Abstract
Traumatic Brain Injury (TBI) is one of the largest health problems in the United States, and affects nearly 2 million people every year. The effects of TBI, including weakness and loss of coordination, can be debilitating and last years after the initial injury. Recovery of motor function is often incomplete. We have developed a method using electrical stimulation of the vagus nerve paired with forelimb use by which we have demonstrated enhanced recovery from ischemic and hemorrhagic stroke. Here we have tested the hypothesis that vagus nerve stimulation (VNS) paired with physical rehabilitation could enhance functional recovery after TBI. We trained rats to pull on a handle to receive a food reward. Following training, they received a controlled-cortical impact (CCI) in the forelimb area of motor cortex opposite the trained forelimb, and were then randomized into two treatment groups. One group of animals received VNS paired with rehabilitative therapy, whereas another group received rehabilitative therapy without VNS. Following CCI, volitional forelimb strength and task success rate in all animals were significantly reduced. VNS paired with rehabilitative therapy over a period of 5 weeks significantly increased recovery of both forelimb strength and success rate on the isometric pull task compared with rehabilitative training without VNS. No significant improvement was observed in the Rehab group. Our findings indicate that VNS paired with rehabilitative therapy enhances functional motor recovery after TBI.
Key words: : motor recovery, neuromodulation, traumatic brain injury, vagal nerve stimulation, vagus nerve stimulation
Introduction
There are more than 200,000 hospitalizations annually due to traumatic brain injury (TBI) in the United States.1 Moderate to severe TBI commonly impairs strength, balance, and coordination in humans.2–4 Thousands of these injuries each year lead to chronic motor dysfunction. Most patients receive physical therapy over a period of several months following a serious brain injury. Although modest improvements are seen in some patients, recovery is often incomplete.4
Vagus nerve stimulation (VNS) has been used clinically to effectively treat many neurological disorders.5–8 Recently, an implementation that uses short bursts of VNS paired with rehabilitative therapy has been demonstrated to enhance functional recovery in animal models of stroke.9–12 In both ischemic and hemorrhagic models of stroke, pairing VNS with rehabilitation improved multiple measures of forelimb performance.9,12 Given the effectiveness of pairing VNS with physical rehabilitation to enhance functional recovery in multiple models of brain injury and neurological disorders, we hypothesized that VNS would also be an effective tool to enhance functional recovery following TBI.
In this study, we trained rats on the isometric pull task, a highly sensitive measure of forelimb function in models of both TBI and stroke.13,14 After training, rats received a controlled-cortical impact (CCI) lesion to model TBI. Animals were returned to training one week after the lesion, and then received 5 weeks of rehabilitative training during which VNS was delivered paired with successful trials. We hypothesized that VNS paired with rehabilitative therapy using the isometric pull task would enhance functional motor recovery following experimental TBI.
Methods
Subjects
Twenty-eight adult female Sprague-Dawley rats weighing between 250 and 300 g were used in this experiment. The rats were housed in a 12:12 h reversed light cycle and were food deprived to no less than 85% of their normal body weight. All handling, housing, surgical procedures, and behavioral training of the rats were approved by the University of Texas Institutional Animal Care and Use Committee.
Behavioral apparatus and software
The behavioral apparatus and software were used as previously described.14 The apparatus consisted of an acrylic box (25.4×30.5×12.1 cm). The box contained a slot in the front right corner that rats could reach through to access the aluminum pull handle. The slot was sized and positioned such that rats could only reach the pull handle using their right forelimb. The pull handle was centered in the slot at a height of 6.35 cm from the cage floor, and the distance of the pull handle to the cage was varied from 1.9 cm inside the cage (relative to the inner cage wall) to 1.9 cm outside the cage. The aluminum pull handle was connected to a force transducer (Vulintus, Inc., Sachse, TX) that could measure pull force with sub-gram accuracy. The force transducers were inspected daily and were recalibrated when necessary. Matlab software (MathWorks, Inc., Natick, MA) was used to control the behavioral apparatus and collect data. A microprocessor controller (Vulintus, Inc., Sachse, TX) sampled the force transducer at a frequency of 100 Hz and sent the information to Matlab software, which displayed the data on the screen, controlled the behavioral session, and saved the data to permanent files.
Behavioral training
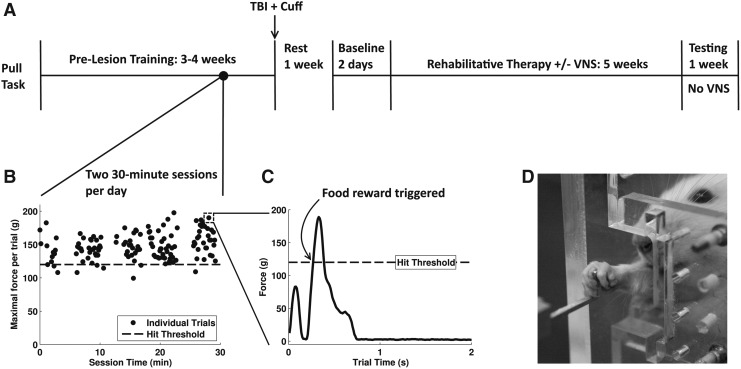
Animals underwent training for two 30-min sessions per day 5 days per week, with at least 2 h between sessions. Shaping procedures were similar to those previously described.13,14 Animals were trained to pull on the handle with 120g of force, and single reward pellets were dispensed following successful trials (45 mg dustless precision pellet, BioServ, Frenchtown, NJ). If rats did not receive 50 pellets per day, they were given 10 g of additional pellets after daily training sessions were completed.
A trial was initiated when at least 10g of force were applied to the pull handle. After initiation the force on the pull handle was sampled for 4 sec. If the force threshold required for a hit was met within a 2-sec window after force initiation, the trial was recorded as a success and a reward pellet was delivered. If the force threshold was not reached within 2 sec, the trial was recorded as a failure and no reward was delivered. Success rate for each day was calculated as the number of hits for that day divided by the total number of trials for the day.
Rats were considered fully trained after they maintained a performance of 85% success at the 120g threshold for 5 consecutive days. None of the animals in this study failed to meet this criterion. After the animals successfully reached this stage, they received a CCI over the forelimb area of left motor cortex to impair use of the trained forelimb. They were then implanted with a vagus nerve cuff and given 7 days to rest before behavioral testing resumed. Because of the severe forelimb deficits resulting from CCI, animals underwent a manual or automated adaptive reshaping procedure during early post-lesion sessions to encourage independent performance of the task. Once a post-lesion baseline assessment could be obtained, which consisted of animals independently initiating at least 50 trials per session for a 2-day period, and at least one successful 120g pull on each day, they were then divided into two therapy groups to receive VNS paired with successful trials during rehabilitative training (VNS+Rehab) or to receive only rehabilitative training but no VNS (Rehab). Due to the duration of the reshaping period, division into experimental groups typically occurred 3 to 4 weeks post-injury. After establishing post-lesion baseline performance and separating animals into experimental groups, rehabilitative training continued for 6 weeks and consisted of training on the pull task with the same parameters that were used during pre-lesion training. (1.9 cm handle distance, 120g force threshold, 2-sec time window). The VNS group did not receive stimulation during the sixth week of training to assess whether the effects of VNS were sustained after stimulation ceased.
Stimulation parameters
VNS onset occurred within 45 msec of each successful trial. The stimulation parameters consisted of a 500-msec train of 15 pulses at 30 Hz. Each biphasic pulse was 0.8 mA in amplitude and 100 μs in phase duration. These parameters were identical to our earlier studies.9–11,15 During the first day of the post-lesion baseline, rats did not have a stimulator cable connected to the headcap. For both Rehab and VNS+Rehab animals, the stimulator cable was connected during the second day of post-lesion baseline assessment, but no stimulation was delivered. Throughout the 6 weeks of therapy, the stimulation cable for the Rehab rats was not connected to a stimulator. During the sixth week, the stimulator cable was not used for either group.
Surgical procedures
Rats were anesthetized with a cocktail of ketamine hydrochloride (50 mg/kg), xylazine (20 mg/kg), and acepromazine (5 mg/kg) injected intramuscularly, and they were given supplemental doses as needed. After placing the rat in the stereotaxic frame, a craniotomy (3 to −2 mm anteroposterior [AP], 0.5 to 4 mm lateral relative to bregma) was performed using rongeurs to expose the motor cortex. A spring-loaded CCI device (Vulintus, Inc., Sachse, TX) set to a velocity of 3 m/sec with an impactor tip of 3 mm in diameter was lowered to the surface of the cortex. Upon triggering, the impactor delivered a downward blow 2 mm in distance and was left in place for 5 sec before being lifted out. The craniotomy was then covered with KwikCast silicone polymer (World Precision Instruments, Sarasota, FL) and sealed with a thin layer of acrylic.
Four bone screws were inserted into the skull at points near the lambdoid suture and over the cerebellum. A two-channel connector was then attached to the cranial screws using acrylic. The rat was then removed from the stereotaxic frame, and was laid in a supine position. An incision was made in the neck, and blunt dissection of the muscles exposed the left cervical vagus nerve. After isolating the vagus nerve from the carotid artery, the nerve was placed inside the stimulating cuff and the cuff was sutured closed. Cuff leads were tunneled subcutaneously and attached to the two-channel connector atop the skull. All incisions were sutured and the exposed two-channel connector was encapsulated in acrylic. A topical antibiotic cream was applied to both incision sites.
Following surgery, rats were provided with 4 mg Rimadyl® and a 350 mL water bottle containing 50 mg of minocycline hydrochloride. This water bottle was removed after one week, and each rat was returned to normal drinking water.
Histology
Following the completion of therapy, animals were perfused with 4% paraformaldehyde, and their brains were removed for processing. Coronal sections 40 μm thick extending through the lesioned area were cut using a cryostat and stained with cresyl violet. Twenty-two of the brains were included in histological analyses to investigate lesion size and damage to both white matter and gray matter.
White matter was measured as the area of the corpus callosum and external capsule in each hemisphere of the brain.14 The total volume of white matter was calculated for each hemisphere, and the ratio was taken as the total volume of white matter in the lesioned hemisphere to the total volume of white matter in the unlesioned hemisphere. The volume of gray matter was similarly calculated, and a ratio of the lesioned to the unlesioned hemisphere was obtained. Histological analysis was completed using ImageJ (National Institutes of Health; imagej.nih.gov) and was performed blind to the experimental group and performance of each animal on the pull task.
Statistical analysis
Behavioral data from each subject was grouped by combining each 5 consecutive days into epochs. Each day consisted of two sessions of data. Post-lesion performance at each epoch was compared across experimental groups, as well as compared with post-lesion baseline performance and the last 5 days of pre-lesion training. Significant differences were determined using repeated-measures analysis of variance (ANOVA), paired t tests, and unpaired t tests. All data are reported as mean±standard error of the mean (SEM). Significant differences between groups are noted in Figures 2 and 4 as *p<0.05. Significant differences for each group from post-lesion are noted by filled markers in Figure 2 and Figure 4. Bonferroni correction was used to calculate a cut-off value for significance as p<0.0083 for therapy time points when compared with post-lesion. Error bars indicate mean±SEM. Matlab software was used for all statistical analyses.
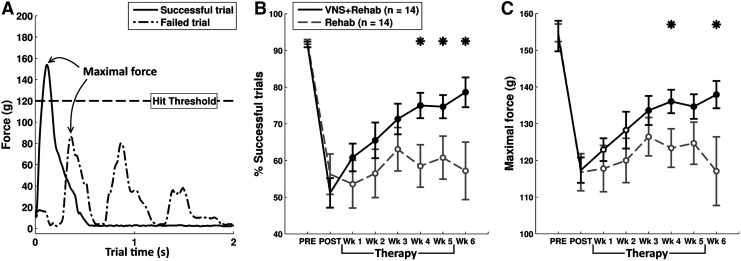
FIG. 2.
VNS paired with rehabilitative training enhances recovery of performance on the isometric pull task. (A) Two example trials are shown to illustrate measures of behavioral performance. Maximal force generated during a trial was one primary measure of performance. (B) The overall success rate of all animals was impaired following CCI. During the therapy period, VNS+Rehab animals demonstrated a significant recovery of success rate, whereas Rehab animals did not. (C) Maximal force of all animals was also impaired following CCI. VNS+Rehab animals demonstrated a significant recovery of force, whereas Rehab animals showed no recovery. Data are plotted as mean±SEM. An * indicates a significant difference between VNS+Rehab and Rehab groups at each time point (p<0.05 with unpaired t tests). Filled circles at each time point indicate a significant difference from the POST time point (Bonferroni-corrected paired t tests p<0.0083). Open circles indicate no significant difference from POST. CCI, controlled-cortical impact; SEM, standard error of the mean; VNS, vagus nerve stimulation.
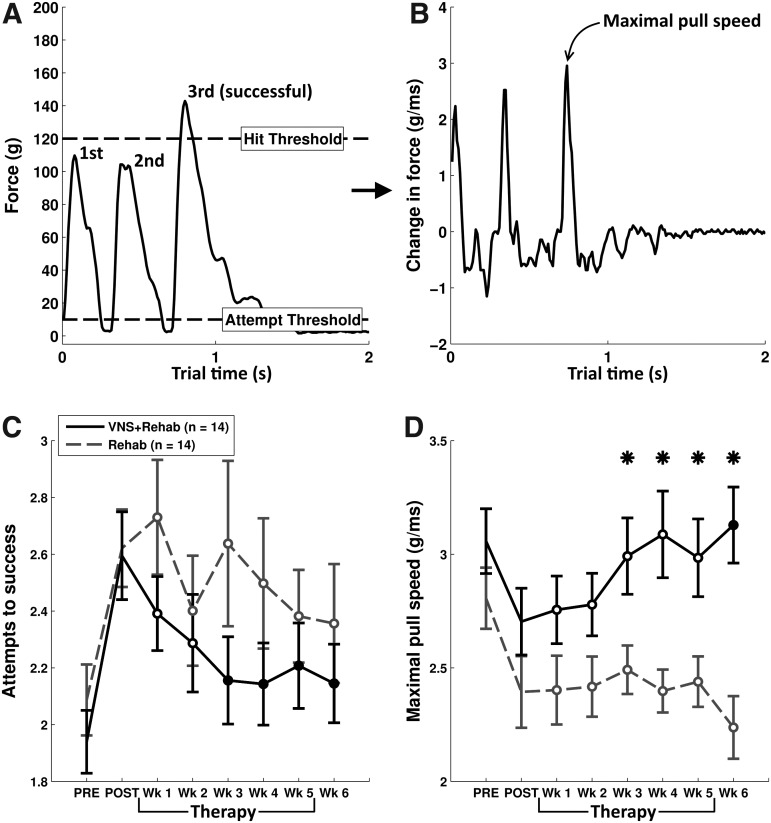
FIG. 4.
A moderate effect of VNS was also observed in the number of attempts to pull 120g and the maximal pull speed of animals. (A) An example pull trial is shown that demonstrates how the number of pull attempts to reach 120g was calculated. (B) To calculate maximal pull speed, we derived the change in force from the original force signal of each trial. (C) Both groups of animals were impaired on the number of attempts to reach 120g following CCI, but a moderate effect of VNS was observed during therapy. (D) Both groups demonstrated a decrease in peak pull speed after CCI. During therapy, there was a significant effect of VNS comparing across the two groups. Data are plotted as mean±SEM. An * indicates a significant difference between VNS+Rehab and Rehab groups at each time point (p<0.05 with unpaired t tests). Filled circles at each time point indicate a significant difference from the POST time point (Bonferroni-corrected paired t tests p<0.0083). Open circles indicate no significant difference from POST. CCI, controlled-cortical impact; SEM, standard error of the mean; VNS, vagus nerve stimulation.
Results
Rats were trained on the isometric pull task
Rats were trained to perform the isometric pull task (Fig. 1).13,14 Training continued until each animal achieved a performance of 5 consecutive days in which average success rate exceeded 85%. Prior to injury, the mean success rate of all rats before injury was 92±0.8% and 92±0.6% in the VNS+Rehab and the Rehab groups, respectively (Fig. 2A,B), and the mean maximal pull force was 153.9±4.2g and 154.7±2.4g for each group (Fig. 2A,C). Additionally, the distribution of maximal force in both groups was similar to that of previously reported animals proficient at the task (Fig. 3, left panels).9 No significant differences in maximal pull force or success rate were observed between groups (unpaired t test of VNS+Rehab vs. Rehab groups; success rate p=0.49; maximal force p=0.85).
FIG. 1.
Rats were trained on the isometric pull task. Following CCI, animals were divided into two groups (VNS+Rehab and Rehab), and received 5 weeks of rehabilitative therapy with or without VNS, followed by one week of further testing. (A) The experimental time line. (B) This plot demonstrates a typical 30-min behavior session. (C) An example individual trial is shown here. This trial shows two individual pull attempts by the animal, and a hit was achieved on the second pull attempt. (D) Rats were trained to reach out of a small window in a booth to pull on an aluminum handle. CCI, controlled-cortical impact; VNS, vagus nerve stimulation.
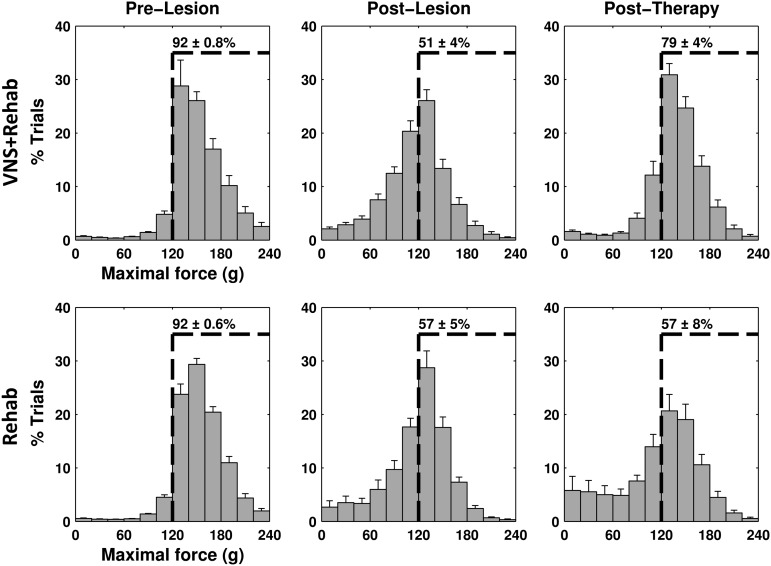
FIG. 3.
The distribution of maximal force generated was significantly altered following CCI and at the end of therapy. Both groups were highly trained on the task before CCI, achieving hit rates above the 85% criterion (left panels). Following CCI, both groups demonstrated a significant impairment in maximal pull force (middle panels). A significantly greater proportion of trials had maximal force below the 120g hit threshold. VNS+Rehab animals demonstrate a significant recovery of maximal pull force (right panels). The proportion of trials exceeding the 120g threshold was significantly greater in the VNS+Rehab group than the Rehab group. The dashed line in each panel indicates 120g, or the force criterion required for a successful trial. The numbers reported on each dashed line indicate the percentage of trials at each time point in the experiment in which the maximal force exceeded 120g. Each value is reported as the mean±SEM. CCI, controlled-cortical impact; SEM, standard error of the mean; VNS, vagus nerve stimulation.
Controlled-cortical impact impairs performance
After rats reached proficiency on the pull task, a CCI was performed over the left motor cortex to impair performance of the trained right forelimb. Following a week of rest, rats underwent reshaping until they could independently perform the task. Rats typically established a post-lesion baseline performance 26±2 calendar days following the lesion. No differences were found between groups in the number of training days required to establish a post-lesion baseline (VNS+Rehab: 13±7 days, Rehab: 13±8 days; unpaired t test p=0.98). CCI significantly impaired performance in both groups (Fig. 2B, VNS+Rehab: 51.2±4%, Rehab: 56.3±5%; paired t test vs. pre-lesion p<0.001 for both groups). Additionally, a shift in the distribution of maximal forces per trial was observed (Fig. 3, middle panels). Following CCI, the distribution of maximal force per trial shifted significantly toward lower peak forces (paired t test of pre-lesion vs. post-lesion, p<0.05 for all force bins below 120g), indicative of weakness in the forelimb. Consequently, the mean maximal pull force used by animals was also significantly reduced in both the VNS+Rehab and Rehab groups (Fig. 2C, VNS+Rehab 117.3±3.5g, Rehab: 116.8±5.1g; paired t test vs. pre-lesion p<0.001 for both groups), and a similar impairment was observed across both groups (post-lesion baseline maximal force of VNS+Rehab vs. Rehab, unpaired t test p=0.93).
Rehabilitative training alone fails to promote significant recovery
We sought to evaluate the ability of rehabilitative training to promote motor recovery from TBI. Following the post-lesion baseline period, rats underwent 6 weeks of rehabilitative training. We first explored whether rats in the Rehab group demonstrated a recovery of success rate with rehabilitative training, but no significant recovery was observed throughout the therapy period (Fig. 2B; repeated measures ANOVA, F(6, 91)=0.25, p=0.96). Additionally, a repeated-measures ANOVA of pull force in the Rehab group revealed that there was no significant recovery of maximal pull force throughout the 6 weeks of post-lesion assessment (Fig. 2C; F(6, 91)=0.39, p=0.88). The distribution of maximal force per trial in the Rehab group at the end of therapy also revealed a significant portion of trials remained sub-threshold (below 120g) even after 6 weeks of training (Fig. 3, bottom right panel, p>= 0.05 for all force bins of Rehab week 6 vs. Rehab post-lesion). This demonstrates that 6 weeks of extensive rehabilitative training was not sufficient to promote significant motor recovery.
VNS delivered during rehabilitative training promotes functional recovery from TBI
We next sought to evaluate whether delivering VNS during rehabilitative training enhances functional recovery from TBI. During the first 5 weeks of post-lesion training, animals in the VNS group received stimulation with each successful trial. During the sixth week VNS animals were disconnected from the stimulator to examine whether the effects of VNS therapy endured even after removal of the stimulation. A repeated-measures ANOVA on overall success rate of the animals during the post-lesion assessment period revealed a significant improvement in the VNS+Rehab group (Fig. 2B; F(6, 91)=5.91, p = <0.001). There was a significant increase in the success rate of VNS+Rehab animals compared with post-lesion baseline performance even after just one week of training (paired t test VNS+Rehab week 1 vs. post-lesion baseline, p=0.006). This recovery was observed throughout the 6-week assessment period (paired t tests VNS+Rehab all weeks vs. post-lesion baseline, all p<0.0083). To compare the success rate across experimental groups, a repeated-measures ANOVA was performed on animals in the VNS+Rehab and Rehab groups during the 6-week post-lesion assessment. This revealed that there was a significant effect of VNS on success rate (F(1, 182)=13.0, p<0.001). Post hoc tests revealed that VNS+Rehab resulted in significantly greater success rates on the pull task than Rehab alone during weeks 4–6 of post-lesion training (unpaired t tests VNS+Rehab vs. Rehab week 4–week 6, all p<0.05). These results further demonstrate that pairing VNS with rehabilitative training enhances recovery of forelimb function after CCI.
To further investigate the effects of pairing VNS with successful trials on recovery of performance, an analysis was also performed on the maximal pull force generated by animals throughout the therapy period. We observed a significant increase in maximal pull force in VNS+Rehab animals over the duration of the post-lesion assessment (Fig. 2C; repeated measures ANOVA, F(6, 91)=4.15, p<0.001). Post hoc tests further revealed improved recovery in VNS+Rehab animals compared with post-lesion from weeks 3 to weeks 6 during the therapy period (paired t tests of VNS+Rehab each week vs. post-lesion baseline, weeks 3–6 p<0.0083). Additionally, repeated-measures ANOVA was performed to examine the effects of VNS on maximal pull force between groups over the 6 weeks of post-lesion training. This test revealed a significant effect of VNS when compared with the Rehab group (F(1, 182)=11.16, p=0.001). Post hoc analyses further revealed that the VNS+Rehab group demonstrated significantly greater pull forces during weeks 4 and 6 of post-lesion assessment compared with the Rehab group (unpaired t tests of VNS+Rehab vs. Rehab, week 4 and 6, p<0.05). No significant difference was observed in maximal pull force between weeks 5 and 6 of the VNS+Rehab group, indicating that the beneficial effects of the VNS paired with successful trials remained at least one week following cessation of VNS (maximal force week 5=134.6±3.3g, week 6=137.9±3.7g, paired t test, p=0.24). The VNS+Rehab group had a significantly greater proportion of trials exceeding the 120g threshold at the end of therapy than the Rehab group (Fig. 3, right panels). These findings indicate that pairing VNS with successful pull trials promotes recovery of force on the pull task.
Reduced speed of force generation contributes to overall weakness and is commonly observed following neurological insult.16 Therefore, we investigated the time required to generate force as another measure of forelimb function after CCI (Fig. 4B,D). We found that there was a significant reduction in maximal speed of force generation in both groups (paired t test of post-lesion baseline vs. pre-lesion, VNS+Rehab p=0.03, Rehab p=0.006). Repeated-measures ANOVA revealed a significant effect of the VNS therapy on maximal pull speed when compared with Rehab animals (F(1, 182)=44.18, p<0.001), and the maximal speed of force generation of VNS+Rehab animals was significantly higher than the Rehab group during weeks 3–6 of the therapy period (unpaired t tests of VNS+Rehab vs. Rehab weeks 3–6, all p<0.05). Therefore, it was observed that pull speed is significantly reduced following TBI, but animals that received VNS paired with rehabilitative therapy had a significantly enhanced recovery of pull speed compared with non-VNS animals.
Due to lower success rates and reduced speed of force generation following CCI, we suspected that animals would require more individual pull attempts during trials to succeed at pulling 120g. We analyzed all pull attempts that exceeded 10g. CCI resulted in an increase in the total number of pull attempts required to reach the 120g threshold in both groups (Fig. 4,C; paired t test of post-lesion baseline vs. pre-lesion, VNS+Rehab p<0.001, Rehab p=0.009). We then explored whether VNS paired with successful trials reduces the number of attempts to generate 120g. A repeated-measures ANOVA revealed an effect of VNS during therapy (F(1, 171)=6.58, p=0.011). Despite this, post hoc tests failed to reveal significant differences between groups at individual time points (unpaired t test of VNS+Rehab vs. Rehab week 1–week 6, all p>= 0.05). These analyses indicate that a modest effect of VNS exists on the number of pull attempts required to reach 120g, but the effect is not strong enough with our sample size to be revealed by post hoc tests at individual time points. Therefore, although a modest effect of VNS exists in this measure of motor function, it is unclear what is the functional significance of this modest effect.
VNS delivered during rehabilitative training does not affect the intensity of rehabilitative training
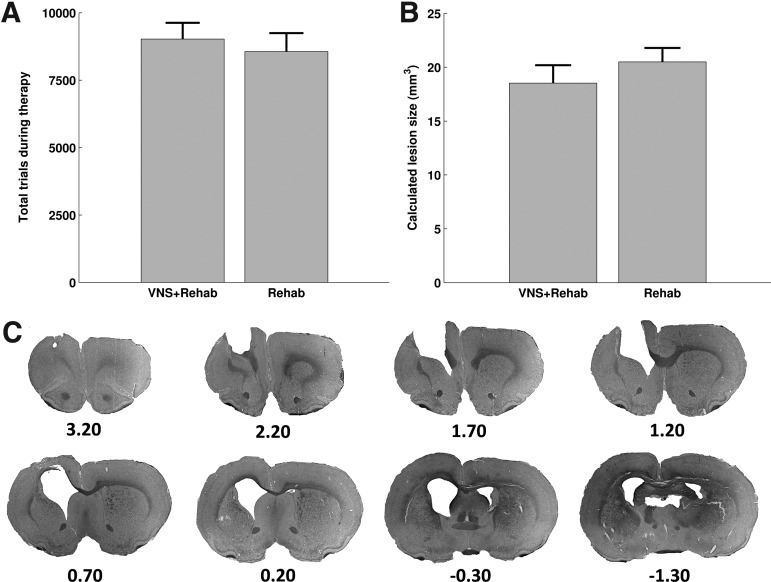
It has been shown that the total number of repetitions during training affects motor recovery after brain injury.17,18 To better understand the enhanced recovery observed in the VNS+Rehab group, we investigated whether it was due to a difference in the total amount of trials performed throughout the post-lesion therapy period. Consistent with previous studies,9,10 no differences were found in the total number of trials performed by animals in the VNS+Rehab and Rehab groups (Fig. 5A; VNS+Rehab: 9044±603 trials, Rehab: 8560±687 trials; unpaired t test VNS+Rehab vs. Rehab, p=0.60). Therefore, the number of repetitions during training cannot explain the significant recovery that was observed in the VNS+Rehab group.
FIG. 5.
Therapy intensity and lesion size cannot account for the enhanced recovery observed in the VNS+Rehab group. (A) Total trials performed during therapy by animals in both groups were not significantly different. (B) The calculated lesion size was not significantly different between groups. (C) Lesion reconstruction of one representative animal. Tissue damage often extended beyond the cortical layers into the corpus callosum and external capsule. Ventricle enlargement was also observed in the lesioned hemisphere, and the ventricular space often merged with the lesion cavity.
VNS delivered during rehabilitative training does not affect lesion size
We examined the extent of tissue damage (Fig. 5B,C) in each brain and sought to derive correlates with behavioral performance in either the Rehab or VNS+Rehab experimental groups. No significant difference in lesion size was found between VNS+Rehab and Rehab animals (VNS+Rehab: 18.5±1.7 mm3, Rehab: 20.5±1.3 mm3; unpaired t test, p=0.36). The ratio of white matter in the lesioned hemisphere compared with that in the unlesioned hemisphere was correlated with performance at the end of therapy (r=0.81, p=0.0027) and the amount of recovery achieved during therapy (r=0.71, p=0.014) in the Rehab group. No correlation was found, however, in the VNS+Rehab group (week 6 maximum force: r=0.15, p=0.65; recovery of force: r=0.08, p=0.83). Additionally, the ratio of gray matter in the lesioned hemisphere to that in the unlesioned hemisphere was also correlated with behavioral performance at the end of therapy in the Rehab group (r=0.61, p=0.046), but no correlation existed for the VNS+Rehab group (r=−0.15, p=0.66). This suggests that motor recovery in the Rehab group may be limited by the extent of the lesion. Alternatively, recovery is independent of lesion extent in the VNS+Rehab group, possibly indicating that plasticity of spared tissue is sufficient to restore function.
Discussion
In this study we tested whether VNS paired with rehabilitative therapy would enhance motor recovery after TBI. Rats were trained to proficiency on the isometric pull task and then received a CCI in motor cortex contralateral to the trained forelimb. CCI resulted in significant impairments of both success rate and pull force. VNS paired with rehabilitative training resulted in significant recovery of these measures of motor function compared with post-injury levels. Conversely, Rehab animals that received the same level of training without VNS did not show any improvement. Both Rehab and VNS+Rehab groups received intensive rehabilitative training following the lesion, with individual animals attempting over 8,500 trials during 5 weeks of therapy. Intensive physical rehabilitation is recognized as the current best possible intervention for TBI patients.19 VNS+Rehab animals demonstrated enhanced recovery beyond any benefits conferred by intensive rehabilitation, and no differences were found in the total number of trials performed or lesion size between the two groups.
Our results for the first time provide evidence that VNS paired with physical rehabilitation improves motor function following TBI. The results of this study support our previous findings that VNS paired with successful trials on a motor task enhances forelimb recovery in models of both ischemic and hemorrhagic stroke.9–12 Further, we have previously demonstrated that VNS paired with a tone-based therapy eliminates the behavioral correlates of noise-induced tinnitus in rats.15 Despite the mechanistically distinct nature of these models of neural injury, VNS paired with a specific rehabilitative regimen promoted recovery in each instance. These results suggest that VNS engages a common mechanism to boost the effects of rehabilitative therapies and enhance neurological recovery.
Cortical plasticity is thought to support normal recovery from brain injury.20–22 Not only does cortical reorganization occur with training,23–25 but the extent of reorganization is correlated with recovery after brain injury.26 VNS paired with skilled motor training drives enhanced cortical reorganization compared with equivalent training without VNS.27 Additionally, pairing VNS with tones substantially reorganizes primary auditory cortex.15,28 This enhanced plasticity in event-related neural circuitry is likely the mechanism that supports VNS-dependent recovery seen in this study as well.
Several pathways and neuromodulatory centers of the brain associated with plasticity are activated by stimulation of the vagus nerve. VNS activates the cholinergic and noradrenergic systems, which are known to be important for plasticity.29–36 VNS-induced release of these neuromodulators in concert with rehabilitative therapy may therefore drive enhanced plasticity to promote recovery. Further studies should evaluate these pathways to help elucidate the mechanisms of VNS-induced recovery.
VNS enhances recovery of motor function in other distinct stimulation paradigms. Smith and colleagues found that unpaired VNS, initiated 2 h post-injury and continuing for 14 days (24 min of total stimulation per day), promoted recovery in a battery of motor tests after a lateral-fluid percussion (LFP) injury.37 In a follow-up study, Smith and associates showed that VNS initiated 24 h after the lesion promotes delayed recovery compared with VNS initiated 2 h post-injury.38 Our results support the findings of Smith and coworkers, and provide further motivation to investigate VNS as a therapy for brain injury. In our study, VNS began 3 to 4 weeks post-injury, and subjects received stimulation only during therapy paired with successful trials (2 min of total stimulation per day). Given that VNS animals significantly recovered using the stimulation paradigm in the current study, and these animals received fewer stimulations than in previous stimulation paradigms, our results provide evidence that VNS paired with rehabilitative training is effective at lower levels and when initiated several weeks after the initial injury. The stimulation parameters used in this study have been shown in previous studies to be most effective when delivered coincident with specific movements during rehabilitative training rather than delayed after training.10,11 Therefore, previous studies demonstrate that pairing VNS with successful trials during training improves motor recovery compared with a matched amount of VNS temporally uncoupled with behavior. This may indicate that the VNS-dependent recovery observed using the paradigm in the current study engages different mechanisms than the VNS-dependent recovery observed by Smith and associates.37,38
Weakness is a problem often reported by patients who have suffered a TBI,3,4 and an increase in strength may be an important factor in recovery. In this study, VNS paired with rehabilitative training enhanced multiple measures of motor recovery after TBI. The VNS+Rehab group demonstrated significantly increased forelimb strength compared with the Rehab group. Additionally, VNS+Rehab animals had higher success rates on the pull task than Rehab animals. Moderate effects of paired VNS therapy were also observed in other measures such as the animals' number of attempts to pull 120g and their speed of force generation. Despite the enhanced recovery that was observed in these measures, a complete recovery to pre-lesion levels was not observed. This indicates that additional optimizations may be made to the therapy to confer further benefits to motor function. Optimizing VNS parameters, such as frequency and pulse-train duration, may confer additional benefit on those behavioral measures that did not fully recover.
The results from this study support the hypothesis that VNS paired with rehabilitation may be a potentially effective therapy for humans with motor dysfunction resulting from TBI. Approximately 5 million Americans are currently living with TBI-related disabilities.39 Although the location and degree of TBIs that patients suffer are diverse, paired VNS therapy may prove useful given the diversity of mechanistically distinct brain injuries that have shown improvement with VNS in animal models. Additionally, pilot clinical trials have demonstrated the safety and efficacy of a VNS-based plasticity therapy for a sensory disorder,40,41 and other clinical trials are currently underway to investigate the effectiveness of this therapy in stroke patients.42,43 The preclinical studies and clinical trials that employ a plasticity-based VNS therapy deliver only 1% of the stimulation used in Food and Drug Administration (FDA)-approved conventional VNS therapy for refractory epilepsy and depression.5,6,44–46 Moreover, intensive rehabilitative therapy, which is presently the best available intervention for TBI patients,19 is optimally effective when initiated early after the onset of injury.47–50 In this study the VNS-paired intervention was initiated between 3 and 4 weeks post-injury, and it endured for 5 weeks. Therefore, the lower stimulation requirements and long therapeutic window demonstrate that paired VNS therapy is a promising candidate for patients who are suffering the long-term effects of brain injury.
In this study, we have shown that pairing VNS with physical rehabilitation enhances recovery of forelimb function and volitional pull strength following an experimental TBI. Our results support previous findings that VNS can promote recovery after brain injury. These provide further motivation for continued studies of VNS delivered during rehabilitation to enhance motor recovery after TBI. Although significantly enhanced recovery was observed, optimization of the therapy may confer additional benefits and greater recovery. With further research, VNS paired therapy holds promise as a candidate for effectively treating patients with severe brain injuries.
Acknowledgments
We would like to acknowledge Andrew Sloan for his technical assistance with the project. We would also like to acknowledge Navid Khodaparast and Anthony Nguyen for the many valuable discussions we had that touched upon the experimental design and data analysis. Tanya Danaphongse, Kate Flanagan, Spruha Shah, Daniel Machuca, Kevin Tran, and Phillip Phan were instrumental in running the behavioral experiments. Kimiya Rahebi constructed all vagus nerve cuff electrodes that were used in this study.
D.T.P. led the project, assisted with the behavioral experiments, performed the surgeries, analyzed the data, and wrote the manuscript. A.N.S. assisted with behavioral experiments and performed surgeries and perfusions. L.J.K. ran behavior experiments, assisted with surgeries, and performed perfusions. C.M.A. and J.L.T. assisted with behavior experiments and performed histological sectioning and analysis. C.C. assisted with behavior experiments and performed surgeries. S.A.H. helped write the manuscript. M.P.K. and R.L.R. both helped write the manuscript and provided feedback throughout the process. All authors read the manuscript and provided feedback.
This project was supported in part by funding from the National Institute of Neurological Disorders and Stroke R01 NS085167.
Author Disclosure Statement
M.P.K. is a consultant and has a financial interest in MicroTransponder Inc., and is listed as an inventor on U.S. patents 8489185 B2 and 8700145 B2. R.L.R. is the owner of Vulintus, Inc. and is listed as an inventor on U.S. patent 8700145 B2. Other authors declare no competing interests.
References
- 1.Faul M., Xu L., Wald M.M., and Coronado V.G. (2010). Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations, and Deaths, 2002–2006. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control, Atlanta, GA [Google Scholar]
- 2.Heitger M.H., Jones R.D., Dalrymple-Alford J.C., Frampton C.M., Ardagh M.W., and Anderson T.J. (2006). Motor deficits and recovery during the first year following mild closed head injury. Brain Injury 20, 807–824 [DOI] [PubMed] [Google Scholar]
- 3.Brown A.W., Malec J.F., Diehl N.N., Englander J., and Cifu D.X. (2007). Impairment at rehabilitation admission and 1 year after moderate-to-severe traumatic brain injury: a prospective multi-centre analysis. Brain Injury 21, 673–680 [DOI] [PubMed] [Google Scholar]
- 4.Walker W.C., and Pickett T.C. (2007). Motor impairment after severe traumatic brain injury: a longitudinal multicenter study. J. Rehabil. Res. Dev. 44, 975–982 [DOI] [PubMed] [Google Scholar]
- 5.Ben‐Menachem E., Mañon‐Espaillat R., Ristanovic R., Wilder B., Stefan H., Mirza W., Tarver W., and Wernicke J. (1994). Vagus nerve stimulation for treatment of partial seizures: 1. A controlled study of effect on seizures. Epilepsia 35, 616–626 [DOI] [PubMed] [Google Scholar]
- 6.DeGiorgio C., Schachter S., Handforth A., Salinsky M., Thompson J., Uthman B., Reed R., Collin S., Tecoma E., and Morris G. (2000). Prospective long‐term study of vagus nerve stimulation for the treatment of refractory seizures. Epilepsia 41, 1195–1200 [DOI] [PubMed] [Google Scholar]
- 7.Sackeim H.A., Rush A.J., George M.S., Marangell L.B., Husain M.M., Nahas Z., Johnson C.R., Seidman S., Giller C., and Haines S. (2001). Vagus nerve stimulation (VNS™) for treatment-resistant depression: efficacy, side effects, and predictors of outcome. Neuropsychopharmacology 25, 713–728 [DOI] [PubMed] [Google Scholar]
- 8.Nemeroff C.B., Mayberg H.S., Krahl S.E., McNamara J., Frazer A., Henry T.R., George M.S., Charney D.S., and Brannan S.K. (2006). VNS therapy in treatment-resistant depression: clinical evidence and putative neurobiological mechanisms. Neuropsychopharmacology 31, 1345–1355 [DOI] [PubMed] [Google Scholar]
- 9.Khodaparast N., Hays S., Sloan A., Hulsey D., Ruiz A., Pantoja M., Rennaker R., II, and Kilgard M. (2013). Vagus nerve stimulation during rehabilitative training improves forelimb strength following ischemic stroke. Neurobiol. Dis. 60, 80–88 [DOI] [PubMed] [Google Scholar]
- 10.Khodaparast N., Hays S.A., Sloan A.M., Fayyaz T., Hulsey D.R., Rennaker R.L., 2nd, and Kilgard M.P. (2014). Vagus nerve stimulation delivered during motor rehabilitation improves recovery in a rat model of stroke. Neurorehabil. Neural Repair 28, 698–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hays S.A., Khodaparast N., Ruiz A., Sloan A.M., Hulsey D.R., Rennaker R., 2nd, and Kilgard M.P. (2014). The timing and amount of vagus nerve stimulation during rehabilitative training affect poststroke recovery of forelimb strength. Neuroreport 25, 676–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hays S.A., Khodaparast N., Hulsey D.R., Ruiz A., Sloan A.M., Rennaker R.L., 2nd, and Kilgard M.P. (2014). Vagus nerve stimulation during rehabilitative training improves functional recovery after intracerebral hemorrhage. Stroke 45, 3097–3100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hays S.A., Khodaparast N., Sloan A.M., Hulsey D.R., Pantoja M., Ruiz A.D., Kilgard M.P., and Rennaker R.L., II (2012). The isometric pull task: a novel automated method for quantifying forelimb force generation in rats. J. Neurosci. Methods 21, 329–337 [DOI] [PubMed] [Google Scholar]
- 14.Pruitt D., Hays S., Schmid A., Choua C., Kim L., Trieu J., Kilgard M.P., and Rennaker R.L. (2014). Controlled-cortical impact reduces volitional forelimb strength in rats. Brain Res. 1582, 91–98 [DOI] [PubMed] [Google Scholar]
- 15.Engineer N.D., Riley J.R., Seale J.D., Vrana W.A., Shetake J.A., Sudanagunta S.P., Borland M.S., and Kilgard M.P. (2011). Reversing pathological neural activity using targeted plasticity. Nature 470, 101–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Canning C.G., Ada L., and O'Dwyer N. (1999). Slowness to develop force contributes to weakness after stroke. Arch. Phys. Med. Rehabil. 80, 66–70 [DOI] [PubMed] [Google Scholar]
- 17.Bütefisch C., Hummelsheim H., Denzler P., and Mauritz K. (1995). Repetitive training of isolated movements improves the outcome of motor rehabilitation of the centrally paretic hand. J. Neurol. Sci. 130, 59–68 [DOI] [PubMed] [Google Scholar]
- 18.Zhu X., Poon W., Chan C.C., and Chan S.S. (2007). Does intensive rehabilitation improve the functional outcome of patients with traumatic brain injury (TBI)? A randomized controlled trial. Brain Injury 21, 681–690 [DOI] [PubMed] [Google Scholar]
- 19.Hellweg S., and Johannes S. (2008). Physiotherapy after traumatic brain injury: a systematic review of the literature. Brain Injury 22, 365–373 [DOI] [PubMed] [Google Scholar]
- 20.Kopp B., Kunkel A., Münickel W., Villringer K., Taub E., and Flor H. (1999). Plasticity in the motor system related to therapy‐induced improvement of movement after stroke. Neuroreport 10, 807–810 [DOI] [PubMed] [Google Scholar]
- 21.Nudo R.J., Plautz E.J., and Frost S.B. (2001). Role of adaptive plasticity in recovery of function after damage to motor cortex. Muscle Nerve 24, 1000–1019 [DOI] [PubMed] [Google Scholar]
- 22.Kilgard M. –P. (2012). Harnessing plasticity to understand learning and treat disease. Trends Neurosci. 35, 715–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kleim J.A., Barbay S., and Nudo R.J. (1998). Functional reorganization of the rat motor cortex following motor skill learning. J. Neurophysiol. 80, 3321–3325 [DOI] [PubMed] [Google Scholar]
- 24.Kleim J.A., Hogg T.M., VandenBerg P.M., Cooper N.R., Bruneau R., and Remple M. (2004). Cortical synaptogenesis and motor map reorganization occur during late, but not early, phase of motor skill learning. J. Neurosci. 24, 628–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Molina-Luna K., Hertler B., Buitrago M.M., and Luft A.R. (2008). Motor learning transiently changes cortical somatotopy. Neuroimage 40, 1748–1754 [DOI] [PubMed] [Google Scholar]
- 26.Ramanathan D., Conner J.M., and Tuszynski M.H. (2006). A form of motor cortical plasticity that correlates with recovery of function after brain injury. Proc. Natl. Acad. Sci. U S A 103, 11370–11375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Porter B.A., Khodaparast N., Fayyaz T., Cheung R.J., Ahmed S.S., Vrana W.A., Rennaker R.L., and Kilgard M.P. (2012). Repeatedly pairing vagus nerve stimulation with a movement reorganizes primary motor cortex. Cerebral Cortex 22, 2365–2374 [DOI] [PubMed] [Google Scholar]
- 28.Shetake J.A., Engineer N.D., Vrana W.A., Wolf J.T., and Kilgard M.P. (2012). Pairing tone trains with vagus nerve stimulation induces temporal plasticity in auditory cortex. Exp. Neurol. 233, 342–349 [DOI] [PubMed] [Google Scholar]
- 29.Bear M.F., and Singer W. (1986). Modulation of visual cortical plasticity by acetylcholine and noradrenaline. Nature 320, 172–176 [DOI] [PubMed] [Google Scholar]
- 30.Kilgard M.P., and Merzenich M.M. (1998). Cortical map reorganization enabled by nucleus basalis activity. Science 279, 1714–1718 [DOI] [PubMed] [Google Scholar]
- 31.Conner J.M., Culberson A., Packowski C., Chiba A.A., and Tuszynski M.H. (2003). Lesions of the basal forebrain cholinergic system impair task acquisition and abolish cortical plasticity associated with motor skill learning. Neuron 38, 819–829 [DOI] [PubMed] [Google Scholar]
- 32.Conner J.M., Chiba A.A., and Tuszynski M.H. (2005). The basal forebrain cholinergic system is essential for cortical plasticity and functional recovery following brain injury. Neuron 46, 173–179 [DOI] [PubMed] [Google Scholar]
- 33.Roosevelt R.W., Smith D.C., Clough R.W., Jensen R.A., and Browning R.A. (2006). Increased extracellular concentrations of norepinephrine in cortex and hippocampus following vagus nerve stimulation in the rat. Brain Res. 1119, 124–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Follesa P., Biggio F., Gorini G., Caria S., Talani G., Dazzi L., Puligheddu M., Marrosu F., and Biggio G. (2007). Vagus nerve stimulation increases norepinephrine concentration and the gene expression of BDNF and bFGF in the rat brain. Brain Res. 1179, 28–34 [DOI] [PubMed] [Google Scholar]
- 35.Ramanathan D., Tuszynski M.H., and Conner J.M. (2009). The basal forebrain cholinergic system is required specifically for behaviorally mediated cortical map plasticity. J. Neurosci. 29, 5992–6000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nichols J., Nichols A., Smirnakis S., Engineer N., Kilgard M., and Atzori M. (2011). Vagus nerve stimulation modulates cortical synchrony and excitability through the activation of muscarinic receptors. Neuroscience 189, 207–214 [DOI] [PubMed] [Google Scholar]
- 37.Smith D.-C., Modglin A.-A., Roosevelt R.-W., Neese S.-L., Jensen R.-A., Browning R.-A., and Clough R.-W. (2005). Electrical stimulation of the vagus nerve enhances cognitive and motor recovery following moderate fluid percussion injury in the rat. J. Neurotrauma 22, 1485–1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith D.C., Tan A.A., Duke A., Neese S.L., Clough R.W., Browning R.A., and Jensen R.A. (2006). Recovery of function after vagus nerve stimulation initiated 24 hours after fluid percussion brain injury. J. Neurotrauma 23, 1549–1560 [DOI] [PubMed] [Google Scholar]
- 39.Thurman D.J., Alverson C., Dunn K.A., Guerrero J., and Sniezek J.E. (1999). Traumatic brain injury in the United States: a public health perspective. J. Head Trauma Rehabil. 14, 602–615 [DOI] [PubMed] [Google Scholar]
- 40.De Ridder D., Vanneste S., Engineer N.D., and Kilgard M.P. (2014). Safety and efficacy of vagus nerve stimulation paired with tones for the treatment of tinnitus: a case series. Neuromodulation 17, 170–179 [DOI] [PubMed] [Google Scholar]
- 41.De Ridder D., Kilgard M., Engineer N., and Vanneste S. (2015). Placebo-controlled vagus nerve stimulation paired with tones in a patient with refractory tinnitus: a case report. Otol. Neurotol. 36, 575–580 [DOI] [PubMed] [Google Scholar]
- 42.Microtransponder. (2014). Paired Vagus Nerve Stimulation (VNS) With Rehabilitation for Upper Limb Function Improvement After Stroke. ClinicalTrials.gov
- 43.Microtransponder. (2014). VNS During Rehabilitation for Improved Upper Limb Motor Function After Stroke. ClinicalTrials.gov
- 44.Morris G.L., 3rd, and Mueller W.M. (1999). Long-term treatment with vagus nerve stimulation in patients with refractory epilepsy. The Vagus Nerve Stimulation Study Group E01-E05. Neurology 53, 1731–1735 [DOI] [PubMed] [Google Scholar]
- 45.Ben-Menachem E., Hellstrom K., Waldton C., and Augustinsson L.E. (1999). Evaluation of refractory epilepsy treated with vagus nerve stimulation for up to 5 years. Neurology 52, 1265–1267 [DOI] [PubMed] [Google Scholar]
- 46.Terry R.S., Jr (2014). Vagus Nerve Stimulation Therapy for Epilepsy. Available at: http://www.intechopen.com/books/epilepsy-topics/vagus-nerve-stimulation-therapy-for-epilepsy
- 47.Cope D.N. (1995). The effectiveness of traumatic brain injury rehabilitation: a review. Brain Injury 9, 649–670 [DOI] [PubMed] [Google Scholar]
- 48.High W.M., Roebuck-Spencer T., Sander A.M., Struchen M.A., and Sherer M. (2006). Early versus later admission to postacute rehabilitation: impact on functional outcome after traumatic brain injury. Arch. Phys. Med. Rehabil. 87, 334–342 [DOI] [PubMed] [Google Scholar]
- 49.Andelic N., Bautz-Holter E., Ronning P., Olafsen K., Sigurdardottir S., Schanke A., Sveen U., Tornas S., Sandhaug M., and Roe C. (2012). Does an early onset and continuous chain of rehabilitation improve the long-term functional outcome of patients with severe traumatic brain injury? J. Neurotrauma 29, 66–74 [DOI] [PubMed] [Google Scholar]
- 50.Thompson J.N., Majumdar J., Sheldrick R., and Morcos F. (2013). Acute neurorehabilitation versus treatment as usual. Br. J. Neurosurg. 27, 24–29 [DOI] [PubMed] [Google Scholar]