials | female>male | 0.003 | 0.854 | 0.5856 |
| MWM swim speed, reversal trials | female>male | 0.039 | 0.544 | 0.4022 |
Shown are the behavioral measures in the current experiment for which a significant main effect of sex was found. p values from analyses of variance, power (1 – β), and calculated effect sizes (Cohen's d) are provided. Cohen's d=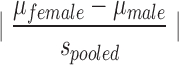 .
.
OF, open field; MWM, Morris water maze.
Females are more active during the first exposure to open field
The OF test provides a wealth of information regarding exploration, anxiety, and motor abilities. Although both male and female mice with TBI had increased distance traveled in the OF on all days tested, there was a main effect of sex on distance traveled on the first day of exposure to the OF arena, the day after sham or CCI procedures, with females having increased activity in the OF arena compared to the male mice. Forty years ago, John Archer noted that although reports are not entirely consistent, the majority of studies on male and female C57BL/6 mice demonstrate greater ambulation in female mice during exposure to a novel OF.40 A more recent review also concluded that female mice are more active than male mice in an OF,41 and estrogen has been shown to increase fear learning, anxiety, and home cage activity, suggesting that the hormone increases arousal.42 However, studies are fundamentally inconsistent. A trend toward or significantly greater OF activity in female C57BL/6 mice is frequently reported,43–49 but almost an equal number of other studies find no differences.50–55 Inconsonant observations regarding sex differences could be the result of variations in specific testing conditions, such as lighting level,56 or subtle aspects of the laboratory environment even when efforts are made to make conditions identical.57 A difference in performance between female and male mice during the first exposure to the OF in our experiment could be a behavioral characteristic that would not be observed in replications, or it could be indicative of an “emotional” reaction by female mice to a novel environment. In support of this suggestion, we also found that female mice were more active in the y-maze (Fig. 5B), also a novel environment. Preinjury habituation or baseline testing may prevent postinjury sex differences that interact and confound interpretation of the experimental question. Additionally, the possibility of sex-related differences should be considered in other assessments that require exposure of mice to a novel environment, such as the novel object recognition test for which sex differences in performance have been noted in C57BL/6 mice.58
Mild or severe traumatic brain injury induces hyperactivity in male and female mice
Significant hyperactive exploration in the OF is a frequently reported effect of CCI delivered over parietal cortex in mice.33,59–63 In the current experiment, hyperactivity in injured mice (Fig. 2A,B) was the result of more-continuous exploration in mice with TBI (fewer starts and stops; Fig. 2C,D), as well as greater speed while mice were mobile (Fig. 2E,F). The precise biological mechanisms by which parietal contusion injuries increase activity in the OF remains unexplained. A comprehensive review of experiments demonstrating hyperactive phenotypes in animals concluded that a variety of experimental manipulations can result in increased locomotion, and the author suggested the existence of a complex control system that modulates and suppresses activity.64 Any lesion or manipulation that results in impairment in this system, located along the axis connecting the olfactory bulb and entorhinal cortex, may result in hyperactive behavior. This control system would especially include the parietal cortex and dorsal hippocampus at the parietal location, which typically sustains significant damage from CCI. Given the significant association between TBI and attention deficit hyperactivity disorder in clinical populations,65 increased study of OF activity and agents that reduce pathological alterations in locomotion could significantly benefit translational TBI research.
Severe traumatic brain injury induces anxiety equally in male and female mice as measured in open field
Anxiety is a common neuropsychiatric symptom post-TBI.66,67 We found that, compared to male mice, female mice exhibited less rearing behavior in the OF (Fig. 3A,B). A recent review noted that measurements of rearing are often used as an indicator of anxiety, but that there is no consensus of how rearing is related to anxiety, or whether rearing simply relates to general exploration.68 Female and male mice did not differ with respect to activity in the center of the OF in this experiment, which is suggested to be indicative of an anxiety-like state in rodents.69,70 Although the number of females suffering from anxiety disorders greatly outnumbers male anxiety patients in the clinical population,71 findings regarding sex differences in rodents on tests of anxiety are notably contradictory. Oddly, most data from studies employing animal models of anxiety suggest an opposite result to the human condition, with male mice displaying greater anxiety-like behaviors than female mice,41 calling into question the relevance of rodent models for the study of sex-related differences for this human neuropsychiatric symptom. Further, as noted previously for the effects of TBI on center-zone activities in OF, any sex differences reported in these measures should be interpreted in the context of possible differences in levels of general arousal.
In the current study, brain-injured mice traveled less distance in the center region (main effect of injury; Fig. 3C,D), corroborating previous findings in C57BL/6 mice when tested in an OF after parietal CCI.33,59,62 However, testing of anxiety in the classic elevated plus maze (EPM) post-TBI yields disparate results. Washington and colleagues72 reported that cortically injured mice spent a greater amount of time in anxiogenic regions of the EPM on the 20th day postinjury and concluded that brain injury results in increased “risk-taking” behaviors in mice. Chauhan and colleagues, however, reported anxiety-like behavior in the EPM at an earlier time point; 24–48 h postinjury.73 It has been noted that data and conclusions regarding the effects of TBI on anxiety in rodents are inconsistent and that additional research is warranted.74 In particular, further studies are needed to determine whether there is a time course postinjury for expression of anxiety-like behavior, whether different testing paradigms yield different conclusions, or whether reduced time in the center of the OF (increased thigmotaxis) is related to hyperlocomotion postinjury and possibly not indicative of increased anxiety at all.
Traumatic brain injury equally impairs motor coordination on the rotarod in males and females
Mice with mild and severe TBI in this experiment confirmed previous reports75–78 of transient impairments in motor coordination as measured on an accelerating rotarod, and here brain-injured males and females had equivalent performance (Fig. 4). In contrast, sex-related differences in sensorimotor function post-TBI, with females faring better than males, have been shown in rats on a beam walk28 and mice using a grid/foot-fault test.31 Beam walking is a more sensitive test of mouse motor coordination post-TBI; performance of brain-injured mice typically improves to sham-level performance on the rotarod within 1 week, whereas a beam-walk task still detects motor deficits up to 4 weeks postinjury.76 Thus, the rotarod test, which reliably evaluates rhythmic motor abilities post-TBI, does not discriminate between sexes postinjury, compared to tasks that place greater challenge on maintaining postural balance and coordination in placement of the extremities.
Females performed poorly compared to males on the y-maze working memory test
The y-maze test of hippocampal-dependent working memory is a brief, convenient test that relies on naturalistic exploration and requires no training, food, or water deprivation. Mice prefer to enter a maze arm that was not previously explored, so the test requires the animals to recall which arm was most recently entered.35 Overall, performance of sham-treated mice was significantly superior to that of animals with severe CCI, as described previously,34 but there was a statistical main effect of sex, with females having poorer performance (Fig. 5A). There is a long-standing controversy in the literature regarding the existence, magnitude, and reliability of sex differences in rodent models of cognition that is beyond the scope of this article. Superior working memory performance of male mice has been observed in the water radial-arm maze,79 but a lack of sex differences in working memory performance has been demonstrated in the y-maze80 and radial arm maze81 in C57BL/6 mice. A meta-analysis in 2005 failed to find any studies demonstrating sex differences in working memory in any mouse strain, although some rat studies affirmed a male advantage.82 Thus, the primary conclusion from the y-maze working memory results in this study is that spontaneous alternation behavior in this paradigm effectively detected cognitive impairments post-TBI. The poorer performance of female mice in this study is unexplained and calls for replication. However, the greater activity of female mice, compared to males (Fig. 5B), may have an effect on attention as the animal navigates and makes choices, thereby contributing to lower spontaneous alternation behavior.
Females swim faster but have equivalent cognitive performance to males on spatial navigation tasks after traumatic brain injury
The MWM is a commonly employed test for cognitive function post-TBI, and our findings are consistent with a history of literature demonstrating the robustness of the test in the detection of learning and memory deficits after CCI in mice.33,72,78,83–86 Female mice in this study swam significantly faster than male mice during both standard training and reversal training trials (Fig. 6A), consistent with our results from OF and y-maze that suggest greater levels of activity in female mice. Faster swim speeds of female mice have been previously reported.87 Although the measure most commonly employed to report cognitive performance in the MWM is latency to find the platform, this measure is confounded if there are significant differences between groups in speed of swimming. Using the distance swam or path length as the measure of cognitive ability, we found no differences in the performance on the MWM between male and female mice. In humans, males perform better than females on a virtual water maze task,88 but it seems that most C57BL/6 mouse studies have concluded a lack of sex differences in spatial task performance in the MWM.44,87,89–92 Cognitive differences between male and female mice post-TBI are largely unexplored. Xiong and colleagues employed an alternative MWM protocol in which the platform was moved to random locations within the target quadrant; male and female mice had equivalent performance in this paradigm post-CCI.31 In rats, a lack of sex differences in MWM performance has also been reported post-CCI.28,93 Further, estrous cycle stage at time of injury had no impact on post-TBI cognitive performance in rats.28 Thus, it appears that use of the MWM for cognitive testing post-TBI does not discriminate between males and females for many species and strains of rodents. However, extrapolation of this conclusion to other cognitive tests would be premature given that sex differences are observed in other behavior tasks. For example, significant differences in performance between C57BL/6 male and female mice have been reported in the novel object recognition test94 and passive-avoidance test95 (but not in the Barnes maze spatial navigation test96,97), and our data suggest poorer performance of female mice on the y-maze test of spontaneous alternation behavior. These tests all rely on natural exploratory activities, and activational effects of estrogen42 may influence attention and cognitive performance in these paradigms.
Implications for inclusion of female animals in pre-clinical traumatic brain injury research
We have demonstrated that gross histological effects and behavioral results from many popular functional assessments post-TBI are largely comparable in age-matched male and female C57BL/6 mice. However, greater activity (distance traveled) of female mice during the first exposure to the OF suggests that a preinjury habituation to the apparatus may minimize sex differences during the first exposure to the novel environment postinjury. We failed to find a sex difference in motor performance as measured by the rotarod, and this may be test specific given that female advantages post-TBI have been previously reported using the more sensitive beam-walk task. Further cognitive testing in males and females post-TBI is warranted. Although this is the second study to demonstrate a lack of sex differences in spatial learning and memory post-CCI in mice,31 we have shown that working memory assessed in a y-maze may be sex dependent, although this sex difference did not preclude an effect of brain injury on cognitive performance on this task. Finally, we have shown that variability in behavioral data from cycling female mice is not greater than that measured in males, and use of sex as a between-subjects factor in statistical analyses should not abrogate or confound a main effect of injury, given that no sex×injury interactions were reported in the current studies. The statement of Mogil and Chanda98 is germane: “If there is no apparent sex difference in a particular experiment, one is no further behind by testing five males and five females and collapsing their data into n=10 than one would be by testing 10 males in the first place.”
Although we attempted to provide results from the most commonly used behavioral tests in pre-clinical TBI research, these experiments ultimately employed a relatively small subset of the behavioral paradigms available in animal research. Descriptions of post-TBI sex differences are warranted in other behavioral domains, in particular for neuropsychiatric symptoms such as anxiety, depression, and aggression. Alternative species and strains may also yield different conclusions. For example, behavioral results from C57BL/6 females is not dependent on estrous cycle stage in OF, rotarod, acoustic startle reflex, and tail flick test for pain, but OF results varied by stage of cycle in Balb/C females.99 These strain differences in effects of circulating hormones on behavior may also have implications for post-TBI functional assessments. Model of TBI must also be considered; CCI-induced injury generally reveals few histopathological differences between males and females,23, 24 whereas other methods of inflicting TBI often reveal sex differences favoring females.21,100,101
Finally, the ultimate goal of translational research is to identify and test potential therapeutic agents for biomedical maladies. Physiological differences between men and women, particularly in pharmacokinetics and pharmacodynamics,102 cannot be ignored in this context. Clinical data indicate that adverse effects of drugs are more common and more severe in women,103 and some pre-clinical studies also suggest differential effects of treatments, including efficacy, on functional improvements in male and female animals post-TBI.29,104,105 However, follow-ups to these studies often do not confirm initial findings of sex differences in response to treatment or lack of efficacy in one sex,30 emphasizing the need for replications of basic research and pre-clinical trials. The data presented here would also benefit from replication in other laboratory environments and using alternative and additional testing procedures. However, at present, these results suggest that there are many functional assessments that, with consideration of sex differences in activity levels, reliably detect behavioral effects of CCI in male and female animals equally without the need to monitor estrous cycle stage at the time of injury and/or testing, and these tests can be employed in the pursuit of potential TBI therapies in both male and female animals.
Acknowledgments
This work was supported by The Center for Neuroscience and Regenerative Medicine (60855-300600-7.01). The opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the Department of Defense, the U.S. government, or the Uniformed Services University of the Health Sciences. The use of trade names does not constitute an official endorsement or approval of the use of such reagents or commercial hardware or software. This document may not be cited for purposes of advertisement. The authors appreciated utilization of the laboratory of Dr. Yumin Zhang for tissue sectioning and the assistance of Jessica Ozl with behavioral testing in a pilot study.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.McIntosh T.K., Smith D.H., Meaney D.F., Kotapka M.J., Gennarelli T.A., and Graham D.I. (1996). Neuropathological sequelae of traumatic brain injury: relationship to neurochemical and biomechanical mechanisms. Lab. Invest. 74, 315–342 [PubMed] [Google Scholar]
- 2.Bramlett H.M., and Dietrich W.D. (2007). Progressive damage after brain and spinal cord injury: pathomechanisms and treatment strategies. Prog. Brain Res. 161, 125–141 [DOI] [PubMed] [Google Scholar]
- 3.Blennow K., Hardy J., and Zetterberg H. (2012). The neuropathology and neurobiology of traumatic brain injury. Neuron 76, 886–899 [DOI] [PubMed] [Google Scholar]
- 4.Kabadi S.V., and Faden A.I. (2014). Neuroprotective strategies for traumatic brain injury: improving clinical translation. Int. J. Mol. Sci. 15, 1216–1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maas A.I., Roozenbeek B., and Manley G.T. (2010). Clinical trials in traumatic brain injury: past experience and current developments. Neurotherapeutics 7, 115–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clayton J.A., and Collins F.S. (2014). Policy: NIH to balance sex in cell and animal studies. Nature 509, 282–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCullough L.D., de Vries G.J., Miller V.M., Becker J.B., Sandberg K., and McCarthy M.M. (2014). NIH initiative to balance sex of animals in preclinical studies: generative questions to guide policy, implementation, and metrics. Biol. Sex Differ. 5, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sandberg K., and Umans J.G.; and the Georgetown Consensus Conference Work Group. (2015). Recommendations concerning the new U.S. National Institutes of Health initiative to balance the sex of cells and animals in preclinical research. FASEB J. 29, 1646–1652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hughes R.N. (2007). Sex does matter: comments on the prevalence of male-only investigations of drug effects on rodent behaviour. Behav. Pharmacol. 18, 583–589 [DOI] [PubMed] [Google Scholar]
- 10.Beery A.K., and Zucker I. (2011). Sex bias in neuroscience and biomedical research. Neurosci. Biobehav. Rev. 35, 565–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prendergast B.J., Onishi K.G., and Zucker I. (2014). Female mice liberated for inclusion in neuroscience and biomedical research. Neurosci. Biobehav. Rev. 40, 1–5 [DOI] [PubMed] [Google Scholar]
- 12.Rogers E., and Wagner A.K. (2006). Gender, sex steroids, and neuroprotection following traumatic brain injury. J. Head Trauma Rehabil. 21, 279–281 [DOI] [PubMed] [Google Scholar]
- 13.Stein D.G. (2008). Progesterone exerts neuroprotective effects after brain injury. Brain Res. Rev. 57, 386–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roof R.L., and Hall E.D. (2000). Gender differences in acute CNS trauma and stroke: neuroprotective effects of estrogen and progesterone. J. Neurotrauma 17, 367–388 [DOI] [PubMed] [Google Scholar]
- 15.Stein D.G. (2007). Sex differences in brain damage and recovery of function: experimental and clinical findings. Prog. Brain Res. 161, 339–351 [DOI] [PubMed] [Google Scholar]
- 16.Slewa-Younan S., van den Berg S., Baguley I.J., Nott M., and Cameron I.D. (2008). Towards an understanding of sex differences in functional outcome following moderate to severe traumatic brain injury: a systematic review. J. Neurol. Neurosurg. Psychiatry 79, 1197–1201 [DOI] [PubMed] [Google Scholar]
- 17.Moore D.W., Ashman T.A., Cantor J.B., Krinick R.J., and Spielman L.A. (2010). Does gender influence cognitive outcome after traumatic brain injury? Neuropsychol. Rehabil. 20, 340–354 [DOI] [PubMed] [Google Scholar]
- 18.Ratcliff J.J., Greenspan A.I., Goldstein F.C., Stringer A.Y., Bushnik T., Hammond F.M., Novack T.A., Whyte J., and Wright D.W. (2007). Gender and traumatic brain injury: do the sexes fare differently? Brain Inj. 21, 1023–1030 [DOI] [PubMed] [Google Scholar]
- 19.Liossi C., and Wood R.L. (2009). Gender as a moderator of cognitive and affective outcome after traumatic brain injury. J. Neuropsychiatry Clin. Neurosci. 21, 43–51 [DOI] [PubMed] [Google Scholar]
- 20.King N.S. (2014). A systematic review of age and gender factors in prolonged post-concussion symptoms after mild head injury. Brain Inj. 28, 1639–1645 [DOI] [PubMed] [Google Scholar]
- 21.Roof R.L., Duvdevani R., and Stein D.G. (1993). Gender influences outcome of brain injury: progesterone plays a protective role. Brain Res. 607, 333–336 [DOI] [PubMed] [Google Scholar]
- 22.Maghool F., Khaksari M., and Siahposht Khachki A. (2013). Differences in brain edema and intracranial pressure following traumatic brain injury across the estrous cycle: involvement of female sex steroid hormones. Brain Res. 1497, 61–72 [DOI] [PubMed] [Google Scholar]
- 23.Bruce-Keller A.J., Dimayuga F.O., Reed J.L., Wang C., Angers R., Wilson M.E., Dimayuga V.M., and Scheff S.W. (2007). Gender and estrogen manipulation do not affect traumatic brain injury in mice. J. Neurotrauma 24, 203–215 [DOI] [PubMed] [Google Scholar]
- 24.Hall E.D., Gibson T.R., and Pavel K.M. (2005). Lack of a gender difference in post-traumatic neurodegeneration in the mouse controlled cortical impact injury model. J. Neurotrauma 22, 669–679 [DOI] [PubMed] [Google Scholar]
- 25.O'Connor C.A., Cernak I., Johnson F., and Vink R. (2007). Effects of progesterone on neurologic and morphologic outcome following diffuse traumatic brain injury in rats. Exp. Neurol. 205, 145–153 [DOI] [PubMed] [Google Scholar]
- 26.Roof R.L., Duvdevani R., Braswell L., and Stein D.G. (1994). Progesterone facilitates cognitive recovery and reduces secondary neuronal loss caused by cortical contusion injury in male rats. Exp. Neurol. 129, 64–69 [DOI] [PubMed] [Google Scholar]
- 27.O'Connor C.A., Cernak I., and Vink R. (2003). Interaction between anesthesia, gender, and functional outcome task following diffuse traumatic brain injury in rats. J. Neurotrauma 20, 533–541 [DOI] [PubMed] [Google Scholar]
- 28.Wagner A.K., Willard L.A., Kline A.E., Wenger M.K., Bolinger B.D., Ren D., Zafonte R.D., and Dixon C.E. (2004). Evaluation of estrous cycle stage and gender on behavioral outcome after experimental traumatic brain injury. Brain Res. 998, 113–121 [DOI] [PubMed] [Google Scholar]
- 29.Wagner A.K., Kline A.E., Ren D., Willard L.A., Wenger M.K., Zafonte R.D., and Dixon C.E. (2007). Gender associations with chronic methylphenidate treatment and behavioral performance following experimental traumatic brain injury. Behav. Brain Res. 181, 200–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Monaco C.M., Mattiola V.V., Folweiler K.A., Tay J.K., Yelleswarapu N.K., Curatolo L.M., Matter A.M., Cheng J.P., and Kline A.E. (2013). Environmental enrichment promotes robust functional and histological benefits in female rats after controlled cortical impact injury. Exp. Neurol. 247, 410–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiong Y., Mahmood A., Lu D., Qu C., Goussev A., Schallert T., and Chopp M. (2007). Role of gender in outcome after traumatic brain injury and therapeutic effect of erythropoietin in mice. Brain Res. 1185, 301–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sorge R.E., Martin L.J., Isbester K.A., Sotocinal S.G., Rosen S., Tuttle A.H., Wieskopf J.S., Acland E.L., Dokova A., Kadoura B., Leger P., Mapplebeck J.C.S., McPhail M., Delaney A., Wigerblad G., Schumann A.P., Quinn T., Frasnelli J., Svensson C.I., Sternberg W.F., and Mogil J.S. (2014). Olfactory exposure to males, including men, causes stress and related analgesia in rodents. Nat. Methods 11, 629–632 [DOI] [PubMed] [Google Scholar]
- 33.Budinich C.S., Tucker L.B., Lowe D., Rosenberger J.G., and McCabe J.T. (2013). Short and long-term motor and behavioral effects of diazoxide and dimethyl sulfoxide administration in the mouse after traumatic brain injury. Pharmacol. Biochem. Behav. 108, 66–73 [DOI] [PubMed] [Google Scholar]
- 34.Tchantchou F., and Zhang Y. (2013). Selective inhibition of alpha/beta-hydrolase domain 6 attenuates neurodegeneration, alleviates blood brain barrier breakdown, and improves functional recovery in a mouse model of traumatic brain injury. J. Neurotrauma 30, 565–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hughes R.N. (2004). The value of spontaneous alternation behavior (SAB) as a test of retention in pharmacological investigations of memory. Neurosci. Biobehav. Rev. 28, 497–505 [DOI] [PubMed] [Google Scholar]
- 36.Morris R. (1984). Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods 11, 47–60 [DOI] [PubMed] [Google Scholar]
- 37.Collins T.J. (2007). ImageJ for microscopy. Biotechniques 43, 25–30 [DOI] [PubMed] [Google Scholar]
- 38.Dash P.K., Orsi S.A., Zhang M., Grill R.J., Pati S., Zhao J., and Moore A.N. (2010). Valproate administered after traumatic brain injury provides neuroprotection and improves cognitive function in rats. PLoS One 5, e11383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ackerly D.D., and Jasieński M. (1990). Size-dependent variation of gender in high density stands of the monoecious annual, Ambrosia artemisiifolia (Asteraceae). Oecologia 82, 474–477 [DOI] [PubMed] [Google Scholar]
- 40.Archer J. (1975). Rodent sex differences in emotional and related behavior. Behav. Biol. 14, 451–479 [DOI] [PubMed] [Google Scholar]
- 41.Kokras N., and Dalla C. (2014). Sex differences in animal models of psychiatric disorders. Br. J. Pharmacol. 171, 4595–4619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morgan M.A., and Pfaff D.W. (2002). Estrogen's effects on activity, anxiety, and fear in two mouse strains. Behav. Brain Res. 132, 85–93 [DOI] [PubMed] [Google Scholar]
- 43.O'Leary T.P., Gunn R.K., and Brown R.E. (2013). What are we measuring when we test strain differences in anxiety in mice? Behav. Genet. 43, 34–50 [DOI] [PubMed] [Google Scholar]
- 44.Voikar V., Koks S., Vasar E., and Rauvala H. (2001). Strain and gender differences in the behavior of mouse lines commonly used in transgenic studies. Physiol. Behav. 72, 271–281 [DOI] [PubMed] [Google Scholar]
- 45.Van Swearingen A.E., Walker Q.D., and Kuhn C.M. (2013). Sex differences in novelty- and psychostimulant-induced behaviors of C57BL/6 mice. Psychopharmacology 225, 707–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Karl T., Duffy L., and Herzog H. (2008). Behavioural profile of a new mouse model for NPY deficiency. Eur. J. Neurosci. 28, 173–180 [DOI] [PubMed] [Google Scholar]
- 47.Avgustinovich D.F., Kovalenko I.L., and Koryakina L.A. (2007). Effects of single episodes of severe stress on the behavior of male and female CBA/Lac and C57BL/6J mice. Neurosci. Behav. Physiol. 37, 731–737 [DOI] [PubMed] [Google Scholar]
- 48.Weil Z.M., Hotchkiss A.K., Gatien M.L., Pieke-Dahl S., and Nelson R.J. (2006). Melatonin receptor (MT1) knockout mice display depression-like behaviors and deficits in sensorimotor gating. Brain Res. Bull. 68, 425–429 [DOI] [PubMed] [Google Scholar]
- 49.Dang M.T., Yokoi F., McNaught K.S., Jengelley T.A., Jackson T., Li J., and Li Y. (2005). Generation and characterization of Dyt1 DeltaGAG knock-in mouse as a model for early-onset dystonia. Exp. Neurol. 196, 452–463 [DOI] [PubMed] [Google Scholar]
- 50.An X.L., Zou J.X., Wu R.Y., Yang Y., Tai F.D., Zeng S.Y., Jia R., Zhang X., Liu E.Q., and Broders H. (2011). Strain and sex differences in anxiety-like and social behaviors in C57BL/6J and BALB/cJ mice. Exper. Anim. 60, 111–123 [DOI] [PubMed] [Google Scholar]
- 51.Rial D., Xikota J.C., Miozzo A., Cruz V.E., Prediger R.D., and Walz R. (2009). Differential gender-related susceptibility to learning and memory deficits in mice submitted to neonatal freezing microgyria model. Brain Res. Bull. 79, 177–181 [DOI] [PubMed] [Google Scholar]
- 52.Bolivar V.J., Caldarone B.J., Reilly A.A., and Flaherty L. (2000). Habituation of activity in an open field: a survey of inbred strains and F1 hybrids. Behav. Genet. 30, 285–293 [DOI] [PubMed] [Google Scholar]
- 53.Bhatnagar S., Nowak N., Babich L., and Bok L. (2004). Deletion of the 5-HT3 receptor differentially affects behavior of males and females in the Porsolt forced swim and defensive withdrawal tests. Behav. Brain Res. 153, 527–535 [DOI] [PubMed] [Google Scholar]
- 54.Davis M.J., Haley T., Duvoisin R.M., and Raber J. (2012). Measures of anxiety, sensorimotor function, and memory in male and female mGluR4(-)/(-) mice. Behav. Brain Res. 229, 21–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kundakovic M., Lim S., Gudsnuk K., and Champagne F.A. (2013). Sex-specific and strain-dependent effects of early life adversity on behavioral and epigenetic outcomes. Front. Psychiatry 4, 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alstott J., and Timberlake W. (2009). Effects of rat sex differences and lighting on locomotor exploration of a circular open field with free-standing central corners and without peripheral walls. Behav. Brain Res. 196, 214–219 [DOI] [PubMed] [Google Scholar]
- 57.Crabbe J.C., Wahlsten D., and Dudek B.C. (1999). Genetics of mouse behavior: interactions with laboratory environment. Science 284, 1670–1672 [DOI] [PubMed] [Google Scholar]
- 58.Bettis T., and Jacobs L.F. (2012). Sex differences in object recognition are modulated by object similarity. Behav. Brain Res. 233, 288–292 [DOI] [PubMed] [Google Scholar]
- 59.Yu F., Wang Z., Tchantchou F., Chiu C.T., Zhang Y., and Chuang D.M. (2012). Lithium ameliorates neurodegeneration, suppresses neuroinflammation, and improves behavioral performance in a mouse model of traumatic brain injury. J. Neurotrauma 29, 362–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wakade C., Sukumari-Ramesh S., Laird M.D., Dhandapani K.M., and Vender J.R. (2010). Delayed reduction in hippocampal postsynaptic density protein-95 expression temporally correlates with cognitive dysfunction following controlled cortical impact in mice. J. Neurosurg. 113, 1195–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kimbler D.E., Shields J., Yanasak N., Vender J.R., and Dhandapani K.M. (2012). Activation of P2X7 promotes cerebral edema and neurological injury after traumatic brain injury in mice. PLoS One 7, e41229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hsieh C.L., Niemi E.C., Wang S.H., Lee C.C., Bingham D., Zhang J., Cozen M.L., Charo I., Huang E.J., Liu J., and Nakamura M.C. (2014). CCR2 deficiency impairs macrophage infiltration and improves cognitive function after traumatic brain injury. J. Neurotrauma 31, 1677–1688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Amenta P.S., Jallo J.I., Tuma R.F., and Elliott M.B. (2012). A cannabinoid type 2 receptor agonist attenuates blood-brain barrier damage and neurodegeneration in a murine model of traumatic brain injury. J. Neurosci. Res. 90, 2293–2305 [DOI] [PubMed] [Google Scholar]
- 64.Viggiano D. (2008). The hyperactive syndrome: metanalysis of genetic alterations, pharmacological treatments and brain lesions which increase locomotor activity. Behav. Brain Res. 194, 1–14 [DOI] [PubMed] [Google Scholar]
- 65.Adeyemo B.O., Biederman J., Zafonte R., Kagan E., Spencer T.J., Uchida M., Kenworthy T., Spencer A.E., and Faraone S.V. (2014). Mild traumatic brain injury and ADHD: a systematic review of the literature and meta-analysis. J. Atten. Disord. 18, 576–584 [DOI] [PubMed] [Google Scholar]
- 66.Mallya S., Sutherland J., Pongracic S., Mainland B., and Ornstein T.J. (2015). The manifestation of anxiety disorders after traumatic brain injury: a review. J Neurotrauma 32, 411–421 [DOI] [PubMed] [Google Scholar]
- 67.Silver J.M., McAllister T.W., and Arciniegas D.B. (2009). Depression and cognitive complaints following mild traumatic brain injury. Am. J. Psychiatry 166, 653–661 [DOI] [PubMed] [Google Scholar]
- 68.Ennaceur A. (2014). Tests of unconditioned anxiety—pitfalls and disappointments. Physiol. Behav. 135, 55–71 [DOI] [PubMed] [Google Scholar]
- 69.Crawley J.N. (1999). Behavioral phenotyping of transgenic and knockout mice: experimental design and evaluation of general health, sensory functions, motor abilities, and specific behavioral tests. Brain Res. 835, 18–26 [DOI] [PubMed] [Google Scholar]
- 70.Prut L., and Belzung C. (2003). The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur. J. Pharmacol. 463, 3–33 [DOI] [PubMed] [Google Scholar]
- 71.Bekker M.H.J., and van Mens-Verhulst J. (2007). Anxiety disorders: sex differences in prevalence, degree, and background, but gender-neutral treatment. Gend. Med. 4, Suppl. B, S178–S193 [DOI] [PubMed] [Google Scholar]
- 72.Washington P.M., Forcelli P.A., Wilkins T., Zapple D.N., Parsadanian M., and Burns M.P. (2012). The effect of injury severity on behavior: a phenotypic study of cognitive and emotional deficits after mild, moderate, and severe controlled cortical impact injury in mice. J. Neurotrauma 29, 2283–2296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chauhan N.B., Gatto R., and Chauhan M.B. (2010). Neuroanatomical correlation of behavioral deficits in the CCI model of TBI. J. Neurosci. Methods 190, 1–9 [DOI] [PubMed] [Google Scholar]
- 74.Malkesman O., Tucker L.B., Ozl J., and McCabe J.T. (2013). Traumatic brain injury—modeling neuropsychiatric symptoms in rodents. Front. Neurol. 4, 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Saatman K.E., Feeko K.J., Pape R.L., and Raghupathi R. (2006). Differential behavioral and histopathological responses to graded cortical impact injury in mice. J. Neurotrauma 23, 1241–1253 [DOI] [PubMed] [Google Scholar]
- 76.Fox G.B., Fan L., Levasseur R.A., and Faden A.I. (1998). Sustained sensory/motor and cognitive deficits with neuronal apoptosis following controlled cortical impact brain injury in the mouse. J. Neurotrauma 15, 599–614 [DOI] [PubMed] [Google Scholar]
- 77.Fox G.B., LeVasseur R.A., and Faden A.I. (1999). Behavioral responses of C57BL/6, FVB/N, and 129/SvEMS mouse strains to traumatic brain injury: implications for gene targeting approaches to neurotrauma. J. Neurotrauma 16, 377–389 [DOI] [PubMed] [Google Scholar]
- 78.Villapol S., Yaszemski A.K., Logan T.T., Sanchez-Lemus E., Saavedra J.M., and Symes A.J. (2012). Candesartan, an angiotensin II AT(1)-receptor blocker and PPAR-gamma agonist, reduces lesion volume and improves motor and memory function after traumatic brain injury in mice. Neuropsychopharmacology 37, 2817–2829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gresack J.E., and Frick K.M. (2003). Male mice exhibit better spatial working and reference memory than females in a water-escape radial arm maze task. Brain Res. 982, 98–107 [DOI] [PubMed] [Google Scholar]
- 80.Typlt M., Mirkowski M., Azzopardi E., Ruettiger L., Ruth P., and Schmid S. (2013). Mice with deficient BK channel function show impaired prepulse inhibition and spatial learning, but normal working and spatial reference memory. PLoS One 8, e81270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stevens L.M., and Brown R.E. (2014). Reference and working memory deficits in the 3xTg-AD mouse between 2 and 15-months of age: a cross-sectional study. Behav. Brain Res. 278C, 496–505 [DOI] [PubMed] [Google Scholar]
- 82.Jonasson Z. (2005). Meta-analysis of sex differences in rodent models of learning and memory: a review of behavioral and biological data. Neurosci. Biobehav. Rev. 28, 811–825 [DOI] [PubMed] [Google Scholar]
- 83.Smith D.H., Soares H.D., Pierce J.S., Perlman K.G., Saatman K.E., Meaney D.F., Dixon C.E., and McIntosh T.K. (1995). A model of parasagittal controlled cortical impact in the mouse: cognitive and histopathologic effects. J. Neurotrauma 12, 169–178 [DOI] [PubMed] [Google Scholar]
- 84.Fox G.B., and Faden A.I. (1998). Traumatic brain injury causes delayed motor and cognitive impairment in a mutant mouse strain known to exhibit delayed Wallerian degeneration. J. Neurosci. Res. 53, 718–727 [DOI] [PubMed] [Google Scholar]
- 85.Yu F., Zhang Y., and Chuang D.M. (2012). Lithium reduces BACE1 overexpression, beta amyloid accumulation, and spatial learning deficits in mice with traumatic brain injury. J. Neurotrauma 29, 2342–2351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yu S., Kaneko Y., Bae E., Stahl C.E., Wang Y., van Loveren H., Sanberg P.R., and Borlongan C.V. (2009). Severity of controlled cortical impact traumatic brain injury in rats and mice dictates degree of behavioral deficits. Brain Res. 1287, 157–163 [DOI] [PubMed] [Google Scholar]
- 87.Frick K.M., Burlingame L.A., Arters J.A., and Berger-Sweeney J. (2000). Reference memory, anxiety and estrous cyclicity in C57BL/6NIA mice are affected by age and sex. Neuroscience 95, 293–307 [DOI] [PubMed] [Google Scholar]
- 88.Driscoll I., Hamilton D.A., Yeo R.A., Brooks W.M., and Sutherland R.J. (2005). Virtual navigation in humans: the impact of age, sex, and hormones on place learning. Horm. Behav. 47, 326–335 [DOI] [PubMed] [Google Scholar]
- 89.Rizk A., Robertson J., and Raber J. (2005). Behavioral performance of tfm mice supports the beneficial role of androgen receptors in spatial learning and memory. Brain Res. 1034, 132–138 [DOI] [PubMed] [Google Scholar]
- 90.Baldan Ramsey L.C., and Pittenger C. (2010). Cued and spatial learning in the water maze: equivalent learning in male and female mice. Neurosci. Lett. 483, 148–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Berger-Sweeney J., Arnold A., Gabeau D., and Mills J. (1995). Sex differences in learning and memory in mice: effects of sequence of testing and cholinergic blockade. Behav. Neurosci. 109, 859–873 [DOI] [PubMed] [Google Scholar]
- 92.Guzmán C.B., Graham K.A., Grace L.A., and Moore A.H. (2009). Sex-dependent effect of cyclooxygenase-2 inhibition on mouse spatial memory. Behav. Brain Res. 199, 355–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wagner A.K., Ren D., Conley Y.P., Ma X., Kerr M.E., Zafonte R.D., Puccio A.M., Marion D.W., and Dixon C.E. (2007). Sex and genetic associations with cerebrospinal fluid dopamine and metabolite production after severe traumatic brain injury. J. Neurosurg. 106, 538–547 [DOI] [PubMed] [Google Scholar]
- 94.Frick K.M., and Gresack J.E. (2003). Sex differences in the behavioral response to spatial and object novelty in adult C57BL/6 mice. Behav. Neurosci. 117, 1283–1291 [DOI] [PubMed] [Google Scholar]
- 95.Li X., Blizzard K.K., Zeng Z., DeVries A.C., Hurn P.D., and McCullough L.D. (2004). Chronic behavioral testing after focal ischemia in the mouse: functional recovery and the effects of gender. Exp. Neurol. 187, 94–104 [DOI] [PubMed] [Google Scholar]
- 96.O'Leary T.P., and Brown R.E. (2012). The effects of apparatus design and test procedure on learning and memory performance of C57BL/6J mice on the Barnes maze. J. Neurosci. Methods 203, 315–324 [DOI] [PubMed] [Google Scholar]
- 97.O'Leary T.P., Savoie V., and Brown R.E. (2011). Learning, memory and search strategies of inbred mouse strains with different visual abilities in the Barnes maze. Behav. Brain Res. 216, 531–542 [DOI] [PubMed] [Google Scholar]
- 98.Mogil J.S., and Chanda M.L. (2005). The case for the inclusion of female subjects in basic science studies of pain. Pain 117, 1–5 [DOI] [PubMed] [Google Scholar]
- 99.Meziane H., Ouagazzal A.-M., Aubert L., Wietrzych M., and Krezel W. (2007). Estrous cycle effects on behavior of C57BL/6J and BALB/cByJ female mice: implications for phenotyping strategies. Genes Brain Behav. 6, 192–200 [DOI] [PubMed] [Google Scholar]
- 100.Roof R.L., and Hall E.D. (2000). Estrogen-related gender difference in survival rate and cortical blood flow after impact-acceleration head injury in rats. J. Neurotrauma 17, 1155–1169 [DOI] [PubMed] [Google Scholar]
- 101.Bramlett H.M., and Dietrich W.D. (2001). Neuropathological protection after traumatic brain injury in intact female rats versus males or ovariectomized females. J. Neurotrauma 18, 891–900 [DOI] [PubMed] [Google Scholar]
- 102.Whitley H., and Lindsey W. (2009). Sex-based differences in drug activity. Am. Fam. Physician 80, 1254–1258 [PubMed] [Google Scholar]
- 103.Nicolson T.J., Mellor H.R., and Roberts R.R. (2010). Gender differences in drug toxicity. Trends Pharmacol. Sci. 31, 108–114 [DOI] [PubMed] [Google Scholar]
- 104.Mannix R., Berglass J., Berkner J., Moleus P., Qiu J., Jantzie L.L., Meehan W.P., III, Stanley R.M., and Robinson S. (2014). Sex differences in the effect of progesterone after controlled cortical impact in adolescent mice: a preliminary study. J. Neurosurg. 121, 1337–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wagner A.K., Kline A.E., Sokoloski J., Zafonte R.D., Capulong E., and Dixon C.E. (2002). Intervention with environmental enrichment after experimental brain trauma enhances cognitive recovery in male but not female rats. Neurosci. Lett. 334, 165–168 [DOI] [PubMed] [Google Scholar]