Abstract
The objective of this study was to investigate the potential causes of high body temperature (Tb) during lactation in mice as a putative limit on energy intake. In particular we explored whether or not offspring contributed to heat retention in mothers while suckling. Tb and physical activity were monitored in 26 female MF1 mice using intraperitoneally implanted transmitters. In addition, maternal behaviour was scored each minute for 8 h d−1 throughout lactation. Mothers that raised larger litters tended to have higher Tb while nursing inside nests (P < 0.05), suggesting that nursing offspring may have influenced heat retention. However, Tb during nursing was not higher than that recorded during other behaviours. In addition, the highest Tb during the observation period was not measured during nursing behaviour. Finally, there was no indication that mothers discontinued suckling because of a progressive rise in their Tb while suckling. Tb throughout lactation was correlated with daily increases in energy intake. Chronic hyperthermia during lactation was not caused by increased heat retention due to surrounding offspring. Other factors, like metabolic heat produced as a by-product of milk production or energy intake may be more important factors. Heat dissipation limits are probably not a phenomenon restricted to lactation.
Food intake at peak lactation in small mammals reaches an asymptote and appears to be limited1. The level of the asymptotic intake is strongly dependent on ambient temperature, with lower ambient temperatures leading to greater food intakes1,2,3,4,5. On the basis of these observations it has been suggested that hyperthermia risk is a potential factor limiting energy intake in lactating mice (the heat dissipation limit theory)5,6,7,8,9,10. Consistent with this hypothesis shaved female MF1 mice increased their food intake and raised bigger pups at weaning11. However, Swiss mice that were shaved during lactation ate more food, but did not significantly increase their milk production or raise heavier pups12,13, and a similar absence of any impact of shaving was observed in hamsters14. Nevertheless, data from larger domesticated animals strongly support the view that hyperthermia risk is a key factor limiting lactation performance15,16. Moreover, direct observations suggest that lactating MF1 mice are chronically hyperthermic compared to non-reproductive and pregnant individuals17,18 and this is consistent with more sporadic body temperature (Tb) measurements reported in several other small rodents during lactation, such as Mongolian gerbils (Meriones unguiculatus)19, Dwarf hamsters (Phodopus campbelli and P. sungorus)20 and Sprague-Dawley rats (Rattus norvegicus)21. These data suggest hyperthermia risk may be a broadly applicable limitation on lactation performance.
High Tb in lactating mice might be a direct consequence of an increase in food consumption, due to the thermic effect of food (also called the heat increment of feeding or specific dynamic action- which reflects the increase in heat production following food ingestion), as well as heat generated during milk synthesis. An alternative hypothesis for the hyperthermia of lactation is that lactating animals face problems dissipating heat because of the surrounding pups when they are nursing, and also the fact the pups need to be sheltered in a nest. Both the pups and the nest adjacent to the nursing mother may affect her ability to dissipate heat. Supporting this viewpoint, in lactating Norway rats (Rattus norvegicus), increases in core Tb and ventrum temperature occurred acutely during nursing bouts either under a warm room temperature at 26 °C or with warmed (39 °C) pups22. An increase in maternal Tb caused rats to terminate nursing bouts22,23. Warming pups resulted in many short nursing bouts in lactating rats (Rattus norvegicus) with unchanged total contact time22. Moreover the time spent nursing increased at lower ambient temperatures. On the other hand, other work suggested that rats did not terminate nursing bouts in response to an increase in core Tb24. In addition during approximately 60% of nursing bouts recorded in lactating rats (Rattus norvegicus), higher Tb were observed during nursing bouts, rather than at the end of the bouts25. Therefore, whether or not surrounding pups have a direct effect on maternal Tb, thereby influencing nursing behaviour remains uncertain. Understanding whether the hyperthermia of lactation is caused mostly by increased heat production or reduced capacity to dissipate heat is important because if it was the former it might point to heat dissipation being a more generally significant factor limiting maximum sustained metabolic rate, as opposed to a lactation specific effect8,10.
The main objective of this work was to evaluate the impact of suckling behaviour by pups on the Tb of their mothers. We made several a priori predictions concerning the patterns of variation in maternal Tb during lactation if it was caused by reduced capacity to dissipate heat due to being surrounded by suckling pups. First, it was predicted that Tb would increase during nursing bouts and the highest Tb of the mothers would be observed at the termination of nursing bouts (Prediction 1). Second, if nursing behaviour was the most important factor causing the increase in maternal Tb in lactation, the highest Tb over a day would be when mothers were nursing, and Tb when nursing would be higher than during other behaviours known to generate heat such as physical activity (Prediction 2). Third, maternal Tb during nursing would vary systematically with litter size, with larger litters causing the mothers to heat up more. Likewise, heavier pups later in lactation might result in more maternal hyperthermia than smaller pups earlier in lactation because the body surface in contact with pups would be greater and these pups would be hotter because they are themselves thermo-competent later in lactation (Prediction 3).
Results
Prediction 1a: Tb will increase towards the end of nursing bouts?
Tb during four nursing bouts selected randomly from a single female mouse show the diversity of patterns of maternal Tb over time when females were nursing (Supplementary Fig. 1). Mean Tb over the last 20 minutes of all recorded nursing bouts that lasted >20 minutes, across all individuals, were compiled in four different periods of lactation (Early: DOL 2–5, Mid: DOL 6–10, Peak: DOL 11–15 and Late: DOL 16–20) (Fig. 1). Nursing Tb varied significantly between the four stages of lactation, but no significant differences were found with suckling time, and there was no stage by suckling time interaction (two way RM ANOVA with ID included as random factor; time, F19,1660 = 1.00, P = 0.46; stage of lactation, F3,1660 = 35.45, P < 0.001, interaction of time and stage, F57,1660 = 0.09, P = 1.00).
Figure 1. Body temperature during the last 20 minutes of nursing bouts.
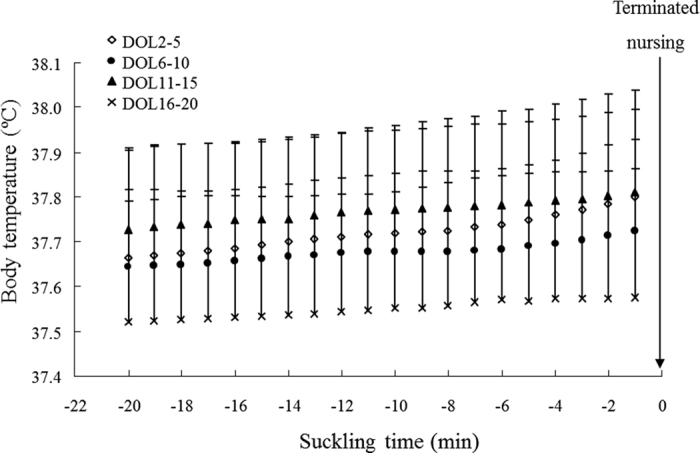
The data were expressed as means + S.D. for four different stages of lactation. Day of lactation (DOL) 2–5 (open-diamond), DOL 6–10 (closed-circle), DOL 11–15 (closed-triangle) and DOL 16–20 (cross). Sample sizes (n) were 18 female mice in early lactation (DOL 2–10) and 26 female mice in late lactation (DOL 11–20) although the means were obtained from 334, 420, 542 and 537 nursing bouts in DOL 2–5, DOL 6–10, DOL 11–15 and DOL 16–20, respectively.
Comparing the different stages of lactation, nursing Tb over the last 20 minutes of suckling was significantly lower in late lactation than that in the other periods (Tukey pairwise comparisons, P < 0.01). In addition, nursing Tb was higher in early lactation and peak lactation compared to mid-lactation (Tukey pairwise comparisons, P < 0.05).
Prediction 1b: Tb at the end of nursing bouts should be higher with longer nursing duration?
The duration (minutes) of all nursing bouts which lasted more than 20 minutes were averaged for each mouse across all nursing bouts on a given day. At the same time, mean Tb at termination of each nursing bout (i.e., Tb in the final minute, from now on called ‘final Tb’) was also collected daily. Final Tb varied significantly with lactating day (Fig. 2, closed symbols, one way ANOVA, F18,1814 = 14.54, P < 0.001). Mean final Tb significantly declined on DOL 19 and 20 compared to the other days (Tukey pairwise comparison, P < 0.001). In addition, mean duration of nursing bouts changed with DOL (Fig. 2, open symbols, one way ANOVA, F18,1814 = 3.90, P < 0.001).Among days of lactation, mean duration of nursing bouts was longer on DOL 2 than that in late lactation between DOL 12 and 19 (Tukey pairwise comparison, P < 0.001). Mean nursing duration on DOL 3 was also longer than DOL 13, 14, 16, 17 and 18 (Tukey pairwise comparison, P < 0.001).
Figure 2. Daily means of final body temperature (Tb) at the end of nursing bouts and duration of nursing bouts.
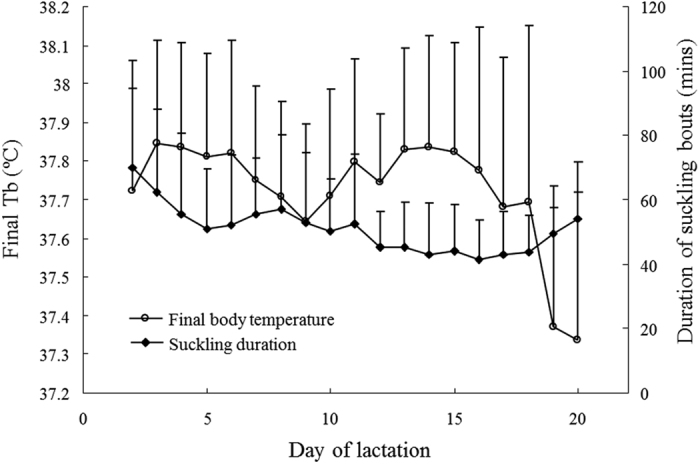
The data are expressed as mean + S.D. (n = 26). Open-circle and closed-diamond symbols represent final Tb and nursing duration, respectively.
Relationships between nursing duration and final Tb are summarised foreach individual mouse (Table 1). Six of the 26 females showed no relationship between nursing duration and final Tb (P > 0.05), however, final Tb significantly decreased with longer duration of nursing bouts for the remaining females (P < 0.05).The same negative trend was found in relationships between mean nursing duration and mean final Tb when examined for the four different stages of lactation (See Fig. 3A for peak lactation). Final Tb was significantly, negatively related with nursing duration during early (y = −0.0074x + 38.229, R2 = 0.326, F1,16 = 7.72, P = 0.013), peak (Fig. 3A: y = −0.0091x + 38.215, R2 = 0.302, F1,24 = 10.38, P = 0.004) and late lactation (y = −0.0136x + 38.19, R2 = 0.218, F1,23 = 6.39, P = 0.019), but this relationship did not reach statistical significance during mid lactation, although a similar trend was visible ( y = −0.0055x + 38.012, R2 = 0.162, F1,16 = 3.09, P = 0.098).
Table 1. Relationships between duration of nursing bouts and final Tb at the end of nursing bouts.
| Mouse ID | Litter size | n | Slope | Intercept | R2 | d.f. | F | |
|---|---|---|---|---|---|---|---|---|
| 1 | 10 | 16 | −0.0135 | 38.492 | 0.156 | 1,14 | 2.596 | |
| 2 | 10 | 32 | −0.0140 | 38.055 | 0.263 | 1,30 | 10.69 | * |
| 3 | 12 | 46 | −0.0085 | 38.21 | 0.13 | 1,44 | 6.583 | |
| 4 | 11 | 23 | −0.0197 | 38.901 | 0.221 | 1,21 | 5.959 | |
| 5 | 9 | 33 | −0.0119 | 38.203 | 0.143 | 1,31 | 5.188 | |
| 6 | 9 | 46 | −0.0110 | 38.18 | 0.206 | 1,44 | 11.43 | * |
| 7 | 14 | 25 | −0.0037 | 37.871 | 0.045 | 1,23 | 1.072 | |
| 8 | 11 | 29 | −0.0137 | 38.212 | 0.25 | 1,27 | 8.978 | |
| 9 | 10 | 88 | −0.0096 | 37.969 | 0.194 | 1,86 | 20.70 | * |
| 10 | 8 | 126 | −0.0053 | 37.708 | 0.042 | 1,124 | 5.455 | |
| 11 | 9 | 97 | −0.0090 | 38.072 | 0.207 | 1,95 | 24.75 | * |
| 12 | 6 | 100 | −0.0068 | 38.033 | 0.189 | 1,98 | 22.82 | * |
| 13 | 8 | 78 | −0.0167 | 38.397 | 0.112 | 1,76 | 9.619 | |
| 14 | 10 | 65 | −0.010 | 38.209 | 0.189 | 1,63 | 14.68 | * |
| 15 | 12 | 96 | −0.016 | 38.53 | 0.24 | 1,94 | 29.7 | * |
| 16 | 5 | 82 | −0.0025 | 37.47 | 0.05 | 1,80 | 4.193 | |
| 17 | 9 | 88 | −0.0039 | 37.89 | 0.054 | 1,86 | 4.901 | |
| 18 | 3 | 89 | −0.0019 | 37.485 | 0.04 | 1,87 | 3.638 | |
| 19 | 8 | 72 | −0.001 | 37.697 | 0.012 | 1,70 | 0.821 | |
| 20 | 7 | 97 | −0.004 | 38.157 | 0.111 | 1,95 | 11.84 | * |
| 21 | 10 | 103 | −0.0051 | 37.979 | 0.065 | 1,101 | 7.042 | |
| 22 | 14 | 79 | −0.0034 | 38.111 | 0.091 | 1,77 | 7.737 | |
| 23 | 8 | 91 | −0.0052 | 37.926 | 0.157 | 1,89 | 16.59 | * |
| 24 | 7 | 76 | −0.0062 | 38.102 | 0.132 | 1,74 | 11.27 | * |
| 25 | 7 | 81 | −0.0021 | 37.681 | 0.023 | 1,79 | 1.885 | |
| 26 | 9 | 75 | −0.0016 | 37.993 | 0.015 | 1,73 | 1.101 |
Using linear regression, slopes, intercepts and R-squared of the relationship between duration of nursing bouts (x-axis) and final Tb at the end of nursing bouts (y-axis) were determined on individual mice. Litter size shows the number of offspring at weaning. Sample size (n) shows the number of nursing bouts recorded during lactation. Degrees of freedom (d.f.), F values for each individual are given in the table.
*Indicates significance at the p < 0.05 level using the Holm-Bonferroni sequential adjustment for multiple testing.
Figure 3.
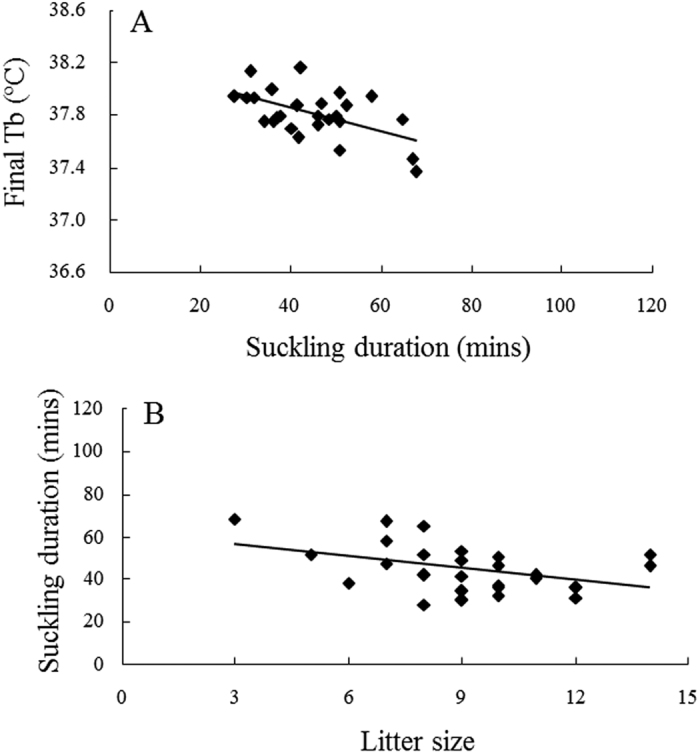
The relationships between nursing duration and final body temperature (A), litter size (B) and litter mass (C) at peak lactation (DOL 11–15, n = 26). Regressions are described by A: y = −0.0091x + 38.215, B: y = −0.0091x + 38.215 and C: y = −0.3793x + 71.314.
In addition to stage of lactation and nursing duration, the effects of other factors such as individuals, litter size and litter mass on final Tb was analysed by GLM. The analysis indicated that all factors had significant influences on final Tb in nursing bouts (nursing duration: F1,1831 = 207.86, P < 0.001; individual: F25,1807 = 16.59, P < 0.001; litter size: F1,1831 = 139.83, P < 0.001; litter mass: F1,1831 = 5.77, P = 0.016).
Furthermore, influences of these factors on nursing duration were investigated using GLM analysis. Nursing duration was significantly related to all the parameters: individuals (F25,1807 = 14.95, P < 0.001), litter size (F1,1831 = 95.24, P < 0.001) and litter mass (F1,1831 = 100.13, P = 0.001). Relationships between nursing duration and litter size or litter mass were explored during the four stages of lactation (Fig. 3B,C respectively show the relationships at peak lactation). Nursing duration was significantly longer in mothers with smaller litters at all four stages of lactation (Early: y = −3.8651x + 92.239, R2 = 0.347, F1,16 = 8.51, P = 0.010; Mid: y = −3.1911x + 79.674, R2 = 0.321, F1,16 = 7.56, P = 0.014; Peak, Fig. 3B: y = −1.8323x + 61.627, R2 = 0.171, F1,24 = 4.96, P = 0.036; Late: y = −1.9357x + 62.526, R2 = 0.259, F1,23 = 8.05, P = 0.009).
Nursing duration was also negatively related to litter mass at all stages of lactation (Early: y = −1.96x + 107.3, R2 = 0.511, F1,16 = 16.7, P < 0.001; Mid: y = −0.8172x + 89.805, R2 = 0.382, F1,16 = 9.75, P = 0.007; Peak, Fig. 3C: y = −0.3793x + 71.314, R2 = 0.252, F1,24 = 8.09, P = 0.009) except late lactation where it marginally failed to reach significance (Late: y = −0.1896x + 61.208, R2 = 0.138, F1,23 = 3.69, P = 0.067). Nursing duration was shorter for mothers with bigger or heavier litters.
Prediction 1c: Mean Tb while nursing inside nest should be higher when total nursing time is longer?
The relationship between total nursing time during the 8-hour observation and mean Tb during nursing inside the nest was examined between individual mice across the whole lactation and at peak lactation (Fig. 4). It was predicted that the more time mice spent nursing inside the nest during behavioural observations, the hotter nursing Tb they would exhibit. However, there was no significant relationship between mean nursing Tb and total time during nursing inside the nest when considering both the entire lactation (Fig. 4A: y = −0.001x + 37.973, R2 = 0.122, F1,24 = 3.42, P = 0.077) or the peak lactation (Fig. 4B: y = −0.0006x + 37.946, R2 = 0.060, F1,24 = 1.54, P = 0.227).
Figure 4. Relationship between mean nursing Tb and total time spent nursing inside the nest (n = 26).
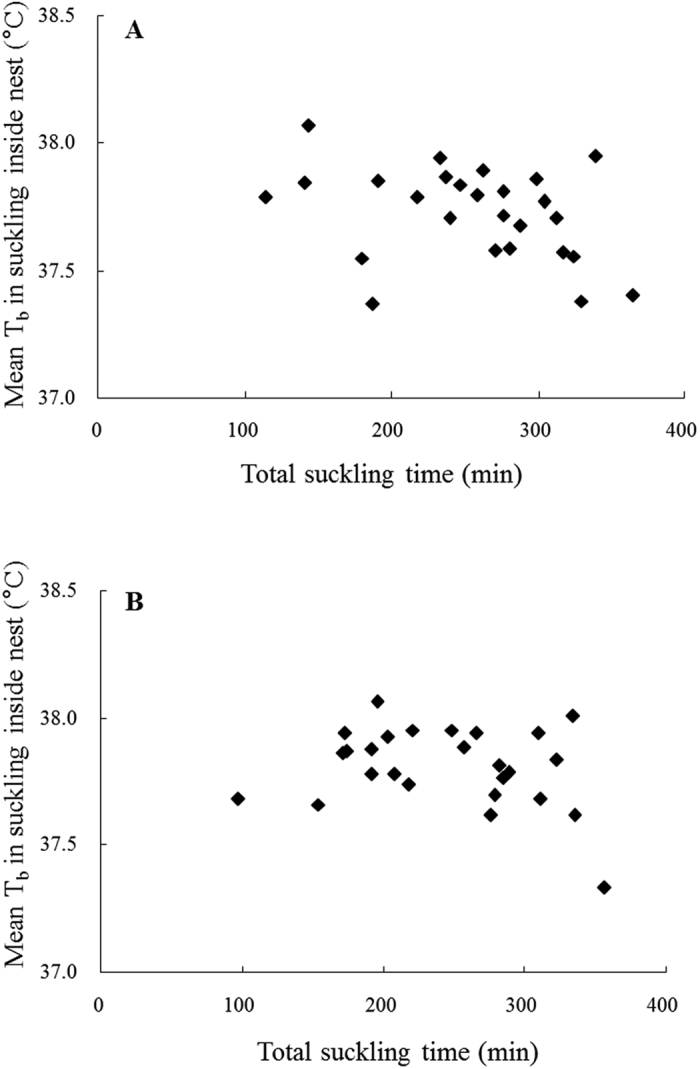
(A) represents means over the whole lactation (DOL 2–20), and (B) represents means over the peak lactation (DOL 11–15). The regressions are described by y = −0.001x + 37.973 (A) and y = −0.0006x + 37.946 (B) and were not significant p > 0.05.
Prediction 2a: Nursing inside nest should generate the hottest Tb?
Mean time spent on different behaviours was calculated on a daily basis across the 26 females. Of the major behaviours in lactating mice, nursing inside nest was a dominant activity during observation hours in daytime (Fig. 5A). Mean time spent on nursing inside nest varied with DOL (one way ANOVA, F18,384 = 4.94, P < 0.001) (Fig. 5A, closed diamonds). Time spent on nursing inside the nest declined significantly from 328.8 ± 53.1 minutes (71.2 ± 10.1% of the 8-hour observation) on DOL 2 to 220.8 ± 91.7 minutes (50.7 ± 19.3%) on DOL 16 (Tukey pairwise comparisons, P < 0.01). In terms of time, eating behaviour was the second most frequent behaviour (Fig. 5A, closed triangles). During the observation hours, eating time also varied with DOL (one way ANOVA, F18,384 = 3.94, P < 0.001) (Fig. 5A, closed triangles). However, its percentage ranged between 10.5 ± 5.5% on DOL 20 and 21.9 ± 7.8% on DOL 9 which was notably smaller than the time spent on nursing inside the nest. In contrast, suckling mice started leaving their nest from DOL 9 and significantly increased time spent on nursing outside the nest as lactation progressed (one way ANOVA, F18,384 = 6.18, P < 0.001, Fig. 5A, open diamonds). Time spent on nursing outside nest was 13.7 ± 30.1 minutes (2.9 ± 6.3%) on DOL 9 and increased to 68.7 ± 90.3 minutes (17.3 ± 23.1%) on DOL 15 and 66.0 ± 73.9 minutes (15.30 ± 17.68%) on DOL 16 (Tukey pairwise comparisons, P < 0.05).
Figure 5. Time spent on various behaviours and their relationship to body temperature.
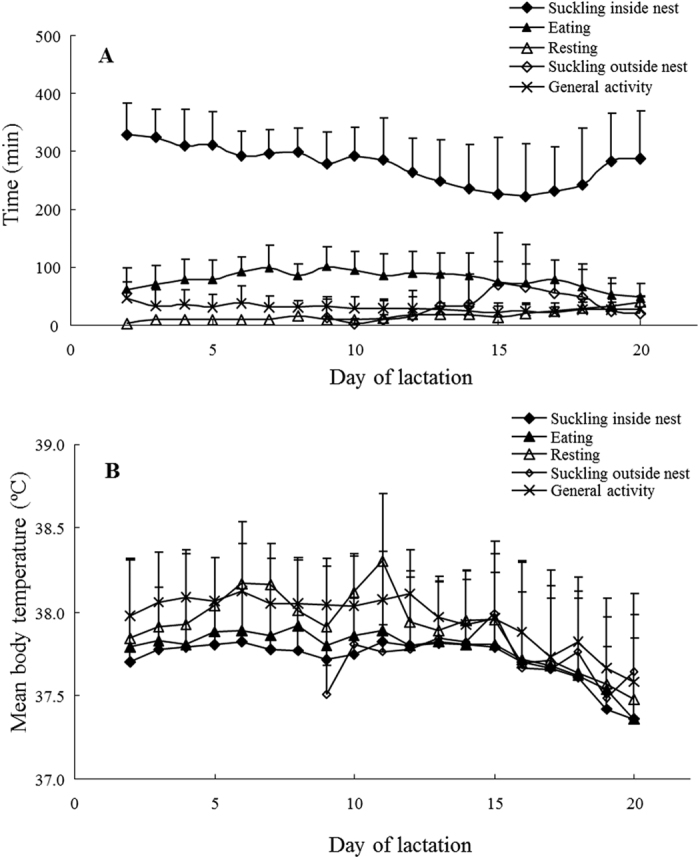
(A) shows the total time spent on different behaviours during the behavioural observation in lactation and (B) shows the daily body temperatures associated with different behaviours. Behaviours are categorised as nursing inside nest (closed-diamond), eating (closed-triangle), resting (open-triangle), nursing outside nest (closed-diamond) and general activity (cross). All data are shown as means + S.D. Sample sizes (n) vary from 18 to 26 mice depending on days of lactation (refer to the details in Supplementary Table 1).
In addition, the total percent nursing time (time spent nursing inside + time spent nursing outside the nest) increased to 69.0 ± 10.8% on DOL 15 and 66.0 ± 9.5% on DOL 16. The total nursing time did not vary significantly with DOL (one way ANOVA F18,384 = 1.55, P = 0.07). Therefore, the proportion of the nursing time to feed offspring was constant throughout lactation, although nest attendance was significantly reduced in late lactation.
Time spent on resting gradually elevated with advanced DOL (one way ANOVA, F18,384 = 3.27, P < 0.001) (Fig. 5A, open triangles) and maximised at 39.6 ± 39.3 minutes (8.75 ± 8.78%) on DOL 20.
Tb while nursing inside the nest significantly declined as lactation advanced (one way ANOVA, F18,383 = 5.86, P < 0.001) (Fig. 5B, closed diamonds). Significant differences in daily changes in nursing Tb were found only between DOL 2 and DOL 19 or 20 (Tukey pairwise comparisons, P < 0.05). Tb varied significantly with behavioural type (nursing inside nest, eating, resting, nursing outside nest and general activity) and DOL (Fig. 5B, two way ANOVA; behavioural type, F4,1481 = 30.82, P < 0.001; DOL, F18,1481 = 16.51, P < 0.001; interaction, F65,1481 = 0.79, P = 0.88). Tb when nursing inside nest was lower than that when resting and during general activity (Tukey pairwise comparisons, P < 0.01). In addition, resting Tb in lactation gave as high a Tb as the other behaviours (Tukey pairwise comparisons, P > 0.05).
Prediction 2b: The highest Tb should be found during nursing bouts?
The highest Tb measured in the observation period was compared to the highest Tb recorded when nursing inside nest through DOL 2–20 (Fig. 6). The highest Tb measured was 38.98 ± 0.43 °C on DOL 14 compared to a maximal Tb of 38.48 ± 0.26 °C during nursing bouts on DOL 12. The difference between the maximal temperatures measured during the whole observation period or nursing bouts only increased as DOL advanced (DOL, F18,764 = 3.66, P < 0.001) although there were no differences between the two highest values in the whole observation and in nursing bouts in early lactation (DOL 2–7) (Tukey pairwise comparisons, P > 0.05). From DOL 8 to DOL 20 except DOL 9, the highest temperatures in the observation hours were significantly higher than those in nursing bouts (Tukey pairwise comparisons, P < 0.05).
Figure 6. Comparison of highest Tb found in nursing bouts and entire observation hours.
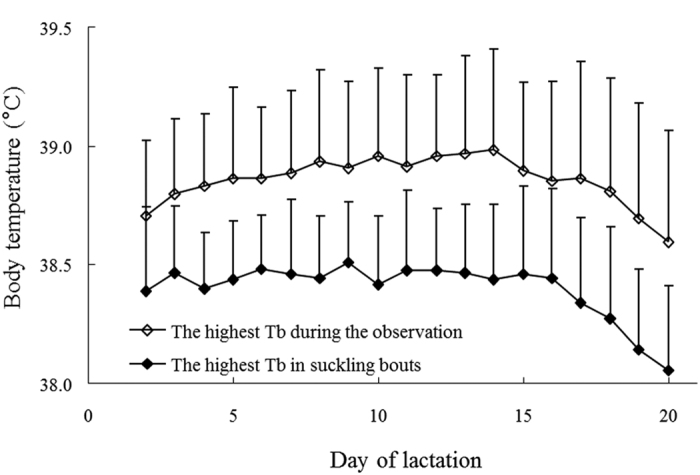
All data are shown as means + S.D. Sample sizes (n) vary from 21 to 26 depending on days of lactation (refer to the details in Supplementary Table 1). Open-diamond represents the highest body temperature observed during the 8-hour behavioural observation between 10.00 h and 18.00 h. Closed-diamond represents the highest body temperature observed in nursing bouts between 10.00 h and 18.00 h.
Prediction 3: Litter size and litter mass will be positively related to nursing Tb.
Tb while nursing inside the nest of individual mice was averaged over the whole lactation or peak lactation. Nursing Tb over the whole of lactation varied from 37.37 °C to 38.07 °C and was significantly, positively related to litter size (Fig. 7A: y = 0.0344x + 37.403, R2 = 0.225, F1,24 = 6.988, P = 0.014). At peak lactation, nursing Tb ranged between 37.33 °C and 38.06 °C and was also significantly related to litter size (Fig. 7B: y = 0.0335x + 37.502, R2 = 0.296, F1,24 = 10.09, P = 0.004).
Figure 7. The relation between litter size and maternal body temperature during nursing inside nest.
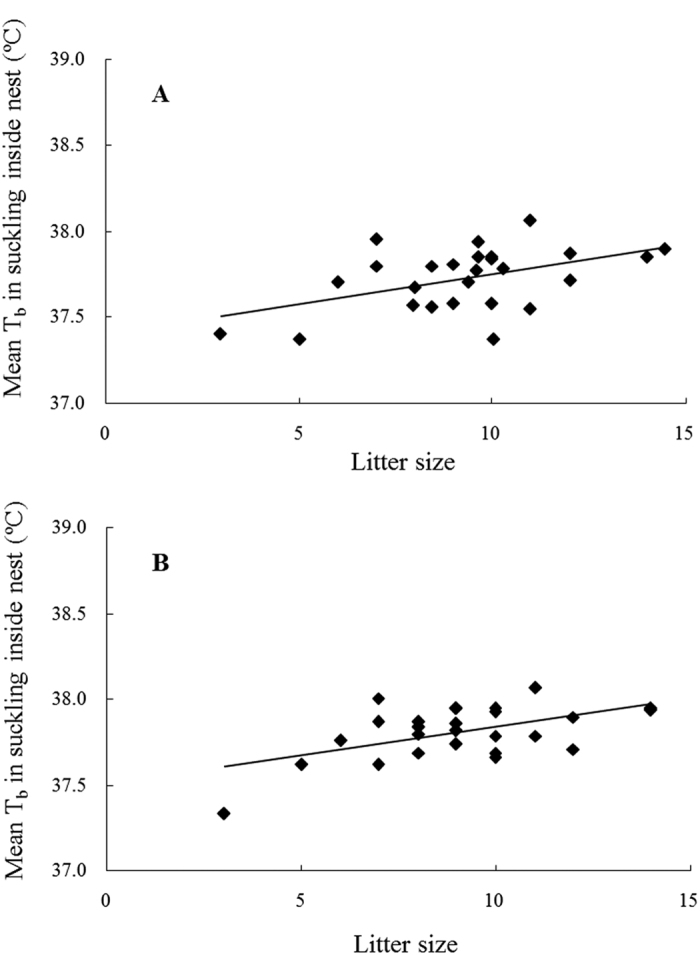
(A) represents means over the whole lactation (DOL 2–20) (y = 0.0344x + 37.403, R2 = 0.225, F1,24 = 6.988, P = 0.014), and (B) represents means over the period of peak lactation (DOL 11–15) (y = 0.0335x + 37.502, R2 = 0.296, F1,24 = 10.09, P = 0.004).
Litter mass varied from 21.73 g to 75.53 g on average over the whole lactation and from 26.61 g to 75.07 g at peak lactation. The positive relationship between nursing Tb and litter mass marginally failed to reach significance when taken as a mean over the whole period of lactation (Fig. 8A: y = 0.0056x + 37.39, R2 = 0.1486, F1,24 = 4.166, P = 0.052), but there was a significant positive relationship between nursing Tb and litter mass at peak lactation (Fig. 8B: y = 0.0064x + 37.366, R2 = 0.347, F1,24 = 12.66, P = 0.002).
Figure 8. The effect of litter mass on maternal body temperature during nursing inside nest.
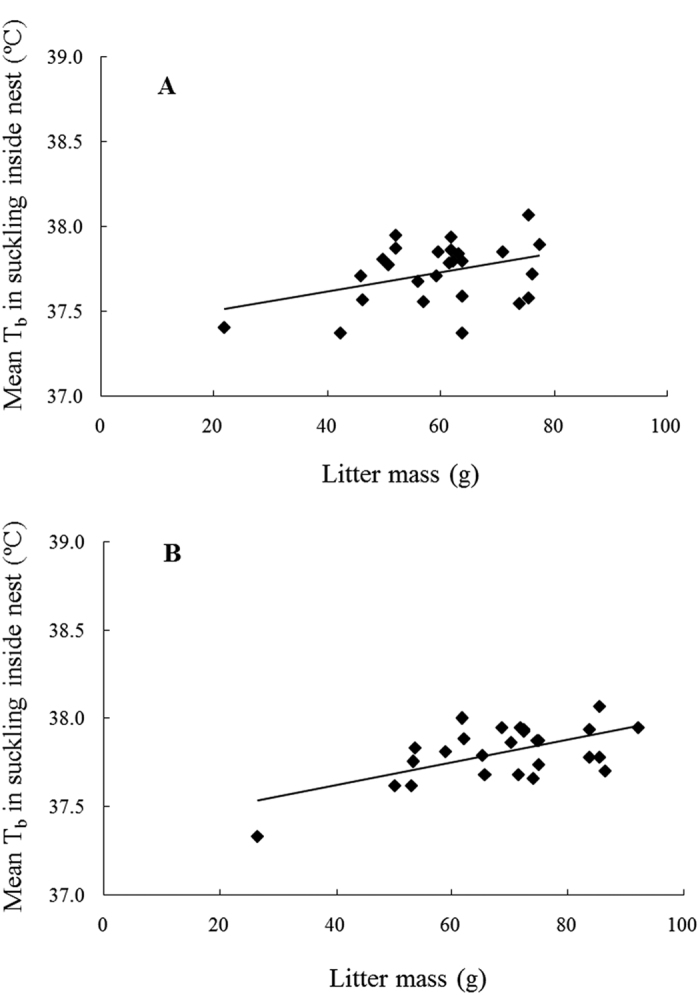
(A) represents means over the whole lactation (DOL 2–20) (y = 0.0056x + 37.39, R2 = 0.1486, F1,24 = 4.166, P = 0.052), and (B) represents means over the peak lactation (DOL 11–15) (y = 0.0064x + 37.366, R2 = 0.347, F1,24 = 12.66, P = 0.002). The sample sizes (n) are 26 mice in the both figures. When the smallest litter size (=3) was removed (n = 25 mice), the regressions changed to A: y = 0.0049x + 37.465, R2 = 0.065, F1,23 = 1.603, P = 0.218 and B: y = 0.0036x + 37.572, R2 = 0.065, F1,23 = 2.939, P = 0.1.
Do eating behaviour and food intake affect elevation in Tb?
Nursing Tb was influenced by litter size and litter mass. However, nursing Tb did not differ from mean Tb related to other behaviours. There might be more important parameters associated with thermoregulation during lactation. First, the effect of energy intake on Tb was investigated as heat is generated through the process of food digestion. Figure 9A shows the daily changes in mean energy intake and mean Tb during lactation across the 26 individuals. Maternal energy intake and mean Tb varied in parallel from DOL 2 until DOL 17. Energy intake increased from DOL 18 onwards due to additional food consumption by growing young. Fluctuations in mean Tb was thus compared with the increase in mean energy intake from DOL 2 until DOL 17 (Fig. 9B). Up to DOL 17, both energy intake and mean Tb were maximised on DOL 14. Mean energy intake varied from 183.01 ± 37.91 kJ day−1 on DOL 2 to 280.55 ± 62.08 kJ day−1 on DOL 14. Mean Tb increased from 37.92 ± 0.21 °C on DOL 2 to 38.12 ± 0.12 °C on DOL 14. Mean Tb was significantly related to mean energy intake during this period of lactation (DOL 2–17) (Fig. 9B: y = 0.0254x + 37.574, R2 = 0.79, F1,14 = 53.74, P < 0.001).
Figure 9. The relationship between mean energy intake and mean Tb.
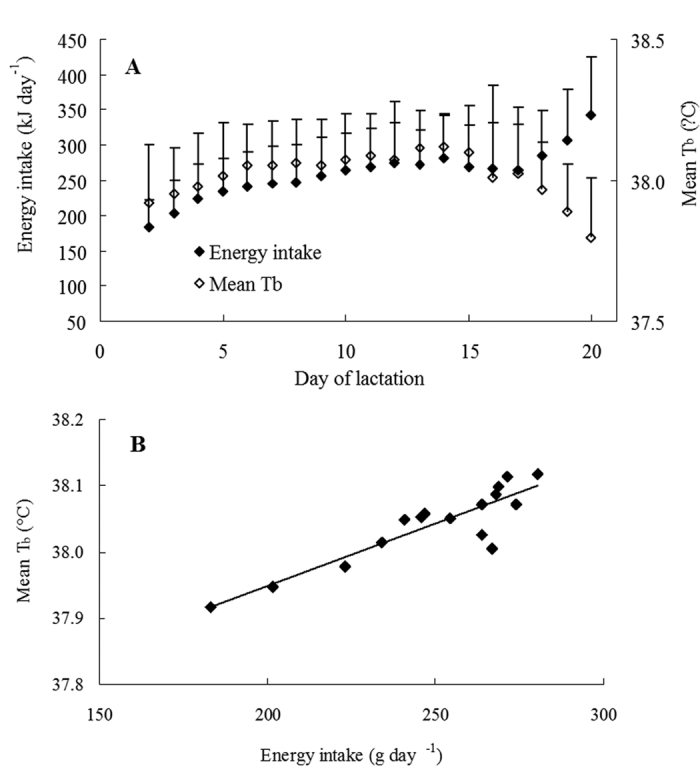
(A) shows daily changes of energy intake and mean Tb averaged across 26 females during lactation. (B) shows the relationship between mean energy intake and mean Tb between DOL 2 and DOL 17 (n = 16 days). The regression is described by y = 0.0254x + 37.574.
Mean Tb when eating might be related to feeding time and to test this total time spent on eating and mean Tb when eating at peak lactation (DOL 11–15) were compared. Lactating mice spent from 33.0 minutes (6.9% of the 8-hour observation) to 166.8 minutes (34.8%) on feeding during the observation hours at peak lactation (n = 26). Mean Tb when eating ranged between 37.51 °C and 38.30 °C (n = 26). There was no significant relationship between eating time and Tb at peak lactation (y = 0.0018x + 37.657, R2 = 0.0998 F1,24 = 1.17, P = 0.29).
Discussion
The heat dissipation hypothesis predicts that the risk of hypothermia during lactation may limit performance. Lactating rodents show a disruption of their Tb rhythms. This is mainly seen as a decrease in the amplitude of the rhythm that is primarily caused by an increase in light phase Tb to levels similar to normal dark phase Tb (Mus musculus18, Rattus norvegicus22, Phodopus sungorus and Phodopus campbelli26). During the light phase, female mice spend most of their time in their nest with their offspring20. Here we tested the hypothesis that the observed hyperthermia is caused by suckling offspring surrounding the female thus limiting her capacity to dissipate heat. If confirmed this would indicate that heat dissipation limits are likely a phenomenon specific to lactation.
We tested three predictions based on the hypothesis. First, if suckling offspring limit the capacity of the female to dissipate heat, we predicted Tb would increase with the duration of nursing bouts and that Tb would reach its maximum at the end of the nursing bout. Contrary to this expectation, nursing Tb did not increase within nursing bouts and final Tb was not positively, but negatively related to nursing bout duration. These results are in agreement with some observations made previously in rats (Rattus norvegicus) where in approximately 60% of nursing bouts, higher Tb was recorded during the bouts rather than at the end of the bouts25, and contradict the idea that females prolong their bouts until they are at near fatal hyperthermia. An association between Tb and nest attendance has been shown in Dwarf hamsters, where females showed increasing Tb while in the nest and a decrease in Tb upon leaving the nest20. Also, in female rats increased duration of suckling bouts were found when females were exposed to cold vs. warmed pups or when mothers were placed in a room with a cold vs. warm ambient temperature22. These observations contradict our results. The differences between our study and these previous studies could reflect real biological differences between the species under investigation. For example, rats are much larger than mice and hence have a lower surface to volume ratio that may make heat dissipation more difficult. Furthermore dwarf hamsters may have better fur insulation than mice also preventing efficient dissipation of heat. On the other hand the differences may reflect methodological differences between studies. In both previous studies transmitters were used to determine the location of the females inside or outside of a nest box and no direct observation of suckling behaviour were made and neither study tested whether there was a consistent rise in Tb over nursing bouts. Our study clearly showed that the highest Tb were not observed while the females were nurturing their offspring inside the nest and that Tb did not increase throughout the nursing bouts as would be expected if suckling offspring were causing female hyperthermia.
During the last few days of lactation, decreases in maternal Tb when nursing inside nest as well as final Tb at the end of nursing bouts were observed, which is consistent with a decrease in mean Tb at the end of lactation18. Dwarf hamsters (P. sungorus and P. campbelli) also declined Tb during the light phase at the later stages of lactation20. At this stage of lactation, the number of offspring surrounding the mothers reduced as they became more mobile and able to obtain solid food by themselves. This might be the reason why Tb in nursing bouts was significantly lower in the last stages of lactation compared to earlier stages of lactation since litter size was significantly related to maternal Tb during nursing inside nest.
The second prediction tested was that maximum Tb over a day should occur during nursing, and Tb should be higher when nursing than during other behaviours known to generate heat. In the current experiment, the maximal Tb measured during the 8 h observation period did not occur while females were nursing, but during other behaviours. On average over the course of lactation the highest Tb were measured while females engaged in physical activity. These results again contradict the idea that females prolong their nursing bouts until they reach near fatal hyperthermia. Furthermore, maximal nursing Tb measured in the present study was lower than the rectal temperature of mice exposed to 34 °C for an hour in the study by Harikai et al.27 although the measuring sites of Tb were different between the two studies. Since rectal temperature is lower than Tb measured in gut28, Tb increased more under the hot condition than in lactation in mice. In the report by Harikai et al.27, mice reduced food consumption, however, lactating mice in this experiment combined hyperthermia with high levels of energy intake. During heat exposure reducing food intake may be a viable option but lactating mice could only do this if they sacrificed milk production which may explain their different responses.
Also, the thermal set point might be elevated in lactating mice since heat production of lactating and non-lactating rats were similar in the cold at 4 °C although only lactating rats could maintain their core Tb29. Similarly, lactating mice in the cold (8 °C) and warm (21 °C) showed no difference in their resting metabolic rate (RMR)4. Mass and activities of brown adipose tissue (BAT) related to non-shivering thermogenesis is decreased in lactating female mice30,31,32.
MF1 mice spent less time on nursing inside the nest on DOL 16 compared to the beginning of lactation, but did not change total contact time (i.e., nursing inside and outside the nest) with their offspring through lactation. In rats, nesting time with offspring was considerably higher on DOL 2 and progressively decreased as lactation advanced22. A similar reduction of mother-young contact time with day of lactation was found in Dwarf hamsters20. In addition, nursing duration was relatively brief when room temperature was increased or mother rats were warmed22. These results imply that peak lactation is the period where the risk of hyperthermia is the greatest for lactating rodents. Although, the fact that maximal Tb did not occur during nursing bouts indicates that offspring huddling around the mother are not the main cause of the hyperthermia at peak lactation.
Lactating mice were hotter when moving around or nest-building than any other behaviour, including nursing, eating and resting. This is consistent with the concept that locomotor activity contributes to Tb change in non-reproductive rodents33,34 and indicates that it may also influence Tb in lactating mice. However, activity levels were considerably reduced throughout lactation18 and locomotor activity is unlikely to cause the prolonged maternal hyperthermia observed in lactating mice.
Another important factor that can cause an increase in Tb is digestion of food. No significant relationship between the time spent eating and Tb was found. The behavioural observations were conducted during the light phase though, while mice predominantly feed at night. When investigating the relationship between total energy intake and mean Tb over the day a significant positive relationship was found. Therefore, the intensive feeding during lactation and associated acute increase in metabolic heat production may lead to elevation of Tb at the peak lactation.
Mothers raising bigger or larger litters were expected to have increased nursing Tb according to our third prediction. Indeed, a positive relationship between litter size or litter mass and maternal Tb while nursing inside the nest was found throughout lactation. This is consistent with the finding that rats raising large litters spent less time on nursing than rats raising small litters22 and a similar positive relationship between litter size and Tb was also found in Dwarf hamsters, but only on lactation day 12 and 1720. These results imply that larger litter size induced a hotter environment for mothers than smaller litter size due to pups limiting the mother’s heat dissipating capacity. The fact that nest attendance time decreased in warmed mother rats compared with unmanipulated mothers also agrees with this interpretation22. If this were the case, one would expect a consistent increase in Tb with nursing duration, which was not observed in the current study. Also, the positive relationship between litter size and litter mass is driven mainly by the smallest litter and when this litter is removed from analysis, no significant relationship remains. An alternative explanation may be that milk stored in the mammary glands was emptied faster from mothers with larger litter sizes resulting in shorter nursing bouts.
Lactating mice show a pronounced increase in energy intake over the course of lactation until a plateau is reached1. This increase in energy intake seemed to parallel changes in Tb and a significant positive relationship between energy intake and Tb was found. Metabolic heat generated from food digestion may thus be an important factor determining female Tb during lactation. In contrast, food deprivation did not affect core Tb during lactation29. Another important factor causing hyperthermia in lactation may be heat generated as a by-product of milk synthesis. Daily growth of litters significantly synchronised with daily changes in maternal Tb18. This might suggest that milk production contributes to heat generation when growth of the litter is taken into account, as milk delivery is positively related to litter mass1,6,35. On the other hand, milk production had no effects on core Tb in mother rats when litter size was adjusted to eight for all litters29. In the current study, litter size widely varied from 3 to 14 at weaning, which might have precipitated the significant relationship between maternal Tb and litter mass.
Conclusion
Nursing behaviour could be a main cause of hyperthermia in lactating mice if nursing offspring significantly enhanced heat retention, and therefore, prevented mothers from dissipating heat. However, our observations of Tb changes while engaging in nursing and other behaviours, showed that Tb did not increase with nursing duration and that the maximum Tb experienced by the mother did not occur during nursing bouts. Nursing offspring and/or nursing behaviour were thus not the crucial factors for maternal hyperthermia, pointing towards a role for heat generation induced by food digestion and/or milk production in causing hyperthermia in lactating mice. Indeed, energy intake and litter mass, which is known to be related to milk energy output, were significantly related to an increase in mean Tb. These data indicate that heat dissipation limits are probably not a phenomenon specific to lactation but more generally applicable across all conditions.
Methods
Behavioural observations
Behavioural observations were conducted using the same female mice (outbred MF1: Mus musculus; Harlan UK Ltd, Bicester, UK) measured in experiments described in Gamo et al.17,18. Mice were implanted with passive transmitters (Vital view) reporting their Tb and physical activity levels at 1 minute intervals. In 2005, individual female mice were observed in the second half of lactation from day 11 to day 20 (date of lactation (DOL) 11 to DOL 20) (n = 8 litters). The observation period was extended to 19 days from DOL 2 until DOL 20 in 2006 (n = 8 litters) and 2007 (n = 10 litters). One transmitter failed midway through lactation and we discontinued observations on this individual after that date. Behaviour monitoring was performed for eight hours a day between 10.00 h and 18.00 h, in the light phase, across all three years. To compare the results from the behaviour observations with the Tb recorded by the VitalView system, animals were visually monitored every minute by direct observation. Behaviours were logged each minute and classified as nursing inside nest, nursing outside nest, eating, grooming, drinking, resting and general activity. A nest was defined as any place where most of pups were located within paper bedding. When the majority of pups were attached to their mothers either inside or outside their nest, maternal behaviour was recorded as nursing. In late lactation, it was occasionally observed that mothers ate food from the hoppers with one or two pups attached. In this case, their behaviour was regarded as eating. Likewise, the effect of a small number of pups (<3) attached to their mothers was ignored when their mothers were pre-dominantly grooming, drinking or moving around. By contrast, resting behaviour was recorded when mothers were sleeping, without any pups attached, either inside or outside their nest.
Food intake was calculated by subtracting the amount of food in the hoppers from that of the previous day. Energy intake was then estimated using estimates of dry mass content and apparent digestibility determined previously in lactating MF1 mice (for a full description of the methods see Gamo et al.17,18).
All procedures concerning animal care and treatment were carried out in accordance with the protocols approved by the ethical committee for the use of experimental animals of the University of Aberdeen, and were licensed by the UK Home Office under PPL 60/3705.
Data collection
Tb and activity counts were correlated with observed behaviours by co-ordinating the recording times. Therefore, for every minute of the 8-hour observation period one of the behavioural categories, with Tb and activity counts was recorded. Uninterrupted nursing bouts lasting longer than 20 minutes were included in the analysis of temperature trends. A total 1,833 nursing bouts were collected across the 26 females over the 19 days of lactation (DOL 2–20). The sample sizes varied depending on day of lactation (Supplementary Table 1).
Total time (minutes) spent nursing was calculated over the whole 8-hour observation period regardless of the nursing bout durations (including bouts <20 min). Mean Tb referring to nursing inside nest was also calculated from all data recorded as nursing inside nest. Likewise, total times (minutes) spent on other behaviours such as eating, resting, nursing outside nest and general activity were calculated. Mean Tb corresponding to each identified behaviour were calculated across all observations. Total time and mean Tb corresponding to all behaviours were averaged daily across 17 female mice during DOL 2–10 and across 25 female mice during DOL 11–20.
Data analysis
All data are expressed as means + standard deviation (S.D.). Prediction 1a: In the analysis only nursing bouts of 20 min and longer were included. Mean Tb was calculated for each nursing bout over the last 20 min of the bout (i.e., suckling time −20 to −1) and compiled for the four stage of lactation (Early: DOL 2–5, Mid: DOL 6–10, Peak: DOL 11–15 and Late: DOL 16–20). Analysis of variance (ANOVA) was used for assessing significant differences in Tb with suckling time or stage of lactation. In this analysis, mother ID was included as a random factor to correct for repeated measures. Furthermore, Tukey post-hoc tests were conducted to compare the different stages of lactation. Prediction 1b: Daily mean duration of nursing bouts was calculated for all nursing bouts lasting 20 min or more (i.e, nursing duration). In addition, daily means for Tb at the termination of these nursing bout (i.e., Tb in the final minute of a nursing bout, from now on called ‘Final Tb’) was calculated. One way ANOVA’s were used to test for differences in duration of nursing bouts or Final Tb with DOL. In these analyses, mother ID was included as a random factor to correct for repeated measures. Linear regressions were used to investigate relationships between nursing duration, final Tb, litter mass and litter size during the different stages of lactation. In addition, generalised linear models (GLM) were run to investigate of several predictors, i.e., DOL, individuals, litter size and litter mass on final Tb and nursing duration. Prediction 1c: Daily means of total duration of nursing during the 8 h observation period and mean Tb while nursing inside the nest were calculated for each individual mother over the whole of lactation or peak lactation. Linear regressions were then applied to test whether there was a relationship between these two variables. Prediction 2a: The time spent on different behaviours, i.e., nursing inside the nest, nursing outside the nest, eating, resting and general activity, was calculated as daily averages for each of the individual mothers. Total time spent on different types of behaviours was also compared with days of lactation by ANOVA followed by Tukey post-hoc tests for each behaviour seperately. In addition, mean Tb recorded during the different behaviours were analysed by two-way ANOVA with DOL and behavioural type as fixed factors. Again mother ID was included in these models to account for repeated measures. Prediction 2b: The highest recorded values of Tb during the whole observation period was compared to the highest Tb recorded during nursing bouts inside the nest for each day of lactation using one way ANOVA with day of lactation as fixed factor and ID as random factor. Prediction 3: Linear regressions were used to examine the relationships between mean nursing Tb and litter size or litter mass. These analysis were performed for mean nursing Tb calculated over the whole period of lactation and nursing Tb during peak lactation separately.
Statistical analyses were carried out using the R programme (R Development Core Team, 2007) and SPSS.
Additional Information
How to cite this article: Gamo, Y. et al. Limits to sustained energy intake XXIV: impact of suckling behaviour on the body temperatures of lactating female mice. Sci. Rep. 6, 25665; doi: 10.1038/srep25665 (2016).
Supplementary Material
Acknowledgments
We would like to thank the animal house staff and all members of the Energetics group for their invaluable help at various stages throughout the project. This work was supported by Natural Environment Research Council grant (NERC, NE/C004159/1). YG was supported by a scholarship from the rotary foundation. LV was supported by a Rubicon grant from the Netherlands Scientific Organisation (NWO).
Footnotes
Author Contributions J.R.S. and Y.G. designed the study. Y.G., A.B., C.T., F.M., K.D., N.J., A.C., H.G., J.M. and J.D. performed the behaviour observations. Y.G., C.H. and S.E.M. performed the surgeries and collected/analysed the body temperature data. Y.G., J.R.S., C.H. and L.V. analysed the data. Y.G., J.R.S., S.E.M., C.H. and L.V. wrote the paper. J.R.S. raised the grant funding for the work to be performed.
References
- Johnson M. S., Thomson S. C. & Speakman J. R. Limits to sustained energy intake. I. Lactation in the laboratory mouse Mus musculus. J. Exp. Biol. 204, 1925–1935 (2001). [DOI] [PubMed] [Google Scholar]
- Hammond K. A. & Diamond J. An experimental test for a ceiling on sustained metabolic rate in lactating mice. Physiol. Zool. 65, 952–977 (1992). [Google Scholar]
- Hammond K. A., Konarzewski M., Torres R. M. & Diamond J. Metabolic ceilings under a combination of peak energy demands. Physiol. Zool. 67, 1479–1506 (1994). [Google Scholar]
- Johnson M. S. & Speakman J. R. Limits to sustained energy intake. V. Effect of cold-exposure during lactation in Mus musculus. J. Exp. Biol. 204, 1967–1977 (2001). [DOI] [PubMed] [Google Scholar]
- Krol E. & Speakman J. R. Limits to sustained energy intake VI. Energetics of lactation in laboratory mice at thermoneutrality. J. Exp. Biol. 206, 4255–4266 (2003). [DOI] [PubMed] [Google Scholar]
- Krol E. & Speakman J. R. Limits to sustained energy intake VII. Milk energy output in laboratory mice at thermoneutrality. J. Exp. Biol. 206, 4267–4281 (2003). [DOI] [PubMed] [Google Scholar]
- Krol E., Johnson M. S. & Speakman J. R. Limits to sustained energy intake VIII. Resting metabolic rate and organ morphology of laboratory mice lactating at thermoneutrality. J. Exp. Biol. 206, 4283–4291 (2003). [DOI] [PubMed] [Google Scholar]
- Speakman J. R. & Krol E. Limits to sustained energy intake. XIII. Recent progress and future perspectives. J. Exp. Biol. 214, 230–241 (2011). [DOI] [PubMed] [Google Scholar]
- Speakman J. R. & Krol E. Maximal heat dissipation capacity and hyperthermia risk: neglected key factors in the ecology of endotherms. J. Anim. Ecol. 79, 726–746 (2010). [DOI] [PubMed] [Google Scholar]
- Speakman J. R. & Krol E. The heat dissipation limit theory and evolution of life histories in endotherms–time to dispose of the disposable soma theory? Integr. Comp. Biol. 50, 793–807 (2010). [DOI] [PubMed] [Google Scholar]
- Krol E., Murphy M. & Speakman J. R. Limits to sustained energy intake. X. Effects of fur removal on reproductive performance in laboratory mice. J. Exp. Biol. 210, 4233–4243 (2007). [DOI] [PubMed] [Google Scholar]
- Zhao Z. J. & Cao J. Effect of fur removal on the thermal conductance and energy budget in lactating Swiss mice. J. Exp. Biol. 212, 2541–2549 (2009). [DOI] [PubMed] [Google Scholar]
- Zhao Z., Chi Q. & Cao J. Milk energy output during peak lactation in shaved Swiss mice. Physiol. Behav. 101, 59–66 (2010). [DOI] [PubMed] [Google Scholar]
- Paul M. J., Tuthill C., Kauffman A. S. & Zucker I. Pelage insulation, litter size, and ambient temperature impact maternal energy intake and offspring development during lactation. Physiol. Behav. 100, 128–134 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman A. Predicted limits for evaporative cooling in heat stress relief of cattle in warm conditions. J. Anim. Sci. 87, 3413–3417 (2009). [DOI] [PubMed] [Google Scholar]
- Renaudeau D., Noblet J. & Dourmad J. Effect of ambient temperature on mammary gland metabolism in lactating sows. J. Anim. Sci. 81, 217–231 (2003). [DOI] [PubMed] [Google Scholar]
- Gamo Y. et al. Limits to sustained energy intake. XVI. Body temperature and physical activity of female mice during pregnancy. J. Exp. Biol. 216, 2328–2338 (2013). [DOI] [PubMed] [Google Scholar]
- Gamo Y. et al. Limits to sustained energy intake. XX. Body temperatures and physical activity of female mice during lactation. J. Exp. Biol. 216, 3751–3761 (2013). [DOI] [PubMed] [Google Scholar]
- Weinandy R. & Gattermann R. Measurement of physiological parameters and activity in a Mongolian gerbil during gravidity and lactation with an implanted transmitter. Physiol. Behav. 58, 811–814 (1995). [DOI] [PubMed] [Google Scholar]
- Scribner S. J. & Wynne-Edwards K. E. Thermal constraints on maternal behavior during reproduction in dwarf hamsters (Phodopus). Physiol. Behav. 55, 897–903 (1994). [DOI] [PubMed] [Google Scholar]
- Fewell J. E. Body temperature regulation in rats near term of pregnancy. Can. J. Physiol. Pharmacol. 73, 364–368 (1995). [DOI] [PubMed] [Google Scholar]
- Leon M., Croskerry P. G. & Smith G. K. Thermal control of mother-young contact in rats. Physiol. Behav. 21, 793–811 (1978). [DOI] [PubMed] [Google Scholar]
- Croskerry P. G., Smith G. K. & Leon M. Thermoregulation and the maternal behaviour of the rat. Nature 273, 299–300 (1978). [DOI] [PubMed] [Google Scholar]
- Woodside B., Pelchat R. & Leon M. Acute elevation of the heat load of mother rats curtails maternal nest bouts. J. Comp. Physiol. Psychol. 94, 61 (1980). [DOI] [PubMed] [Google Scholar]
- Leon M., Adels L. & Coopersmith R. Thermal limitation of mother–young contact in Norway rats. Dev. Psychobiol. 18, 85–105 (1985). [DOI] [PubMed] [Google Scholar]
- Scribner S. J. & Wynne-Edwards K. E. Disruption of body temperature and behavior rhythms during reproduction in dwarf hamsters (Phodopus). Physiol. Behav. 55, 361–369 (1994). [DOI] [PubMed] [Google Scholar]
- Harikai N. et al. Dynamic Responses to Acute Heat Stress between 34. DEG. C. and 38.5. DEG. C. and Characteristics of Heat Stress Response in Mice. Biological and Pharmaceutical Bulletin 26, 701–708 (2003). [DOI] [PubMed] [Google Scholar]
- Kelly G. Body temperature variability (Part 1): a review of the history of body temperature and its variability due to site selection, biological rhythms, fitness, and aging. Alternative medicine review 11, 278 (2006). [PubMed] [Google Scholar]
- Adels L. E. & Leon M. Thermal control of mother-young contact in Norway rats: factors mediating the chronic elevation of maternal temperature. Physiol. Behav. 36, 183–196 (1986). [DOI] [PubMed] [Google Scholar]
- Trayhurn P. Decreased capacity for non-shivering thermogenesis during lactation in mice. Pflügers Archiv 398, 264–265 (1983). [DOI] [PubMed] [Google Scholar]
- Trayhurn P. & Jennings G. Functional atrophy of brown adipose tissue during lactation in mice. Effects of lactation and weaning on mitochondrial GDP binding and uncoupling protein. Biochem. J. 248, 273–276 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krol E., Martin S. A., Huhtaniemi I. T., Douglas A. & Speakman J. R. Negative correlation between milk production and brown adipose tissue gene expression in lactating mice. J. Exp. Biol. 214, 4160–4170 (2011). [DOI] [PubMed] [Google Scholar]
- Refinetti R. Contribution of locomotor activity to the generation of the daily rhythm of body temperature in golden hamsters. Physiol. Behav. 56, 829–831 (1994). [DOI] [PubMed] [Google Scholar]
- Weinert D. & Waterhouse J. Diurnally changing effects of locomotor activity on body temperature in laboratory mice. Physiol. Behav. 63, 837–843 (1998). [DOI] [PubMed] [Google Scholar]
- Schubert K. A. et al. Maternal energy allocation to offspring increases with environmental quality in house mice. Am. Nat. 173, 831–840 (2009). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


