Abstract
Defects in the mitochondrial respiratory chain (RC) underlie a spectrum of human conditions, ranging from devastating inborn errors of metabolism to aging. We performed a genome-wide, Cas9-mediated screen to identify factors that are protective during RC inhibition. Our results highlight the hypoxia response, an endogenous program evolved to adapt to limiting oxygen availability. Genetic or small molecule activation of the hypoxia response is protective against mitochondrial toxicity in cultured cells and zebrafish models. Chronic hypoxia leads to a marked improvement in survival, body weight, body temperature, behavior, neuropathology and disease biomarkers in a genetic mouse model of Leigh syndrome, the most common pediatric manifestation of mitochondrial disease. Further preclinical studies are required to assess whether hypoxic exposure can be developed into a safe and effective treatment for human diseases associated with mitochondrial dysfunction.
Mitochondria are ancient organelles that are essential for normal physiology and health. The respiratory chain (RC) is crucial to mitochondrial function and generates approximately 90% of cellular ATP via oxidative phosphorylation (1). In the oxidative step, four large protein complexes transfer electrons from NADH (the reduced form of nicotinamide adenine dinucleotide) or FADH (the reduced form of flavin adenine dinucleotide) to oxygen while generating a proton gradient. Approximately 90% of the oxygen we breathe is utilized as a substrate for the RC (1). In the phosphorylation step, the proton gradient is dissipated by a fifth and final complex to generate ATP. Numerous additional chemical reactions and transport processes are intimately coupled to the redox and proton pumping activities of the RC.
A spectrum of human diseases result from a faulty RC (2-4). Virtually all age-related disorders, including type 2 diabetes, neurodegeneration, and sarcopenia, are accompanied by a quantitative decline in the activity of the mitochondrial RC. The aging process itself is associated with a gradual decrease of oxidative phosphorylation in multiple tissues. Monogenic disorders of the mitochondrial RC represent the largest class of inborn errors of metabolism. To date, lesions in over 150 genes, encoded by the nuclear or mitochondrial (mtDNA) genomes, have been identified as disease-causing. Mutations in these genes lead to a biochemical deficiency of one or more of the RC complexes, resulting in either tissue-specific or multisystem disease with devastating effects on human health. Patients with RC disorders can present with blindness, deafness, gray or white matter brain disease, cardiomyopathy, skeletal muscle myopathy, GI dysmotility, anemia, ataxia, liver disease and kidney disease.
Management of these disorders remains challenging (5, 6). While individual mutations are rare, the overall disease burden of mitochondrial disease is significant with an estimated prevalence of 1:4300 live births (7). Therefore, a general and effective therapeutic is needed. The current mainstay of managing mitochondrial disease involves the use of vitamin co-factors (CoQ, α-lipoic acid, riboflavin, L-carnitine) (8). Other proposed strategies include the use of small molecule bypass of defective RC components, using electron carriers such as idebenone, and antioxidants. None of these approaches have demonstrated efficacy in randomized controlled clinical trials.
Several lines of evidence point to the existence of endogenous coping mechanisms for mitochondrial dysfunction. It is notable that mitochondrial disorders can be highly tissue-specific and episodic (2, 9). These disorders are often triggered by drugs, alcohol, or viral illnesses, implying that a genetic lesion is not always sufficient to cause cellular dysfunction, but rather that the lesion may need to be compounded with an environmental insult. Such observations suggest the existence of cellular pathways or environments that buffer against mitochondrial lesions.
A genome-wide screen to spotlight suppressors of mitochondrial disease
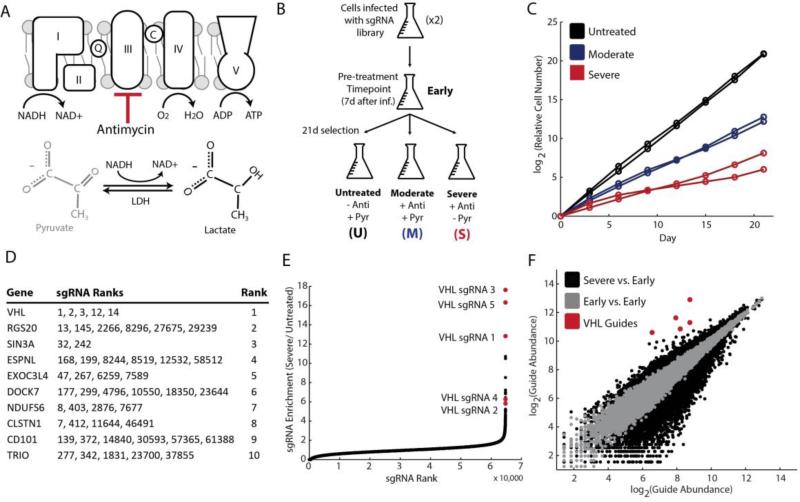
We modeled mitochondrial disease in the human leukemic suspension cell line, K562, and performed a Cas9-mediated knockout screen (10, 11). We used the natural product, antimycin, as a complex III inhibitor of the respiratory chain. In the presence of antimycin, the respiratory chain is unable to oxidize high energy reducing equivalents to power ATP production; however, cytoplasmic lactate dehydrogenase maintains NAD+ redox balance. Removal of pyruvate exacerbates reductive stress, further preventing cell proliferation (12). We modeled mitochondrial disease with the addition of antimycin alone (moderate disease) or antimycin in combination with removal of pyruvate (severe disease), using cell growth as a proxy for disease magnitude (Fig. 1A). We infected K562 cells with a ~65,000 single guideRNA (sgRNA) library, targeting ~18,000 genes (10). After one week of genome editing, we transferred the pool of knockout cells to experimental conditions of untreated, moderate disease and severe disease states (Fig. 1B). We collected samples for an enrichment screen by allowing the knockout pool to grow in selection conditions for three weeks. The relative growth between untreated and moderate disease conditions was 300-fold and between untreated and severe disease conditions was 7,000-fold (Fig. 1C).
Figure 1. Genome-scale Cas9-mediated knockout screen identifies VHL inhibition as protective during states of mitochondrial dysfunction.
(A) Mitochondrial disease was modeled with the addition of the complex III inhibitor, antimycin (moderate disease) or antimycin and removal of pyruvate (severe disease). (B) K562 cells were infected in duplicate with the genome-scale Cas9-mediated knockout library, and separated into conditions of untreated, moderate disease or severe disease. Samples were taken at a pre-treatment time point, as well as after three weeks of selection. (C) Growth curves for cumulative differences in growth rates in different experimental conditions for both infection replicates. (D) RIGER output based on enrichment of sgRNAs in severe disease condition relative to pre-treatment conditions. Each row denotes a single gene, with ranks of corresponding sgRNAs in middle column. Ranks for individual sgRNAs are out of ~65,000 total sgRNAs in library. (E) sgRNA enrichment magnitude vs. rank, with most enriched sgRNA shown to the far right. sgRNAs corresponding to VHL in red. (F) Guide abundance in pre-treatment conditions (Infection 1 vs. Infection 2) shown in grey for each sgRNA, representative of experimental noise. Guide abundance in severe disease condition vs. pre-treatment condition in black, with VHL sgRNAs in red.
As expected, three weeks of genome editing in untreated cells led to a significant depletion of sgRNAs corresponding to essential genes, including those related to transcription, translation, and splicing (fig. S1). Nearly 20% of the 500 most essential genes were mitochondrial proteins, especially mitochondrial ribosomal proteins and electron transport chain subunits (table S1). As mitochondrial proteins make up approximately 5% of the proteome (13), this enrichment highlights the dramatic effects of mitochondrial dysfunction on viability.
Of the ~18,000 genes tested, the knockout screen identified inhibition of the Von Hippel-Lindau (VHL) factor as the most effective genetic suppressor of mitochondrial disease, in both the moderate and severe disease conditions (Fig. 1D). RIGER analysis ranked VHL knockout cells as the most enriched over time in both infection replicates corresponding to severe and moderate disease (table S2). The five sgRNAs spanning all three exons of VHL ranked 1, 2, 3, 12 and 14 out of ~65,000 total guides for enrichment in disease conditions relative to pre-treatment conditions (Fig. 1D-F, fig. S2-3). Furthermore, the most significant VHL sgRNAs were enriched greater than 20-fold in disease states (fig. S4). Of note, VHL knockout cells were also enriched in untreated conditions over time, reflecting an overall effect on cell growth. However, this enrichment was significantly less than in disease conditions.
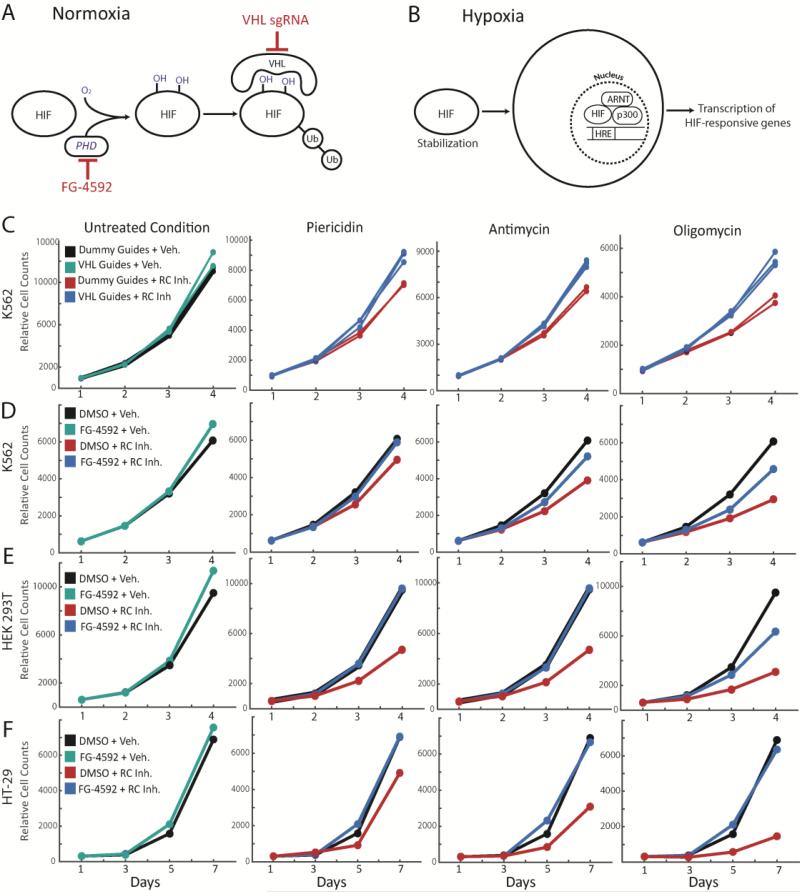
VHL activity is a key regulator of the hypoxic response pathway (14-15). Organisms have evolved elaborate mechanisms to adapt to fluctuating oxygen tensions and extreme environments. In normoxic conditions, the hypoxia inducible transcription factors (HIF) are constitutively made and hydroxylated by the prolyl-hydroxylase (PHD) enzymes (Fig. 2A) (16-18). The hydroxylated form is recognized by the ubiquitin ligase, VHL, and targeted for degradation. In response to environmental hypoxia, the PHD reaction does not take place, allowing HIF stabilization and activation of the hypoxia transcriptional program (Fig. 2B). VHL-knockout cells show HIF stabilization, even during normoxic conditions, thereby bypassing cellular oxygen sensing mechanisms (18-20). Our screen suggested that harnessing innate responses to hypoxia may be protective in the setting of inherited mitochondrial disease.
Figure 2. Genetic or small molecule activation of the HIF response is protective against multiple forms of RC inhibition, in multiple cell types.
(A) Schematic for HIF degradation during normoxia. (B) Schematic for induction of hypoxia transcriptional program during hypoxia. (C) Growth curves for K562 VHL-knockout cells (cyan, blue) or non-targeting sgRNA cells (black, red) for untreated or disease conditions (mean shown). Disease conditions correspond to inhibition of Complex I (piericidin), Complex III (antimycin) or Complex V (oligomycin). Growth curves for (D) K562 cells, (E) HEK293T cells and (F) HT-29 cells ± FG-4592, in combination with untreated or disease conditions (inhibition of complex I, III and V). All time points were measured in duplicate and all growth curves are representative of 2-3 independent experiments (mean shown). All final cell counts of FG-treated rescue (or VHL-KO rescue in 2A) in presence of RC inhibitor were statistically significant (one-sided t-test p-value < 0.05).
Genetic and small molecule proof-of-concept in cellular models
We validated and characterized the hypoxic response as a therapeutic target by testing the ability of VHL-knockout cells to withstand respiratory chain dysfunction. VHL-knockout cells showed increased cell proliferation in the presence of antimycin relative to non-targeting (dummy) sgRNA-modified cells (Fig. 2C). Furthermore, there was perfect correspondence between the degree of VHL sgRNA enrichment in the CRISPR screen and the rescue effect size of individual sgRNAs (fig. S5), likely reflecting differences in genome editing efficiencies. VHL-knockout cells were also more resistant to Complex I inhibition by piericidin and complex V (ATP synthase) inhibition by oligomycin, demonstrating the potentially broad utility of our therapeutic approach (Fig. 2C).
We next explored small molecules as tools for triggering the hypoxia response. While a VHL-inhibitor has been reported (21), it is not cell permeable. PHD inhibitors have been developed as investigational drugs for anemia and ischemic disorders (22). FG-4592 is currently in Phase III clinical trials for the treatment of anemia of chronic kidney disease and acts by upregulating the canonical marker of the hypoxia response, erythropoietin (Epo). We reasoned that FG-4592 treatment would mimic VHL-knockout, thus triggering a broader hypoxia transcriptional program. Normal growth rates were minimally increased by FG-4592. Complex I, III or V inhibition stunts cell growth, but not death (fig. S6) in most cell lines, including HT-29s, HEK 293Ts and K562s. Administering ~50μM FG-4592 in advance and during respiratory chain dysfunction nearly or completely rescued this growth defect, in a dose-dependent manner (Fig 2D-F, fig. S7). The nearly full rescue of the disease state across different cell lines and across chemical lesions highlights the general utility of our approach.
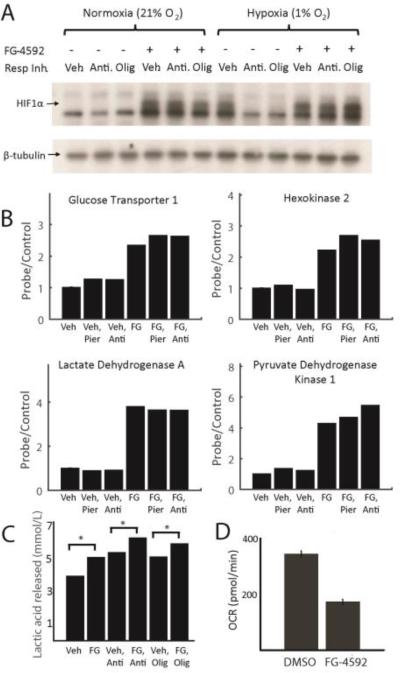
We characterized the rescue mechanism of FG-4592, by studying its effect on the hypoxia response and energy metabolism. While HIF1α is undetectable during normoxic exposure, treatment with FG-4592 stabilized the transcription factor even during normoxia. It has previously been noted that a paradox exists between severe mitochondrial dysfunction and cellular sensing of hypoxia (23). In cell culture, full inhibition of the RC prevents HIF stabilization, even under low oxygen conditions that would otherwise trigger the hypoxia response (23-24). Of note, FG-4592 treatment bypassed this paradox and enabled HIF1α stabilization in the face of mitochondrial dysfunction, during states of normoxia or hypoxia (Fig. 3A). Further work is needed to determine if the paradox contributes significantly to disease pathology, or whether it is simply a feature of severe respiratory chain blockade in cultured cells.
Figure 3. FG-4592 causes normoxic stabilization of HIF1α and rewires energy metabolism.
(A) Immunoblot showing HIF1α ± RC inhibition with antimycin or oligomycin, ± FG-4592 under normoxia (21% O2) or hypoxia (1% O2). RC inhibition prevents HIF1α stabilization during hypoxia. FG-4592 administration overcomes this paradox and stabilizes HIF1α even during normoxia. Immunoblot is representative of independent experiments done in duplicate in HT-29 cells. (B) Normalized expression for known HIF targets glucose transporter 1 (GLUT1), hexokinase 2 (HK2), lactate dehydrogenase A (LDHA), and pyruvate dehydrogenase kinase 1 (PDK1) +/− RC inhibition, +/− FG-4592 in HT-29 cells. Data shown as mean of two independent experiments and normalized so vehicle-treated expression (probe/control) is 1. (C) Mean concentration of lactic acid secreted by cells treated with FG-4592 or DMSO ± RC inhibitors as proxy for anaerobic glycolytic flux. Data shown for HEK293T cells (without pyruvate to eliminate contribution from LDH reaction) and is representative of at least two independent experiments (D) Basal oxygen consumption rates for HEK293T cells treated with FG-4592 or DMSO for > 24h, averaged across three independent experiments (Mean ± S.E.). (one-sided t-test p-value < 0.05 for all pairwise comparisons ± FG-4592 in figures 3B-3D).
The HIF transcriptional response is believed to be protective during states of hypoxia, at least in part by shifting the cell's reliance away from mitochondrial oxidative energy metabolism. The HIF1α response can preserve energy supply at low oxygen tensions in a redox neutral manner. Indeed, treatment with FG-4592 for 24h, upregulated transcription of glycolytic enzymes (Fig. 3B, fig. S8) such as the glucose transporter 1 (GLUT1), hexokinase 2 (HK2), and lactate dehydrogenase (LDHA). HIF1α activation is also known to shunt the carbon supply away from the TCA cycle and towards the LDH reaction (25-28), as evidenced by the significant upregulation of pyruvate dehydrogenase kinase (PDK1) (Fig. 3B). Although lesions to the respiratory chain and hypoxia can in principle limit respiratory chain activity, cells do not mount the hypoxia response upon RC inhibition as the signal is lacking. However, FG-4592 treatment artificially triggers the hypoxia transcriptional program, even during normoxic conditions (Fig. 3B, fig. S8).
To corroborate the transcriptional changes, we also measured lactic acid production and oxygen consumption as proxies of glycolysis and oxidative phosphorylation. Glycolysis was somewhat increased by RC inhibition, likely as a result of allosteric mechanisms (Fig. 3C). Treatment with FG-4592 increased glycolysis by nearly 25% in HEK293T cells under basal conditions and beyond allosteric mechanisms in response to RC inhibition. Furthermore, basal oxygen consumption was decreased by approximately 2-fold with FG-4592 treatment (Fig. 3D). This may be protective in the setting of mitochondrial dysfunction, as it may limit the amount of ROS produced by impaired electron transport.
Genetic and small molecule proof-of-concept in zebrafish models
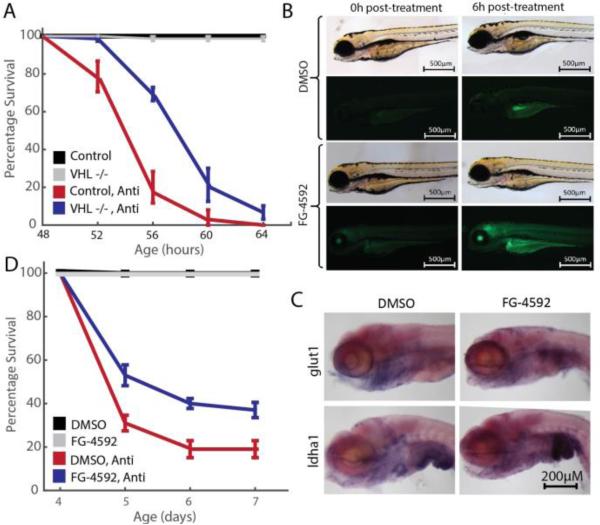
To further establish proof-of-concept, we asked whether genetic or small molecule activation of the hypoxia response would be protective against respiratory chain poisoning in zebrafish embryos. vhl-null zebrafish continuously upregulate the hypoxia response throughout early development (29). Just as vhl-knockout cells are protected against mitochondrial dysfunction, we asked whether vhl-null zebrafish might be more resilient to RC poisoning. Zebrafish embryos exhibit sensitivity to multiple, specific RC inhibitors including antimycin (30-32). We demonstrated a significant improvement in the survival of vhl-null embryos exposed to 2.5nM antimycin compared to heterozygous and wild-type controls (Fig. 4A).
Figure 4. vhl knockout or FG-4592 treatment activates the HIF response in zebrafish embryos and alleviates death caused by respiratory chain inhibition.
(A) 48hpf vhl-null zebrafish are less sensitive to RC inhibition than control (WT and Het) fish, n ≥ 75 per treatment, p < 0.001 by Mantel-Cox test. (B) FG-4592 treatment activates expression of HIF-responsive promoter in Tg(phd3::EGFP) embryos. Images are shown for embryos treated with either DMSO or 2.5μM FG-4592 from 96 to 102hpf. Embryos were assayed for GFP expression at 0 hours post treatment (hptx) and 6hptx. DMSO treatment fails to activate GFP expression beyond autofluorescence in Tg(phd3::EGFP) transgenic embryos, while FG-4592 robustly initiates GFP expression by 6hptx. (C) Known Hif targets, glut1 and ldha1 are overexpressed in 96hpf zebrafish embryos treated with FG-4592 for 6h. (D) Exposure to FG-4592 rescues antimycin-induced zebrafish embryonic death. Respiratory chain inhibition by 2.5nM antimycin in 4dpf (days post fertilization) embryos results in significant death within the first 24 hours of treatment. Co-exposure of antimycin with FG-4592 (2.5μM) doubles embryo survival, while FG-4592 alone has no impact. n=75 per treatment, p < 0.0001 by Mantel-Cox test.
We then extended our small molecule approach to the zebrafish model of RC dysfunction. A previously generated zebrafish reporter strain Tg(phd3::EGFP) expresses GFP under the control of a HIF-responsive promoter, thereby enabling in vivo assessment of activating the hypoxia transcriptional response (33, 34). FG-4592 treatment of Tg(phd3::EGFP) embryos at 96hpf resulted in a time-dependent increase in fluorescence of individual reporter fish (Fig. 4B). Furthermore, in situ hybridization for the glycolytic HIF targets glut1 and ldha1, demonstrated significant upregulation upon FG-4592 treatment (Fig. 4C), confirming that FG-4592 engages the zebrafish prolyl-hydroxylases to trigger the hypoxia transcriptional program. We then demonstrated that co-treatment of embryos with FG-4592 rescued antimycin-induced death by nearly 2-fold (Fig. 4D). The genetic and small molecule experiments in zebrafish provide proof of concept that activation of the hypoxia program can protect against insults to the mitochondrial respiratory chain.
Hypoxic breathing alleviates disease and extends lifespan in a mouse model of Leigh syndrome
The cellular and zebrafish models provided proof-of-concept that individual components of the cellular response to hypoxia are protective against mitochondrial toxins. While small molecules are capable of activating specific branches of the hypoxia program, we reasoned that they may not have as broad and potent an effect as the naturally evolved, whole-body physiological response to hypoxia itself. Moreover, small molecule drugs for activating the hypoxia response are currently in clinical trials for kidney disease and anemia. However, a large fraction of mitochondrial disease originates in the central nervous system, and our preliminary pharmacokinetic studies suggested limited blood-brain-barrier penetration of these drugs in mice. Higher doses resulted in whole-body toxicity. As mammals have evolved a complex homeostatic program to adapt to low oxygen tensions in their environment, we reasoned that a similarly broad hypoxic stress response might protect animal models of mitochondrial disease.
Leigh syndrome is the most common pediatric form of mitochondrial disease. Though relatively healthy at birth, patients develop irreversible neurodegeneration by two years of age (6). They suffer bilaterally symmetric lesions in the brain stem and basal ganglia, with marked gliosis. Most patients die between the ages of 3-16 months. To date, over 75 different genes have been implicated in this syndrome, with Complex I deficiency being the most frequent biochemical cause of disease. One of the more severe recessive forms of Leigh syndrome is caused by inactivation of the NDUFS4 gene, which codes for NADH:ubiquinone oxidoreductase subunit S4. Recently, a mouse model that recapitulates many features of Leigh Syndrome was generated by disruption of the murine Ndufs4 gene (35). Ndufs4 knockout (KO) mice breathing ambient air (21% O2) display retarded growth rates, impaired visual acuity and a delayed startle response. Their body temperature falls progressively until reaching 32C, shortly before death at 50-60 days of age. Diseased mice also display locomotor deficits and failure to thrive by 50d. Their neuropathology closely resembles clinical findings, with a substantial inflammatory response in the brainstem and cerebellum.
We first performed experiments to confirm that Ndufs4 KO mice can tolerate a brief exposure to environmental hypoxia and that they activate a hypoxic response in a manner similar to WT mice. We exposed 4 wild type (WT) mice and 4 KO mice to 8.5% O2 at sea level pressure for 6h. Acute exposure of wild type mice to hypoxia triggers HIF stabilization, resulting in Epo transcription and translation. After a 6h exposure, we measured Epo protein levels in plasma and showed that both WT and KO mice upregulated Epo production by approximately 40-fold (fig. S9). These results indicate that the KO mice mount a hypoxic transcriptional response and that the RC inhibition-HIF stabilization paradox does not extend to this disease setting.
We next tested whether chronic exposure to moderate environmental hypoxia – breathing 11% O2, a level known to be tolerated by humans (equivalent to 4,500m altitude) – could alleviate the disease phenotype in the Ndufs4 KO mice. Environmental hypoxia was generated by adjusting the relative concentration of nitrogen and oxygen in the input gas mixture. This created environmental oxygen tensions similar to those found in the high mountain communities of Nepal and Peru (36). Continuous gas flow and CO2 absorption by CaOH2 within the 11% O2 hypoxic chamber maintained CO2 levels below 0.4% with continuous monitoring. A control ambient environment for breathing 21% O2 was created with an identical chamber set-up. Ndufs4 KO and control mice were continuously exposed to breathing at normoxia or 11% hypoxia after enrollment in the experiment, excluding brief exposure to normoxia for behavior tests and maintenance 3 times per week. Untreated Ndufs4 KO mice typically begin to show disease progression after approximately 30 days of post-birth air exposure, which is about ten days after weaning. Since hypoxia-related vascular responses (constriction of pulmonary circulation, dilation of ductus arteriosus) occur in early post-natal development, we initiated chronic hypoxic exposure treatments after the mice were 30 days old.
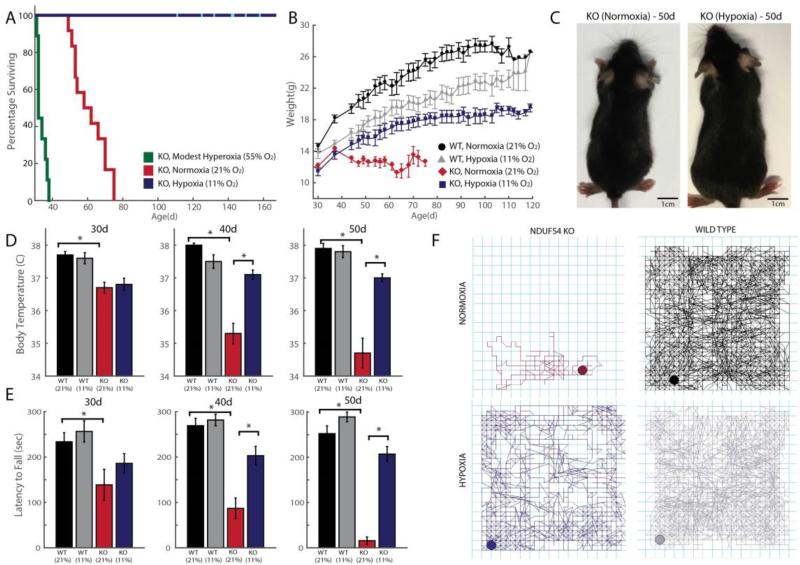
Remarkably, chronic hypoxia prevented the development of many disease symptoms in the Ndufs4 KO mice and significantly extended their survival. All normoxia-exposed Ndufs4 KO mice either fulfilled criteria for humane euthanasia or died at a median age of ~60d with none surviving past 75d (Fig. 5A). In contrast, there were no deaths in Ndufs4 KO mice that were chronically breathing 11% O2. Several mice showed a mild clasping phenotype at ages greater than 120d. At the time of submission of this manuscript, the oldest KO mice breathing 11% O2 were 170d old.
Figure 5. Chronic hypoxia extends lifespan and alleviates disease in a mouse model of Leigh syndrome, whereas chronic hyperoxia exacerbates disease.
(A) Ndufs4 KO mice of both genders were chronically exposed to hypoxia (11% O2), normoxia (21% O2) or hyperoxia (55% O2), at 30d of age and survival was recorded (n = 12, n = 12, n = 9 mice respectively). Cyan bars represent current age of hypoxic KO mice. (B) Body weights were measured in WT and KO mice exposed to normoxia or hypoxia, three times a week upon enrollment in the study. Weights are shown as mean ± S.E. (C) Representative images of 50d-old KO mice exposed to normoxia or hypoxia. (D) Body temperature was measured in KO mice exposed to normoxia or hypoxia at age ~30d, 40d and 50d. Temperatures are shown as mean ± S.E. (n ≥ 7 for all groups) (E) Latency to fall on an accelerating rod was measured as median values of triplicate trials per mouse for WT and KO mice, exposed to normoxia or hypoxia at different ages (n ≥ 7 for all groups). (F) Representative 1h locomotor activity traces of sick, normoxia-treated KO mice and age-matched hypoxia-treated KO mice, as well as controls. All data shown as normoxia KO (maroon), hypoxia KO (blue), normoxia WT (black) and hypoxia WT (grey). *denotes t-test p-value < 0.05.
Hypoxia-treated mice showed an improvement in body weight gain, core temperature maintenance, and neurologic behavior. All Ndufs4 KO mice continued to gain weight between 30-37d of age (Fig 5B-C). At this stage, untreated KO mice lose weight, become hypothermic and die. In contrast, Ndufs4 KO mice breathing 11% O2 gained weight for several weeks, at which point body weight gain slowed, similar to the growth kinetics of WT mice. The growth rate of hypoxia-treated KO mice matched that of WT mice breathing 11% O2 upon treatment, suggesting that the primary cause of weight loss was alleviated by hypoxic exposure. At 30d of age, untreated Ndufs4 KO mice have similar core body temperatures to control mice. However by 50d, there is nearly a 4°C drop in temperature (Fig. 5D). KO mice chronically breathing 11% O2 showed no reduction of core body temperature. Thus, chronic hypoxic breathing improves the underlying metabolic phenotype that directly or indirectly results in alterations of energy and nutrient metabolism.
Ndufs4 KO mice, as well as patients suffering from Leigh syndrome, exhibit striking defects in locomotor activity. Ataxia and failure to thrive are hallmarks of mitochondrial dysfunction. Behavioral tests were performed at 10d intervals in normoxia and hypoxia-treated, WT and KO mice. The rotarod test (37) measures the ability of mice to maintain grip strength, balance and resist fatigue on an accelerating, rotating rod. At 30 days of age, KO mice breathing air display a slight depression in the median time they can stay on a rotarod (Fig. 5E). This ability declines by 40d and at 50d, untreated KO mice are no longer able to stay on the rod for more than a few seconds, due to a combination of muscular weakness, inability to balance and loss of visual activity. Hypoxia-treated WT mice performed similarly to normoxia-treated control mice. KO mice breathing 11% O2 displayed a near complete rescue of this locomotor phenotype. As a further neurological – behavioral test, spontaneous locomotor activity was measured as total distance traveled within an hour (Fig. 5F). Untreated KO mice show drastically reduced spontaneous locomotor activity and jump counts. These defects are significantly rescued in hypoxia-treated mice, however only to 50% of the values of normoxic WT mice (fig. S10).
Modest hyperoxic breathing exacerbates disease in a mouse model of Leigh syndrome
The striking therapeutic effect of hypoxia led us to investigate whether oxygen itself was a key molecular parameter determining Leigh syndrome progression. Thus, we asked whether the converse environmental scenario of chronic mild hyperoxic exposure (55% O2) would affect the disease. We exposed WT and Ndufs4 KO mice to 55% normobaric oxygen starting at 30d of age. We found that breathing 55% oxygen had no effect on survival of the WT mice. In contrast, all Ndufs4 KO mice breathing 55% oxygen died within 2 to 11 days of this exposure; this is significantly earlier than Ndufs4 KO mice breathing ambient air (21% O2), which typically succumb within 3-4 weeks after experimental exposures begin (Fig. 5A). The reduced survival of the KO mice breathing 55% O2, along with the markedly extended healthspan of the KO mice that were breathing 11% O2, points to the essential and previously unappreciated role of arterial oxygen tension in determining the progression of mitochondrial disease. This suggests that patients with mitochondrial disease may be highly sensitive to oxygen toxicity.
Circulating biomarkers and histopathology in a hypoxia-treated mouse model of Leigh syndrome
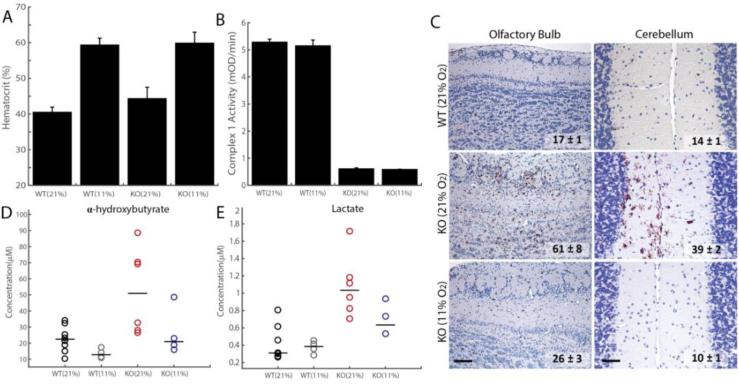
We further characterized Ndufs4 KO mice following treatment with chronic hypoxia. As expected, the hematocrit in these mice is elevated from 40% during normoxia to ~60% during hypoxia, indicating EPO target engagement by hypoxic breathing (Fig. 6A). Given how effective hypoxia is in treating these mice, we asked whether they still retain a deficiency in complex I activity. Indeed, we find that although Ndufs4 KO mice appear healthy following hypoxia treatment, brain complex I activity remains dramatically reduced to the same levels as untreated Ndufs4 KO mice (Fig. 6B).
Figure 6. Hypoxia exposure of Ndufs4 KO mice alleviates metabolic disease markers, as well as neuropathology, without rescuing Complex I activity.
(A) Hematocrit values for WT and KO mice treated with normoxia or hypoxia for ~3 weeks (n = 3-4 per group, test p-value < 0.05 for normoxia vs. hypoxia for both WT and KO). (B) Complex I Activity is significantly reduced in KO mice relative to WT mice, in both normoxic and hypoxic conditions (n = 3-4 per group, t-test p-value < 0.01). (C) Representative images for immunostaining against the inflammatory marker, Iba-1, in the olfactory bulb and cerebellum of Ndufs4 KO mice treated with hypoxia or normoxia and WT mice exposed to normoxia breathing. The number of Iba-1 positive cells per 10 random fields of view shown for each treatment group (Mean ± S.E., t-test p-value < 0.01 for normoxic vs. hypoxic KO, n = 3-4 per group). Scale bar is 200 microns for OB and 50 microns for cerebellum. (D) Plasma α-HB levels in WT and KO mice, exposed to hypoxia or normoxia (n = 4-8 per group). Median shown as horizontal bar. (E) Plasma lactate in WT and KO mice, exposed to hypoxia or normoxia (n = 4-8 per group). Median shown as horizontal bar.
Normoxia-treated knockout mice exhibited substantial neuronal degeneration. Lesions were accompanied by Iba-1+ microglial proliferation within olfactory lobes, cerebellum and brainstem as documented elsewhere (38). In contrast, knockout mice breathing 11% O2 exhibited minimal to no lesions (Fig. 6C), and were virtually indistinguishable histologically from WT controls.
Recently, α-hydroxybutyrate (α-HB) was identified as a novel circulating plasma marker of Leigh syndrome (39). Consistent with this, we found that α-HB is elevated in the plasma of Ndufs4 KO mice breathing ambient air, but not in Ndufs4 KO mice treated with chronic hypoxia (Fig. 6D). Similarly, plasma lactate levels were increased in Ndufs4 KO mice breathing ambient air between 50-65d of age, whereas this increase was partially prevented by 11% hypoxic exposure (Fig. 6E).
Collectively, these studies confirm that chronic hypoxic exposure to breathing 11% O2 activates the endogenous hypoxic response. Hypoxia does not correct the proximal lesion within mitochondrial complex I, but rather prevents the onset of subsequent biochemical and histopathological defects.
Discussion
The preclinical studies described here suggest that hypoxic gas mixtures may be useful in treating or preventing mitochondrial disorders. This approach is seemingly counterintuitive, since oxygen is a key substrate for the respiratory chain. However, hypoxia activates an evolutionarily-conserved adaptive program that allows mammals to cope with limiting oxygen levels. This program decreases an organism's reliance on mitochondrial oxidative metabolism. Such adaptive programs are not necessarily triggered by mitochondrial disease, as the hypoxic signal is absent. Moreover, hypoxia leads to a state in which oxygen delivery and consumption are simultaneously reduced, whereas in mitochondrial disease, oxygen delivery continues in the face of impaired utilization. Such a mismatch between delivery and utilization, potentially contributes to oxygen toxicity. Hence, hypoxia may represent nature's solution for overcoming mitochondrial disease pathology, both by triggering innate adaptive programs and by simultaneously limiting the substrate for oxygen toxicity.
Multiple cellular and systemic mechanisms are likely acting in concert to provide the therapeutic effect we observe in mice. First, hypoxia may be triggering the HIF-dependent transcriptional program that is known to activate key biochemical pathways – including glycolysis for redox neutral ATP production and decreased flux through PDH to prevent mitochondrial ROS generation. Second, breathing 11% O2 reduces the provision of oxygen to the cell – oxygen that would otherwise be available as a substrate for free radical production or aberrant signaling. Many enzymes are designed to operate under reducing conditions and are highly sensitive to ambient oxygen levels. Hypoxia may establish a new oxygen set point that is better suited to the cellular environment created by impaired RC activity, whereas hyperoxia may create an environment that is less favorable. Third, it is likely that hypoxia is also operating at the level of organ systems physiology (e.g., O2 delivery and CO2 clearance by the cardiovascular system, endocrine function, immune signaling), which are inherently non-cell autonomous and previously reported to be altered in humans living at high altitudes. Future studies will fully decipher the role of HIF, and cellular vs. cell-autonomous mechanisms underlying the in vivo therapeutic benefit.
Importantly, our results may hold therapeutic potential for patients with mitochondrial disease. However, before the chronic hypoxia strategy described here can be evaluated in the clinical setting, additional pre-clinical studies are needed to establish its safety and efficacy. First, studies of additional mouse models will determine the generalizability of this approach to other rare mitochondrial disorders, common disorders of mitochondrial dysfunction, and disorders of oxidative stress. Second, in the current study, we have utilized a treatment regimen consisting of chronic 11% inspired oxygen. Healthy humans can acclimate to high altitudes such as those encountered in Mount Blanc, Peru, and Nepal, where ambient oxygen tensions are comparable to those used in our experiments, but it is possible these conditions would not be well tolerated by patients. Future studies should thus evaluate whether intermediate oxygen levels between 11%-20% are effective in mouse disease models. Third, subsequent work will also allow us to determine the quantitative extent of lifespan extension observed in our mouse models. Finally, if intermittent hypoxia proves as effective as chronic hypoxia, it may allow for a nighttime therapy for which face masks and sleeping tents have already been devised by the sports industry.
An important principle in the management of mitochondrial disease is to avoid exposures – such as aminoglycoside or tetracycline antibiotics – that are known to be toxic to mitochondria. Patients with mitochondrial disease (like many other patients) are routinely treated with supplemental oxygen and breathe high levels of inspired oxygen during general anesthesia, recovery from surgery and during intensive care. Retrospective or prospective studies may help to extend our observations to humans, and if confirmed, would imply that caution should be exercised in O2 exposure and that administering supplemental oxygen should be limited to those instances in which it is clinically indicated.
At present, how lesions in the respiratory chain lead to such diverse pathology remains a mystery. Given the striking therapeutic efficacy of hypoxic breathing and detrimental effect of moderate hyperoxia, we propose that aberrant oxygen metabolism, signaling, or toxicity lies at the heart of mitochondrial pathogenesis. The identification of such a critical parameter suggests that a real understanding of mitochondrial pathogenesis is within reach.
Finally, it is notable that the aging process and virtually all age-related degenerative diseases are associated with secondary mitochondrial dysfunction and oxidative stress (40). While antioxidants have been proposed as a strategy to alleviate these disorders by scavenging free radicals, our work suggests that simply limiting the substrate for oxygen toxicity may prove more effective. Moreover, hypoxia can trigger an adaptive program designed to decrease our body's reliance on mitochondrial oxidative metabolism. It is conceivable that breathing hypoxic gas mixtures may prevent the onset of more common disorders.
Supplementary Material
Acknowledgments
We thank William Kaelin Jr. and members of the Mootha lab for valuable feedback; Rohit Sharma, Michele Ferrari, Arlin Rogers and the MGH animal facility for assistance with experiments; Freek van Eeden for Tg(phd3 ::EGFP) zebrafish. IHJ is supported by the Department of Energy Computational Science Graduate Fellowship Program (DE-FG02-97ER25308). FZ is supported by the National Institutes of Health through NIMH (5DP1-MH100706, 1R01-MH110049) and NIDDK (5R01DK097768-03), a Waterman Award from the National Science Foundation, the New York Stem Cell, Simons, Paul G. Allen Family, and Vallee Foundations and B. Metcalfe. FZ is a New York Stem Cell Foundation Robertson Investigator. WG is supported by NIH Grants R01DK090311 and R24OD017870 and is a Pew Scholar in the Biomedical Sciences. This work was supported by a gift from the Marriott Mitochondrial Disorders Research Fund (VKM) and a gift in memory of Daniel Garland (VKM). VKM is an Investigator of the Howard Hughes Medical Institute. VKM is a founder of and paid scientific advisor for Raze Therapeutics. WG is a paid consultant for FATE Therapeutics. FZ is a founder of and a scientific advisor for Editas Medicine and is a scientific advisor for Horizon Discovery. VKM, IHJ, LZ, and WMZ are listed as inventors on a patent application filed by Massachusetts General Hospital related to technology reported in this paper on the use of hypoxia and the hypoxia response in the treatment of mitochondrial dysfunction. FZ, OS, and NES are listed as inventors on a patent application (PCT/US2013/074800) filed by The Broad Institute/MIT related to the genome-scale CRISPR knockout screening technology used in this study. The CRISPR knockout library is available through a Uniform Biological MTA from Addgene. The Ndufs4 KO mice were a kind gift of Richard Palmiter and are available under a material transfer agreement with the University of Washington, Seattle.
Footnotes
References and Notes
- 1.Rich P. Chemiosmotic coupling: the cost of living. Nature. 2003;421:583. doi: 10.1038/421583a. [DOI] [PubMed] [Google Scholar]
- 2.Vafai SB, Mootha VK. Mitochondrial disorders as windows into an ancient organelle. Nature. 2012;491:374–383. doi: 10.1038/nature11707. [DOI] [PubMed] [Google Scholar]
- 3.Koopman WJ, Willems PH, Smeitink JA. Monogenic mitochondrial disorders. N. Engl. J. Med. 2012;366:1132–1141. doi: 10.1056/NEJMra1012478. [DOI] [PubMed] [Google Scholar]
- 4.Nunnari J, Suomalainen A. Mitochondria: in sickness and in health. Cell. 2012;148:1145–1159. doi: 10.1016/j.cell.2012.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pfeffer G, Majamaa K, Turnbull DM, Thorburn D, Chinnery PF. Treatment of mitochondrial disorders. Cochrane Database Syst Rev. 2012;4:CD004426. doi: 10.1002/14651858.CD004426.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lake NJ, Bird MJ, Isohanni P, Paetau A. Leigh Syndrome: Neuropathology and Pathogenesis. Journal of Neuropathology & Experimental Neurology. 2015;74:482–492. doi: 10.1097/NEN.0000000000000195. [DOI] [PubMed] [Google Scholar]
- 7.Gorman GS, et al. Prevalence of nuclear and mitochondrial DNA mutations related to adult mitochondrial disease. Annals of Neurology. 2015;77:753–759. doi: 10.1002/ana.24362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parikh S, et al. Diagnosis and management of mitochondrial disease: a consensus statement from the Mitochondrial Medicine Society. Genetics in Medicine. 2015;17:689–701. doi: 10.1038/gim.2014.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haas RH, et al. Mitochondrial disease: a practical approach for primary care physicians. Pediatrics. 2007;120:1326–1333. doi: 10.1542/peds.2007-0391. [DOI] [PubMed] [Google Scholar]
- 10.Shalem O, et al. Genome-scale CRISPR-Cas9 knockout screening in human cells. Science. 2014;343:84–87. doi: 10.1126/science.1247005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang T, Wei JJ, Sabatini DM, Lander ES. Genetic screens in human cells using the CRISPR-Cas9 system. Science. 2014;343:80–84. doi: 10.1126/science.1246981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.King MP, Attardi G. Human cells lacking mtDNA: repopulation with exogenous mitochondria by complementation. Science. 1989;246:500–503. doi: 10.1126/science.2814477. [DOI] [PubMed] [Google Scholar]
- 13.Calvo SE, Clauser KR, Mootha VK. MitoCarta2. 0: an updated inventory of mammalian mitochondrial proteins. Nucleic Acids Research. 2016 doi: 10.1093/nar/gkv1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohh M, et al. Ubiquitination of hypoxia-inducible factor requires direct binding to the β-domain of the von Hippel-Lindau protein. Nature Cell Biol. 2000;2:423–427. doi: 10.1038/35017054. [DOI] [PubMed] [Google Scholar]
- 15.Robinson CM, Ohh M. The multifaceted von Hippel–Lindau tumour suppressor protein. FEBS Letters. 2014;588:2704–2711. doi: 10.1016/j.febslet.2014.02.026. [DOI] [PubMed] [Google Scholar]
- 16.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl. Acad. Sci. U.S.A. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Molecular Cell. 2010;40:294–309. doi: 10.1016/j.molcel.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ivan M, et al. Biochemical purification and pharmacological inhibition of a mammalian prolyl hydroxylase acting on hypoxia-inducible factor. Proc. Natl. Acad. Sci. U.S.A. 2002;99:13459–13464. doi: 10.1073/pnas.192342099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iliopoulos O, Levy AP, Jiang C, Kaelin WG, Jr., Goldberg MA. Negative regulation of hypoxia-inducible genes by the von Hippel-Lindau protein. Proc. Natl. Acad. Sci. U.S.A. 1996;93:10595–10599. doi: 10.1073/pnas.93.20.10595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maxwell PH, et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 21.Buckley DL, et al. Targeting the von Hippel-Lindau E3 ubiquitin ligase using small molecules to disrupt the VHL/HIF-1α interaction. J. Am. Chem. Soc. 2012;134:4465–4468. doi: 10.1021/ja209924v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rabinowitz MH. Inhibition of hypoxia-inducible factor prolyl hydroxylase domain oxygen sensors: tricking the body into mounting orchestrated survival and repair responses. J. Med. Chem. 2013;56:9369–9402. doi: 10.1021/jm400386j. [DOI] [PubMed] [Google Scholar]
- 23.Chandel NS, et al. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc. Natl. Acad. Sci. U.S.A. 1998;95:11715–11720. doi: 10.1073/pnas.95.20.11715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chua YL, et al. Stabilization of hypoxia-inducible factor-1α protein in hypoxia occurs independently of mitochondrial reactive oxygen species production. J. Biol. Chem. 2010;285:31277–31284. doi: 10.1074/jbc.M110.158485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Papandreou I, Cairns RA, Fontana L, Lim AL, Denko NC. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 3. 187-197. 2006 doi: 10.1016/j.cmet.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 26.Tello D, et al. Induction of the Mitochondrial NDUFA4L2 Protein by HIF-1α decreases oxygen consumption by inhibiting complex I activity. Cell Metab. 2011;14:768–779. doi: 10.1016/j.cmet.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 27.Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3:177–185. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 28.Simon MC. Coming up for air: HIF-1 and mitochondrial oxygen consumption. Cell Metab. 2006;3:150–151. doi: 10.1016/j.cmet.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 29.van Rooijen E, et al. Zebrafish mutants in the von Hippel-Lindau tumor suppressor display a hypoxic response and recapitulate key aspects of Chuvash polycythemia. Blood. 2009;113:6449–6460. doi: 10.1182/blood-2008-07-167890. [DOI] [PubMed] [Google Scholar]
- 30.Pinho BR, et al. How mitochondrial dysfunction affects zebrafish development and cardiovascular function: an in vivo model for testing mitochondria-targeted drugs. Br. J. Pharmacol. 2013;169:1072–1090. doi: 10.1111/bph.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stackley KD, Beeson CC, Rahn JJ, Chan SS. Bioenergetic profiling of zebrafish embryonic development. PLOS One. 2011;6:e25652. doi: 10.1371/journal.pone.0025652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harris JM, et al. Glucose metabolism impacts the spatiotemporal onset and magnitude of HSC induction in vivo. Blood. 2013;121:2483–2493. doi: 10.1182/blood-2012-12-471201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Santhakumar K, et al. A zebrafish model to study and therapeutically manipulate hypoxia signaling in tumorigenesis. Cancer Res. 2012;72:4017–4027. doi: 10.1158/0008-5472.CAN-11-3148. [DOI] [PubMed] [Google Scholar]
- 34.Chowdhury R, et al. Selective small molecule probes for the hypoxia inducible factor (HIF) prolyl hydroxylases. ACS Chem Biol. 2013;8:1488–1496. doi: 10.1021/cb400088q. [DOI] [PubMed] [Google Scholar]
- 35.Kruse SE, et al. Mice with mitochondrial complex I deficiency develop a fatal encephalomyopathy. Cell Metabolism. 2008;7:312–320. doi: 10.1016/j.cmet.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pawson IG. Growth and development in high altitude populations: a review of Ethiopian, Peruvian, and Nepalese studies. Proceedings of the Royal Society of London B: Biological Sciences. 1976;194:83–98. doi: 10.1098/rspb.1976.0067. [DOI] [PubMed] [Google Scholar]
- 37.Caston J, Jones N, Stelz T. Role of preoperative and postoperative sensorimotor training on restoration of the equilibrium behavior in adult mice following cerebellectomy. Neurobiology of Learning and Memory. 1995;64:195–202. doi: 10.1006/nlme.1995.0002. [DOI] [PubMed] [Google Scholar]
- 38.Quintana A, Kruse SE, Kapur RP, Sanz E, Palmiter RD. Complex I deficiency due to loss of Ndufs4 in the brain results in progressive encephalopathy resembling Leigh syndrome. Proc. Natl. Acad. Sci. U.S.A. 2010;107:10996–11001. doi: 10.1073/pnas.1006214107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Legault JT, et al. A metabolic signature of mitochondrial dysfunction revealed through a monogenic form of Leigh Syndrome. Cell Reports. 2015;13:981–989. doi: 10.1016/j.celrep.2015.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 41.Luo B, et al. Highly parallel identification of essential genes in cancer cells. Proc. Natl. Acad. Sci. U.S.A. 2008;105:20380–20385. doi: 10.1073/pnas.0810485105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pagliarini DJ, et al. A mitochondrial protein compendium elucidates complex I disease biology. Cell. 2008;134:112–123. doi: 10.1016/j.cell.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Subramanian A, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. U.S.A. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnson SC, et al. mTOR inhibition alleviates mitochondrial disease in a mouse model of Leigh syndrome. Science. 2013;342:1524–1528. doi: 10.1126/science.1244360. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.