Abstract
Objective: To describe longitudinal effects of feeding volume and type of milk on fecal calprotectin (f-CP) in very low–birth weight (VLBW) infants.
Study Design: Prospective data were collected across Neonatal Intensive Care Unit (NICU) admission for 6 weeks or until discharge in 75 VLBW neonates. The mean gestational age on entry into the study was 29 weeks.
Results: Seventy-four (99%) mothers provided expressed milk in varying amounts. Twenty-three mothers (31%) provided exclusive mother's own milk (MOM) throughout. Preterm infant formula (PIF) and pasteurized donor milk were added to feedings of remaining infants. Pooled MOM was analyzed weekly for levels of a panel of cytokines, chemokines, and growth factors, and secretory Immunoglobulin A (sIgA) so that the exact amount of exposure to the gut of these milk bioactives could be estimated. f-CP levels ranged from 160 to 350 μg/g stool. Total feeding volume was positively associated with f-CP, controlling for infant weight, and f-CP levels rose across time. Exclusive MOM feedings for the entire measurement period were associated with rising levels of f-CP, but mixed feedings (MOM with added PIF or pasteurized donor milk (PDM) did not show this increase over time.
Conclusion: The presence of f-CP may represent a response to milk volumes and MOM, which represents normal development rather than always implicating pathological inflammation in the VLBW infant.
Introduction
Calprotectin (CP) is a 36.5-kDa heterotrimer found in neutrophil granules and monocytes.1,2 CP is involved in intracellular signal transduction, immunoglobulin production, and regulation of the inflammatory system. CP also has an antifungal and bacteriostatic activity when it binds to zinc.1,3 CP is a clinical marker in infectious, inflammatory, autoimmune, and malignant conditions.1 In particular, the fecal level of calprotectin (f-CP) has been studied as a noninvasive biomarker of gut inflammation. A cutoff level of 50 mg/kg has been established for inflammatory bowel conditions in adults and children older than 4 years of age.3
In the past decade, several studies have explored the utility of f-CP as a biomarker for gut inflammation in the neonate. The time course and properties of f-CP in both preterm and term infants have been examined to establish a cutoff level for the diagnosis of gastrointestinal inflammation. However, the presence of f-CP does not always indicate pathologic gut inflammation. f-CP concentrations are much higher in both healthy full-term and preterm infants than in children and adults,3–8 are higher in preterm than in term infants,6 and are present at high levels in meconium.6 In the first weeks of life, preterm and term infants display a wide range of inter- and intraindividual variation in f-CP levels.3,8 Infants with necrotizing enterocolitis (NEC) have much higher f-CP levels than healthy infants3,9–11 and levels are positively correlated with severity of NEC.9,11 f-CP levels are associated with gastrointestinal distress in preterm infants, with levels higher than 363 μg/g stool as a cutoff for the presence of mild enteropathy.12 The presence of f-CP in preterm and term infant stools might be an indication of gut microbiota maturation, as neutrophils migrate to the gut in response to the developing microbial colonization. Protection against pathogenic microbes might be facilitated through this mechanism as the commensal microbiota develops.
The possible relationships between type of feeding and f-CP levels in the preterm infant have not been well characterized and the data are conflicting.8,13–15 The current recommendation for feeding of preterm infants is to provide mother's own milk (MOM), fortified with protein, minerals, and vitamins for all infants weighing <1,500 g at birth. If MOM is not available or is contraindicated, pasteurized donor milk (PDM) should be used.16 Preterm infants fed MOM receive many immune factors, cells, and microbiota that are not present in formula or in donor milk. We hypothesized that in very low–birth weight (VLBW) infants, human milk consumption would correlate positively with f-CP levels. Our objective was to further clarify the effect and type of feeding, as well as biological factors present in human preterm milk, on f-CP levels in VLBW neonates.
Materials and Methods
VLBW infants were recruited after admission to the Neonatal Intensive Care Unit (NICU) at a single tertiary care hospital. Mothers consented to participate in a parent study, which involved completing a demographic form, and collection by the nurses of small (0.5 mL) daily aliquots of expressed MOM or PDM and stool samples from infants' diapers, collected weekly. Every effort was made to enroll them as soon as possible after admission, without coercion and in consideration of parental stress and confusion. The mean day of life for entry into the first 7-day study period was 5.4 ± 0.04 days and the mean corrected gestational age (cGA) at that time was 29 ± 0.36 weeks. If their entry into the study was after 8 days of life (N = 16), their data were entered into the second week of measurement. If an infant was entered after 14 days, we did not include him or her in the study. Once they were enrolled, data were collected from the entry into the study in weekly increments for 6 weeks. These infants were receiving MOM, preterm infant formula (PIF), or PDM per unit policy, and the MOM that was pooled during this first interval contained colostrum in almost every case.
The demographic form and medical record data provided information about the maternal history, pregnancy, labor, and the birth of the infant. VLBW infants admitted to the NICU at the study site were fed according to established evidence-based feeding protocol. As per unit policy, all mothers were encouraged to provide expressed milk for their infants unless there was a medical contraindication. Enteral feedings were initiated as soon as infants achieved cardiorespiratory stability, usually within 48 hours of life. For infants of mothers wishing to provide exclusive MOM, enteral feedings were withheld for up to 72 hours to allow for the provision of maternal colostrum as first feeding. If maternal colostrum was not available after 72 hours, consent for donor milk feeding was obtained. Feedings were advanced by a maximum of 20 mL/kg/day as tolerated. Oral feedings were begun at 32–34 weeks if infants were stable from a respiratory standpoint and showed appropriate developmental cues. If the mother was lactating, and wished to feed the baby at breast, feedings at the breast were introduced before bottle feedings.
If mothers were unwilling or unable to provide adequate volumes of MOM, then PDM was fed with mothers' consent until 32–34 weeks cGA, at which time cow's milk-based PIF was introduced. The first 60 maternal milk samples were labeled and fed to the infant in the same order in which they were collected. The first 60 pumpings were chosen to roughly correspond with the first week to 10 days of milk expression. This was to ensure that the baby received all of the colostrum and early transitional milk felt to contain large amounts of immune components as soon as possible after birth. After the first 60 pumped milk samples had been consumed, the infants were fed freshly pumped milk when available, then thawed and prepared frozen MOM in the absence of fresh milk. Both MOM and PDM were fortified with a cow's milk-based human milk fortifier (HMF; Similac) when infants reached an enteral feeding volume of 80–100 mL/kg/day.
Precise volumes of MOM, PIF, fortifier, and PDM fed to the infant were documented in the electronic medical record. The daily samples of MOM and PDM were collected in tuberculin syringes before the addition of fortifier, frozen at −20°C, and processed in the laboratory within 2–7 days. The milk was then thawed to room temperature, and the daily samples were pooled each week. Pooled milk was centrifuged at 2,000 g for 10 minutes at 4°C and the fat layer removed and discarded. The whey supernatant was then removed and syringe filtered using a Millipore Low protein binding Durapore® (PVDF) 0.45-μm filter (Merck Millipore, Cork, Ireland). The filtered whey was then placed in a labeled Eppendorf tube and frozen at −80°C until analysis for cytokines, chemokines, and growth factors (CCGF) by MAGPIX, using Millipore kits. The CCGF analyzed were IL-4, IL-6, IL-10, IL-8, TNF-α, MIP-1α, IP-10, and MCP-1. Milk secretory Immunoglobulin A (sIgA) was measured in diluted whey by enzyme-linked immunosorbent assay (ELISA) using a kit from ALPCO (Salem, NH). The stool samples were scooped into cryovials at the hospital and stored at room temperature until transported to the laboratory for processing, usually within 3 days. f-CP is stable for 7 days when stored at room temperature.3 The samples were then frozen at −80°C until analysis. One hundred milligrams of stool was weighed, placed in a 15-mL conical tube, and agitated with a wooden stirrer. An extraction buffer was added, the sample vortexed to form a fine slurry, and then placed on a shaker for 25 minutes. One milliliter was removed and centrifuged at 10,000 g for 20 minutes. The supernatant was removed for analysis by ELISA (Eurospital, Trieste, Italy). The f-CP was expressed as μg/g of stool. Every assay included a standard curve and quality controls, all samples were done in duplicate and the intra-assay coefficients of variation were <10%.
Linear mixed model analyses were performed to test the association of milk consumption, type of feeding, and milk bioactive components with the repeated measures of log-transformed f-CP controlling for infant weight, and for effects of exclusive MOM feeding across the entire NICU stay compared with mixed feedings. Unstructured correlation was chosen so that no assumption was made about the correlation structure among the repeated measures.
Results
Participants
While 83 infants were recruited, 2 infants died, 3 withdrew or were dropped, and 3 had missing data due to transfer or late enrollment. Data were available for 75 VLBW infants (birth weight <1,500 g) enrolled in the study. Due to discharges, enrollment later than first week of life (N = 16), or transfers, there were different numbers for each week for feeding status and f-CP (ranging from 53 in week 1 to 68 in weeks 2 and 4). Sample collection and data abstraction continued for 6 weeks or until hospital discharge or transfer. There were seven sets of twins, and one set of triplets. Demographic and clinical data for the study sample are displayed in Table 1. All but eight infants received antibiotics during their NICU hospitalization, and the mean number of days on antibiotics was 6.8 ± 0.73 days. The mean number of days until full enteral feeding for the sample was 12.6 days.
Table 1.
Description of the Sample Infant Characteristics
| Race (n = 75), N (%) | |
| Caucasian | 25 (33.3) |
| African American | 30 (40) |
| Hispanic white | 14 (18.6) |
| Hispanic black | 1 (1.4) |
| Asian/Pacific Islander | 2 (2.7) |
| Other (race not reported) | 3 (4) |
| Birth characteristics (n = 75), mean ± SD | |
| Infant birth weight (g) | 1,077.7 ± 219.5 |
| Apgar at 1 minute | 6.0 ± 1.92 |
| Apgar at 5 minutes | 7.5 ± 1.52 |
| Gestational age | 28.3 ± 2.39 |
| Weights, mean ± SD | |
| Weight at 6 weeks of age (g) (n = 64) | 1,867.2 ± 317.6 |
| Weight at discharge (g) (n = 70) | 2,695.7 ± 911.5 |
| Morbidity (N = 77), N (%) | |
| PFO or PDA | 23 (30) |
| Other cardiac problems | 8 (10.5) |
| CLD | 4 (5.3) |
| IUGR | 11 (14.5) |
| ROP | 14 (18.4) |
| Sepsis | 11 (14.5) |
| IVH | 9 (11.8) |
| NEC | 3 (3.9) |
| Deaths | 2 (2.6) |
CLD, chronic lung disease; IUGR, intrauterine growth restriction; IVH, intraventricular hemorrhage; NEC, necrotizing enterocolitis; PDA, patent ductus arteriosus; PFO, patent foramen ovale; ROP, retinopathy of prematurity; SD, standard deviation.
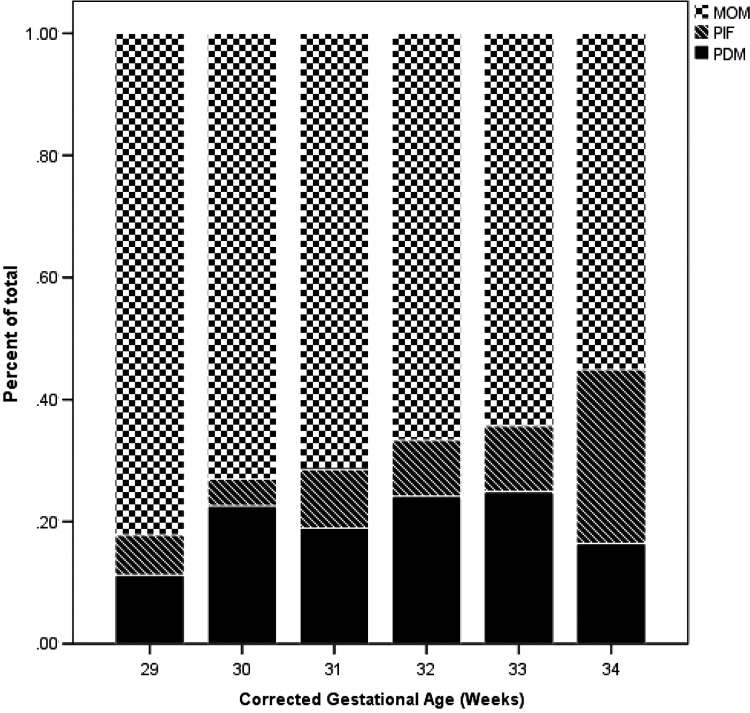
All but one mother in the study pumped some MOM for their infants, although the amounts varied, and therefore, the proportions of MOM to total milk fed differed each week. Twenty-three mothers (31%) fed exclusive MOM with no PDM or PIF added across the entire admission. HMF was added to this pumped milk per unit policy. Figure 1 depicts the mean percentages of the three different types of milk by gestational age across time, only for the mixed feeding group. Over time the percentage of feeding that was MOM declined and the percentage of formula increased. After the third week, not all mothers were able to provide fresh MOM and frozen MOM was used for feeding when available.
FIG. 1.
Comparison of contributions of MOM, PDM, and PIF total milk volume across time. MOM, mother's own milk; PDM, pasteurized donor milk; PIF, preterm infant formula.
f-CP levels
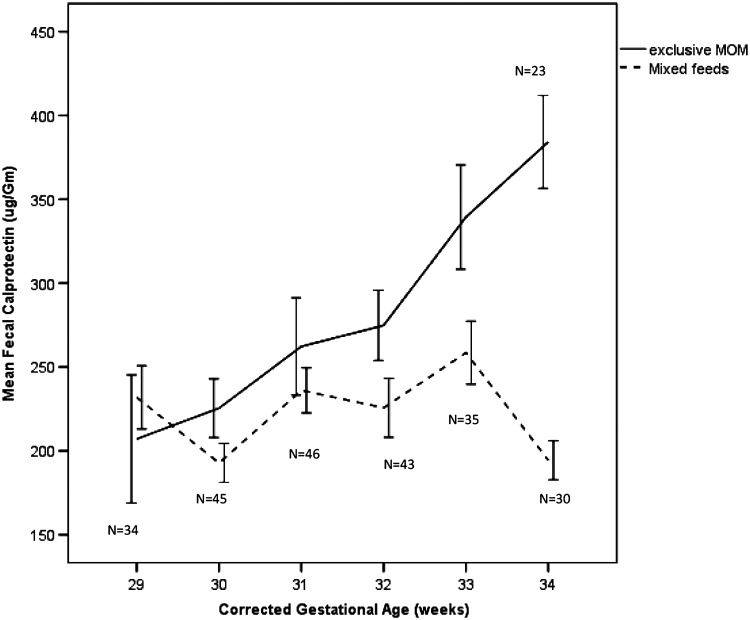
Levels of f-CP in the stool samples increased from collection week 1–6. This increase was significant when analyzed using a mixed model approach [F(1,59) = 244, p < 0.001]. There was a significant relationship between total volume of milk fed across time and f-CP (log transformed) [F(1,72) = 7.2, p < 0.009] after controlling for infant weight. There was a significant difference in f-CP levels over time based on the type of feedings given (Fig. 2). Infants fed exclusive MOM with no other types of feedings (except added fortifier) across the measurement period had rising f-CP levels, but those who were fed combinations of MOM, PIF, and PDM had lower and fairly level concentrations of f-CP across time that declined further at around 33 weeks cGA. The difference in log transformed CP by week was significant [F(5,228) = 2.79, p < 0.018], and the difference between MOM and mixed feedings was significant [F(1,54) = 4.83, p < 0.03], but the group by time difference was only marginally significant (p = 0.09), probably due to the smaller sample size at weeks 5 and 6 and the fact that the groups diverged from each other in the latter weeks of measurement.
FIG. 2.
Mean levels of fecal calprotectin across time in very low–birth weight infants by corrected gestational age in exclusive MOM feeding compared to mixed feeding.
We did not find a relationship between f-CP and days on antibiotics in our sample of VLBW infants or with maternal prenatal antibiotics or steroids (data not shown). We did find that infants born by vaginal delivery (22%) had higher mean f-CP levels (333 μg/mL ±74 standard error of the mean [SEM]) in the first measurement week compared to C-section delivered infants (178 μg/mL ±23 SEM) (t = 2.48, p < 0.02). The difference in f-CP did not remain statistically significant in subsequent weeks.
There were no significant relationships between levels of CCGF and sIgA in milk and f-CP levels in the mixed models (data not shown). There was a significant correlation between total milk IP-10 ingested and f-CP at 5 (r = 0.42, p = 0.007) and 6 weeks (r = 0.51, p = 0.005) of age. Days of antibiotics were highly inversely correlated with gestational age (r = −0.59, p < 0.001), but there was no relationship between days on antibiotics and f-CP at any point in time.
Discussion
We found that levels of f-CP increased over time in these VLBW infants and were positively associated with the volumes of enteral feedings, while controlling for infant weight. Rouge et al. also found that enteral feeding was associated with higher levels of f-CP across time in 47 preterm infants, measured every 2 weeks until discharge.8 They further reported that f-CP levels were also related to the stool microbiome. f-CP was correlated with colonization by Clostridium and Staphylococcus and infants of mothers who received antibiotics prenatally. Infants who had received antibiotics in the NICU had lower levels of f-CP. We did not find this relationship with days on antibiotics and f-CP in our sample of VLBW infants, but only six of our infants did not receive antibiotics.
The type of feeding made a significant difference in levels of f-CP over time, with infants receiving only MOM showing an increase in f-CP compared to infants with mixed feedings. f-CP may be important in the development and tolerance of the commensal gut microbiome and response to pathogenic microbes. MOM contains a unique pioneer microbiota, and both MOM and PDM contain many unique oligosaccharides that provide nutritional support for the commensal microbiome.17 It should also be noted that early human breast milk contains many millions of cells, predominantly neutrophils18 and monocytes, as well as chemotactic factors such as IL-8, and hence, increasing levels of f-CP in the infant gut could be in response to milk cytokines and milk microbiota.
Most of the studies on feeding and f-CP have been done with term infants. Savino et al. reported that the median f-CP level was significantly higher in breast-fed versus formula-fed term infants.19 Dorosko et al., in a prospective longitudinal study, compared f-CP levels in healthy 0- to 6-month-old U.S. infants who were exclusively breastfed or given mixed feeding; levels were significantly higher in the exclusively breastfed group.14 Li et al. reported that the median f-CP level was significantly higher in breastfed term infants than in nonbreastfed infants when measured up to 5 months of age. f-CP was negatively correlated with age in both kinds of feeding.15 Other studies of term infants have not found a difference in f-CP by breastfeeding status.5,20 It is likely that the dynamics of f-CP in preterm infants may differ than in term infants.
The few studies that have looked at the feeding type and f-CP in preterm infants report inconsistent results. Yang et al. reported that f-CP levels were not related to gestational age or feeding regimens in VLBW infants.21 In contrast, Zoppelli et al. examined 1,899 fecal samples from 206 VLBW infants and found that f-CP levels were dependent on gestational and postnatal age with particularly low levels in extremely premature infants.13 Zoppelli et al. measured over 28 days and had 66 cases of NEC or other gastrointestinal disease. They also excluded infants who had received antibiotic treatment after postnatal day 8. In addition, the majority of their babies below 32 weeks of gestational age received formula milk. Thus, this population is not really comparable to the data reported here,
We report that f-CP levels rose across time through the sixth week of data collection in exclusively MOM-fed VLBW infants compared to infants who received mixed feedings who showed a decrease. The time course showed that f-CP levels showed the greatest difference between exclusive MOM-fed and mixed-fed infants between 32 and 34 weeks cGA. Notably, this is when many of these mixed-fed infants began to receive proportionately less MOM. It is also around the time that HMF is added to feedings. Our sample may also be unique in the high proportion of mothers who pumped MOM for their infants, either exclusively or predominantly during the first few weeks of life. Our recruitment plan in the parent study was to over-recruit women who intended to pump breast milk. The surprise was that so many of these women committed to providing milk for their infants, many of them throughout the NICU stay, even when supplementation was needed. This may have been partly due to being engaged as a participant in a study of breastfeeding, which may have reinforced their desires and capacity to continue to provide breast milk for their small vulnerable infants.
One possibility is that association of f-CP levels with both volume of feedings and MOM suggests that f-CP levels may be related to gastrointestinal maturation. However, we do not have established biomarkers of gut maturation in the current study that would support this possibility. Neutrophil infiltration to the gut is a normal part of gut development in the VLBW infant and is associated with a regulated inflammatory process as the microbiota becomes established. The levels of f-CP measured in the current study were not exceptionally high and may reflect normal neutrophil and macrophage infiltration as part of maturation of the gut immune axis.
The association of IP-10 with f-CP at 5 and 6 weeks, when f-CP levels are continuing to rise in exclusively MOM-fed VLBW infants, is intriguing. IP-10 is a chemokine that may act to support intestinal immune response by causing migration and activation of T cells in the neonatal gut.22 Neutrophils, which are the major source of f-CP, have been shown to actually produce IP-10 in mouse models after stimulation with TLR9.23 TLR9 binds to pathogen-associated molecular patterns on infectious microbes, which may contribute to recognition of virulent organisms in the neonatal gut.
Conclusion
The current study adds to our understandings of the natural course of f-CP in VLBW infants. Exclusive MOM feeding in VLBW leads to increasing levels of f-CP over time. This may reflect an f-CP normal pattern of f-CP change over time than when PIF or PDM is added. There was an association between the IP-10 levels in MOM and the increases in f-CP observed at 5 and 6 weeks of age in these infants. Additional studies are needed to compare the changing levels of f-CP in premature infants compared with full-term infants, over a longer period of time, to understand the significance of f-CP levels in stool in the developing intestine of premature infants and full-term infants and to determine the relationship between feeding and f-CP levels comparing MOM with mixed feeding for both the premature and the full-term infant.
Acknowledgments
Appreciation to NICU nurses who helped in collection of these data. Funding sources: NIH (1R21NR013094).
Disclosure Statement
No competing financial interests exist.
References
- 1.Johne B, Fagerhol MK, Lyberg T, et al. Functional and clinical aspects of the myelomonocyte protein calprotectin. Mol Pathol 1997;50:113–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dale I, Fagerhol MK, Naesgaard I. Purification and partial characterization of a highly immunogenic human leukocyte protein, the L1 antigen. Eur J Biochem 1983;134:1–6 [DOI] [PubMed] [Google Scholar]
- 3.Kapel N, Campeotto F, Kalach N, et al. Faecal calprotectin in term and preterm neonates. J Pediatr Gastroenterol Nutr 2010;51:542–547 [DOI] [PubMed] [Google Scholar]
- 4.Oord T, Hornung N. Fecal calprotectin in healthy children. Scand J Clin Lab Invest 2014;74:254–258 [DOI] [PubMed] [Google Scholar]
- 5.Campeotto F, Butel MJ, Kalach N, et al. High faecal calprotectin concentrations in newborn infants. Arch Dis Child Fetal Neonatal Ed 2004;89:F353–F355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laforgia N, Baldassarre ME, Pontrelli G, et al. Calprotectin levels in meconium. Acta Paediatr 2003;92:463–466 [DOI] [PubMed] [Google Scholar]
- 7.Josefsson S, Bunn SK, Domellof M. Fecal calprotectin in very low birth weight infants. J Pediatr Gastroenterol Nutr 2007;44:407–413 [DOI] [PubMed] [Google Scholar]
- 8.Rouge C, Butel MJ, Piloquet H, et al. Fecal calprotectin excretion in preterm infants during the neonatal period. PLoS One 2010;5:e11083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aydemir G, Cekmez F, Tanju IA, et al. Increased fecal calprotectin in preterm infants with necrotizing enterocolitis. Clin Lab 2012;58:841–844 [PubMed] [Google Scholar]
- 10.Aydemir O, Aydemir C, Sarikabadayi YU, et al. Fecal calprotectin levels are increased in infants with necrotizing enterocolitis. J Matern Fetal Neonatal Med 2012;25:2237–2241 [DOI] [PubMed] [Google Scholar]
- 11.Albanna EA, Ahmed HS, Awad HA. Stool calprotectin in necrotizing enterocolitis. J Clin Neonatol 2014;3:16–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campeotto F, Baldassarre M, Butel MJ, et al. Fecal calprotectin: Cutoff values for identifying intestinal distress in preterm infants. J Pediatr Gastroenterol Nutr 2009;48:507–510 [DOI] [PubMed] [Google Scholar]
- 13.Zoppelli L, Guttel C, Bittrich HJ, et al. Fecal calprotectin concentrations in premature infants have a lower limit and show postnatal and gestational age dependence. Neonatology 2012;102:68–74 [DOI] [PubMed] [Google Scholar]
- 14.Dorosko SM, Mackenzie T, Connor RI. Fecal calprotectin concentrations are higher in exclusively breastfed infants compared to those who are mixed-fed. Breastfeed Med 2008;3:117–119 [DOI] [PubMed] [Google Scholar]
- 15.Li F, Ma J, Geng S, et al. Comparison of the different kinds of feeding on the level of fecal calprotectin. Early Hum Dev 2014;90:471–475 [DOI] [PubMed] [Google Scholar]
- 16.Gartner LM, Morton J, Lawrence RA, et al. Breastfeeding and the use of human milk. Pediatrics 2005;115:496–506 [DOI] [PubMed] [Google Scholar]
- 17.Jeurink PV, van Bergenhenegouwen J, Jimenez E, et al. Human milk: A source of more life than we imagine. Benef Microbes 2013;4:17–30 [DOI] [PubMed] [Google Scholar]
- 18.Xanthou M. Human milk cells. Acta Paediatr 1997;86:1288–1290 [DOI] [PubMed] [Google Scholar]
- 19.Savino F, Castagno E, Calabrese R, et al. High faecal calprotectin levels in healthy, exclusively breast-fed infants. Neonatology 2010;97:299–304 [DOI] [PubMed] [Google Scholar]
- 20.Oswari H, Prayitno L, Dwipoerwantoro PG, et al. Comparison of stool microbiota compositions, stool alpha1-antitrypsin and calprotectin concentrations, and diarrhoeal morbidity of Indonesian infants fed breast milk or probiotic/prebiotic-supplemented formula. J Paediatr Child Health 2013;49:1032–1039 [DOI] [PubMed] [Google Scholar]
- 21.Yang Q, Smith PB, Goldberg RN, et al. Dynamic change of fecal calprotectin in very low birth weight infants during the first month of life. Neonatology 2008;94:267–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takahata Y, Takada H, Nomura A, et al. Detection of interferon-gamma-inducible chemokines in human milk. Acta Paediatr 2003;92:659–665 [DOI] [PubMed] [Google Scholar]
- 23.Ericson JA, Duffau P, Yasuda K, et al. Gene expression during the generation and activation of mouse neutrophils: Implication of novel functional and regulatory pathways. PLoS One 2014;9:e108553. [DOI] [PMC free article] [PubMed] [Google Scholar]