Abstract
Traumatic brain injury (TBI) is closely and bi-directionally linked with alcohol use, as by some estimates intoxication is the direct or indirect cause of one-third to one-half of all TBI cases. Alcohol use following injury can reduce the efficacy of rehabilitation and increase the chances for additional injury. Finally, TBI itself may be a risk factor for the development of alcohol use disorders. Children who suffer TBIs have poorer life outcomes and more risk of substance abuse. We used a standardized closed-head injury to model mild traumatic brain injuries. We found that mice injured as juveniles but not during adulthood exhibited much greater alcohol self-administration in adulthood. Further, this phenomenon was limited to female mice. Using behavioral testing, including conditioned place preference assays, we showed that early injuries increase the rewarding properties of alcohol. Environmental enrichment administered after injury reduced axonal degeneration and prevented the increase in drinking behavior. Additionally, brain-derived neurotrophic factor gene expression, which was reduced by TBI, was normalized by environmental enrichment. Together, these results suggest a novel model of alterations in reward circuitry following trauma during development.
Key words: : alcohol, BDNF, development, environmental enrichment, sex differences, traumatic brain injury
Introduction
In the United States alone, each year approximately 1.7 million individuals are treated in hospitals for a traumatic brain injury (TBI).1,2 The Centers for Disease Control and Prevention have estimated that there are likely an additional 3.8 million untreated concussions in the U.S. annually.3 A large subset of these injuries occurs in young children. Children who suffer TBIs early in life are at a much greater risk for alcohol abuse disorders later in life and this is of particular importance in light of the data linking post-TBI drinking to poorer long-term outcomes.4–7 Alcohol use and misuse are closely and bi-directionally linked to TBI; by some estimates, intoxication is the direct or indirect cause of one-third to one-half of all TBI cases.8,9 There also is mounting clinical and experimental evidence that TBI increases problem alcohol intake.9–12 Problem drinking also greatly increases the chances for additional TBI later in life, which can produce much greater impairments in previously injured patients.4 Children who suffer TBI are less likely to complete their education, find employment, and marry, and are more likely to suffer from poor health, neurological symptoms, and psychiatric ailments, which interact with, or are exacerbated by, alcohol misuse.4,13,14 Therefore targeting the substance abuse issues in TBI patients can have significant and long-term health benefits.
Human clinical findings have indicated that TBIs early in life have long-term deleterious consequences and exacerbate the response to subsequent injuries.15 Alcohol consumption is both a major risk factor for TBI and can decrease the effectiveness of rehabilitation.8 Additionally, some epidemiological studies have reported that TBI can increase problem alcohol use later in life.15 However, the underlying neuronal mechanisms associated with increased drinking in clinical TBI patients are difficult to determine, given the heterogeneity of injury severity and type, age at injury, prior history of substance abuse, sex differences, and myriad psychosocial factors. In order to begin to address these issues, we have developed a model of mild closed-head TBI that we administered in juvenile and adult mice in order to determine whether TBI increases spontaneous alcohol self-administration when the first exposure to alcohol occurs in adulthood, and whether early life injuries would exacerbate this behavioral response to injury.
Methods
Experiments in this report utilized Swiss-Webster mice bred at Ohio State University (OSU) and derived from breeders purchased from Charles River (Wilmington, MA). Pups were weaned at 21 days of age and housed in standard conditions in groups of 3–5 same-sex conspecifics (unless otherwise specified) in a standard mouse cage (32 × 16 × 12 cm). Mice had ad libitum access to food (Harlan-Teklad #8640, Madison, WI) and filtered tap water and housed in a 14:10 light–dark cycle. All procedures were approved by the OSU Institutional Animal Care and Use Committee and were conducted in accordance with National Institute of Health guidelines.
TBI procedure
A mild closed-head injury was induced using an Impact One device (Leica Biosystems, Richmond, IL). For juvenile mice, 21-day-old mice were secured in a stereotaxic frame and the skull was exposed using aseptic surgical technique. The impactor was placed gently on the surface of the skull (-1 AP, 1-ML relative to bregma) and then the stainless steel impactor tip (round; 2 mm in diameter) was driven into the closed skull to a depth of 1 mm at 3 m/sec (dwell time, 30 msec). This treatment reliably produces mild TBI without skull fracture, hemorrhage, or mortality. The sham procedure was identical except the impactor is lowered into contact with the skull and then retracted. The skin was closed with nylon sutures. Surgeries on 60-day-old adults were identical except that the coordinates were −2AP, −2ML and the impactor tip was 3 mm in diameter.
Alcohol self-administration
Mice were given daily access to two bottles, one containing water and the other ethanol diluted in water. The ethanol concentration (v/v) was increased every 4 days; mice received 3%, 6%, 10%, and finally 20% ethanol. Fluid intake and body weight were assessed every 2 days. The positions of the bottles were changed every 2 days to control for position preferences. Average ethanol consumption per day was obtained for each ethanol concentration. To obtain a measure of ethanol consumption that corrected for individual differences in mouse size, grams of ethanol consumed per kilogram of body weight per day were calculated for each mouse. As a measure of relative ethanol preference, an ethanol preference ratio was calculated by dividing the total ethanol solution consumed by total fluid (ethanol plus water) consumption. Using the same paradigm, mice also were given access to escalating concentrations of the bitter tastant quinine (0.1 mM and 0.3 mM) and water sweetened with saccharine (1.6 mM and 3.2 mM).
Loss of righting reflex
Mice were injected with 4 g/kg of a 20% ethanol solution intraperitoneally (IP)16 and the latency to the loss of righting reflex was visually recorded. Following loss of righting reflex, mice were placed on their backs in a V-shaped container and return of righting reflex was recorded when the mice could right themselves twice in 1 min.
Conditioned place preference
Plastic chambers measuring 42 × 30 × 18 cm were divided in half by a separator wall. Chambers were designed with different tactile (grid canvas floor of different materials and shape) and visual cues on the walls (thin stripes vs. checkerboard pattern). On Day 1, mice were injected with saline (10mL/kg; IP) and allowed to explore the two sides freely for 5 min in order to habituate to the apparatus and determine side bias. Mice were then assigned to receive alcohol on one of the sides of the chamber in such a manner as to minimize initial side biases. Mice were injected with ethanol (2 g/kg of a 20% ethanol solution IP) on Days 2, 4, 6, and 8 of conditioning and were confined to the assigned side of the chamber for 5 min daily. On Days 3, 5, 7, and 9, mice were placed in the opposite side and injected with saline. On day 10 mice were injected with saline and allowed to freely explore the chambers and the time spent in both the alcohol-conditioned and saline-conditioned sides was recorded. Following conditioned place preference (CPP) testing, mice were returned to their home cages for 4 days and then were injected again with either ethanol (2 g/kg) or saline, and then perfused 2 h later for analysis of immediate early gene expression.
Environmental enrichment
Mice were housed in larger rat-sized cages (45 × 25 × 20 cm) 5–8 mice/cage with a running wheel, chew toys, Habitrail, extra nestlets, and deeper bedding. Toys and wheels were changed weekly throughout the experiment.
Real-time polymerase chain reaction
Fresh brains were removed, placed in RNAlater RNA storage buffer and then prefrontal cortex from the injured hemisphere was dissected out. RNA was extracted using Trizol reagent (Thermo-Fisher Scientific, Waltham, MA), reverse transcribed with M-MLV and quantitative polymerase chain reaction (PCR) conducted with Taqman.
Tissue processing
Mice were overdosed with sodium pentobarbital and transcardially perfused with 4% paraformaldehyde following injury. To assess degeneration of neuronal processes, we performed silver staining using a NeuroSilver Kit (FD Neurotechnologies, Columbia, MD) according to the manufacturer's instructions. Sections were cut at 40 μm on a cryostat, stained with the silver staining kit, mounted on gel covered slides and then coverslipped with permount. C-Fos immunoreactive cells were labeled using an antibody obtained from Santa Cruz Biotechnology (SC-52; Dallas, TX) and visualized with ABC and DAB (Vector Laboratories, Burlingame, CA). Fluoro-Jade C staining was conducted according to our previously published methods.17
Microscopy
Axonal degeneration was assessed by qualitatively rating the number and distribution of argyrophilic axonal segments in the corpus callosum and other forebrain white matter tracts by an observer blinded to the experimental conditions. Multiple sections (at least three) were scanned at 10× magnification and then regions of degeneration were viewed at 40×. The degree of staining was then scored on a 4-point scale (0 = few axonal profiles, 3 = dense axonal degeneration throughout white matter tracts and the corpus callosum bilaterally).17 C-Fos immunoreactive cells were counted bilaterally following acquisition of z stacks consisting of 10 sets of 4 μm slices per region. Stacks from the accumbens core and shell, central nucleus of the amygdala, and periaqueductal gray were flattened and cells counted using the Image J cell counter. Cell counts were conducted in defined regions of interest (ROIs) for each region (accumbens, ROI = 0.072 mm2; amygdala, ROI = 0.04 mm2; periaqueductal gray, ROI = 0.22 mm2) and are reported as cells per mm2. Our analysis did not reveal significant hemisphere differences in c-Fos numbers; therefore, the cell count is averaged across hemispheres for each animal, and the counts are reported as the average across groups. An investigator blinded to experimental conditions performed all analyses.
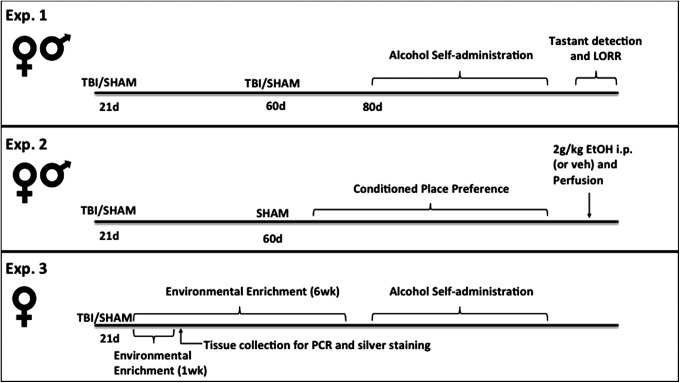
Protocol (Fig. 1)
FIG. 1.
Experimental timeline. Experiment 1 investigated whether juvenile and/or adult TBI altered alcohol self-administration, detection of tastants, or behavioral sensitivity to alcohol in adulthood. Experiment 2 tested whether juvenile injury enhanced the hedonic response to (2 g/kg) alcohol in the conditioned place preference paradigm and the effects of alcohol on immediate early gene expression in adulthood. In Experiment 3, environmental enrichment immediately after juvenile injury was used as a proxy for early intervention, and drinking behavior and injury outcomes were assessed.
Experiment 1
The effect of juvenile and adult TBI on drinking behavior in male and female mice. Male and female mice (n = 8–10 per group), underwent a TBI (or sham) procedure at 21 days of age, 60 days of age, or both time-points, resulting in the following groups: TBI/sham (injury only at 21 days), TBI/TBI (injuries at both time-points), sham/TBI (injury only at 60 days), or sham/sham (control). Beginning at the age of 80 days, mice were assessed for alcohol self-administration, followed by tastant detection and loss of righting reflex (LORR) testing.
Experiment 2
Effects of early TBI on CPP and immediate early gene expression following alcohol administration. Male and female (n = 7–9 per group) mice underwent a single TBI (or sham) procedure at 21 days of age, then all mice received a sham procedure at 60 days and were assessed for alcohol CPP beginning on Day 80. At the end of the experiment, all mice were injected with 2 g/kg ethanol (or saline control) and perfused 2 h later.
Experiment 3
Effects of environmental enrichment on drinking behavior and injury outcome. Female mice (n = 6 per group) underwent a TBI (or sham) procedure at 21 days of age and were immediately placed in environmental enrichment (or standard) housing. One cohort was sacrificed 1 week after enrichment for tissue collection for PCR or silver staining, an additional cohort was maintained in enrichment for 6 weeks, then single housed for 1 week before testing for alcohol self-administration.
Statistical analysis
Alcohol self-administration was analyzed via a four-factor repeated measures analysis of variance (ANOVA). CPP data were analyzed via paired t-tests. All other animal data were analyzed via ANOVA and all results were considered significant at p ≤ 0.05.
Results
Experiment 1: Juvenile TBI increases alcohol drinking
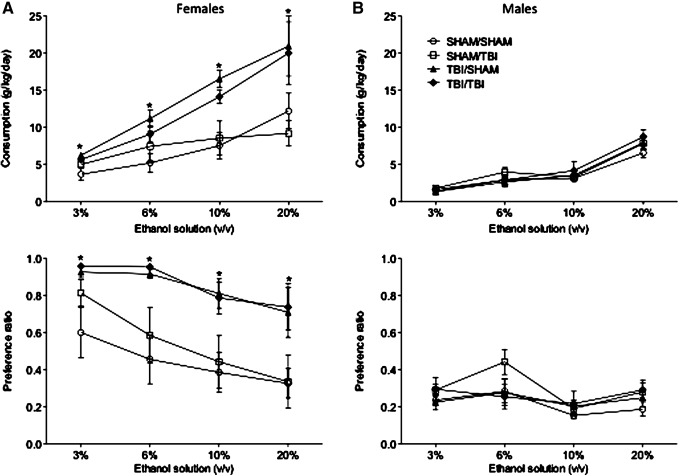
We injured animals of both sexes either at 21 days of age, 60 days of age, or during both developmental periods and assessed spontaneous alcohol consumption in adulthood. Surprisingly, juvenile TBI (21 days) significantly increased alcohol consumption in adulthood in a 2-bottle choice test but only in females (F3,63 = 4.152; p = 0.01; η2 = 0.16; Fig. 2A). Alcohol consumption and the ratio of alcohol:water were both significantly increased in female mice that were injured at 21 days. Conversely, there was no effect of juvenile TBI on drinking behavior in males (p > 0.05; Fig. 2B). Finally, injuries during early adulthood (60 days) either alone or in combination with juvenile injuries did not alter alcohol consumption in either sex (p > 0.05 in all cases).
FIG. 2.
Traumatic brain injury (TBI)-induced ethanol consumption. Male and female mice underwent SHAM or TBI in early life (21 days), adulthood (60 days), or at both time-points. (A) A two-bottle preference test conducted with increasing concentrations of ethanol indicated increased consumption for ethanol at all concentrations tested in adult females who experienced an early life TBI. SHAM surgery did not increase ethanol intake, and adult TBI did not increase ethanol consumption/preference. (B) In contrast, TBI did not increase ethanol preference in males at any concentration tested. An asterisk (*) indicates significant group difference (p < 0.05) at the indicated ethanol concentration.
To determine if enhanced alcohol consumption in injured females was mediated by alterations in the ability to detect tastants, we performed additional two-bottle choice experiments with two concentrations of quinine and two concentrations of saccharine (Table 1). Neither juvenile nor adult TBI altered intake or the tastant:water ratio of any stimulus. Next, we tested whether the kinetics of the alcohol response were altered after TBI by performing a loss of righting reflex test. Again, injuries did not alter the latency to loss of righting reflex or the total time unconscious (p > 0.05 in all cases). Taken together, these data suggest that enhanced alcohol self-administration following TBI is not mediated by either a reduced ability to detect various tastants or long-term changes in alcohol metabolism. These results led us to investigate injury-induced changes in the rewarding properties of alcohol.
Table 1.
Tastant Consumption and Loss of Righting Reflex
| Measure | Sham/Sham | Sham/Injury | Injury/Sham | Injury/Injury |
|---|---|---|---|---|
| Quinine | ||||
| 0.1 mM Preference | 0.58 (0.13) | 0.85 (0.07) | 0.63 (0.16) | 0.71 (0.12) |
| 0.1 mM Consumption | 0.26 (0.05) | 0.35 (0.06) | 0.32 (0.07) | 0.42 (0.01) |
| 0.3 mM Preference | 0.49 (0.13) | 0.60 (0.18) | 0.41 (0.13) | 0.66 (0.16) |
| 0.3 mM Consumption | 0.22 (0.05) | 0.35 (0.07) | 0.25 (0.08) | 0.35 (0.06) |
| Saccharine | ||||
| 1.6 mM Preference | 0.84 (0.06) | 0.83 (0.08) | 0.72 (0.09) | 0.91 (0.06) |
| 1.6 mM Consumption | 0.47 (0.02) | 0.53 (0.02) | 0.52 (0.03) | 0.50 (0.01) |
| 3.2 mM Preference | 0.73 (0.06) | 0.79 (0.08) | 0.68 (0.05) | 0.91 (0.04) |
| 3.2 mM Consumption | 0.48 (0.02) | 0.57 (0.05) | 0.51 (0.02) | 0.50 (0.04) |
| Loss of righting reflex | ||||
| Latency (sec) | 116.86 (17.53) | 98.67 (11.76) | 115.17 (8.07) | 101.00 (12.29) |
| Time unconscious (min) | 91.26 (13.99) | 101.17 (9.92) | 92.16 (15.86) | 117.58 (10.81) |
Preference and consumption of bitter (quinine) and sweet (saccharine) tastants were assessed in two bottle preference tests following sham or traumatic brain injury in adolescence and/or adulthood. No significant differences are reported between groups for either tastant at the concentrations tested. Data for tastant measures are presented as group mean (± standard error of the mean [SEM]), preference is determined for each tastant relative to water, consumption is reported as mL/g body mass. The loss of righting reflex (LORR) was measured following a 4g/kg (intraperitoneal) injection of 20% ethanol. Latency to LORR is reported in seconds (± SEM), and total time spent unconscious is reported in minutes (± SEM). No significant differences are reported between groups for LORR measures.
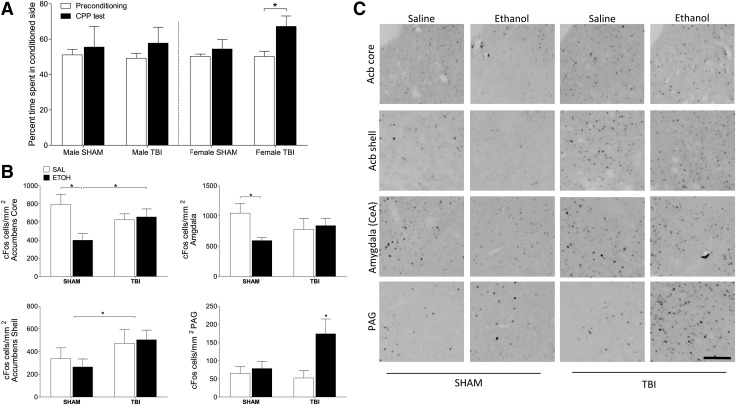
Experiment 2: Alcohol produces CPP only in injured female mice
To assess whether TBI during juvenile development alters the reinforcing properties of alcohol, we conducted a CPP test, a well-validated assay of drug reward. As there was no effect of adult injuries on alcohol preference, we chose to focus on only the juvenile injuries in this experiment. CPP responses to alcohol were only evident in female mice that had been injured at 21 days of age (t11 = −3.374; p = 0.006; η2 = 0.48; Fig. 3A), whereas sham injured females and males of both injury groups showed no CPP relative to the baseline test. In order to examine the neurological substrates of this phenomenon, we injected the female mice that had undergone CPP with either alcohol or saline and collected tissue 2 h later to assess expression of the immediate early gene product c-Fos (Fig. 3B, 3C). Juvenile TBI altered the pattern of c-Fos expression across the reward pathway but in females only. In female mice, in the core of the nucleus accumbens, for instance, ethanol reduced c-Fos expression in sham-injured animals (F1,22 = 1.396; p = 0.048; η2 = 0.13) but had no effect in injured animals. In contrast, TBI increased c-Fos expression in the shell of the accumbens in injured female mice, regardless of ethanol treatment (F1,25 = 4.061; p = 0.05; η2 = 0.14). In the central amygdala, alcohol also reduced c-Fos expression in sham-injured mice but did not alter c-Fos in injured females (F1,23 = 3.939; p = 0.05; η2 = 0.13).
FIG. 3.
Traumatic brain injury (TBI) induces ethanol conditioned place preference (CPP) in females. Adult male and female mice underwent CPP testing following early life TBI or SHAM surgery. (A) Relative to pre-conditioning baseline testing, females that had a TBI were the only group that spent significantly more time in the ethanol-conditioned side of the chamber during the CPP test. (B, C) Immunohistochemistry and quantitative analysis of cFos expression following a (2 g/kg) injection of ethanol, or saline in females. TBI and ethanol injection produced significant differences in cFos expression in the accumbens core, shell, central nucleus of the amygdala, and periaqueductal gray. An asterisk (*) indicates significant differences between indicated groups (p < 0.05). Scale bar = 100 μm.
Finally, in the periaqueductal gray, alcohol greatly increased c-Fos expression but only in injured females (F1,16 = 3.763; p = 0.028; η2 = 0.21). In contrast, there were no effects of either juvenile TBI or alcohol on c-Fos expression in the accumbens, amygdala, or periaqueductal gray in male mice (p > 0.05 in all cases; Table 2). Taken together, these data indicate that juvenile TBI alters both the behavioral response to alcohol conditioning and the pattern of neuronal activation associated with alcohol seeking and reward, but does so only in female mice.
Table 2.
Expression of c-Fos in Male Mice
| Group | Accumbens core | Accumbens shell | Amygdala central/nucleus | Periaqueductal gray |
|---|---|---|---|---|
| SHAM + Saline | 637.73 (116.55) | 342.59 (61.23) | 615.00 (133.43) | 59.85 (4.97) |
| SHAM + Ethanol | 513.89 (105.16) | 290.51 (76.96) | 560.42 (56.50) | 100.00 (14.76) |
| TBI + Saline | 470.43 (127.14) | 345.83 (96.34) | 775.00 (164.74) | 89.09 (9.55) |
| TBI + Ethanol | 478.17 (64.82) | 258.93 (47.41) | 729.17 (138.40) | 98.49 (32.19) |
Traumatic brain injury (TBI)/SHAM male mice were injected with ethanol (2 g/kg) or saline, and brain tissue harvested 2 h later. Quantitative analysis of c-Fos–positive cells in the nucleus accumbens, amygdala, and periaqueductal gray revealed no significant surgery or injection effects; all p > 0.05. Data are presented as group mean (± standard error of the mean).
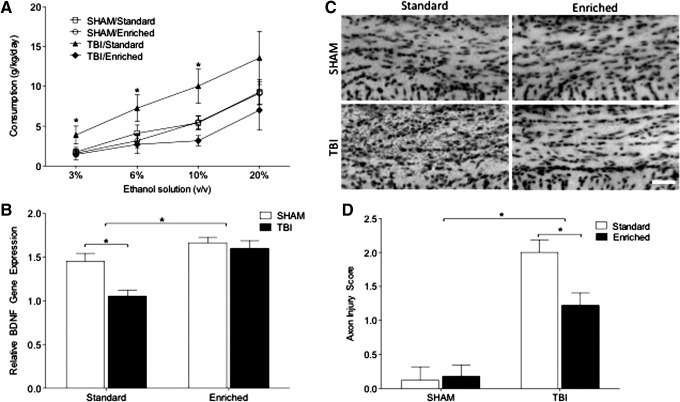
Experiment 3: Environmental enrichment prevents TBI-induced increases in drinking, reduces axonal degeneration, and increases brain-derived neurotrophic factor gene expression
Given that injuries early in life greatly increased alcohol consumption in female mice, we next sought to determine whether environmental enrichment, a manipulation that both modulates addiction-related behaviors and protects the nervous system from TBI-induced damage, would reduce the increase in drinking behavior. We housed female mice in enriched environments immediately following TBI for 6 weeks before separating the animals to examine alcohol self-administration. Remarkably, environmental enrichment abolished the enhancement of drinking behavior induced by juvenile TBI (F1,19 = 3.840; p = 0.026; η2 = 0.37; Fig. 4A). In a separate cohort of mice housed for only 1 week after TBI in enriched conditions, we extracted mRNA from the prefrontal cortex and identified that TBI significantly reduced cortical brain-derived neurotrophic factor (BDNF) expression. In contrast, environmental enrichment increased BDNF gene expression, relative to standard housing, and specifically prevented its reduction following TBI (F1,13 = 23.374; p < 0.0001; η2 = 0.47; Fig. 4B). Next, we examined whether environmental enrichment would reduce neuronal degeneration following TBI. One week of environmental enrichment immediately following juvenile TBI significantly reduced the degree of axonal degeneration in the forebrain as indicated by silver staining (F1,33 = 5.212; p = 0.029; η2 = 0.05; Fig. 4C, 4D). Finally, examination of Fluoro-Jade C staining revealed no evidence of neuronal cell death (data not shown).
FIG. 4.
Post-traumatic brain injury (TBI) environmental enrichment reduces post-traumatic drinking. Female mice underwent early-life SHAM or TBI surgery and were placed in an enriched environment, or standard housing. (A) While standard housing produced increased ethanol consumption following TBI, environmental enrichment significantly reduced ethanol preference to the levels consumed by SHAM-injured mice (B) Environmental enrichment significantly increased cortical brain-derived neurotrophic factor (BDNF) gene expression and normalized BDNF expression following TBI. (C, D) Early life TBI resulted in axonal degeneration, as assessed via silver staining; however, the extent of degeneration was significantly reduced by environmental enrichment. An asterisk (*) indicates significant group differences (p < 0.05). Scale bar = 20 μm.
Discussion
This work provides both mechanistic insight and a powerful animal model to study the biology of post-traumatic alcohol misuse. In contrast to our hypothesis that juvenile and adult injuries would interact to alter adult behavior, we show that mild TBIs that occur during juvenile development, but not in adulthood, produce long lasting increases in alcohol self-administration and alter the reinforcing properties of alcohol. Further, this effect is limited to females, as male mice that underwent an identical surgical procedure did not display altered drinking behavior in adulthood, CPP to intraperitoneal alcohol, or changes in c-Fos expression. Critically, the changes in drinking behavior could be rescued by housing mice in an enriched environment following injury, a manipulation that also reduced axonal degeneration and normalized BDNF gene expression.
An increase in alcohol self-administration in rodents can be caused by a number of different proximate mechanisms, including alterations in the senses of taste or smell, changes in alcohol pharmacokinetics, and alterations in the rewarding and/or aversive properties of ethanol.18 The data contained in this manuscript strongly implicate alterations in reward-related circuitry following juvenile TBI. Female mice injured at 21 days of age did not differ from sham-injured mice in self-administration of bitter or sweetened liquids and exhibited similar responses in the loss of righting reflex assay, indicating that sensory or pharmacokinetic changes are unlikely to mediate the enhanced drinking behavior in injured females.16 In contrast, intraperitoneal alcohol, which bypasses oral or olfactory cues, induced CPP only in injured female mice.19 Taken together, these data strongly suggest that juvenile TBI increases the rewarding properties of alcohol. An analysis of immediate early gene expression following alcohol or vehicle administration indicated that early life injuries significantly alter both the basal and alcohol-stimulated activational state of several structures associated with drug reward, including the nucleus accumbens, central nucleus of the amygdala, and periaqueductal gray, but does so only in female mice.
We used injury paradigms that were as similar as possible for the injuries at 21 and 60 days of age; however, as there is significant hardening of the skull by early adulthood, it is possible that the actual force transmitted through the brain varied between developmental periods. In any case, TBIs in children are a key public health issue as TBI is the most common cause of death and disability in children.1 Juvenile girls are experiencing increasing rates of TBI in organized sports, particularly soccer.20 Many pathophysiological responses to TBI produce greater impairment in younger populations, and outcomes are generally inversely proportional to the age of injury.21 This developmental effect occurs despite the generally larger capacity for plasticity and repair in younger brains but may be secondary to the disruption of neurodevelopmental processes.22,23
The dopamine system is positioned to serve as a key mediator of TBI-induced alterations in reward processing. Dopaminergic agonists have shown some clinical efficacy in alleviating cognitive and mood dysfunction following TBI.24–27 Further dopaminergic dysfunction, indicated by reduced dopamine transporter binding following TBI, has been documented with single-photon emission computed tomography.28 In experimental studies, tissue dopamine content appears to rise acutely after TBI.29–31 This pattern of responses is highly consistent with the alterations in the dopaminergic system following adolescent but not adult exposure to binge-like levels of ethanol.32,33
Exposure to alcohol during early adolescence strongly drives dopamine release. However, adolescent alcohol appears to blunt dopaminergic signaling and responses to alcohol in adulthood. Specifically, mice that were exposed to alcohol in adolescence spontaneously drank more, and when re-exposed to alcohol in adulthood, exhibited reduced c-Fos activation in the nucleus accumbens core and dopamine efflux.33,34 Importantly, this effect was highly age specific as exposure to alcohol during later developmental stages reduced both the immediate dopamine efflux and the sensitization of the dopaminergic system to later ethanol exposure.32 It seems possible, therefore, that the injury-induced surge of dopamine release throughout the forebrain sensitizes reward circuitry in a similar manner. Combining these data with the evidence for later long-term hypodopaminergia following TBI strongly suggests that increases in alcohol drinking behavior are mediated by three overlapping events. Dopamine efflux directly following injury during juvenile development alters long-term regulation of dopaminergic tone, which in turn reduces the alcohol-induced dopamine release in adulthood. Then, injury-induced dopamine dysfunction further reduces dopaminergic activity. Taken together, these events would produce a brain that is highly vulnerable to high levels of alcohol consumption.32,35,36 Conversely, environmental enrichment, which has been shown to normalize dopaminergic tone following brain injury, blocked enhanced drinking.37
Although the neuroprotective effect of environmental enrichment on TBI outcome is well documented, this is the first report indicating environmental enrichment as a method for reducing post-TBI alcohol consumption. Consistent with previous reports, BDNF gene expression in the ipsilateral prefrontal cortex of injured females was significantly reduced by TBI in standard-housed mice (importantly, this cohort of mice was not exposed to alcohol). However, environmental enrichment both increased BDNF in sham-injured mice and prevented injury-induced reductions in BDNF. Importantly, BDNF concentrations in peripheral blood are reduced in alcoholic patients but are higher during periods of abstinence.38,39 In experimental animals, BDNF expression is negatively associated with alcohol self-administration and CPP.40 Further, chronic alcohol intake reduces BDNF gene expression.40 In this study, cortical BDNF express was reduced in injured mice prior to exposure to any alcohol, suggesting that the reduced BDNF expression associated with chronic drinking may lead to an even further reduction of BDNF expression over time. Additionally, BDNF appears to oppose some of the alterations in neurotransmitter signaling that sensitize the brain to drugs of abuse, indicating that reductions in BDNF may exacerbate dopaminergic dysfunction induced by TBI.41,42
To the best of our knowledge, this is the first paper to report sex differences in post-traumatic drinking behavior. Here, female mice injured during early development drank much more than other females or males in any surgical condition. A greater understanding of the underlying biology of this phenomenon is of paramount importance given the epidemiological findings that TBI incidence may be increasing in young women and these patients may suffer worse outcomes.43 Further, women are particularly vulnerable to medical consequences of heavy drinking, including liver disease, infertility, and mortality.44,45 It is important to note that in this study, females drank more than males regardless of surgical manipulation. This is consistent with other reports in laboratory rodents, where females self-administer more alcohol under both continuous and binge-like access conditions and in operant procedures.46–49 Additionally, female rodents appear to find ethanol more rewarding, as indicated by greater CPP and enhanced dopamine efflux following ethanol administration.50,51 Whether this higher basal preference for alcohol in female rodents is permissive and/or necessary for increases in drinking after juvenile TBI remains an open question. In any case, sex steroid hormones modulate alcohol intake and sex differences in alcohol abuse first appear around the time of puberty in both rodents and humans, and drinking behavior is modulated by sex steroid homones.52,53 Further study into the role of organizational and activational effects of sex steroids in mediating the effects of TBI on adult alcohol intake is warranted.
TBI is intricately interwoven with alcohol use and abuse, as alcohol is a precipitating factor in many injuries and alcohol abuse can reduce the effectiveness of rehabilitation. Further, brain injuries in childhood reduce health, life satisfaction, and career prospects, while increasing the chances of incarceration. The data presented here indicate that although juvenile TBI has persistent and potentially deleterious consequences across the life span, these consequences are not necessarily fixed at the time of injury. Environmental enrichment reduced axonal degeneration, normalized BDNF gene expression, and prevented the increase in drinking following TBI. Although rodent environmental enrichment is probably a relatively poor proxy for intensive cognitive and behavioral rehabilitation it does suggest that there is an opportunity for long-term benefits of early and prolonged intervention following TBI in young patients. Further, the model of TBI that we used in this study produced only subtle neuronal pathology and left the animals appearing grossly normal. There was no evidence of neuronal cell bodies dying and axonal degeneration was relatively mild. These data highlight the potential for long-term adverse consequences of comparatively mild TBIs and indicate that much greater detection and intervention efforts are necessary in this population.
In conclusion, a relatively mild TBI that resulted in minimal functional impairments produced increases in alcohol intake and responses to alcohol in the reward system many weeks later, and did so only in juvenile females. Thus, this animal model can provide us a powerful tool to investigate the neurobiology underlying alterations in drinking behavior, sex differences, and the protective effects of environmental enrichment.
Acknowledgments
Support for this work was provided by an OSU Neurological Institute Pilot Award and additional support for behavioral research was provided by NS045758. The authors gratefully acknowledge Drs. Kevin Foust, Georgia Bishop, and Jacqueline Barker for their advice and assistance. Additionally, the authors thank Katie Centner, Lindsey Freeman, Sam Colachis, Joe Abraham, Ben Sarac, Vanessa Wenger, Maya Prabhu, and Sam Nichols for their hard work and support.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Langlois J.A., Rutland-Brown W., and Wald M.M. (2006). The epidemiology and impact of traumatic brain injury: a brief overview. J. Head Trauma Rehabil. 21, 375–378 [DOI] [PubMed] [Google Scholar]
- 2.Faul M.D., Wald M.M., Xu L., and Coronado V.G. (2010). Traumatic brain injury in the United States; emergency department visits, hospitalizations, and deaths, 2002–2006. Centers for Disease Control and Prevention: Atlanta, GA [Google Scholar]
- 3.Navarro R.R. (2011). Protective equipment and the prevention of concussion—what is the evidence? Current Sports Med. Reports 10, 27–31 [DOI] [PubMed] [Google Scholar]
- 4.Corrigan J.D., Bogner J., Mellick D., Bushnik T., Dams-O'Connor K., Hammond F.M., Hart T., and Kolakowsky-Hayner S. (2013). Prior history of traumatic brain injury among persons in the Traumatic Brain Injury Model Systems National Database. Arch. Phys. Medicine Rehabil. 94, 1940–1950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kreutzer J.S., Witol A.D., and Marwitz J.H. (1996). Alcohol and drug use among young persons with traumatic brain injury. J. Learn. Disabil. 29, 643–651 [DOI] [PubMed] [Google Scholar]
- 6.Ruff R.M., Marshall L.F., Klauber M.R., Blunt B.A., Grant I., Foulkes M.A., Eisenberg H., Jane J., and Marmarou A. (1990). Alcohol abuse and neurological outcome of the severely head injured. J. Head Trauma Rehabil. 5, 21–31 [Google Scholar]
- 7.McKinlay A., Corrigan J., Horwood L.J., and Fergusson D.M. (2014). Substance abuse and criminal activities following traumatic brain injury in childhood, adolescence, and early adulthood. J. Head Trauma Rehabil. 29, 498–506 [DOI] [PubMed] [Google Scholar]
- 8.Corrigan J.D. (1995). Substance abuse as a mediating factor in outcome from traumatic brain injury. Arch. Phys. Med. Rehabil. 76, 302–309 [DOI] [PubMed] [Google Scholar]
- 9.Bjork J.M. and Grant S.J. (2009). Does traumatic brain injury increase risk for substance abuse? J. Neurotrauma 26, 1077–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adams R.S., Larson M.J., Corrigan J.D., Horgan C.M., and Williams T.V. (2012). Frequent binge drinking after combat-acquired traumatic brain injury among active duty military personnel with a past year combat deployment. J. Head Trauma Rehabil. 27, 349–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lowing J.L., Susick L.L., Caruso J.P., Provenzano A.M., Raghupathi R., and Conti A.C. (2014). Experimental traumatic brain injury alters ethanol consumption and sensitivity. J. Neurotrauma 31, 1700–1710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corrigan J.D., Cuthbert J.P., Harrison-Felix C., Whiteneck G.G., Bell J.M., Miller A.C., Coronado V.G., and Pretz C.R. (2014). US population estimates of health and social outcomes 5 years after rehabilitation for traumatic brain injury. J. Head Trauma Rehabil. 29, E1–E9 [DOI] [PubMed] [Google Scholar]
- 13.Karver C.L., Wade S.L., Cassedy A., Taylor H.G., Stancin T., Yeates K.O., and Walz N.C. (2012). Age at injury and long-term behavior problems after traumatic brain injury in young children. Rehabil. Psychol. 57, 256–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kreutzer J.S., Wehman P.H., Harris J.A., Burns C.T., and Young H.F. (1991). Substance abuse and crime patterns among persons with traumatic brain injury referred for supported employment. Brain Inj. 5, 177–187 [DOI] [PubMed] [Google Scholar]
- 15.Corrigan J.D., Bogner J., and Holloman C. (2012). Lifetime history of traumatic brain injury among persons with substance use disorders. Brain Inj. 26, 139–150 [DOI] [PubMed] [Google Scholar]
- 16.Crabbe J.C., Gray D.K., Young E.R., Janowsky J.S., and Rigter H. (1981). Initial sensitivity and tolerance to ethanol in mice: correlations among open field activity, hypothermia, and loss of righting reflex. Behav. Neural Biol. 33, 188–203 [DOI] [PubMed] [Google Scholar]
- 17.Weil Z.M., Gaier K.R., and Karelina K. (2014). Injury timing alters metabolic, inflammatory and functional outcomes following repeated mild traumatic brain injury. Neurobiol. Dis. 70, 108–116 [DOI] [PubMed] [Google Scholar]
- 18.McBride W.J. and Li T.K. (1998). Animal models of alcoholism: neurobiology of high alcohol-drinking behavior in rodents. Crit. Rev. Neurobiol. 12, 339–369 [DOI] [PubMed] [Google Scholar]
- 19.Reid L.D., Hunter G.A., Beaman C.M., and Hubbell C.L. (1985). Toward understanding ethanol's capacity to be reinforcing: a conditioned place preference following injections of ethanol. Pharmacol. Biochem. Behav. 22, 483–487 [DOI] [PubMed] [Google Scholar]
- 20.Rosenthal J.A., Foraker R.E., Collins C.L., and Comstock R.D. (2014). National High School Athlete Concussion Rates From 2005–2006 to 2011–2012. Am. J. Sports Med. 42, 1710–1715 [DOI] [PubMed] [Google Scholar]
- 21.Kochanek P.M., Clark R.S., Ruppel R.A., Adelson P.D., Bell M.J., Whalen M.J., Robertson C.L., Satchell M.A., Seidberg N.A., Marion D.W., and Jenkins L.W. (2000). Biochemical, cellular, and molecular mechanisms in the evolution of secondary damage after severe traumatic brain injury in infants and children: Lessons learned from the bedside. Pediatric Crit. Care Med. 1, 4–19 [DOI] [PubMed] [Google Scholar]
- 22.Gerber P. and Coffman K. (2007). Nonaccidental head trauma in infants. Child Nerv. Syst. 23, 499–507 [DOI] [PubMed] [Google Scholar]
- 23.Kramer M.E., Chiu C.Y., Walz N.C., Holland S.K., Yuan W., Karunanayaka P., and Wade S.L. (2008). Long-term neural processing of attention following early childhood traumatic brain injury: fMRI and neurobehavioral outcomes. J. Int. Neuropsychol. Soc. 14, 424–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neurobehavioral Guidelines Working, G., Warden D.L., Gordon B., McAllister T.W., Silver J.M., Barth J.T., Bruns J., Drake A., Gentry T., Jagoda A., Katz D.I., Kraus J., Labbate L.A., Ryan L.M., Sparling M.B., Walters B., Whyte J., Zapata A., and Zitnay G. (2006). Guidelines for the pharmacologic treatment of neurobehavioral sequelae of traumatic brain injury. J. Neurotrauma 23, 1468–1501 [DOI] [PubMed] [Google Scholar]
- 25.Whyte J., Hart T., Schuster K., Fleming M., Polansky M., and Coslett H.B. (1997). Effects of methylphenidate on attentional function after traumatic brain injury. A randomized, placebo-controlled trial. Am. J. Phys. Med. Rehabil. 76, 440–450 [DOI] [PubMed] [Google Scholar]
- 26.Whyte J., Hart T., Vaccaro M., Grieb-Neff P., Risser A., Polansky M., and Coslett H.B. (2004). Effects of methylphenidate on attention deficits after traumatic brain injury: a multidimensional, randomized, controlled trial. Am. J. Phys. Med. Rehabil. 83, 401–420 [DOI] [PubMed] [Google Scholar]
- 27.Goldstein L.B. (2003). Neuropharmacology of TBI-induced plasticity. Brain Inj. 17, 685–694 [DOI] [PubMed] [Google Scholar]
- 28.Donnemiller E., Brenneis C., Wissel J., Scherfler C., Poewe W., Riccabona G., and Wenning G.K. (2000). Impaired dopaminergic neurotransmission in patients with traumatic brain injury: a SPECT study using 123I-beta-CIT and 123I-IBZM. Eur. J. Nuclear Med. 27, 1410–1414 [DOI] [PubMed] [Google Scholar]
- 29.McIntosh T.K., Yu T., and Gennarelli T.A. (1994). Alterations in regional brain catecholamine concentrations after experimental brain injury in the rat. J. Neurochem. 63, 1426–1433 [DOI] [PubMed] [Google Scholar]
- 30.Huger F. and Patrick G. (1979). Effect of concussive head injury on central catecholamine levels and synthesis rates in rat brain regions. J. Neurochem. 33, 89–95 [DOI] [PubMed] [Google Scholar]
- 31.Massucci J.L., Kline A.E., Ma X., Zafonte R.D., and Dixon C.E. (2004). Time dependent alterations in dopamine tissue levels and metabolism after experimental traumatic brain injury in rats. Neurosci. Lett. 372, 127–131 [DOI] [PubMed] [Google Scholar]
- 32.Zandy S.L., Matthews D.B., Tokunaga S., Miller A.D., Blaha C.D., and Mittleman G. (2015). Reduced dopamine release in the nucleus accumbens core of adult rats following adolescent binge alcohol exposure: age and dose-dependent analysis. Psychopharmacology 232, 777–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Philpot R.M., Wecker L., and Kirstein C.L. (2009). Repeated ethanol exposure during adolescence alters the developmental trajectory of dopaminergic output from the nucleus accumbens septi. Int. J. Dev. Neurosci. 27, 805–815 [DOI] [PubMed] [Google Scholar]
- 34.Pascual M., Boix J., Felipo V., and Guerri C. (2009). Repeated alcohol administration during adolescence causes changes in the mesolimbic dopaminergic and glutamatergic systems and promotes alcohol intake in the adult rat. J. Neurochem. 108, 920–931 [DOI] [PubMed] [Google Scholar]
- 35.Bales J.W., Wagner A.K., Kline A.E., and Dixon C.E. (2009). Persistent cognitive dysfunction after traumatic brain injury: a dopamine hypothesis. Neurosci. Biobehav. Rev. 33, 981–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.George S.R., Fan T., Ng G.Y., Jung S.Y., O'Dowd B.F., and Naranjo C.A. (1995). Low endogenous dopamine function in brain predisposes to high alcohol preference and consumption: reversal by increasing synaptic dopamine. J. Pharmacol. Exp. Ther. 273, 373–379 [PubMed] [Google Scholar]
- 37.Wagner A.K., Chen X., Kline A.E., Li Y., Zafonte R.D., and Dixon C.E. (2005). Gender and environmental enrichment impact dopamine transporter expression after experimental traumatic brain injury. Exper. Neurol. 195, 475–483 [DOI] [PubMed] [Google Scholar]
- 38.Zanardini R., Fontana A., Pagano R., Mazzaro E., Bergamasco F., Romagnosi G., Gennarelli M., and Bocchio-Chiavetto L. (2011). Alterations of brain-derived neurotrophic factor serum levels in patients with alcohol dependence. Alcohol. Clin. Exp. Res. 35, 1529–1533 [DOI] [PubMed] [Google Scholar]
- 39.Huang M.C., Chen C.H., Liu H.C., Chen C.C., Ho C.C., and Leu S.J. (2011). Differential patterns of serum brain-derived neurotrophic factor levels in alcoholic patients with and without delirium tremens during acute withdrawal. Alcohol. Clin. Exp. Res. 35, 126–131 [DOI] [PubMed] [Google Scholar]
- 40.Machens H.G., Pabst A., Dreyer M., Gliemroth J., Gorg S., Bahlmann L., Klaus S., Kaun M., Kru Ger S., and Mailander P. (2006). C3a levels and occurrence of subdermal vascular thrombosis are age-related in deep second-degree burn wounds. Surgery 139, 550–555 [DOI] [PubMed] [Google Scholar]
- 41.McGough N.N., He D.Y., Logrip M.L., Jeanblanc J., Phamluong K., Luong K., Kharazia V., Janak P.H., and Ron D. (2004). RACK1 and brain-derived neurotrophic factor: a homeostatic pathway that regulates alcohol addiction. J. Neurosci. 24, 10542–10552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barker J.M., Taylor J.R., De Vries T.J., and Peters J. (2014). Brain-derived neurotrophic factor and addiction: pathological versus therapeutic effects on drug seeking. Brain Res. 2014. November 4; [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Farace E. and Alves W.M. (2000). Do women fare worse? A metaanalysis of gender differences in outcome after traumatic brain injury. Neurosurg. Focus 8, 1–8 [DOI] [PubMed] [Google Scholar]
- 44.Epstein E.E., Fischer-Elber K., and Al-Otaiba Z. (2007). Women, aging, and alcohol use disorders. J. Women Aging 19, 31–48 [DOI] [PubMed] [Google Scholar]
- 45.Ceylan-Isik A.F., McBride S.M., and Ren J. (2010). Sex difference in alcoholism: who is at a greater risk for development of alcoholic complication? Life Sci. 87, 133–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lancaster F.E., Brown T.D., Coker K.L., Elliott J.A., and Wren S.B. (1996). Sex differences in alcohol preference and drinking patterns emerge during the early postpubertal period. Alcohol. Clin. Exp. Res. 20, 1043–1049 [DOI] [PubMed] [Google Scholar]
- 47.Cailhol S. and Mormede P. (2001). Sex and strain differences in ethanol drinking: effects of gonadectomy. Alcohol. Clin. Exp. Res. 25, 594–599 [PubMed] [Google Scholar]
- 48.Middaugh L.D., Kelley B.M., Bandy A.L.E., and McGroarty K.K. (1999). Ethanol consumption by C57BL/6 mice: Influence of gender and procedural variables. Alcohol 17, 175–183 [DOI] [PubMed] [Google Scholar]
- 49.Barker J.M., Torregrossa M.M., Arnold A.P., and Taylor J.R. (2010). Dissociation of genetic and hormonal influences on sex differences in alcoholism-related behaviors. J. Neurosci. 30, 9140–9144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Torres O.V., Walker E.M., Beas B.S., and O'Dell L.E. (2014). Female rats display enhanced rewarding effects of ethanol that are hormone eependent. Alcohol. Clin. Exp. Res. 38, 108–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blanchard B.A., Steindorf S., Wang S., and Glick S.D. (1993). Sex-differences in ethanol-induced dopamine release in nucleus-accumbens and in ethanol-consumption in rats. Alcohol. Clin. Exp. Res. 17, 968–973 [DOI] [PubMed] [Google Scholar]
- 52.Witt E.D. (2007). Puberty, hormones, and sex differences in alcohol abuse and dependence. Neurotoxicol. Teratol. 29, 81–95 [DOI] [PubMed] [Google Scholar]
- 53.Patton G.C., McMorris B.J., Toumbourou J.W., Hemphill S.A., Donath S., and Catalano R.F. (2004). Puberty and the onset of substance use and abuse. Pediatrics 114, E300–E306 [DOI] [PMC free article] [PubMed] [Google Scholar]