Abstract
Background
To compare outcomes of open and endovascular repair of aortocaval fistulas (ACFs) in the setting of abdominal aortic aneurysms (AAAs).
Methods
A literature review was undertaken on Pubmed from 1999 to 2014 to identify reported cases of both endovascular and open repair of ACF, including the index case, presented here. Primary outcomes for endovascular repair were: complications, presence of endoleak, and death. Primary outcomes for open repair were: complications and death.
Results
Forty articles were reviewed with a total of 67 patients, including the index case. Endovascular approach was used in 26 patients (39%). Endoleaks were present in 50%, whereas similarly 46% of patients had a reported complication. Five deaths (19%) occurred in the endovascular group. Open repair was performed in 41 cases (61%). The rate of complication and the death in open repair were 36% and 12%, respectively (P = 0.327 and P = 0.910, respectively) compared with endovascular. Mean follow-up was 7.7 months for the endovascular group and 8.5 months in the open group.
Conclusions
Previous demonstrations of high morbidity and mortality with open repair of ACF in the setting of AAA have motivated endovascular approaches. However, endoleaks are a significant problem and were present in 50% of ACF cases. The continued presence of an endoleak in the setting of an ACF may result in persistence of the ACF, unlikely thrombosis of the endoleak, and continued sac enlargement. Endovascular repair presents theoretical benefit, yet is not associated with a reduced rate of complication or death versus open repair in this contemporary review.
Introduction
Aortocaval fistulas (ACFs) are a rare entity. Although the most common cause is penetrating trauma, in the setting of abdominal aortic aneurysms (AAAs), the incidence is <1%.1 Among ruptured AAA, ACF is noted in 2–7% of cases.2 ACF was first described by Symes in 1831, and over a century later, Dr. Cooley reported the first successful repair in 1955.3 Dr. Woolley in 1995 reported the first case of aortic exclusion used in the treatment of an intraoperatively diagnosed ACF. At the dawn of the endovascular age, most ACF had been repaired primarily in an open fashion and the literature accounted the surgical mortality to be as high as 16–66%.4,5 However, after Beveridge et al.6 in 1998 reported the first endovascular repair of ACF, many have published reports of ACF describe endovascular management. We sought to review the contemporary outcomes and complications of both methods, present a successful case of open repair, and determine if endovascular repair was associated with improved outcomes.
Case Report
A 55-year-old African-American man with a 42-pack-year smoking history, coronary artery disease, and ongoing dyspnea was referred for an AAA. The patient underwent CT angiography which identified a 6.8-cm infrarenal AAA as well as bilateral iliac aneurysms, measuring 5.0 and 3.5 cm. More important, it also revealed a large ACF (Fig. 1). Previous echocardiograms showed a declining left ventricular ejection fraction (current left ventricular ejection fraction = 35%) and a dilated right ventricle with a pulmonary artery pressure of 70 mm Hg. A right heart catheterization was performed which demonstrated several residual 50–70% coronary stenosis but no lesions which would benefit from further percutaneous revascularization. The coronary arteriography also confirmed a massively elevated right heart filling pressure of 25 mm Hg. His overall deteriorating clinical condition was thought to be secondary to his progressive high-output heart failure and was taken semielectively for an open repair as the obligate delay for a fenestrated device to address his juxtarenal AAA would place him at undue risk for further functional decline. A pulmonary catheter was placed and transesophageal echocardiography performed (Fig. 2). Repair was approached via a generous midline incision during which time massive edema was noted throughout retroperitoneum. There was a thrill in the vena cava, and as the cava was compressed in that area, normalization of the right ventricular hemodynamics occurred. The aortoiliacs were controlled, and the aneurysm sac opened revealing a large ACF. Venous bleeding was controlled manually with compression within the aneurysm sac, and the ACF repaired primarily with running Prolene sutures from within the aortic wall (Video 1). Estimated blood loss was 2000 mL, and the patient received 750 mL of Cell Saver transfusion and 2 units of banked blood. After ACF closure, the patient's pulmonary artery pressures immediately decreased from 61 of 42 to 23 of 15. His heart rate was maintained; however, the patient was placed on a nitroglycerin drip for hypertension. The aortic and iliac aneurysms were repaired with a bifurcated Dacron graft, each distal limb to the iliac bifurcations. His postoperative course was uncomplicated. His cardiac output decreased from 12.0 L/min preoperatively to 5.6 L/min postoperatively. He was discharged home on postoperative day 7. At his 2-month check, he lost 70 pounds, and he was beginning to return previous activities such as swimming and weightlifting. At 1 year of follow-up, his echocardiogram demonstrates favorable remodeling and improved ejection fraction.
Fig. 1.
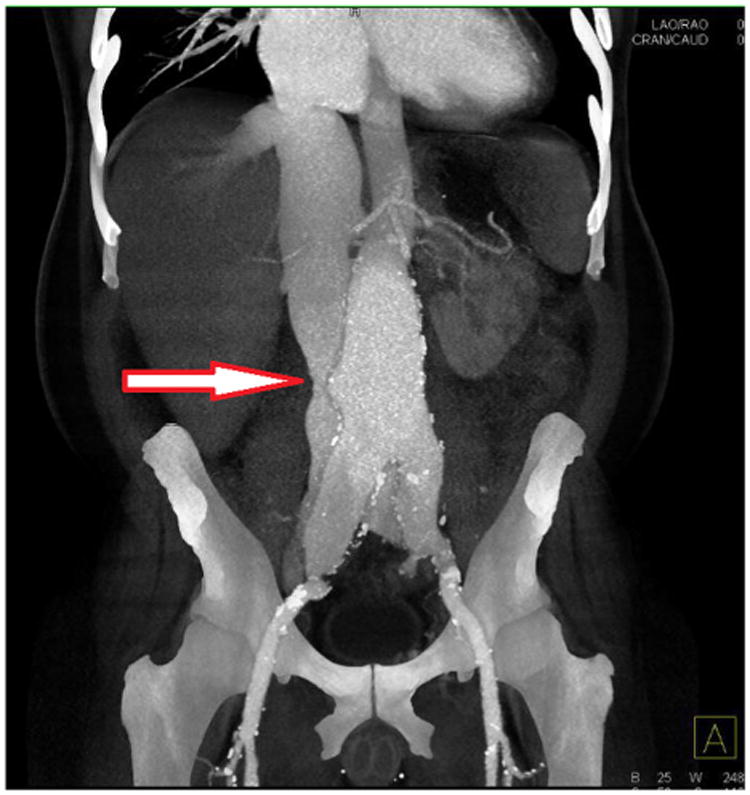
Three-dimensional reconstruction of CT angiography. White arrow, large aortocaval fistula.
Fig. 2.

Intraoperative transesophageal echocardiogram. Massively dilated right atrium.
Methods
A contemporary literature review was undertaken on Pubmed from 1999 to 2014 using search terms ACF, aortoiliac fistula, and aortovenous fistula to identify reported cases of both endovascular and open repair of ACF. We elected to limit the search over the last 15 years because we postulated that after 1999, surgeons had the option to choose between open and endovascular repair. Only cases involving nontraumatic aortocaval, aortoilial, or iliocaval fistulas were included. The case presentation and demographics were summarized and follow-up recorded. In addition, only articles identifying presence or absence of endoleak were included for the endovascular group. Overall, the patients with ACF were divided in 2 categories for comparison according to the treatment received: the endovascular repair group (n = 26) and the open repair group (n = 41). Primary outcomes for the endovascular repair were: complications, presence of group, and the death. Most authors included endoleaks as a complication if further intervention was required. Primary outcomes for open repair were: complications and death. Rupture was defined as violation of the retroperitoneum and excluded simply the presence of an ACF. Statistical analysis was performed using single-tailed Fisher's exact test for categorical variables given sample size and expected contingency tables.
Results
Demographics
Forty articles were reviewed totaling 67 patients, including our patient presented above. The average age was 68 and 70 years in open and endovascular groups, respectively (Table I). Most patients were men (96%). Most patients presented with acute onset of abdominal or back pain, seen in 75.6% open and 46.1% endovascular repairs. Of note, 24% of patients did present with shortness of breath 19.5% in open group and 30.7% in the endovascular group (P = 0.223).
Table I. Demographics and presentation.
| Open | Endo | Analysis | |
|---|---|---|---|
|
|
|
|
|
| n = 41, n (%) | n = 26, n (%) | (P value) | |
| Demographic | |||
| Age (years) | 68 (44–85) | 70 (56–80) | – |
| Male gender | 40 (97.5) | 24 (92.3) | 0.332 |
| Presentation | |||
| Shortness of breath | 8 (19.5) | 8 (30.7) | 0.223 |
| Leg swelling | 3 (7.3) | 10 (38.4) | 0.002 |
| Abdominal bruit | 2 (4.8) | 1 (3.8) | 0.668 |
| Pulsatile mass | 3 (7.3) | 2 (7.7) | 0.709 |
| Abdominal/back pain | 31 (75.6) | 12 (46.1) | 0.015 |
| Hemorrhagic shock | 7 (17) | 3 (11.5) | 0.418 |
| Ruptured | 25 (60.9) | 6 (23.1) | 0.002 |
Anatomy
Most patients had infrarenal ACFs; 87.8% open and 84.6% endovascular (Table II). One patient in the open group had a juxtarenal ACF. Iliocaval and aortoilial fistulas were less common. Five patients, 2 in the endovascular group and 3 in the open group, were noted to have either an inflammatory aneurysm or retroperitoneal fibrosis. There were 25 (60.9%) ruptured aneurysms in the open group and 6 (23.1%) in the endovascular group.
Table II. Anatomy.
| Open | Endo | ||
|---|---|---|---|
|
|
|
||
| n = 41, n (%) | n = 26, n (%) | P value | |
| ACF | 36 (87.8) | 22 (84.6%) | |
| Infrarenal ACF | 35 (85.3) | 22 (84.6) | 0.673 |
| Juxtarenal ACF | 1 (2.4) | 0 (0) | 0.612 |
| Iliocaval fistula | 2 (4.8) | 3 (11.5) | 0.291 |
| Aortoiliac fistula | 3 (7.3) | 1 (3.8) | 0.494 |
| Inflammatory/retroperitoneal fibrosis | 3 (7.3) | 2 (7.7) | 0.710 |
| Ruptured | 25 (60.9) | 6 (23.1) | 0.024 |
Bold indicates P < 0.05 for open versus endo.
Technique
Table III reviews the intraoperative approach to all 67 patients. Most patients undergoing an open repair had primary repair of the ACF from within the sac. Espinel et al.11 and Madani et al.46 used a retrograde balloon occlusion of the inferior vena cava (IVC) for venous control before primarily repairing the ACF. Four patients were repaired with a patch angioplasty of the IVC.8,9,12 Endovascular repair included several endograft types: Boston Scientific (Natick, MA), Gore (Flagstaff, CA), Cook (Bloomington, IN), Edwards Lifescience (Irvine, CA), Medtronic (Minneapolis, MN), and La Maitre (Burlington, MA).
Table III. Intraoperative approach.
| Open | |
|---|---|
| Tsolakis 19997 | Repaired primarily within sac3, IVC ligation1 |
| Ferrari 20008 | Patch angioplasty |
| Takazawa 20019 (2 cases) | Patch angioplasty |
| Gandini 200210 | Repaired primarily within sac |
| Espinel 200611 | Retrograde balloon occlusion of IVC, repaired primarily within sac |
| Iriz 200612 | Patch angioplasty |
| Maeda 200713 (5 cases) | All repaired primarily within sac |
| Laxdal 200714 | Repaired primarily within sac |
| Takaseya 200715 | Repaired primarily within sac |
| Siepe 200916 | IVC stent followed by open primary repair |
| Pevec 201017 | Repaired primarily within sac |
| Kondo 201118 (2 cases) | 1. Aortic exclusion (tight closure of aortic sac) |
| 2. Repaired primarily within sac | |
| Oda 201119 | Repaired primarily within sac |
| Kotsikoris 201220 | All repaired primarily within sac |
| Laporte 201221 | Repaired primarily within sac |
| Unosawa 201322 | Repaired primarily within sac |
| Nakazawa 201423 | Repaired primarily within sac |
| Madani 201446 | Retrograde balloon occlusion of IVC, Repaired primarily within sac |
| Orion 2014 | Repaired primarily within sac |
|
| |
| Endovascular | |
|
| |
| Umscheid 200024 | Vanguard bifurcated (Boston Scientific) |
| Lau 200125 | Aorto-uni, custom |
| Duxbury 200226 | Gore limb Excluder |
| Vetrhus 200527 (2 cases) | Tri-Fab Cook2 |
| Godart 200628 | Amplatz duct occluder |
| Hetzel 200629 | Lifepath iliac extension (Edwards Lifesciences) |
| Kopp 200630 | Zenith bifurcated Cook |
| Fukuda 200731 | Aorto-uni, custom |
| Leon 200732 | Gore excluder |
| Kwon 200833 | Gore excluder |
| Juszkat 200934 | Zenith iliac extension |
| Guzzardi 201035 | Gore excluder, IVC filter |
| Melas 201136 | Aorto-uni La Maitre |
| LaBarbera 201137 | Gore bifurcated, converted to aorto-uni |
| Akwei 201138 (4 cases) | 1. Tri-Fab Cook |
| 2. Zenith aorto-uni | |
| 3. Bi-Fab Cook | |
| 4. Zenith Cook aorto-uni | |
| Yuminaga 201239 | Zenith Cook with Tri-Fab limb extension |
| Rapacciuolo 201240 | Amplatzer plug then delayed Talent stent graft (Medtronic) |
| Shah 201241 | C3 Gore deployed within sac and extended proximally |
| Bernstein 201342 | Gore excluder intra-aortic, delayed Gore TAG intracaval |
| van de Luijtgaarden 201343 | Endurant (Medtronic) |
| Silveira 201444 | Gore excluder intra-aortic, Gore aortic cuff intracaval |
| Nakad 201445 | Endurant (Medtronic) |
Outcomes
There were 15 of 41 patients (36%) in the open group who had a postoperative complication (Table IV). These included renal failure, respiratory failure, deep venous thrombosis, bowel obstruction, acalculous cholecystitis, pseudomembranous colitis, wound infection, paralysis, and lower-extremity ischemia. There were 5 mortalities (12% mortality rate among reported open cases). Thirteen patients (50%, P = 0.910, vs. open repair) of those undergoing an endovascular approach had a reported complication (Table V). When only considering endoleaks, 13 patients had a reported endoleak, either at initial surgery or in a delayed fashion. Of the 13 endoleaks, 5 (38%) were intervened on and 6 (46%) resolved spontaneously. Five deaths (19%) occurred in the endovascular group of which none had a ruptured aneurysm. One case died 3 months postoperative and may have been unrelated to the surgery.27 The remaining deaths included multiorgan failure after pneumonia, worsening acidosis, and massive pulmonary embolism. Mean follow-up was 7.7 months for the endovascular group and 8.8 months in the open group (Tables IV and V).
Table IV. Open repair–postoperative.
| Author | Complication | Follow-up (months) |
|---|---|---|
| Tsolakis 19997 | Death in 3 patients (1 also had spinal ischemia) | None |
| Ferrari 20008 | Acalculous cholecystitis, pseudomembranous colitis | 10 |
| Takazawa 20019 (2 cases) | Renal failure in 1 patient, cholecystitis in 1 patient | None |
| Gandini 200210 | Recurrence of ACF and converted to endovascular repair | 24 |
| Espinel 200611 | None | None |
| Iriz 200612 | Renal failure | 0.75 |
| Maeda 200713 (5 cases) | Death in 1 patient, paraplegia in 1 patient | None |
| Laxdal 200714 | Lower-extremity ischemia, respiratory failure | 12 |
| Takaseya 200715 | None | None |
| Siepe 200916 | Renal failure, respiratory failure, atrial fibrillation, wound infection | None |
| Pevec 201017 | None | None |
| Kondo 201118 (2 cases) | Bowel obstruction in 1 patient | 1 |
| Oda 201119 | None | None |
| Kotsikoris 201220 | Deep vein thrombosis, death in 1 patient | Average 18.5 |
| Laporte 201221 | None | None |
| Unosawa 201322 | None | 12 |
| Nakazawa 201423 | None | 30 |
| Madani 201446 | None | None |
| Orion 2014 | None | 12 |
| Mean follow-up | 8.8 |
Table V. Endovascular repair–postoperative.
| Author | Complication | Endoleak | Follow-up (months) |
|---|---|---|---|
| Umscheid 200024 | Compression of right iliac limb | Yes | 6 |
| Lau 200125 | Stent occlusion, leg ischemia | Yes | 12 |
| Duxbury 200226 | Prolapse into ACF, IVC thrombus | No | 1 |
| Vetrhus 200527 (2 cases) | 1. None | 1. Yes | 1. 12 |
| 2. Death at 3 months | 2. No | 2. 3 | |
| Godart 200628 | None | No | 6 |
| Hetzel 200629 | None | No | None |
| Kopp 200630 | Inferior mesenteric artery coiled | Yes | 6 |
| Fukuda 200731 | None | No | 48 |
| Leon 200732 | None | No | 1.25 |
| Kwon 200833 | None | No | 5 |
| Juszkat 200934 | None | No | None |
| Guzzardi 201035 | None | Yes | 12 |
| Melas 201136 | Enlarging sac requiring IVC stent graft | Yes | 36 |
| LaBarbera 201137 | Persistent type II, Amplatzer VSD occluder to ACF and Inferior mesenteric artery coiled | Yes | 1 |
| Akwei 201138 (4 cases) | 1. Death day 3 | 1. No | 1. None |
| 2. Death day 1 | 2. Yes | 2. None | |
| 3. Death hour 1 | 3. No | 3. None | |
| 4. Persistent type Ia and sac enlargement, extended with Palmaz | 4. Yes | 4. 12 | |
| Yuminaga 201239 | None | Yes | 1.5 |
| Rapacciuolo 201240 | None | No | 1 |
| Shah 201241 | None | Yes | 1 |
| Bernstein 201342 | Complete thrombosis of left iliac veins and near thrombosis of IVC, ischemic colitis, death at day 35 | Yes | None |
| van de Luijtgaarden 201343 | Persistent ACF after 1 year | Yes | 12 |
| Silveira 201444 | None | No | 24 |
| Nakad 201445 | None | No | None |
| Mean follow-up | 7.7 |
Discussion
Although ACFs can be traumatic or iatrogenic in nature, the majority are primary because of the spontaneous erosion of an AAA into the neighboring venous structures. Previous to the dispersion of endovascular aneurysm repair (EVAR), these patients were primarily repaired in an open fashion. After 1998, surgeons have increasingly elected for an endovascular method, proposing a decreased risk of complications and death. However, endovascular repairs are not without their own significant morbidity as demonstrated in this contemporary analysis.
Endoleaks are a major concern for these patients. Vetrhus et al.27 proposed that endovascular repair is justified with only a small risk of endoleak. We found an endoleak rate of 50% which is much higher than that reported by Antonio et al.47 in 2009. Of the reported endoleaks, less than half resolved spontaneously and certainly this high proportion is connected to the concurrent ACF promoting patency of the aortovenous circuit. Although many would argue that type II endoleaks can be monitored closely, when the patient has an ACF, perhaps these should be addressed during their initial hospital stay, particularly in cases in virulent presentation. LaBarbera et al.37 obtained CT angiography on postoperative day 9 showing persistent ACF and type II endoleak and subsequently decided to pursue coil embolization of the inferior mesenteric artery, sac and placement of Amplatzer VSD occluder across the ACF on postoperative day 13. It is more concerning that some endoleaks are occasionally not appreciated on completion angiogram. Melas et al.36 presented such a case, and the patient represented at 6 months with stable sac but a larger sized ACF and increasing signs of heart failure. This begs the argument that in cases where the potential for endoleak is high and the patient's cardiac performance is at risk, the completion angiogram should not be taken as a reliable guide and adjunctive imaging such as early CT or duplex should be routine.25 In the face of an increased incidence of endoleak, some surgeons have modified their endovascular approach. Godart et al.28 describe placing an Amplatzer duct occluder transfemorally for a persistent ACF after endovascular repair. Perhaps the penultimate repair is the placement of an intravenous stent graft as described by Melas et al.36 whereby they deployed an UniFit (Le Maitre Vascular, Burlington, MA) stent graft to seal the shunt from within the IVC (in addition to aortic repair). Bernstein et al.42 encountered thrombosed iliac veins after EVAR repair of ACF for which they recanalized the iliac system and reconstructed it in a “kissing” configuration using a Gore Excluder (Gore, Flagstaff, CA) with Extension and a Gore TAG thoracic endoprosthesis in the IVC. There is continued apprehension for deep venous thrombosis in endovenous stenting of the caval side of the ACF, and the question of anticoagulation is a difficult one both long term and immediately postoperative. There are little data to guide us on anticoagulation for endovenous stenting in the setting of an endoleak and ACF, but it is concerning that anticoagulation would persist the untoward aortovenous circuit.
It is intuitive that endovascular surgery, which places less physical stress on the body, may be more appropriate for patients who are unlikely to survive an open surgery. However, this statement requires examination. The most obvious choice might be those patients who have a ruptured aneurysm or those patients with decompensated heart failure. Although the Immediate Management of the Patient with Rupture: Open versus Endovascular Repair (IMPROVE) aneurysm trial48 reported that endovascular repair was not associated with significant reduction in 30-day mortality in patients with ruptured AAAs, many proponents feel that if patients can be treated by EVAR, survival is superior. The limiting factor in many cases of open repair for ruptured aneurysms with ACF is that the institution does not offer the appropriate endovascular graft “off the shelf.” Siepe et al.16 presented a hybrid approach in 2009. The patient presented with malperfusion and rapidly deteriorated into shock; 2 aortic stent grafts (Medtronic Inc, Fidley, MN) were placed into the IVC first then after hemodynamics stabilized, transitioned to an open repair. The juxtarenal anatomy excluded it from an endovascular repair of the AAA, but the idea of a hybrid approach is noteworthy and may be a viable option to stabilize the “symptomatic” ACF.
In addition, those patients with inflammatory aneurysms or retroperitoneal fibrosis and ACF deserve mention. There were 5 patients in this review. It has been reported that ruptured inflammatory aneurysms have a high incidence of ACF.49 Certainly with inflammatory aneurysms or retroperitoneal fibrosis, an open dissection proves more difficult with increased bleeding risk. Injuries to the IVC or ureters have also been reported complications of inflammatory aneurysms. Thus, these patients may be best served by endovascular repair if possible.
There still remain patients who are not ruptured and are relatively fit for open surgery. The ongoing concern would be management of the overloaded right ventricle and pulmonary hypertension. Most ACF patients will have some degree of heart failure, but hemodynamic changes caused by the ACF are completely reversed after ACF closure and latitude for operative risk to be improved through surgery is a realizable goal. In most, systemic vascular resistance increases whereas central venous pressure, pulmonary artery pressure, stroke volume, and cardiac output all decrease.50 Thus, if patients are not limited by significant coronary artery disease, then even moderate heart failure alone should not preclude a patient from open repair.
When considering a high reported mortality rate for open repair of ACF, many surgeons still look to endovascular repair first; whether the AAA is ruptured or not. Five deaths were reported with EVAR and ACF, which represented 19% of the endovascular group. However, one was 3 months postoperative and may have been unrelated to the surgery.27 Furthermore, Akwei et al.38 reported 4 endovascular cases of which 3 died, but all three of those deaths had been admitted to the hospital as many as 6 days before the diagnosis of the ACF. Thus, delay in diagnosis and treatment likely played a factor in their outcome, and these patients were not likely to fare better with open surgery. Therefore, if a delayed diagnosis is not a factor, the 30-day mortality is adjusted to 3.8% instead of the crude 19% estimate. This is far less than the 12% mortality rate of open repair demonstrated herein. Indeed the improvement in mortality rate of the ACF condition, we have revealed is duly noted for endovascular and open repair, and compares quite favorably to the historical literature.
Conclusions
Forty papers were reviewed with a total of 67 patients with AAA and ACF, the largest series of ACF known to date. Open surgery remains a viable option to patients who are relatively fit; however, endovascular repair may provide a lower risk of 30-day mortality. Nonetheless, a high incidence and persistence of endoleak with the endovascular approach requires caution. The continued presence of an endoleak in the setting of an ACF may result in several untoward outcomes: probable persistence of the ACF, ongoing heart failure risk, unlikely thrombosis of endoleaks, disseminated intravascular coagulation, and even continued sac enlargement. We would agree for these patients that endoleak management should be aggressively pursued. Numerous techniques have been employed for this complication including endovenous stenting and Amplatzer duct occluders, but longer follow-up is needed. Overall, the endovascular approach demonstrates merit and is a viable option for many ACF. Furthermore, timely diagnosis of ACF to manage intraoperative cardiac performance and to recognize the unique nature of endoleak risk to the patient after ACF repair may be central to promoting the improved mortality risk of ACF we appreciated in this modern assessment of outcomes.
Supplementary Material
Acknowledgments
There are no sources of financial support or competitive relationships that pertain to this article.
Footnotes
Supplementary Data: Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.avsg.2015.09.006.
References
- 1.Brightwell RE, Pegna V, Boyne N. Aortocaval fistula: current management strategies. ANZ J Surg. 2013;83:31–5. doi: 10.1111/j.1445-2197.2012.06294.x. [DOI] [PubMed] [Google Scholar]
- 2.Schmidt R, Bruns C, Walter M, et al. Aorto-caval fistula—an uncommon complication of infrarenal aortic aneurysms. Thorac Cardiovasc Surg. 1994;42:208–11. doi: 10.1055/s-2007-1016489. [DOI] [PubMed] [Google Scholar]
- 3.Woolley DS, Spence RK. Aortocaval fistula treated by aortic exclusion. J Vasc Surg. 1995;22:639–42. doi: 10.1016/s0741-5214(95)70053-6. [DOI] [PubMed] [Google Scholar]
- 4.Alexander JJ, Imbembo AL. Aorto-vena cava fistula. Surgery. 1989;105:1–12. [PubMed] [Google Scholar]
- 5.Davidovic L, Dragas M, Cvetkovic S, et al. Twenty years of experience in the treatment of spontaneous aorto-venous fistulas in a developing country. World J Surg. 2011;35:1829–34. doi: 10.1007/s00268-011-1128-1. [DOI] [PubMed] [Google Scholar]
- 6.Beveridge CJ, Pleass HC, Chamberlain J, et al. Aortoiliac aneurysm with arteriocaval fistula treated by a bifurcated endovascular stent-graft. Cardiovasc Intervent Radiol. 1998;21(3):244–6. doi: 10.1007/s002709900253. [DOI] [PubMed] [Google Scholar]
- 7.Tsolakis JA, Papadoulas S, Kakkos SK, et al. Aortocaval fistula in ruptured aneurysms. Eur J Vasc Endovasc Surg. 1999;17:390–3. doi: 10.1053/ejvs.1998.0777. [DOI] [PubMed] [Google Scholar]
- 8.Ferrari M, Bonanomi G, Fossati N, et al. Surgical management of inflammatory aortic aneurysm associated with occult aortocaval fistula. Surgery. 2000;127:234–6. doi: 10.1067/msy.2000.102755. [DOI] [PubMed] [Google Scholar]
- 9.Takazawa A, Sakahashi H, Toyama A. Surgical repair of a concealed aortocaval fistula associated with an abdominal aortic aneurysm: report of two cases. Surg Today. 2001;31:842–4. doi: 10.1007/s005950170062. [DOI] [PubMed] [Google Scholar]
- 10.Gandini R, Ippoliti A, Pampana E, et al. Emergency endograft placement for recurrent aortocaval fistula after conventional AAA repair. J Endovasc Ther. 2002;9:208–11. doi: 10.1177/152660280200900212. [DOI] [PubMed] [Google Scholar]
- 11.Espinel CF, Calligaro KD, Dougherty MJ. Percutaneous balloon occlusion of the inferior vena cava as an adjunct for treating ruptured type IV thoracoabdominal aneurysm and aortocaval fistula. J Vasc Surg. 2006;43(4):834–5. doi: 10.1016/j.jvs.2005.12.052. [DOI] [PubMed] [Google Scholar]
- 12.Iriz E, Oxdogan ME, Erer D, et al. A giant aortocaval fistula due to abdominal aortic aneurysm. Int J Cardiol. 2006;112:78–80. doi: 10.1016/j.ijcard.2006.03.075. [DOI] [PubMed] [Google Scholar]
- 13.Maeda H, Umezawa H, Goshima M, et al. Surgery for ruptured abdominal aortic aneurysm with an aortocaval and iliac vein fistula. Surg Today. 2007;37:445–8. doi: 10.1007/s00595-006-3429-9. [DOI] [PubMed] [Google Scholar]
- 14.Laxdal E, Sovik K, Pedersen G, et al. Inflammatory infrarenal aortic aneurysm with aortocaval fistula. Ann Vasc Surg. 2007;21:633–6. doi: 10.1016/j.avsg.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 15.Takaseya T, Hiromatsu S, Akashi H, et al. A case of unilateral leg edema due to abdominal aortic aneurysm with aortocaval fistula. Ann Thorac Cardiovasc Surg. 2007;13:135–8. [PubMed] [Google Scholar]
- 16.Siepe M, Koeppe S, Euringer W, et al. Aortocaval fistula from acute rupture of an abdominal aortic aneurysm treated with a hybride approach. J Vasc Surg. 2009;49:1574–6. doi: 10.1016/j.jvs.2008.12.074. [DOI] [PubMed] [Google Scholar]
- 17.Pevec WC, Lee ES, Lamba R. Symptomatic, acute aortocaval fistula complicating an infrarenal aortic aneurysm. J Vasc Surg. 2010;51:475. doi: 10.1016/j.jvs.2009.03.053. [DOI] [PubMed] [Google Scholar]
- 18.Kondo N, Takahashi K, Takeuchi S, et al. Surgical repair of arteriovenous fistula associated with infrarenal aorto-iliac aneurysm: report of two contrasting cases. Ann Vasc Dis. 2011;4:150–3. doi: 10.3400/avd.cr.10.01037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oda T, Yasunaga H, Hosokawa Y, et al. Preoperative computed tomographic diagnosis of an aortocaval fistula associated with aneurysm of the abdominal aorta. Ann Thorac Cardiovasc Surg. 2011;17:531–3. doi: 10.5761/atcs.cr.10.01626. [DOI] [PubMed] [Google Scholar]
- 20.Kotsikoris I, Papas TT, Papanas N, et al. Aortocaval fistula formation due to ruptured abdominal aortic aneurysms: a 12-year series. Vasc Endovascular Surg. 2012;46:26–9. doi: 10.1177/1538574411418842. [DOI] [PubMed] [Google Scholar]
- 21.Laporte F, Olivier A, Groben L, et al. Aortocaval fistula: an uncommon cause of paradoxical embolism. J Cardiovasc Med (Hagerstown) 2012;13:68–71. doi: 10.2459/JCM.0b013e32834039d7. [DOI] [PubMed] [Google Scholar]
- 22.Unosawa S, Kimura H, Niino T. Surgical repair of ruptured abdominal aortic aneurysm with non-bleeding aortocaval fistula. Ann Vasc Dis. 2013;6:209–11. doi: 10.3400/avd.cr.12.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakazawa S, Mohara J, Takahashi T, et al. Aortocaval fistula associated with ruptured abdominal aortic aneurysm. Ann Vasc Surg. 2014;28:1793. doi: 10.1016/j.avsg.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 24.Umscheid T, Stelter WJ. Endovascular treatment of an aortic aneurysm ruptured into the inferior vena cava. J Endovasc Ther. 2000;7:31–5. doi: 10.1177/152660280000700105. [DOI] [PubMed] [Google Scholar]
- 25.Lau LL, O'Reilly MJ, Johnston LC, et al. Endovascular stentgraft repair of primary aortocaval fistula with an abdominal aortoiliac aneurysm. J Vasc Surg. 2001;33:425–8. doi: 10.1067/mva.2001.111485. [DOI] [PubMed] [Google Scholar]
- 26.Duxbury MS, Wells IP, Roobottom C, et al. Endovascular repair of spontaneous no-aneurysmal aortocaval fistula. Eur J Vasc Endovasc Surg. 2002;24:276–8. doi: 10.1053/ejvs.2002.1708. [DOI] [PubMed] [Google Scholar]
- 27.Vetrhus M, McWilliams R, Tan CK, et al. Endovascular repair of abdominal aortic aneurysms with aortocaval fistula. Eur J Vasc Endovasc Surg. 2005;30:640–3. doi: 10.1016/j.ejvs.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 28.Godart F, Haulon S, Houmany M, et al. Transcatheter closure of aortocaval fistula with the amplatzer duct occluder. J Endovasc Ther. 2005;12:134–7. doi: 10.1583/04-1332.1. [DOI] [PubMed] [Google Scholar]
- 29.Hetzel G, Gabriel P, Rompel O, et al. Aortocaval fistula after stent-graft repair. J Endovasc Ther. 2006;13:117–20. doi: 10.1583/05-1558MR.1. [DOI] [PubMed] [Google Scholar]
- 30.Kopp R, Weidenhagen R, Hoffmann R, et al. Immediate endovascular treatment of an aortoiliac aneurysm ruptured into the inferior vena cava. Ann Vasc Surg. 2006;20:525–8. doi: 10.1007/s10016-006-9061-8. [DOI] [PubMed] [Google Scholar]
- 31.Fukuda I, Minakawa M, Fukui K, et al. Management of an aorto-caval fistula from a ruptured aortic false aneurysm using a covered stent graft. Interact Cardiovasc Thorac Surg. 2007;6:682–4. doi: 10.1510/icvts.2007.153064. [DOI] [PubMed] [Google Scholar]
- 32.Leon LR, Jr, Arslan B, Ley E, et al. Endovascular therapy of spontaneous aortocaval fistulae associated with abdominal aortic aneurysms. Vascular. 2007;15:35–40. doi: 10.2310/6670.2007.00011. [DOI] [PubMed] [Google Scholar]
- 33.Kwon SH, Oh JH, Park SJ, et al. Endovascular repair of a spontaneous right common iliac artery—inferior vena cava fistula due to infrarenal aortoiliac aneurysm. Vasc Endovascular Surg. 2008;42:279–83. doi: 10.1177/1538574407312649. [DOI] [PubMed] [Google Scholar]
- 34.Juszkat R, Pukacki F, Zarzecka A, et al. Endovascular treatment of ruptured abdominal aneurysm into the inferior vena cava in patient after stent graft placement. Cardiovasc Intervent Radiol. 2009;32:776–80. doi: 10.1007/s00270-009-9525-7. [DOI] [PubMed] [Google Scholar]
- 35.Guzzardi G, Fossaceca R, Divenuto I, et al. Endovascular treatment of ruptured abdominal aortic aneurysm with aortocaval fistula. Cardiovasc Intervent Radiol. 2010;33:853–6. doi: 10.1007/s00270-009-9640-5. [DOI] [PubMed] [Google Scholar]
- 36.Melas N, Saratzis A, Saratzis N, et al. Inferior vena cava stent-graft placement to treat endoleak associated with an aortocaval fistula. J Endovasc Ther. 2011;18:250–4. doi: 10.1583/10-3296.1. [DOI] [PubMed] [Google Scholar]
- 37.LaBarbera M, Nathanson D, Hui P. Percutaneous closure of aortocaval fistula using the amplatzer muscular VSD occlude. J Invasive Cardiol. 2011;23:343–4. [PubMed] [Google Scholar]
- 38.Akwei S, Altaf N, Tennant W, et al. Emergency endovascular repair of aortocaval fistula—a single center experience. Vasc Endovascular Surg. 2011;45:442–6. doi: 10.1177/1538574411407087. [DOI] [PubMed] [Google Scholar]
- 39.Yuminaga Y, Mohabbat W, Nguyen T, et al. Simultaneous endovascular repair of an iatrogenic carotid-jugular fistula and a large iliocaval fistula presenting with multiorgan failure: a case report. J Med Case Rep. 2012;6:33. doi: 10.1186/1752-1947-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rapacciuolo A, De Angelis MC, di Pietro E, et al. Percutaneous treatment of a aorto-caval fistula in a old high risk patient. BMC Surg. 2012;12(Suppl 1):S32. doi: 10.1186/1471-2482-12-S1-S32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shah TR, Parikh P, Borkon M, et al. Endovascular repair of contained abdominal aortic aneurysm rupture with aortocaval fistula presenting with high-output heart failure. Vasc Endovascular Surg. 2013;47:51–6. doi: 10.1177/1538574412462633. [DOI] [PubMed] [Google Scholar]
- 42.Bernstein J, Jimenez JC. Inferior vena cava thrombosis following endovascular repair of acute aortocaval fistula: a word of caution. Vasc Endovascular Surg. 2013;47:467–9. doi: 10.1177/1538574413490839. [DOI] [PubMed] [Google Scholar]
- 43.van de Luijtgaarden KM, Bastos Goncalves F, Rouwet EV, et al. Conservative management of persistent aortocaval fistula after endovascular aortic repair. J Vasc Surg. 2013;58:1080–3. doi: 10.1016/j.jvs.2012.10.138. [DOI] [PubMed] [Google Scholar]
- 44.Silveira PG, Cunha JR, Barbosa Lima GB, et al. Endovascular treatment of ruptured abdominal aortic aneurysm with aortocaval fistula based on aortic and inferior vena cava stent-graft placement. Ann Vasc Surg. 2014;28:1933. doi: 10.1016/j.avsg.2014.06.073. [DOI] [PubMed] [Google Scholar]
- 45.Nakad G, Abi Chedid G, Osman R. Endovascular treatment of major abdominal arteriovenous fistula: a systematic review. Vasc Endovascular Surg. 2014;48:388–95. doi: 10.1177/1538574414540485. [DOI] [PubMed] [Google Scholar]
- 46.Madani A, Leung S, Obrand D. Open repair of inflammatory abdominal aortic aneurysm and aortocaval fistula using retrograde balloon occlusion. Vasc Endovascular Surg. 2014;48(1):80–2. doi: 10.1177/1538574413508828. [DOI] [PubMed] [Google Scholar]
- 47.Antoniou GA, Koutsias S, Karathanos C, et al. Endovascular stent-graft repair of major abdominal arteriovenous fistula: a systematic review. J Endovasc Ther. 2009;16:514–23. doi: 10.1583/09-2725.1. [DOI] [PubMed] [Google Scholar]
- 48.Powell JT, Sweeting MJ, Thompson MM, et al. Endovascular or open repair strategy for ruptured abdominal aortic aneurysm: 30 day outcomes from IMPROVE randomized trial. BMJ. 2014;348:f7661. doi: 10.1136/bmj.f7661. [DOI] [PubMed] [Google Scholar]
- 49.Tambyraja AL, Murie JA, Chalmers RT. Ruptured inflammatory abdominal aortic aneurysm: insights in clinical management and outcome. J Vasc Surg. 2004;39(2):400–3. doi: 10.1016/j.jvs.2003.07.029. [DOI] [PubMed] [Google Scholar]
- 50.Neema PK, Ramakrishnan S, Sinha PK, et al. Anesthetic implications of surgical repair of an aortocaval fistula. J Cardiothorac Vasc Anesth. 2003;17:236–9. doi: 10.1053/jcan.2003.55. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


