Abstract
Torsades de pointes (TdP) is a life-threatening arrhythmia associated with prolongation of the corrected QT (QTc) interval on the electrocardiogram. More than 100 drugs available in Canada, including widely used antibiotics, antidepressants, cardiovascular drugs and many others, may cause QTc interval prolongation and TdP. Risk factors for TdP include QTc interval >500 ms, increase in QTc interval ≥60 ms from the pretreatment value, advanced age, female sex, acute myocardial infarction, heart failure with reduced ejection fraction, hypokalemia, hypomagnesemia, hypocalcemia, bradycardia, treatment with diuretics and elevated plasma concentrations of QTc interval–prolonging drugs due to drug interactions, inadequate dose adjustment of renally eliminated drugs in patients with kidney disease and rapid intravenous administration. Pharmacokinetic drug interactions associated with the highest risk of TdP include antifungal agents, macrolide antibiotics (except azithromycin) and drugs to treat human immunodeficiency virus interacting with amiodarone, disopyramide, dofetilide or pimozide. Other important pharmacokinetic interactions include antidepressants (bupropion, duloxetine, fluoxetine, paroxetine) interacting with flecainide, quinidine or thioridazine. Pharmacists play an important role in minimizing the risk of drug-induced QTc interval prolongation and TdP through knowledge of drugs that are associated with a known or possible risk of TdP, individualized assessment of risk of drug-induced QTc interval prolongation, awareness of drug interactions most likely to result in TdP and attention to dose reduction of renally eliminated QTc interval-prolonging drugs in patients with kidney disease. Treatment of hemodynamically stable TdP consists of discontinuation of the offending drug(s), correction of electrolyte abnormalities and administration of intravenous magnesium sulfate 1 to 2 g.
Knowledge into Practice.
Torsades de pointes (TdP) may result in sudden cardiac death. More than 100 drugs available in Canada, including widely used antibiotics, antidepressants, cardiovascular drugs and many others, may cause QTc interval prolongation and TdP.
Pharmacists can play an important role in minimizing the risk of drug-induced QTc interval prolongation and TdP through knowledge of drugs associated with TdP, assessment of risk of QTc interval prolongation via the QTc interval prolongation risk score, awareness of drug interactions most likely to result in TdP and attention to dose reduction of renally eliminated QTc interval–prolonging drugs in patients with kidney disease. Analysis of these factors informs the pharmacist’s clinical judgment regarding the need for intervention for patients receiving potentially QTc interval–prolonging medications.
Mise En Pratique Des Connaissances.
Les torsades de pointes peuvent causer un arrêt cardiaque soudain. Plus d’une centaine de médicaments en vente au Canada peuvent provoquer un allongement de l’intervalle QTc et des TdP, y compris des antibiotiques, antidépresseurs, médicaments cardiovasculaires et autres produits largement utilisés.
Les pharmaciens peuvent jouer un rôle important pour atténuer les risques d’allongement de l’intervalle QTc et de TdP provoqués par des médicaments, grâce à leurs connaissances sur les médicaments entraînant des TdP, à l’évaluation des risques au moyen d’une échelle de risque d’allongement de l’intervalle QTc, à la connaissance des interactions médicamenteuses qui sont susceptibles d’entraîner des TdP et à la réduction posologique des médicaments qui allongent l’intervalle QTc et qui sont éliminés par les reins chez les patients atteints d’une néphropathie. Le pharmacien fonde son jugement clinique sur l’analyse de ces facteurs pour déterminer les cas qui nécessitent une intervention chez les patients recevant des médicaments pouvant allonger l’intervalle QTc
Drug-induced prolongation of the QT interval on the electrocardiogram (ECG) increases the risk of the life-threatening ventricular arrhythmia known as torsades de pointes (TdP). Over the past 20 years, several drugs, including terfenadine, astemizole, cisapride and grepafloxacin, have been withdrawn from the Canadian market as a result of causing deaths due to TdP.1 However, more than 100 drugs with the potential to cause TdP remain available in Canada.2,3 Pharmacists should be aware of drugs that can cause QT interval prolongation and TdP, risk factors and methods for reducing the risk. The objectives of this article are to 1) describe and define the QT interval and TdP, 2) identify commonly used drugs that may cause QT interval prolongation and TdP, 3) describe risk factors for QT interval prolongation and TdP and 4) provide practical recommendations for pharmacists regarding methods of reducing the risk of QT interval prolongation and TdP and treating TdP.
ECG and the QT interval
The ECG is a noninvasive method of evaluating the heart’s electrical activity and represents the phases of the atrial and ventricular action potentials (Figure 1). The P wave represents depolarization of the atria, which precedes and is necessary for atrial contraction. The QRS complex represents depolarization of the ventricles, which precedes and is necessary for ventricular contraction. The QRS complex corresponds to phase 0 and phase 1 of the ventricular action potential. Phase 0 represents ventricular depolarization. The T wave represents the final component (phase 3) of ventricular repolarization (Figure 1). However, ventricular repolarization begins as soon as phase 0 depolarization ends and is complete at the end of phase 3. Therefore, the entire period of ventricular repolarization is represented by the interval from the Q wave to the end of the T wave, known as the QT interval (Figure 2).4 As phase 3 ventricular repolarization becomes prolonged, the left ventricle becomes more susceptible to premature electrical impulses known as early afterdepolarizations. When an early afterdepolarization occurs during the latter portion of an extended phase 3 ventricular repolarization, represented on the ECG by a prolonged QT interval, this can trigger TdP. Therefore, the longer the QT interval, the greater the likelihood of TdP.
Figure 1.
Relationship between the electrocardiogram and the ventricular action potential
Upper frame: Major waves and complexes on the electrocardiogram. P wave represents atrial depolarization; QRS complex represents ventricular depolarization and the initial portion of ventricular repolarization; T wave represents the final phase of ventricular repolarization; the interval from the Q wave to the end of the T wave (QT interval) represents the complete period of ventricular repolarization. Lower frame: Phases of the ventricular action potential: phase 0 represents ventricular depolarization, which occurs as a result of rapid flow of sodium into the cardiac myocyte; phase 1 represents the initial phase of ventricular repolarization, due to transient movement of potassium out of the cell; phase 2 represents the plateau phase of ventricular repolarization, due to movement of sodium and calcium into the cell; phase 3 represents the final phase of ventricular repolarization, resulting from movement of potassium out of the cell; phase 4 represents the resting phase, maintained by the sodium-potassium pump.
Figure 2.
The QT interval
(Left) Normal electrocardiogram. (Right) Prolonged QT interval. Reprinted with permission from Trinkley KE, Page RL II, Lien H, et al. QT interval prolongation and the risk of torsades de pointes: essentials for clinicians. Curr Med Res Opin 2013;29:1719-26. Publisher: Taylor & Francis, Ltd, www.tandfonline.com.
The QT interval varies as heart rate varies. As the heart rate increases, the QT interval shortens and vice versa. Therefore, to be certain that changes in QT interval actually represent changes in ventricular repolarization, rather than simply changes in heart rate, the QT interval must be corrected to account for heart rate variations. The heart rate–adjusted QT interval is known as the corrected QT (QTc) interval. The most common QT interval correction equation, and that which is used in routine clinical practice, is Bazett’s formula5:
where QTc is the heart rate–corrected QT interval and RR interval is the interval between the R waves on the ECG; the RR interval is the heart rate but expressed in milliseconds or seconds of time on the ECG. The normal QTc interval in adults is 0.36 to 0.47 s (360–470 ms) in men and 0.36 to 0.48 s (360–480 ms) in women.6
Torsades de pointes
TdP is a polymorphic ventricular tachycardia (VT) associated with QTc interval prolongation. The majority of patients who experience VT, which is most commonly associated with myocardial infarction, myocardial ischemia or heart failure, exhibit monomorphic VT; that is, the QRS complexes manifest a consistent shape and amplitude (morphology). TdP is a polymorphic arrhythmia; that is, the morphology of the QRS complexes is variable and not constant. TdP was initially described in the 1960s by the French physician François Dessertenne and was termed twisting of the points because the “points” of the QRS complexes on the ECG appeared to “twist” around the isoelectric baseline (Figure 3).7
Figure 3.
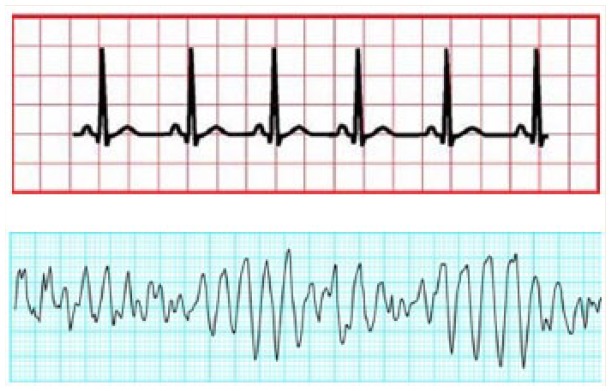
Torsades de pointes compared with normal sinus rhythm
(Top) Normal sinus rhythm. (Bottom) Torsades de pointes. Reprinted with permission from Tisdale JE. Review of cardiac arrhythmias and rhythm interpretation. In: Wiggins BS, Sanoski CA, editors Emergency cardiovascular pharmacotherapy. A point of care guide. Bethesda (MD): American Society of Health-System Pharmacists; 2012. pp 23, 38.
TdP results in heart rates of 160 to 240 beats per minute.6 Symptoms of TdP are primarily related to the rapid heart rate and the resulting effects on blood pressure and cardiac output2 and include palpitations, dizziness, lightheadedness, shortness of breath, near-syncope and syncope. In some cases, TdP may be nonsustained and terminate spontaneously. However, TdP often degenerates rapidly into ventricular fibrillation, resulting in sudden cardiac death. Therefore, TdP can be a catastrophic occurrence. Consequently, strategies for minimizing the risk of TdP are important.
QTc interval prolongation and the risk of TdP
QTc interval prolongation may be congenital or acquired. Congenital long QT syndrome (LQTS), for which as many as 13 distinct genetic mutations have been identified,8 occurs in approximately 1 in 2000 live births.9 Acquired QTc interval prolongation is most often caused by drugs.2
QTc interval prolongation increases the risk of TdP, particularly when the QTc interval exceeds 500 ms.10-12 The majority of reported or published cases of TdP have occurred in patients with a QTc interval >500 ms, and TdP is rare when the QTc interval is less than 500 ms.13 The risk of TdP is also increased when the QTc interval becomes prolonged greater than 60 ms compared with the pretreatment value.13
The incidence of TdP in the general population is unknown. In a study conducted in Sweden, the annualized incidence of TdP in the general population was estimated to be 4 in 100,000.14 Assuming a similar incidence in Canada, this translates to approximately 1400 TdP cases annually. The incidence of TdP associated with specific QTc interval–prolonging drugs ranges from 2% to 12% depending on the drug, dose administered and the presence of other risk factors.
Hospitalized patients, particularly those in intensive care units (ICUs), are at higher risk of developing QTc interval prolongation and TdP because of a greater preponderance of risk factors.6 The incidence of QTc interval prolongation in patients in ICUs ranges from 24% to 28%15,16; as many as 18% of patients in ICUs are admitted with preexisting QTc interval prolongation. Among 154 patients in adult ICUs and progressive care units during a 2-month period, 1 case of TdP (0.6%) was reported.16
Drugs associated with TdP
More than 100 drugs available in Canada are associated with a known or possible risk of TdP. An excellent source of information regarding drugs that may cause TdP is provided at https://www.crediblemeds.org. Anyone can access the QT drugs list on this website; registration is necessary and requires a username and password, but access is free of charge. This website categorizes drugs that may cause TdP into “Known,” “Possible” and “Conditional” risk and “Drugs to avoid in congenital LQTS.”3 Definitions of these categories are provided in Table 1. Based on these definitions, a list of drugs with a known or possible risk of TdP is provided in Table 2.
Table 1.
Categories of drugs that may cause TdP3
| Category | Definition |
|---|---|
| Known risk | Substantial evidence supports the conclusion that these drugs prolong the QT interval AND are clearly associated with a risk of TdP, even when taken as directed in official labeling. |
| Possible risk | Substantial evidence supports the conclusion that these drugs can cause QT interval prolongation BUT there is insufficient evidence at this time that these drugs, when used as directed in official labeling, are associated with a risk of causing TdP. |
| Conditional risk | Substantial evidence supports the conclusion that these drugs are associated with a risk of TdP BUT only under certain conditions (e.g., excessive dose, hypokalemia, congenital LQTS or by causing a drug-drug interaction that results in excessive QT interval prolongation). |
| Drugs to avoid in congenital LQTS | Substantial evidence supports the conclusion that these drugs pose a risk of TdP for patients with congenital LQTS. Drugs on this list include those in the above 3 risk categories and other drugs that do not prolong the QT interval per se but have a theoretical risk of causing arrhythmia that is based on their known stimulant actions on the heart. |
LQTS, long QT syndrome; TdP, torsades de pointes.
Table 2.
Drugs associated with a known or possible risk of torsades de pointes3
| Drug class | Known risk | Possible risk |
|---|---|---|
| Alpha-blocker | Alfuzosin | |
| Anesthetic, general | Propofol Sevoflurane |
|
| Antiarrhythmic | Amiodarone Disopyramide Dofetilide Flecainide Ibutilide Procainamide Quinidine Sotalol |
|
| Anticonvulsant | Felbamate | |
| Antidepressant | Citalopram Escitalopram |
Clomipramine Desipramine Imipramine Lithium Mirtazapine Nortriptyline Trimipramine Venlafaxine |
| Anticancer | Arsenic trioxide Eribulin Vandetanib |
Bortezomib Bosutinib Certinib Crizotinib Dabrafenib Dasatanib Lapatanib Nilotinib Pazopanib Sorafenib Sunitinib Vemurafenib Tamoxifen Panobinostat Vorinostat |
| Antiemetic | Ondansetron Droperidol |
Dolasetron Granisteron Promethazine |
| Antifungal | Fluconazole Pentamidine |
|
| Antihypertensive | Isradipine Moexipril/hydrochlorothiazide Nicardipine |
|
| Antimalarial | Chloroquine Halofantrine |
Artenimol/piperaquine |
| Antipsychotics | Chlorpromazine Haloperidol Pimozide Thioridazine |
Aripiprazole ClozapineIloperidone Olanzapine Paloperidone Quetiapine Risperidone Sertindole Ziprasidone |
| Antibiotic | Azithromycin Clarithromycin Erythromycin Ciprofloxacin Levofloxacin Moxifloxacin |
Bedaquiline Gemifloxacin Norfloxacin Ofloxacin Telavancin Telithromycin |
| Antiviral | Atazanavir Foscarnet Rilpivirine Saquinavir |
|
| Antispasmodic | Mirabegron | |
| Cholinesterase inhibitor | Donepezil | |
| Dopamine agonist | Apomorphine | |
| Estrogen agonist/antagonist | Toremifene | |
| Gonadotropin receptor agonist/antagonist | Leuprolide | |
| Gonadotropin-releasing hormone agonist/antagonist | Degarelix | |
| Histamine H2 receptor antagonist | Famotidine | |
| Immunosuppressant | Tacrolimus | |
| Illicit drugs | Cocaine | |
| Muscle relaxants | Tizanidine Tolterodine |
|
| Norepinephrine reuptake inhibitor | Atomoxetine | |
| Oxytocic | Oxytocin | |
| Opiates | Methadone | |
| Phosphodiesterase 3 inhibitors | Anagrelide Cilostazol |
|
| Phosphodiesterase 5 inhibitors | Vardenafil | |
| Progesterone antagonist | Mifepristone | |
| Sedative | Dexmedetomidine | |
| Somatostatin analog | Pasireotide | |
| Sphingosine phosphate receptor modulator | Fingolimod |
QTc interval–prolonging drugs are commonly prescribed. Several drugs that cause TdP are among the top 200 medications prescribed annually in the United States, including azithromycin, trazodone, sertraline, fluconazole, citalopram, escitalopram, ciprofloxacin, venlafaxine, aripiprazole, pantoprazole, risperidone and paroxetine.17 In an analysis of nearly 5 million outpatients, 23% filled prescriptions for QTc interval–prolonging drugs.18
Risk factors for drug-induced QTc interval prolongation and TdP
Risk factors are important for the development of QTc interval prolongation and TdP. In comparison with patients who have no risk factors, the odds ratio for QTc interval prolongation in patients with 1 risk factor is 3.2 (95% confidence [CI] 2.1–5.5); the odds ratio increases markedly in patients with 2 or ≥3 risk factors (7.3 [4.6–11.7] and 9.2 [4.9–17.4], respectively).19 In an analysis of 144 published articles describing 249 patients with TdP associated with noncardiovascular drugs, nearly 100% had ≥1 risk factor and 71% had ≥2 risk factors.20 Therefore, risk factor assessment is critical for evaluating the risk of QTc interval prolongation and TdP,6 and risk factor modification, where possible, is important for reducing the risk of drug-induced TdP.
Risk factors for drug-induced TdP are presented in Table 3. A QTc interval >500 ms and/or prolongation of the QTc interval ≥60 ms are risk factors. Women are at higher risk of TdP than men; this is most likely related to the fact that testosterone shortens QTc intervals and is protective against QTc interval prolongation in men.21 In addition, some evidence indicates that estrogen may lengthen QTc intervals in women.22 Older patients (generally >65 years of age) are at higher risk for drug-induced TdP,23,24 which may be related to declining serum testosterone concentrations in men25 and lower serum progesterone concentrations in women.26 Acute myocardial infarction prolongs ventricular repolarization during the infarction; however, QTc intervals are not permanently prolonged and return to baseline when the acute myocardial ischemia has resolved. Heart failure due to reduced ejection fraction is associated with lengthening of the QTc interval and increases the risk of TdP by 2- to 3-fold compared with that in patients with normal left ventricular function.27-30 Hypokalemia, hypomagnesemia and hypocalcemia increase the risk for TdP. Therapy with diuretics also increases the risk, most likely by provoking electrolyte abnormalities. Some evidence indicates that concomitant administration of ≥2 QTc interval–prolonging drugs may increase the risk. Conditions that lead to elevated plasma concentrations of QTc interval–prolonging drugs increase the risk of drug-induced TdP, including pharmacokinetic drug interactions (Table 4), inadequate dose adjustment of renally eliminated QTc interval–prolonging drugs in patients with acute kidney injury or chronic kidney disease (Table 5) and rapid infusion of intravenously administered QTc interval–prolonging medications. Some patients may have a genetic predisposition to experiencing drug-induced TdP; genetic polymorphisms known to be associated with some forms of the congenital LQTS may be present in 10% to 15% of patients who experience drug-induced TdP.31
Table 3.
| • QTc interval >500 ms • Increase in QTc interval >60 ms compared with pretreatment value • Advanced age • Female sex • Acute myocardial infarction • Heart failure with reduced ejection fraction • Hypokalemia • Hypomagnesemia • Hypocalcemia • Bradycardia • Treatment with diuretics • Concurrent administration of >1 QTc interval–prolonging drugs • Elevated plasma concentrations of QTc interval–prolonging drugs ○ Inadequate dose adjustment of renally eliminated drug in patients with acute kidney injury or chronic kidney disease ○ Rapid intravenous infusion of QTc interval–prolonging drug ○ Drug interaction(s) • Possible genetic predisposition |
Table 4.
Pharmacokinetic drug interactions associated with the highest risk of drug-induced QTc interval prolongation and torsades de pointes
| Precipitant drug | Mechanism | QTc interval–prolonging drug |
|---|---|---|
| Antifungal agents: Itraconazole Ketoconazole Posaconazole Voriconazole |
Inhibition of CYP 3A4 | Amiodarone Disopyramide Dofetilide Pimozide |
| Macrolide antibiotics*: Erythromycin Clarithromycin Telithromycin |
Inhibition of CYP 3A4 | Amiodarone Disopyramide Dofetilide Pimozide |
| HIV drugs: Atazanavir Darunivir/ritonavir Fosamprenavir Indinavir Nelfinavir Ritonavir Saquinavir Tipranavir |
Inhibition of CYP 3A4 | Amiodarone Disopyramide Dofetilide Pimozide |
| Antidepressants: Bupropion Duloxetine Fluoxetine Paroxetine |
Inhibition of CYP 2D6 | Flecainide Quinidine Thioridazine |
| Others: Terbinafine |
Inhibition of CYP 2D6 | Flecainide Quinidine Thioridazine |
Not azithromycin.
CYP, hepatic cytochrome P-450 enzyme; HIV, human immunodeficiency virus.
Table 5.
Drugs known to cause torsades de pointes that require dose adjustment in patients with acute kidney injury or chronic kidney disease
| • Ciprofloxacin • Disopyramide • Dofetilide • Eribulin • Flecainide • Fluconazole • Levofloxacin • Procainamide • Sotalol • Vandetanib |
When is pharmacist intervention necessary?
Pharmacists may minimize the risk of drug-induced QTc interval prolongation and TdP primarily through attention to risk factors. Methods of reducing the risk of drug-induced TdP are listed in Table 6. It is recognized that some community pharmacists may not have access to some information, such as the current or pretreatment QTc interval, left ventricular ejection fraction and/or history of TdP or sudden cardiac death, but these factors are listed in case this information is available. Areas in which pharmacists may have the most impact on reducing the risk of QTc interval prolongation/TdP include maintaining normal serum electrolyte concentrations, avoidance or mitigation of high-risk drug interactions (Table 4) and appropriate dose adjustment of renally eliminated QTc interval–prolonging drugs in patients with kidney disease.
Table 6.
Methods of reducing the risk of drug-induced torsades de pointes
| • Where possible, avoid use of QTc interval–prolonging drugs in patients known to have pretreatment QTc intervals >450 ms. • Discontinue QTc interval–prolonging drug(s) if QTc interval prolongs to >500 ms. • Reduce dose or discontinue QTc interval–prolonging drug(s) if the QTc interval increases ≥60 ms from pretreatment value. • Maintain serum potassium concentration within normal range. • Maintain serum magnesium concentration within normal range. • Maintain serum calcium concentration within normal range. • Where possible, avoid the use of QTc interval–prolonging drugs in patients with heart failure and a left ventricular ejection fraction <20%. • Avoid important drug interactions (Table 4). • Adjust doses of renally eliminated QTc interval–prolonging drugs in patients with acute kidney injury or chronic kidney disease (Table 5). • Avoid rapid intravenous administration of QTc interval–prolonging drugs. • Where possible, avoid concomitant administration of >1 QTc interval–prolonging drug. • Avoid use of QTc interval–prolonging drugs in patients with a history of drug-induced torsades de pointes or those who have previously been bresuscitated from an episode of sudden cardiac death. • Avoid use of QTc interval–prolonging drugs in patients who have been diagnosed with one of the congenital long QT syndromes. |
Potential drug interactions are common in patients taking QTc interval–prolonging medications. Of approximately 1.1 million outpatients who filled prescriptions for QTc interval–prolonging drugs, 9.4% filled overlapping prescriptions for 2 or more of those drugs or for a QTc interval–prolonging agent and a drug that inhibits its clearance.18 Pharmacokinetic drug interactions with the highest likelihood of leading to QTc interval prolongation/TdP are listed in Table 4. Particular attention to and avoidance of these drug interactions is important for risk minimization. Pharmacokinetic interactions not listed in this table are much less likely to result in QTc interval prolongation or TdP. In addition, as mentioned previously, avoidance of concomitant use of ≥2 QTc interval–prolonging drugs, to prevent additive lengthening of the QTc interval, is also recommended whenever possible. Appropriate dose adjustment of renally eliminated QTc interval–prolonging drugs is extremely important for minimizing the risk of drug-induced QTc interval prolongation/TdP in patients with kidney disease. QTc interval–prolonging drugs for which dose adjustment is required in patients with acute kidney injury and chronic kidney disease are listed in Table 5.
As risk factors are important for the development of QTc interval prolongation/TdP, quantification of risk may be helpful in targeting patients at greatest need of pharmacist intervention/monitoring. A risk score for predicting the development of QTc interval prolongation in patients hospitalized in cardiac care units has been developed and validated (Table 7).32 Roughly 50% of patients with a risk score ≥7, in the moderate-to-high range, proceeded to develop QTc interval prolongation. This risk score was incorporated into a clinical decision support computer alert; when a patient was admitted to the cardiac care units, a computer alert was generated when the patient was prescribed a QTc interval–prolonging drug and the calculated risk score indicated that the patient was at moderate or high (but not low) risk of developing QTc interval prolongation. Upon receiving the alert, the pharmacist entering the order for the QTc interval–prolonging medication contacted the physician to discuss obtaining more frequent ECGs for QTc interval monitoring, assuring the correct dose and maintenance of adequate serum electrolyte concentrations or substituting a drug that does not prolong the QTc interval, where possible and appropriate. This computer alert process resulted in modification of prescribing of noncardiovascular QTc interval–prolonging drugs and significantly reduced the risk of QTc interval prolongation in patients in these cardiac care units.33
Table 7.
| Risk factor | Points |
|---|---|
| Age ≥68 years | 1 |
| Female | 1 |
| Loop diuretic | 1 |
| Serum potassium ≤3.5 mmol/L | 2 |
| Presenting QTc interval ≥450 ms | 2 |
| Acute myocardial infarction† | 2 |
| Heart failure with reduced ejection fraction | 3 |
| 1 QTc interval-prolonging drug‡ | 3 |
| ≥2 QTc interval-prolonging drugs‡ | 3 |
| Sepsis† | 3 |
| Maximum score | 21 |
Risk score category: low risk = <7; moderate risk = 7 to 10; high risk = ≥11.
During acute event/disease; QTc interval generally returns to normal following resolution.
Three points for taking 1 QTc interval–prolonging drug; 3 additional points for taking ≥2 QTc interval–prolonging drugs (for a total of 6 points).
While this risk score was developed and validated in a population of patients hospitalized in cardiac care units, it may be of value to community pharmacists and hospital pharmacists not practising in cardiac care units. Pharmacists may use this risk score to assess the risk of QTc interval prolongation in patients receiving QTc interval–prolonging medications. Using this risk score, awareness of the drug interactions listed in Table 4 and paying diligence to appropriate adjustment of drug doses in patients with kidney disease, pharmacists can determine the need to contact prescribers to discuss the risk pertaining to individual patients or, in the case or pharmacist-prescribers, make monitoring and prescribing decisions. If the patient’s QTc interval risk is low and there are no important drug interactions or dose adjustments necessary, then pharmacist intervention is unnecessary. If the risk is moderate or high, then pharmacist intervention in the form of communication with the prescribing physician may be of benefit. In this situation, pharmacists should discuss with the physician the importance of maintaining normal serum potassium, magnesium and calcium concentrations; ECG monitoring for determination of QTc intervals when appropriate and feasible; and, for patients with a risk score in the high range, whether it is possible to prescribe an alternative, non–QTc interval–prolonging drug for the therapeutic indication. It should be emphasized that this risk score does not take into account pharmacokinetic drug interactions or appropriate dosing of renally eliminated QTc interval–prolonging drugs. If the patient is taking a drug combination listed in Table 4, or is receiving a drug in Table 5 for which the dose has not been appropriately adjusted for acute kidney injury/chronic kidney disease, then pharmacist communication with the prescribing physician is warranted irrespective of the calculated QTc interval risk score.
Recommendations regarding the appropriate frequency of ECG monitoring for determination of the QTc interval in community-based patients receiving QTc interval–prolonging drugs have not been widely promulgated. Patients who require therapy with methadone should undergo a pretreatment ECG to determine QTc interval, another at 30 days following initiation of therapy and annually thereafter.34 To guide pharmacists’ discussions with prescribers or decision-making by pharmacist-prescribers, it seems reasonable to recommend that patients with a QTc risk score ≥7 and those taking drug combinations in Table 4 for whom therapy cannot be altered should have an ECG to determine QTc interval when the plasma concentration of the QTc interval–prolonging drug is estimated to be at steady state, that is, 5 half-lives following the initiation of therapy.4 For hospitalized patients receiving therapy with QTc interval–prolonging drugs, it is recommended that the QTc interval be documented prior to initiation of therapy and at least every 8 to 12 hours following the initiation of therapy, dose increase or overdose.6 If QTc interval prolongation is observed, then more frequent monitoring is recommended.
Management of TdP
A treatment algorithm for TdP is presented in Figure 4. Drugs known to prolong the QTc interval should be discontinued immediately. Serum potassium and/or magnesium should be replaced if the patient is hypokalemic or hypomagnesemic. If the patient is hemodynamically unstable (systolic blood pressure <90 mmHg, heart rate >150 bpm, unconscious or losing consciousness and/or experiencing chest pain), asynchronous defibrillation should be performed, rather than synchronized electrical cardioversion, as synchronization of shocks to the QRS complex/T wave is often impossible in patients with polymorphic ventricular arrhythmias.35,36 In patients with hemodynamically stable TdP, intravenous magnesium 1 to 2 g administered over 15 minutes may terminate the arrhythmia, regardless of whether the patient is hypomagnesemic or has a normal serum magnesium concentration.37,38 In patients unresponsive to intravenous magnesium, intravenous isoproterenol or rapid overdrive pacing via a temporary transvenous pacemaker may be used if the patient has TdP that is associated with bradycardia. Increasing the heart rate via isoproterenol or rapid pacing facilitates restoration of sinus rhythm. In patients with hemodynamically stable TdP unresponsive to magnesium and, where appropriate, isoproterenol or rapid pacing, sedation followed by elective defibrillation may be indicated. Sotalol-associated TdP that is unresponsive to conventional therapy has been successfully managed with hemodialysis39 or peritoneal dialysis.40
Figure 4.
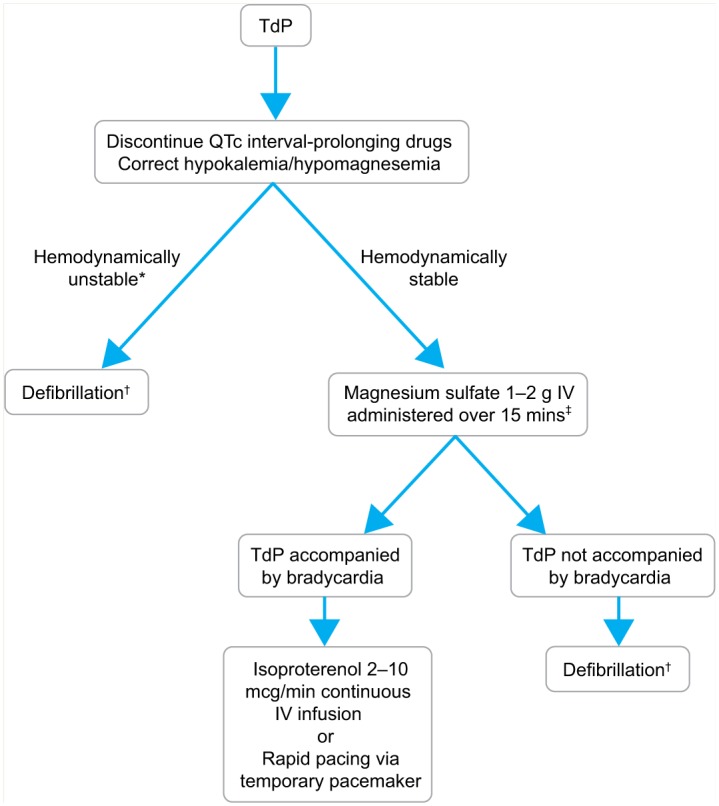
Algorithm for management of torsades de pointes
*Often defined as 1 or more of the following: systolic blood pressure <90 mmHg, heart rate > 150 beats per minute, unconscious or losing consciousness or chest pain.
†Polymorphic arrhythmias do not permit synchronization; therefore, defibrillation is recommended, rather than synchronized direct current cardioversion.35,36 Administer sedation when possible.
‡Even if patient is not hypomagnesemic
IV, intravenous; TdP, torsades de pointes.
Conclusion
In summary, more than 100 drugs available in Canada may cause QTc interval prolongation, which increases the risk of sudden cardiac death due to TdP. Risk factors for drug-induced QTc interval prolongation and TdP include older age, female sex, acute myocardial infarction, heart failure with reduced ejection fraction, hypokalemia, hypomagnesemia, hypocalcemia, bradycardia, diuretic therapy, concomitant therapy with multiple QTc interval–prolonging drugs, elevated plasma concentrations of QTc interval–prolonging drugs (due to drug interactions or inadequate dose adjustment of renally eliminated drugs in patients with kidney disease) and a possible genetic predisposition. Pharmacists may play an important role in minimizing the risk of drug-induced QTc interval prolongation and TdP through knowledge of drugs that are associated with TdP, assessment of risk with the QTc interval prolongation risk score, awareness of drug interactions most likely to result in TdP and attention to dose reduction of renally eliminated QTc interval–prolonging drugs in patients with kidney disease.■
Footnotes
Declaration of Conflicting Interests:The author is a volunteer member of the Advisory Board for the QT Drug Lists for the website www.crediblemeds.org. The author declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding:The author received no financial support for the research, authorship and/or publication of this article.
References
- 1. Lexchin J. Drug withdrawals from the Canadian market for safety reasons, 1963-2004. CMAJ 2005;172:765-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tisdale JE. Ventricular arrhythmias. In: Tisdale JE, Miller DA, editors Drug-induced diseases. Prevention, detection and management. 2nd ed. Bethesda (MD): American Society of Health-Systems Pharmacists; 2010. pp 485-515. [Google Scholar]
- 3. Woosley RL, Romero KA. Welcome to CredibleMeds. Available: www.Crediblemeds.org (accessed Nov. 6, 2015).
- 4. Trinkley KE, Page RL, II, Lien H, et al. QT interval prolongation and the risk of torsades de pointes: essentials for clinicians. Curr Med Res Opin 2013;29:1719-26. [DOI] [PubMed] [Google Scholar]
- 5. Bazett HC. An analysis of time relationships of the electrocardiogram. Heart 1920;7:353-70. [Google Scholar]
- 6. Drew BJ, Ackerman MJ, Funk M, et al. ; on behalf of the American Heart Association Acute Cardiac Care Committee of the Council on Clinical Cardiology, the Council on Cardiovascular Nursing and the American College of Cardiology Foundation. Prevention of torsades de pointes in hospital settings: a scientific statement from the American Heart Association and the American College of Cardiology Foundation. Circulation 2010;121:1047-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dessertenne F. La tachycardie ventriculaire à deux foyers opposes variables. Arch Mal Coeur Vaiss 1966;59:263-72. [PubMed] [Google Scholar]
- 8. Goldenberg I, Zareba W, Moss AJ. The long QT syndrome. Curr Probl Cardiol 2008;33:629-94. [DOI] [PubMed] [Google Scholar]
- 9. Mozaffarian D, Benjamin EJ, Go AS, et al. ; on behalf of the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation 2015;131:e29-e322. [DOI] [PubMed] [Google Scholar]
- 10. Moss AJ, Schwartz PJ, Crampton RS, et al. The long QT syndrome: prospective longitudinal study of 328 families. Circulation 1991;84:1136-44. [DOI] [PubMed] [Google Scholar]
- 11. Sharma ND, Rosman HS, Padhi ID, et al. Torsades de pointes associated with intravenous haloperidol in critically ill patients. Am J Cardiol 1998;81:238-40. [DOI] [PubMed] [Google Scholar]
- 12. Pratt CM, Singh SN, Al-Khalidi HR, et al. The efficacy of azimilide in the treatment of atrial fibrillation in the presence of left ventricular dysfunction: results from the Azimilide Postinfarct Survival Evaluation (ALIVE) trial. J Am Coll Cardiol 2004;43:1211-1216. [DOI] [PubMed] [Google Scholar]
- 13. US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER). Guidance for Industry. E14 Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs. Rockville (MD): US Department of Health and Human Services; 2005. [Google Scholar]
- 14. Darpö B. Spectrum of drugs prolonging QT interval and the incidence of torsades de pointes. Eur Heart J Suppl 2001;3(suppl K):K70-K80. [Google Scholar]
- 15. Tisdale JE, Wroblewski HA, Overholser BR, et al. Prevalence of QT interval prolongation in patients admitted to cardiac care units and frequency of subsequent administration of QT-interval prolonging drugs. Drug Saf 2012;35:459-70. [DOI] [PubMed] [Google Scholar]
- 16. Pickham D, Helfenbein E, Shinn JA, et al. High prevalence of corrected QT interval prolongation in acutely ill patients is associated with mortality: results of the QT in Practice (QTIP) study. Crit Care Med 2012;40:394-9. [DOI] [PubMed] [Google Scholar]
- 17. RxList. Top 200 drugs list. Available: www.rxlist.com/script/main/hp.asp (accessed Sept. 22, 2015). [Google Scholar]
- 18. Curtis LH, Østbye T, Sendersky V, et al. Prescription of QT-prolonging drugs in a cohort of about 5 million outpatients. Am J Med 2003;114:135-41. [DOI] [PubMed] [Google Scholar]
- 19. Pickham D, Helfenbein E, Shinn JA, et al. How many patients need QT interval monitoring in critical care units? Preliminary report of the QT in Practice study. J Electrocardiol 2010;43:572-76. [DOI] [PubMed] [Google Scholar]
- 20. Zeltser D, Justo D, Halkin A, et al. Torsade de pointes due to noncardiac drugs: most patients have easily identifiable risk factors. Medicine 2003;82:282-90. [DOI] [PubMed] [Google Scholar]
- 21. Zhang Y, Ouyang P, Post WS, et al. Sex-steroid hormones and electrocardiographic QT-interval duration: findings from the third National Health and Nutrition Examination Survey and the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol 2011;174:403-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Carnethon MR, Anthony MS, Cascio WE, et al. A prospective evaluation of the risk of QT prolongation with hormone replacement therapy: the atherosclerosis risk in communities study. Ann Epidemiol 2003;13:530-6. [DOI] [PubMed] [Google Scholar]
- 23. Pratt CM, Al-Khalidi HR, Brum JM, et al. Cumulative experience of azimilide-associated torsades de pointes ventricular tachycardia in the 19 clinical studies comprising the azimilide database. J Am Coll Cardiol 2006;48:471-7. [DOI] [PubMed] [Google Scholar]
- 24. Shaffer D, Singer S, Korvick J, et al. Concomitant risk factors in reports of torsades de pointes associated with macrolide use: review of the United States Food and Drug Administration Adverse Event Reporting System. Clin Infect Dis 2002;35:197-200. [DOI] [PubMed] [Google Scholar]
- 25. Charbit B, Christin-Maître S, Démolis JL, et al. Effects of testosterone on ventricular repolarization in hypogonadic men. Am J Cardiol 2009;103:887-90. [DOI] [PubMed] [Google Scholar]
- 26. Kadish AH, Greenland P, Limacher MC, et al. Estrogen and progestin use and the QT interval in postmenopausal women. Ann Noninvas Electrocardiol 2004;9:366-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ellenbogen KA, Stambler BS, Wood MA, et al. Efficacy of intravenous ibutilide for rapid termination of atrial fibrillation and atrial flutter: a dose-response study. J Am Coll Cardiol 1996;28:130-6. [DOI] [PubMed] [Google Scholar]
- 28. Stambler BS, Wood MA, Ellenbogen KA, et al. Efficacy and safety of repeated intravenous doses of ibutilide for rapid conversion of atrial flutter or fibrillation. Ibutilide Repeat Dose Study Investigators. Circulation 1996;94:1613-21. [DOI] [PubMed] [Google Scholar]
- 29. Singh S, Zoble RG, Yellen L, et al. Efficacy and safety of oral dofetilide in converting to and maintaining sinus rhythm in patients with chronic atrial fibrillation or atrial flutter. The Symptomatic Atrial Fibrillation Investigative research on Dofetilide (SAFIRE-D) study. Circulation 2000;102:2385-90. [DOI] [PubMed] [Google Scholar]
- 30. Torp-Pedersen C, Moller M, Bloch-Thomsen PE, et al. Dofetilide in patients with congestive heart failure and left ventricular dysfunction. Danish Investigations of Arrhythmia and Mortality on Dofetilide Study Group. N Engl J Med 1999;341:857-65. [DOI] [PubMed] [Google Scholar]
- 31. Yang P, Kanki H, Yang T, et al. Allelic variants in long-QT disease genes in patients with drug-associated torsades de pointes. Circulation 2002;105:1943-8. [DOI] [PubMed] [Google Scholar]
- 32. Tisdale JE, Jaynes HA, Kingery JR, et al. Development and validation of a risk score to predict QT interval prolongation in hospitalized patients. Circ Cardiovasc Qual Outcomes 2013;6:479-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tisdale JE, Jaynes HA, Kingery JR, et al. Effectiveness of a clinical decision support system for reducing the risk of QT interval prolongation in hospitalized patients. Circ Cardiovasc Qual Outcomes 2014;7:381-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Krantz MJ, Martin J, Stimmel B, et al. QTc interval screening in methadone treatment. Ann Intern Med 2009;150:387-95. [DOI] [PubMed] [Google Scholar]
- 35. Neumar RW, Otto CW, Link MS, et al. Part 8: adult advanced cardiovascular life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2010;122(suppl 3):S729-67. [DOI] [PubMed] [Google Scholar]
- 36. Link MS, Berkow LC, Kudenchuk PJ, et al. Part 7: adult advanced cardiovascular life support. 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2015;132(suppl 2):S444-S464. [DOI] [PubMed] [Google Scholar]
- 37. Manz M, Pfeiffer D, Jung W, et al. Intravenous treatment with magnesium in recurrent persistent ventricular tachycardia. New Trends Arrhythmias 1991;7:437-42. [Google Scholar]
- 38. Tzivoni D, Banai S, Schuger C, et al. Treatment of torsade de pointes with magnesium sulfate. Circulation 1988;77:392-7. [DOI] [PubMed] [Google Scholar]
- 39. Singh SN, Lazin A, Cohen A, et al. Sotalol-induced torsades de pointes successfully treated with hemodialysis after failure of conventional therapy. Am Heart J 1991;121:601-2. [DOI] [PubMed] [Google Scholar]
- 40. Tang S, Lo CY, Lo WK, et al. Sotalol-induced torsade de pointes in a CAPD patient—successful treatment with intermittent peritoneal dialysis (letter). Perit Dial Int 1997;17:207-8. [PubMed] [Google Scholar]




