Abstract
Objectives
Capsule colonoscopy is an additional screening modality for colorectal cancer. Second-generation capsule colonoscopy (CC2) may have improved efficacy in the detection of colon adenomas as compared with prior devices. The purpose of this study was to evaluate the performance of CC2 in the detection of polyps in symptomatic and screening patients in the USA.
Design
Prospective, multicentre study.
Setting and participants
Two academic medical centres and two private practice facilities, evaluating patients with indications for colonoscopy.
Methods
Patients underwent capsule colonoscopy procedure using magnesium citrate as a boost, followed by colonoscopy on the same day. The main outcome measurement was accuracy of CC2 for the detection of colorectal polyps ≥6 and ≥10 mm as compared with conventional colonoscopy.
Results
51 patients were enrolled, 50 of whom had CC2 and colonoscopy examinations and were included in the accuracy analysis. 30% and 14% of patients had polyps ≥6 and ≥10 mm, respectively. For lesions ≥10 mm identified on conventional colonoscopy, CC2 sensitivity was 100% (95% CI 56.1% to 100%) with a specificity of 93.0% (79.9% to 98.2%). For polyps ≥6 mm, the CC2 sensitivity was 93.3% (66.0% to 99.7%) and the specificity was 80.0% (62.5% to 90.9%). There was a 61% adequate cleansing rate with 64% of CC2 procedures being complete.
Conclusions
In the initial US experience with CC2 there was adequate sensitivity for detecting patients with polyps ≥6 mm in size. Magnesium citrate was inadequate as a boost agent.
Trial registration number
Keywords: COLONOSCOPY, GASTROINTESINAL ENDOSCOPY, COLONIC POLYPS, COLONIC ADENOMAS
Summary box.
What is already known about this subject
-
▸
Capsule colonoscopy has been used in colorectal screening and symptomatic populations for the detection of colonic polyps.
-
▸
Second-generation capsule colonoscopy (CC2) has a high sensitivity for colon polyps using conventional colonoscopy as the gold standard and appears superior to the first generation.
-
▸
Limited data exist in the USA regarding the accuracy of CC2, in particular in mixed screening and diagnostic populations.
-
▸
The ideal bowel preparation for CC2 is still under development.
What are the new findings in this study
-
▸
This was the first study to use CC2 in the US population and is the only US study to assess its sensitivity and specificity using conventional colonoscopy as the gold standard in a mixed screening and symptomatic population.
-
▸
CC2 had a high sensitivity for the detection of ≥6 and ≥10 mm polyps in this population and an acceptable specificity.
-
▸
As used in this study, magnesium citrate appeared to be an inadequate ‘boost’ for CC2.
How might it impact on clinical practice in foreseeable future
-
▸
CC2 has adequate sensitivity for use in patients who are at elevated risk of complications from or decline standard colorectal cancer screening, or who have had an incomplete colonoscopy.
Introduction
Capsule colonoscopy is a technology in progress which may serve as an additional minimally invasive modality for colon cancer screening.1–5 The technology may be particularly helpful in patients who decline or are at elevated risk of complications from standard colonoscopy or who have had an incomplete colonoscopy.6–9 The limitations of first-generation capsule colonoscopy have been well documented and include low sensitivity and specificity for the detection of adenomas and cancer, suboptimal bowel cleansing, and incomplete study rates.10 In a European multicentre study, the sensitivities for the detection of adenomas ≥10 mm and cancer were only 64% and 74%, respectively.11
The second-generation capsule features several technological advancements. These include a motion-dependent variable frame rate (4 and 35 frames per second when the capsule is stationary and in motion, respectively), capsule power management, imaging enhancements (field of view, image quality), and an improved software interface.9 Recently, second-generation capsule colonoscopy (CC2) was evaluated in a screening population in 16 centres in the USA and Israel and was found to have high sensitivity for the detection of patients with conventional adenomas ≥6 mm in size, but suboptimal sensitivity for the detection of patients whose only lesions ≥6 mm in size were in the serrated class, although a subsequent Japanese study showed good CC2 sensitivity for flat and sessile serrated polyps.12 13
The study described here is the only other evaluation of CC2 performed in the USA and was conducted as a preliminary evaluation prior to the above-mentioned trial.12 This preliminary trial tested a different bowel cleansing regimen and is the only US trial to assess capsule performance in a mixed symptomatic and screening population. We now present the results of the preliminary investigation, since this study adds to the available evidence regarding the performance of the second-generation capsule in the detection of polyps.
Materials and methods
The second-generation capsule system tested in the study consists of the ingestible CC2 sensors attached to the abdomen which receive capsule images, a portable data recorder and CC2 software which processes the images for display and review on a computer workstation.14 The specific goals of this study included the initial exploration of the performance of CC2 in a population enriched for polyps, as well as the assessment of magnesium citrate as a ‘boost’ to facilitate study completion and maintain adequate cleansing. The primary end point of the study was the accuracy of the detection of colorectal polyps ≥6 and ≥10 mm as compared with conventional colonoscopy. The study was approved by the Institutional Review Boards with human protections oversight at each of the study centres. This trial was registered on http://clinicaltrials.gov (number NCT01087528).
Study design
The study was conducted at four US endoscopy centres, including two academic medical centres and two private practice facilities, from July 2009 to March 2010. The investigation was designed for 50 participants to participate. Participants were contacted who were previously scheduled for conventional colonoscopy with screening, surveillance, or diagnostic indications. Eligible participants who agreed to participate gave informed consent and underwent the CC2 procedure, followed by the colonoscopy procedure on the same day. The CC2 video was interpreted by a gastroenterologist at each study site who was experienced at reading capsule studies and had participated in previous trials with the first-generation colon capsule. The conventional colonoscopy was performed by a different experienced colonoscopist, and the individual reading the CC2 video and the individual performing the colonoscopy were blinded to the results of the other study.
Participant inclusion criteria
Eligible participants were between the ages of 18 and 70, and with indications for conventional colonoscopy, including clinical symptoms (eg, rectal bleeding, haematochezia, melena, positive faecal occult blood test), recent change in bowel habits patients ≥50 years, a polyp ≥10 mm on a prior radiographic test or sigmoidoscopy, a personal history of polyp(s) ≥6 mm in size that was removed at least 3 years ago, or colorectal cancer screening if age was ≥60 years.
Participant exclusion criteria
Patients were excluded if they had known significant medical conditions (eg, congestive heart failure, renal disease), allergy, or other known contraindications to the medications used in the study, a cardiac pacemaker or other implanted electromedical device, or a scheduled MRI examination within 7 days after capsule ingestion. Additional reasons for exclusion include factors associated with an increased risk for capsule retention such as dysphagia or swallowing disorder, prior gastrointestinal tract surgery (if judged a risk by the investigators), Crohn's disease, intestinal tumours, radiation enteritis, incomplete colonoscopy due to obstruction, non-steroidal anti-inflammatory drug (NSAID) enteropathy, and known gastrointestinal motility disorders including delayed gastric emptying. Also excluded were participants with any condition precluding compliance with the study or device instructions, current participation in another clinical study and women who are either pregnant or of childbearing potential and not practising medically acceptable methods of contraception.
The CC2 procedure
The study participants ingested clear liquids on the day prior to the capsule procedure and colonoscopy. Patients ingested 4 L of polyethylene glycol-electrolyte lavage solution, split as 2 L the evening before and 2 L on the morning of the examination day with completion at least 45 min prior to capsule ingestion. The capsule was ingested at the study centre. An initial ‘boost’ was administered after the capsule had exited the stomach to propel the capsule and promote adequate cleansing, which consisted of 8 ounces of magnesium citrate to facilitate capsule propulsion. Exit from the stomach was determined by automated detection and notification by the digital recorder, or real-time assessment of images by the physician investigator.15 Metoclopramide 10 mg could be administered orally if the capsule was delayed in the stomach. A second boost consisting of a 5-ounce dose of magnesium citrate was administered if the examination was not complete 3 h after the first boost. A dose of simethicone with the boosts could be used to reduce image decrement by bubbles. If the capsule was not expelled 5 h after the first boost, a 10 mg bisacodyl suppository was administered. The study participant recorded the time the capsule exited the body which was used to calculate the total transit time. The procedure was complete when the capsule was expelled, or the system was disconnected if the capsule was not expelled 8 h after ingestion.
Capsule video review
The CC2 videos were read by an experienced capsule reader at the study site who was blinded to the results of the colonoscopy, and within 1 week of the CC2 procedure. The colon preparation was scored as excellent, good, fair, or poor using a validated grading system in five colon segments (caecum, ascending, transverse, descending/sigmoid, and rectum).16 An overall cleansing score was also assessed. The colon preparation was graded as ‘adequate’ with a score of either excellent or good. The size of the identified polyps was measured using a software tool developed for this purpose. The location was estimated during video reading using landmarks in the video that were based on anatomical structures (caecum, hepatic flexure, splenic flexure, and rectum) and software which displayed the approximate position of the capsule in the abdomen–pelvis region.
Colonoscopy procedure
Conventional colonoscopy was performed on the same day as the capsule study, even if the capsule examination was not yet complete, and with the performing gastroenterologist blinded to the results of the capsule study. A minimal withdrawal time of 6 min was used. The caecal landmarks were noted and photographed in each case, and the withdrawal time was recorded. The colon preparation was scored as excellent, good, fair or poor using a standard grading system in five colon segments (caecum, ascending, transverse, descending/sigmoid, and rectum) with colonoscopy washings factoring into the grading. An overall cleansing score was also assessed. The colon prep was graded as ‘adequate’ with a score of either excellent or good. All colonoscopies were video recorded for future reference. Each detected polyp was documented by location using endoscopic landmarks, by size with comparison to an open forceps of 9 mm diameter, and by endoscopic features.17 All polyps were sent for pathological assessment locally at the study centres. For polyps identified on CC2 study, but not on colonoscopy, a second colonoscopy was left to the discretion of the site investigator and the patient's referring provider.18 19
Polyp matching in the CC2 examination and colonoscopy
Polyps were examined in each patient and were considered as matching if the calculated size ranges for the CC2 and colonoscopy studies overlapped, and if the location estimates were in the same or adjacent colon segment. The size range for each identified polyp for both studies was calculated as the observed size±50%. Thus, if a polyp measured 12 mm by colonoscopy, its±50% range would be 6–18 mm. If a polyp in the same or an adjacent segment by CC2 capsule was 8 mm, its±50% range would be 4–12 mm.20 If the locations were in agreement and the two size ranges overlap, the polyps would be a potential match. The larger of the two observed sizes was used to assign polyps to the groups ≤5, ≥6 and/or ≥10 mm. If CC2 did not visualise a segment, polyps identified by colonoscopy in that segment could give a false negative result for capsule.
Pathology assessment
Conventional adenomas included lesions classified by histology as tubular, tubulovillous or villous adenomas. Lesions classified as sessile serrated polyps (adenomas) or hyperplastic polyps were grouped as serrated class lesions.
Patient safety and follow-up
All participants were interviewed at each study visit and by telephone 7–14 days after the procedures to assess for well-being and for adverse events. An adverse event was any undesirable experience (sign, symptom, illness, abnormal laboratory value or other medical event) that appeared or worsened during the study. Serious adverse events were those resulting in death, life-threatening experience, hospitalisation, congenital anomaly or birth defect, or medical or surgical intervention to prevent permanent impairment.
Statistical considerations
The purpose of the study was to permit the initial exploration of the performance of CC2 in a population enriched for polyps and to assess the performance of magnesium citrate as a boost to ensure study completion. The sample size was set at 50 patients to allow for adequate participation at each of the study centres. The primary outcome of the study was the per-patient accuracy of the detection of colorectal polyps ≥6 and ≥10 mm as compared with conventional colonoscopy. The secondary end points included the assessment of the diagnostic yield for detecting colonic lesions, colon cleansing level in different colon segments, capsule excretion times and completion rate, physician reading time, and the assessment of adverse events.
Results
These are the final results of clinicaltrials.gov number NCT01087528. A total of 51 patients were enrolled (figure 1), with a mean age of 60.2 years (range 32–70), of whom 55% (n=28) were female. The mean weight was 84 kg (58–146 kg) and the mean height was 173 cm (150–193 cm). One participant was excluded from the analysis, who withdrew before capsule ingestion. The indications for colonoscopy included average-risk screening (n=28), polyp surveillance (n=11), change in bowel habits (n=16), rectal bleeding (n=7), abdominal pain (n=6) and positive faecal occult blood test (n=1); some patients had more than one indication.
Figure 1.
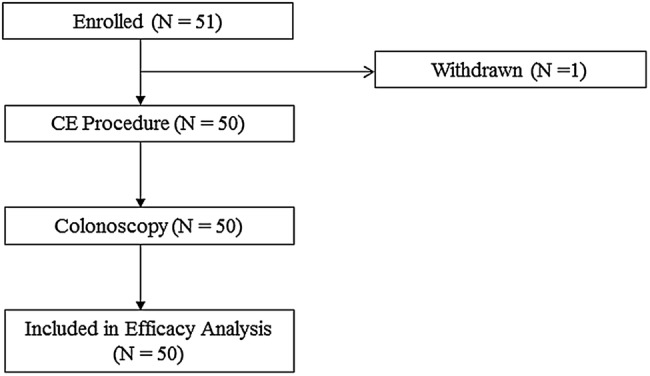
Colon capsule evaluation: patient flow.
For ≥6 mm polyps, 30% of the study population was positive (15/50), while 14% were positive for ≥10 mm polyps (7/50). For polyps 6 mm or larger identified on conventional colonoscopy, the overall CC2 per-patient sensitivity in an intention to diagnose analysis was 93.3% (95% CI 66.0% to 99.7%) and the specificity was 80% (95% CI 62.5% to 90.9%). For lesions 10 mm or larger, the overall CC2 per-patient sensitivity was 100% (95% CI 56.1% to 100.00%) with a specificity of 93.0% (95% CI 79.9% to 98.2%). Table 1 shows the intention to diagnose the performance of the CC2 examination for the detection of polyps ≥6 mm and polyps ≥10. The single ≥6 mm false negative was the result of an incomplete capsule examination. The patient had four ≥6 mm rectal polyps identified by conventional colonoscopy, but the capsule was prematurely removed from the sigmoid colon after 9 h and 4 min, prior to entering the rectum.
Table 1.
Intention to diagnose per-patient performance of second-generation capsule colonoscopy in the detection of polyps
| Polyp size (mm) | Polyp prevalence n (%) | CC2 per segment matching analysis |
|
|---|---|---|---|
| (N=50 patients) | Sensitivity % (95% CI) |
Specificity % (95% CI) |
|
| ≥6 | 15 (30%) | 93.3 (66.0 to 99.7) | 80.0 (62.5 to 90.9) |
| ≥10 | 7 (14%) | 100.0 (56.1 to 100.0) | 93.0 (79.9 to 98.2) |
CC2, second-generation capsule colonoscopy.
There were no cancers detected by either method in this study. Diverticulosis was detected in 82% and 61% of the patients by CC2 and colonoscopy, respectively. In two participants, vascular ectasias were observed only in the capsule study. There were too few polyps to perform a meaningful assessment of polyps by histologic subtype. As noted, repeat colonoscopy was left to the discretion of the site investigator in patients with polyps detected by CC2, but not by colonoscopy. One patient had multiple polyps detected by CC2, including 23 and 12 mm lesions in the ascending colon (figure 2 A, B), but only a 9 mm polyp in the descending colon was noted in the original colonoscopy; this was the only patient who underwent a second conventional colonoscopy to confirm CC2 findings. The second colonoscopy confirmed the CC2 findings, demonstrating a 20 and a 10 mm polyp in the ascending and transverse colon, respectively (figure 2 C, D). This patient was considered as a true positive for CC2 in polyp detection. There were seven additional cases where CC2 identified a ≥6 mm polyp and a second colonoscopy was not performed. Although it is impossible to determine the effect of absent follow-up on specificity, if the results from the recent US trial are representative of this study, approximately 42% of initial false positives would have been converted to true positives after a second colonoscopy.12 In our study, 2–3 additional patients with ≥6 mm polyps on CC2 would have been converted to true positives, with a resulting specificity of 84.8–87.5%. It was also shown by Rex et al12 that the second colonoscopy does not identify all lesions that appear as very convincing polyps by CC2, so the true specificity may be even higher.
Figure 2.
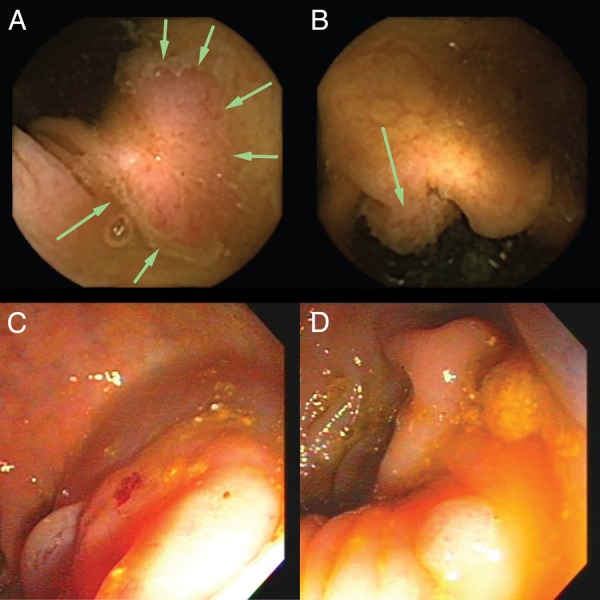
(A–D) Colon capsule images of polyps that were missed on initial colonoscopy. Capsule images of a 23 mm flat polyp (A) and a 12 mm sessile polyp (B), both of which were located in the ascending colon in a single patient. Neither polyp was identified on the initial colonoscopy, which prompted a second colonoscopy, where the matching lesions were identified (C and D, respectively).
The overall cleansing level was graded ‘adequate’ in 61% (95% CI 46% to 75%) of patients (table 2). The segmental scores were graded as adequate in 43% in the rectum, 83% in the sigmoid/descending colon, 59% in the transverse colon, 61% in the ascending colon, and 63% in the caecum. At colonoscopy, an ‘adequate’ overall cleansing was achieved in 86% (95% CI 73% to 94%), and segmental scores were adequate in 92% in the rectum, 86% in the sigmoid/descending colon, 82% in the transverse colon, 88% in the ascending colon and 88% in the caecum.
Table 2.
Percentage of studies with adequate bowel cleansing level by colon segment
| Colon segment | Capsule study % Adequate (95% CI) |
Colonoscopy % Adequate (95% CI) |
|---|---|---|
| Overall assessment | 61% (46% to 75%) | 86% (73% to 94%) |
| Rectum | 43% (31% to 67%) | 92% (81% to 98%) |
| Descending colon | 83% (67% to 93%) | 86% (73% to 94%) |
| Transverse colon | 59% (42% to 74%) | 82% (69% to 91%) |
| Ascending colon | 61% (46% to 75%) | 88% (75% to 95%) |
| Caecum | 63% (47% to 76%) | 88% (75% to 95%) |
The bowel cleansing level was assessed by the gastroenterologist performing the capsule study or colonoscopy; a preparation grading of excellent or good was considered ‘adequate’. At colonoscopy, the grading was performed after washings, if used.
Approximately, two-thirds (64%, n=32) of the capsule procedures were complete within 8 h, before the colonoscopy was performed. For incomplete studies, the last capsule images were recorded in the small bowel (n=1), caecum (n=1), ascending colon (n=5), transverse colon (n=1), descending colon (n=2), sigmoid colon (n=7) and rectum (n=1). CC2 excretion was <4 h in 16% of patients, 4–6 h in 26%, 6–8 h in 16% and ≥8 h in 42%. Across all patients, there were a total of 10 polyps that were ≥10 mm, 16 in the 6–9 mm size range and 116 that were ≤5 mm detected by colonoscopy. For capsule, 11 polyps were observed to be ≥10 mm, 22 were identified in the 6–9 mm range and 66 were ≤5 mm (table 3).
Table 3.
Size and distribution of all polyps detected across the 48 participants by second-generation capsule colonoscopy and colonoscopy
| CC2 | Colonoscopy | |
|---|---|---|
| Polyps by size (mm) | ||
| ≤5 | 66 (66.7%) | 116 (81.6%) |
| 6–9 | 22 (22.2%) | 16 (11.3%) |
| ≥10 | 11 (11.1%) | 10 (7.0%) |
| Polyps by location | ||
| Caecum | 14 (14.1%) | 8 (5.6%) |
| Ascending | 18 (18.2%) | 26 (18.3%) |
| Transverse | 5 (5.1%) | 27 (19.0%) |
| Descending | 26 (26.3%) | 23 (16.2%) |
| Sigmoid | 9 (9.1%) | 14 (9.9%) |
| Rectum | 27 (27.3%) | 44 (31.0%) |
| Total | 99 | 142 |
CC2, second-generation capsule colonoscopy.
The capsule reading times were 20–29 min in 3 patients, 30–39 min in 9 patients, 40–49 min in 10 patients, 50–59 min in 9 patients and ≥60 min in 19 patients. The overall reading time was <60 min in 62% of patients.
There were no serious adverse events in the study. Two patients reported minor adverse events, including one with mild abdominal bruising, and one with moderate abdominal pain, both of which were considered unlikely to be related to the study by the investigators.
Discussion
We report the initial US experience with the second-generation colon capsule for the evaluation of colorectal polyp detection and the only US study to assess the performance in a mixed screening and symptomatic population. The specific goal of this study was to explore the performance of CC2 in a population enriched for polyps, and to evaluate the efficacy of magnesium citrate as a ‘boost’ agent to facilitate the study completion. The sensitivity of the CC2 examination for polyp detection was similar, although numerically higher, to that reported in the subsequent large multinational trial, despite a lower percentage of complete transit studies, and inferior bowel prep on average.12 With the use of magnesium citrate as the boost, the overall quality of the preparation was inferior to that in the subsequent large multinational trial,12 which used oral sulfate solution as the boost, accounting for the improved completion rate and superior colon prep.
In comparison, the specificity in the current study was approximately equivalent to other studies, with the exception of a lower specificity for polyps ≥6 mm than the study by Rex et al.12 21 22 This may reflect difficulties in matching polyps, or the fact that colonoscopists in the large multicentre trial were non-blinded after performance of the initial colonoscopy with the opportunity to verify that polyps detected by capsule were true positives rather than false positives.12 In addition, in our study, the use of the magnesium citrate as a boosting and prep agent may have influenced the specificity. Finally, the presence of symptomatic patients in this study may have prompted readers to more aggressively report polyps.
Capsule colonoscopy is an approach to minimally invasive colon cancer screening, which is a technology in evolution.1 2 4 13 23–25 CC2 is an improvement over the first-generation device, driven by a wider imaging field of view, a variable image acquisition rate, an improved software interface, and further modifications of the bowel prep and capsule boost regimen. The performance challenges to achieve higher rates of study completion and adequate visualisation remain. Technologic innovations will continue to improve certain aspects of these challenges. Capsule colonoscopy will be evaluated against optical colonoscopy and other non-invasive approaches, such as CT colonography and stool-based tests (eg, faecal immunochemical test (FIT) and DNA assays).8 20 26 27 Currently, the niche for capsule colonoscopy in the US includes patients who are at elevated risk of complications from or decline standard screening, or who have had an incomplete colonoscopy—which may expand the pool of those screened for colorectal cancer.
Study strengths include the first US investigation of the performance of the second-generation capsule, in a prospective study design, and same day colonoscopy. Our study was a multicentre study with academic and private medical facilities, with experienced gastroenterologists. Incomplete capsule examinations were considered as complete for the purpose of matching with conventional colonoscopy, thereby providing a disadvantage for CC2 sensitivity. This is the only US study to use magnesium citrate as a ‘boost’ and to assess capsule performance in a mixed screening and symptomatic population.
The primary limitation was the limited sample size inherent in a preliminary investigation. Consequently, it is difficult to ascertain whether the numerically higher sensitivity in this study as compared with the only other US CC2 trial, which included strictly screening patients, is related to the presence of symptomatic patients, or other causes.12 As a result, the sensitivity documented in this study may not be generalisable to the typical screening population. The use of magnesium citrate as the boosting agent was a limitation, as the cleansing level was graded adequate in only two-thirds of patients, although this finding was important for the design of subsequent studies; the low level of adequate cleansing in the rectum also makes the results in this segment less reliable. CC2 completion rates may not be reflective of clinical practice; the conventional colonoscopy was performed after approximately 8 h, which prematurely terminated the examination in some cases. Finally, CC2 ‘false positives’ were generally not confirmed by a second colonoscopy, which likely adversely affected the specificity.
In conclusion, in the initial US experience in a limited population of patients with features designed to enrich their prevalence of colon polyps, CC2 had adequate sensitivity for detecting patients with polyps ≥6 mm in size. Several trials have now shown good sensitivity for the detection of polyps in this size range by the second-generation capsule.12 21 22 28 Herein, magnesium citrate was relatively ineffective as a boost agent for capsule colonoscopy.
Footnotes
Contributors: DRM, PRM, DPR, and DKR substantially contributed to the conception or design of the work, its acquisition, gave critical revisions of the article, and final approval of the version to be published. DRM and DKR were involved in the analysis and interpretation of the data, and drafting the article.
Funding: This study was funded in part by Given Imaging, Inc, now part of Medtronic.
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: The study was approved by the Institutional Review Boards with human protections oversight at each of the study centres.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Wang A, Banerjee S, Barth BA, et al. , ASGE Technology Committee. Wireless capsule endoscopy. Gastrointest Endosc 2013;78:805–15. doi:10.1016/j.gie.2013.06.026 [DOI] [PubMed] [Google Scholar]
- 2.Slawinski PR, Obstein KL, Valdastri P. Emerging issues and future developments in capsule endoscopy. Tech Gastrointest Endosc 2015;17:40–6. doi:10.1016/j.tgie.2015.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Gossum A. Wireless capsule endoscopy of the large intestine: a review with future projections. Curr Opin Gastroenterol 2014;30:472–6. doi:10.1097/MOG.0000000000000103 [DOI] [PubMed] [Google Scholar]
- 4.Tal AO, Vermehren J, Albert JG. Colon capsule endoscopy: current status and future directions. World J Gastroenterol 2014;20:16596–602. doi:10.3748/wjg.v20.i44.16596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology 2008;134:1570–95. doi:10.1053/j.gastro.2008.02.002 [DOI] [PubMed] [Google Scholar]
- 6.Lowe AW, Moseley RH. Covering the cover. Gastroenterology 2015;148:865–7. doi:10.1053/j.gastro.2015.03.03125824350 [Google Scholar]
- 7.Adrián-de-Ganzo Z, Alarcón-Fernandez O, Ramos L, et al. Uptake of colon capsule endoscopy vs colonoscopy for screening relatives of patients with colorectal cancer. Clin Gastroenterol Hepatol 2015;13:2293–301.e1. doi:10.1016/j.cgh.2015.06.032 [DOI] [PubMed] [Google Scholar]
- 8.Spada C, Hassan C, Barbaro B, et al. Colon capsule versus CT colonography in patients with incomplete colonoscopy: a prospective, comparative trial. Gut 2015;64:272–81. doi:10.1136/gutjnl-2013-306550 [DOI] [PubMed] [Google Scholar]
- 9.Spada C, Hassan C, Costamagna G. Colon capsule endoscopy. Gastrointest Endosc Clin N Am 2015;25:387–401. doi:10.1016/j.giec.2014.11.007 [DOI] [PubMed] [Google Scholar]
- 10.Bretthauer M. The capsule and colorectal-cancer screening--the crux of the matter. N Engl J Med 2009;361:300–1. doi:10.1056/NEJMe0903924 [DOI] [PubMed] [Google Scholar]
- 11.Van Gossum A, Munoz-Navas M, Fernandez-Urien I, et al. Capsule endoscopy versus colonoscopy for the detection of polyps and cancer. N Engl J Med 2009;361:264–70. doi:10.1056/NEJMoa0806347 [DOI] [PubMed] [Google Scholar]
- 12.Rex DK, Adler SN, Aisenberg J, et al. Accuracy of capsule colonoscopy in detecting colorectal polyps in a screening population. Gastroenterology 2015;148:948–957.e2. doi:10.1053/j.gastro.2015.01.025 [DOI] [PubMed] [Google Scholar]
- 13.Saito Y, Saito S, Oka S, et al. Evaluation of the clinical efficacy of colon capsule endoscopy in the detection of lesions of the colon: prospective, multicenter, open study. Gastrointest Endosc 2015;82:861–9. doi:10.1016/j.gie.2015.02.004 [DOI] [PubMed] [Google Scholar]
- 14.Eliakim R. The PillCam COLON capsule for colon cancer screening: comparison between the first- and second-generation capsules. Hosp Pract (1995) 2010;38:110–16. doi:10.3810/hp.2010.06.303 [DOI] [PubMed] [Google Scholar]
- 15.Adler S, Hassan C, Metzger Y, et al. Accuracy of automatic detection of small-bowel mucosa by second-generation colon capsule endoscopy. Gastrointest Endosc 2012;76:1170–4. doi:10.1016/j.gie.2012.07.034 [DOI] [PubMed] [Google Scholar]
- 16.Leighton JA, Rex DK. A grading scale to evaluate colon cleansing for the PillCam COLON capsule: a reliability study. Endoscopy 2011;43:123–7. doi:10.1055/s-0030-1255916 [DOI] [PubMed] [Google Scholar]
- 17.Rex DK, Rabinovitz R. Variable interpretation of polyp size by using open forceps by experienced colonoscopists. Gastrointest Endosc 2014;79:402–7. doi:10.1016/j.gie.2013.08.030 [DOI] [PubMed] [Google Scholar]
- 18.Rex DK, Cutler CS, Lemmel GT, et al. Colonoscopic miss rates of adenomas determined by back-to-back colonoscopies. Gastroenterology 1997;112:24–8. doi:10.1016/S0016-5085(97)70214-2 [DOI] [PubMed] [Google Scholar]
- 19.Rex DK, Schoenfeld PS, Cohen J, et al. Quality indicators for colonoscopy. Gastrointest Endosc 2015;81:31–53. doi:10.1016/j.gie.2014.07.058 [DOI] [PubMed] [Google Scholar]
- 20.Tierney WM. Colonoscopy versus capsule: sharing the spotlight. Gastroenterology 2015;148:892–4. doi:10.1053/j.gastro.2015.03.020 [DOI] [PubMed] [Google Scholar]
- 21.Eliakim R, Yassin K, Niv Y, et al. Prospective multicenter performance evaluation of the second-generation colon capsule compared with colonoscopy. Endoscopy 2009;41:1026–31. doi:10.1055/s-0029-1215360 [DOI] [PubMed] [Google Scholar]
- 22.Spada C, Hassan C, Munoz-Navas M, et al. Second-generation colon capsule endoscopy compared with colonoscopy. Gastrointest Endosc 2011;74:581–589.e1. doi:10.1016/j.gie.2011.03.1125 [DOI] [PubMed] [Google Scholar]
- 23.Health Quality Ontario. Colon capsule endoscopy for the detection of colorectal polyps: an evidence-based analysis. Ont Health Technol Assess Ser 2015;15:1–39. eCollection 2015 [PMC free article] [PubMed] [Google Scholar]
- 24.Palimaka S, Blackhouse G, Goeree R. Colon capsule endoscopy for the detection of colorectal polyps: an economic analysis. Ont Health Technol Assess Ser 2015;15:1–43. eCollection 2015 [PMC free article] [PubMed] [Google Scholar]
- 25.Spada C, Barbaro F, Andrisani G, et al. Colon capsule endoscopy: what we know and what we would like to know. World J Gastroenterol 2014;20:16948–55. doi:10.3748/wjg.v20.i45.16948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spada C, Hassan C, Costamagna G. Colon capsule endoscopy in colorectal cancer screening: a rude awakening from a beautiful dream? Clin Gastroenterol Hepatol 2015;13:2302–4. doi:10.1016/j.cgh.2015.08.027 [DOI] [PubMed] [Google Scholar]
- 27.Rondonotti E, Pennazio M. Colorectal polyp diagnosis: results with the second-generation colon capsule (CCE-2). Colorectal Dis 2015;17(Suppl 1):31–5. doi:10.1111/codi.12819 [DOI] [PubMed] [Google Scholar]
- 28.Holleran G, Leen R, O'Morain C, et al. Colon capsule endoscopy as possible filter test for colonoscopy selection in a screening population with positive fecal immunology. Endoscopy 2014;46:473–8. doi:10.1055/s-0034-1365402 [DOI] [PubMed] [Google Scholar]


