Abstract
Background
Septic shock is a major healthcare problem with a high mortality rate that might be caused by immunosuppression. Programmed cell death receptor-1 (PD-1) and programmed cell death receptor ligand-1 (PD-L1), which are co-inhibitory receptor molecules, participate in sepsis-induced immunosuppression. In this study, we investigated which PD-1-related molecules can be used to evaluate the risk stratification and prognosis of septic patients. Furthermore, we explored the prognostic significance of a combination of ideal predictors and conventional clinical risk parameters in septic shock patients.
Methods
In total, 29 healthy controls, 59 septic patients, and 76 septic shock patients were enrolled in this study. Considering that the focus of the research was on the second phase of sepsis, blood samples were obtained at days 3–4 after the onset of systemic inflammatory response syndrome (SIRS). PD-1 and PD-L1 expression were measured on circulating CD4+ T cells, CD8+ T cells, and monocytes (PD-L1 only) by flow cytometry.
Results
Our results showed that only monocyte PD-L1 expression gradually increased, based on the increasing severity of disease (P < 0.001). Similarly, multivariate logistic regression analysis showed that only monocyte PD-L1 expression was an independent predictor of 28-day mortality in septic shock patients. Area under the receiver operating characteristic curve analysis of the combination of monocyte PD-L1 expression and conventional clinical risk parameters indicated a more significant prognostic ability than analysis of each parameter alone.
Conclusion
Our study demonstrated that, among PD-1-related molecules, only monocyte PD-L1 expression after 3–4 days of sepsis was associated with risk stratification and mortality in septic patients. Furthermore, measurement of monocyte PD-L1 expression was a promising independent prognostic marker for septic shock patients.
Keywords: PD-L1, Immunosuppression, Septic shock, Mortality
Background
Septic shock is a major healthcare problem with a high mortality rate [1]. Early and aggressive support treatment has not improved survival in patients with septic shock [2]. Postmortem studies of septic patients highlighted that immunosuppression may be a key cause of increased mortality [3, 4]. Indeed, the majority of nonsurviving patients with septic shock died in the stage of immunosuppression [5]. The immunosuppression phase is characterized by increased lymphocyte apoptosis, increased numbers of regulatory T cells, increased suppression of cytokine production, and decreased human leukocyte antigen-DR (HLA-DR) expression [6–9]. Programmed cell death receptor-1 (PD-1) and programmed cell death receptor ligand-1 (PD-L1), which are co-inhibitory receptor molecules, play major roles in sepsis-induced immunosuppression [4].
PD-1 is expressed on activated T cells, natural killer cells, and B cells [10]. Its ligand, PD-L1, is broadly expressed on hematopoietic and nonhematopoietic cells [11]. The PD-1/PD-L1 pathway exerts inhibitory effects by regulating T-cell activation, tolerance, and immunopathology [12, 13]. Although many studies have explored the roles of the PD-1/PD-L1 pathway in septic animals and patients, few studies have explored the relationship between PD-1-related molecules and the risk stratification of septic patients, and evaluated which PD-1-related molecules are useful biomarkers to predict mortality during the immunosuppressive phase of septic shock. In addition, most previous reports of PD-1-related molecules during sepsis focused on single measurements of immunosuppression. The correlation of PD-1-related molecules with conventional clinical risk parameters may be more useful for predicting 28-day mortality. In this study, we evaluated whether PD-1 and PD-L1 expression on circulating CD4+ T cells, CD8+ T cells, and monocytes (PD-L1 only) at days 3–4 of the onset of sepsis can be used to evaluate the risk stratification and prognosis of septic patients. Furthermore, we explored the prognostic significance of a combination of ideal predictors and conventional clinical risk parameters in septic shock patients.
Methods
Patients
The patients in this study were from the emergency department (ED) of Beijing Chao-yang Hospital. There are about 250,000 ED admissions per year in this university teaching hospital. Patients admitted to the ED at days 1–2 after the onset of signs of systemic inflammatory response syndrome (SIRS) were evaluated for possible enrollment according to the inclusion and exclusion criteria. The eligible patients were treated according to the international guidelines for management of septic shock [2]. The beginning of vasopressive therapy was defined as the onset of the septic shock. Considering the investigation’s focus on the second phase of sepsis, blood samples were obtained at days 3–4 after the onset of SIRS [5, 14].
According to the diagnostic criteria of the 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference [15], sepsis was defined by an identifiable site of infection, which was evidence of a systemic inflammatory response (SIRS) manifested by at least two of the following criteria: (a) body temperature >38.5 °C or <36 °C; (b) heart rate >90 beats per minute; (c) respiratory rate >20 breaths per minute or PaCO2 <32 mmHg; and (d) white cell count >12,000/mm3 or <4000/mm3, or percentage of immature neutrophils >10 %. Septic shock was defined as sepsis-induced hypotension despite adequate fluid resuscitation. Sepsis-induced hypotension was defined as a systolic blood pressure (SBP) <90 mmHg or mean arterial pressure (MAP) <70 mmHg or SBP decrease >40 mmHg or less than two standard deviations below normal for age in the absence of other causes of hypotension. According to the criteria of the International Sepsis Forum Consensus Conference on Definitions of Infection [16], the infection was defined on the basis of clinical features, laboratory findings, and an imaging test. The exclusion criteria were: (a) age less than 18 years; (b) patients with HIV infection or cancer if they were treated with a high dose of corticoids or if they presented with an aplasia (polymorphonuclear neutrophil count of less than 0.5 G/L); (c) patients who died within 2 days of the onset of septic shock; (d) the signs of SIRS occurred more than 3 days prior to admission, or patients were transferred from another hospital; and (e) patients who declined to consent.
Data collection
Clinical characteristics of patients, including age, gender, past medical history, vital signs, and results of correlative laboratory examinations, were recorded at days 3–4 after the onset of septic shock. Sepsis-related organ failure assessment (SOFA) score and simplified acute physiology score II (SAPS II) were calculated according to related clinical and demographic data. This study was approved by the Beijing Chao-yang Hospital Ethics Committee. Written informed consent was obtained from the patients and volunteers. Consents for patients who were unable to provide consent were provided by first-degree relatives. During follow-up the following data were collected: comorbidities (chronic obstructive pulmonary diseases, congestive heart failure, cerebrovascular disease, diabetes), and the outcome after 28 days (survival or death).
Flow cytometry
Samples of peripheral blood were collected in ethylenediamine tetraacetic acid (EDTA) anticoagulant tubes. The samples were transported to the laboratory at 4 °C within 2 h. Erythrocytes were lysed and cells were stained by a researcher who was blinded to the clinical data. Antibodies were purchased from BD Bioscience (San Jose, CA, USA) or eBioscience (San Diego, CA, USA). According to the manufacturer’s recommendations monoclonal antibodies and their isotype controls were used: BV421-labeled anti-PD1 (5 μl, clone EH12.1), PE-labeled anti-PD-L1 (20 μl, clone M1H1), APC-H7 labeled anti-CD3 (5 μl, clone SK7), FITC-labeled anti-CD4 (5 μl clone OKT4), FITC-labeled anti-CD8 (20 μl, clone RPA-T8), and APC-H7-labeled anti-CD14 (5 μl, clone MφP9) per 100 μl of whole blood. Samples were run on a Gallios™ Flow Cytometer (Beckman Coulter, Inc.) and analyzed using Gallios Software Version 1.0 (Beckman Coulter, Inc.). Lymphocytes were gated by forward scatter (FSC) and side scatter (SSC), and T-cells subsets were further identified by CD3+ and CD4+ staining. Monocytes were identified by CD14+ staining (Fig. 1). At least 3000 cells were analyzed from each sample. The threshold was defined using an isotype control. Results are expressed as percentages and the mean of fluorescence intensities (MFI).
Fig. 1.
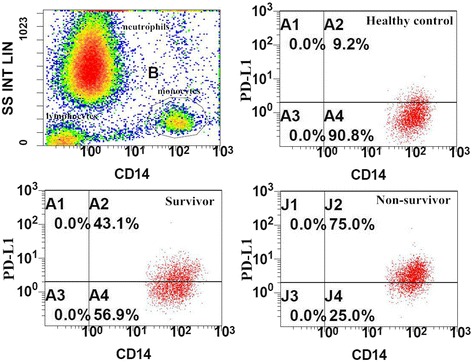
Representative flow dot plots of monocyte gating strategy and the percentage of programmed cell death receptor ligand-1 (PD-L1) on monocytes in different groups. The monocytes (gate B) were gated in the dot plots of side-scatter characteristics (SS) vs CD14. The multiple dot plots of CD14 vs PD-L1 are representative of the percentage of monocytes in healthy control, survivor, and nonsurvivor groups
Statistical analysis
The baseline characteristics were described as frequencies, percentages, median and inter-quartile ranges. Comparisons between groups were made using the Pearson χ2 test for categorical data and the Mann-Whitney test for continuous variables. For multi-group comparisons, the Kruskal-Wallis test was applied. Age, white blood cell (WBC), lymphocytes, SOFA score, and SAPS II were stratified using cutoff values based on the population median [5]. PD-1 and PD-L1 expressions were stratified using the optimal threshold indicated by the receiver operating characteristic (ROC) curve. Using cutoff values determined by ROC curves, Kaplan-Meier survival curves were established, and the log-rank test was applied for the comparisons of survival distributions. Binary logistic regression was used to identify the variables associated with 28-day mortality in patients with septic shock. Variables with P < 0.15 in univariate analysis were conserved in the model. All statistical tests were two-tailed, and P < 0.05 was considered statistically significant. All data were analyzed using SPSS 19.0 software (SPSS Inc., Chicago, IL, USA).
Results
Patient characteristics
Initially, 29 health controls, 59 septic patients, and 87 septic shock patients were admitted to the ED of Beijing Chao-yang Hospital from June 2014 to September 2015. However, 11 septic shock patients were excluded because they died within 2 days of the onset of septic shock. Thus, 76 septic shock patients were enrolled in this study. Septic patients were divided into the sepsis subgroup and septic shock subgroup according to the disease severity. The demographic and clinical characteristics of the patients are shown in Tables 1 and 2. A comparison of survivors and nonsurvivors showed significant differences in the number of lymphocytes, SOFA score, SAPS II, and comorbidities. Higher SOFA score and SAPS II indicated a high level of severity. The major types of infection were pneumonia (53.9 %) and urinary system infection (USI) (23.7 %).
Table 1.
Baseline characteristics of the patients
| Parameters | Control | Sepsis | Septic shock | P value |
|---|---|---|---|---|
| Number | 29 | 59 | 76 | – |
| Age (years) | 68 (66–75) | 71 (66–78) | 71 (61–78) | 0.497 |
| Male, n (%) | 18 (62.1 %) | 32 (54.2 %) | 37 (48.7 %) | 0.458 |
| WBC (×109/L) | 6.9 (5.6–7.9) | 13.3 (10.9–17.8) | 14.9 (11.8–17.2) | <0.001 |
| Lymphocyte (×109/L) | 2.8 (2.4–3.1) | 1.06 (0.73–1.63) | 0.76 (0.54–1.07) | <0.001 |
| SOFA score | 0 | 5 (3–7) | 11 (9–14) | <0.001 |
| SAPS II | 12 (12–15) | 26 (24–32) | 53 (46–60) | <0.001 |
| Percentage of PD-1+/CD4+ T cells (%) | 26.2 (23.2–29.9) | 34.1 (28.4–44.4) | 38.2 (29.2–47.7) | <0.001 |
| MFI of PD-1 on CD4+ T cells | 6.3 (5.8–6.8) | 7.1 (5.2–9.2) | 7.5 (6.0–9.4) | 0.008 |
| Percentage of PD-1+/CD8+ T cells (%) | 22.6 (18.7–28.2) | 31.6 (23.9–46.6) | 36.5 (27.3–51.3) | <0.001 |
| MFI of PD-1 on CD8+ T cells | 5.9 (4.8–6.6) | 6.4 (4.2–7.5) | 6.6 (4.9–8.4) | 0.181 |
| Percentage of PD-L1+/CD4+ T cells (%) | 18.2 (12.3–22.6) | 21.5 (16.5–30.5) | 21.0 (9.3–30.6) | 0.021 |
| MFI of PD-L1 on CD4+ T cells | 1.8 (1.6–2.2) | 1.8 (1.6–2.6) | 1.8 (1.5–2.0) | 0.230 |
| Percentage of PD-L1+/CD8+ T cells (%) | 22.6 (17.1–26.2) | 18.8 (14.9–37.6) | 19.5 (10.3–37.4) | 0.645 |
| MFI of PD-L1 on CD8+ T cells | 1.5 (1.4–1.7) | 1.6 (1.2–2.1) | 1.5 (1.4–1.8) | 0.596 |
| Percentage of monocytes expressing PD-L1 (%) | 12.9 (10.4–15.3) | 29.2 (12.1–43.9) | 35.9 (20.4–54.7) | <0.001 |
| MFI of PD-L1 on monocytes | 3.4 (3.0–3.9) | 4.5 (2.2–7.5) | 8.3 (7.7–9.7) | <0.001 |
| Type of infection, n (%) | ||||
| Pneumonia | 0 | 30 (50.8 %) | 41 (53.9 %) | 0.731 |
| IAI | 0 | 7 (11.8 %) | 11 (14.5 %) | 0.658 |
| CNSI | 0 | 5 (8.5 %) | 6 (7.9 %) | 0.903 |
| USI | 0 | 17 (28.8 %) | 18 (23.7 %) | 0.500 |
| Number of comorbidities | ||||
| ≥1, n (%) | 0 | 11 (18.6 %) | 39 (51.3 %) | <0.001 |
| 28-day mortality, n (%) | 0 | 10 (16.9 %) | 27 (35.5 %) | 0.016 |
Data are shown as median and interquartile range unless otherwise indicated. Kruskal-Wallis one-way analysis of variance was performed for multi-group comparisons
CNSI central nervous system infection, IAI intra-abdominal infection, MFI mean of fluorescence intensities, PD-1 Programmed cell death receptor-1, PD-L1 programmed cell death receptor ligand-1, SAPS II simplified acute physiology score II, SOFA sepsis-related organ failure assessment, USI urinary system infection, WBC white blood cells
Table 2.
Baseline characteristics of the patients with septic shock
| Parameters | Survivors | Nonsurvivors | Overall population | P value |
|---|---|---|---|---|
| Number | 49 | 27 | 76 | – |
| Age (years) | 73 (62–78) | 70 (61–78) | 71 (61–78) | 0.765 |
| Male, n (%) | 22 (44.9 %) | 15 (55.6 %) | 37 (48.7 %) | 0.792 |
| WBC (×109/L) | 14.9 (12.4–16.9) | 13.3 (10.9–17.8) | 14.9 (11.8–17.2) | 0.641 |
| Lymphocyte (×109/L) | 0.78 (0.59–1.24) | 0.65 (0.52–0.82) | 0.76 (0.54–1.07) | 0.046 |
| SOFA score | 11 (9–12) | 14 (12–17) | 11 (9–14) | <0.001 |
| SAPS II | 50 (43–56) | 60 (53–66) | 53 (46–60) | <0.001 |
| Percentage of PD-1+/CD4+ T cells (%) | 35.1 (28.5–44.8) | 42.9 (34.9–49.0) | 38.2 (29.2–47.7) | 0.034 |
| MFI of PD-1 on CD4+ T cells | 7.0 (5.7–8.9) | 8.8 (7.1–9.6) | 7.5 (6.0–9.4) | 0.036 |
| Percentage of PD-1+/CD8+ T cells (%) | 33.0 (24.8–44.3) | 47.1 (30.3–53.9) | 36.5 (27.3–51.3) | 0.032 |
| MFI of PD-1 on CD8+ T cells | 5.9 (4.7–8.1) | 7.8 (5.4–9.8) | 6.6 (4.9–8.4) | 0.033 |
| Percentage of PD-L1+/CD4+ T cells (%) | 16.4 (8.6–28.4) | 22.1 (12.0–34.0) | 21.0 (9.3–30.6) | 0.295 |
| MFI of PD-L1 on CD4+ T cells | 1.9 (1.5–2.0) | 1.8 (1.6–2.2) | 1.8 (1.5–2.0) | 0.961 |
| Percentage of PD-L1+/CD8+ T cells (%) | 23.2 (10.8–38.9) | 12.9 (9.9–32.5) | 19.5 (10.3–37.4) | 0.204 |
| MFI of PD-L1 on CD8+ T cells | 1.6 (1.4–1.9) | 1.5 (1.4–1.7) | 1.5 (1.4–1.8) | 0.378 |
| Percentage of monocytes expressing PD-L1 (%) | 28.9 (17.9–43.6) | 53.9 (31.8–72.9) | 35.9 (20.4–54.7) | 0.001 |
| MFI of PD-L1 on monocytes | 8.2 (7.5–9.0) | 9.1 (8.1–11.3) | 8.3 (7.7–9.7) | 0.012 |
| Type of infection, n (%) | ||||
| Pneumonia | 27 (55.1 %) | 14 (51.9 %) | 41 (53.9 %) | 0.786 |
| IAI | 8 (16.3 %) | 3 (11.1 %) | 11 (14.5 %) | 0.781 |
| CNSI | 4 (8.2 %) | 2 (7.4 %) | 6 (7.9 %) | 0.743 |
| USI | 10 (20.4 %) | 8 (29.6 %) | 18 (23.7 %) | 0.366 |
| Number of comorbidities | ||||
| ≥1, n (%) | 21 (42.9 %) | 18 (66.7 %) | 39 (51.3 %) | 0.047 |
Data are shown as median and interquartile range unless otherwise indicated
CNSI central nervous system infection, IAI intra-abdominal infection, MFI mean of fluorescence intensities, PD-1 Programmed cell death receptor-1, PD-L1 programmed cell death receptor ligand-1, SAPS II simplified acute physiology score II, SOFA sepsis-related organ failure assessment, USI urinary system infection, WBC white blood cells
Comparison of median levels of PD-1 and PD-L1 expression
PD-1 and PD-L1 expression was measured on circulating CD4+ T cells, CD8+ T cells, and monocytes (PD-L1 only) at days 3–4 after the onset of SIRS (Table 1). Compared with healthy controls, the percentages of circulating PD-1/CD4+ T cells, PD-1/CD8+ T cells, and monocytes expressing PD-L1 were significantly higher in septic patients and septic shock patients (P < 0.05). Interestingly, only the percentages of monocytes expressing PD-L1 were obviously different between septic patients and septic shock patients (P < 0.05). Similar results were also observed when expressed as MFI.
Correlation of PD-L1 expression with SOFA score or SAPS II in all patients
Spearman correlation analysis showed that the percentage of monocytes expressing PD-L1 in septic patients was positively correlated with SAPS II (r = 0.638; P < 0.001) and SOFA score (r = 0.654; P < 0.001). MFI of PD-L1 on monocytes was also positively correlated with SAPS II (r = 0.653; P < 0.001) and SOFA score (r = 0.582; P < 0.001).
PD-1 and PD-L1 expression levels in survivors and nonsurvivors in septic shock patients
Septic shock patients were divided into survivors and nonsurvivors according to the 28-day mortality. In nonsurvivors, the percentages of circulating PD-1/CD4+ T cells, PD-1/CD8+ T cells, and monocytes expressing PD-L1 were significantly higher in comparison with survivors (Fig. 2; P < 0.05). Similar results were also observed when expressed as MFI (Fig. 2; P < 0.05).
Fig. 2.
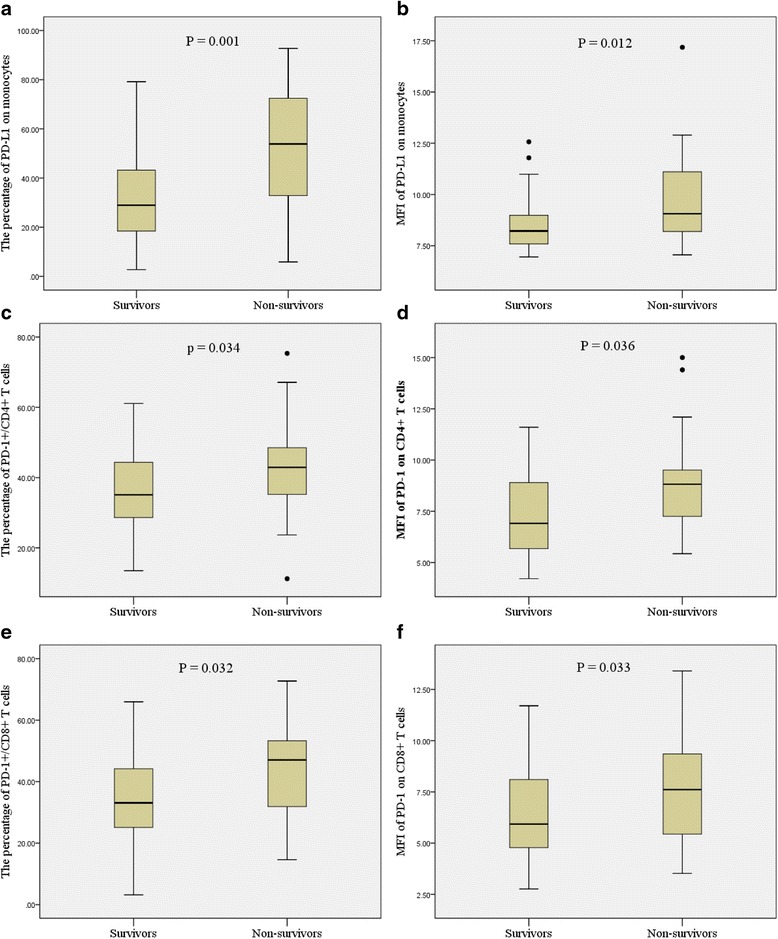
Box-plot representation of programmed cell death receptor-1 (PD-1)-related molecules. Data are shown as box plot with medians (lines inside boxes), 25th and 75th quartiles (limits of boxes); whiskers indicate the range. Programmed cell death receptor ligand-1 (PD-L1) expression on monocytes and PD-1 expression on T cells (CD4+ and CD8+ T cells) were significantly increased in nonsurvivors (n = 27) in comparison with survivors (n = 49) (a–f). MFI mean of fluorescence intensities
Value of PD-L1 expression for predicting 28-day mortality in septic shock patients
The ROC curve analysis (area under the curve (AUC)) showed that the percentage of monocytes expressing PD-L1 for predicting 28-day mortality was 0.729 (Table 3 and Fig. 3). ROC curve analysis showed that 44.2 % of monocytes expressing PD-L1 was the optimal threshold for predicting 28-day mortality in patients with septic shock. Using cutoff values determined by ROC, patients with a percentage of monocytes expressing PD-L1 higher than 44.2 % had a lower probability of survival at day 28 than patients with lower PD-L1 levels (Fig. 4; P < 0.001).
Table 3.
Area under the curve of various parameters for predicting 28-day mortality in patients with septic shock
| Variable | AUC | P value | 95 % Confidence interval | |
|---|---|---|---|---|
| Lower limit | Upper limit | |||
| Percentage of monocytes expressing PD-L1 | 0.729 | 0.001 | 0.607 | 0.852 |
| MFI of PD-L1 on monocytes | 0.681 | 0.009 | 0.548 | 0.813 |
| SAPS II | 0.768 | <0.001 | 0.653 | 0.883 |
| SOFA score | 0.736 | 0.001 | 0.610 | 0.863 |
| Percentage of PD-L1 on monocytes + SAPS II | 0.891 | <0.001 | 0.807 | 0.976 |
| MFI of PD-L1 on monocytes + SAPS II | 0.881 | <0.001 | 0.797 | 0.965 |
| Percentage of PD-L1 on monocytes + SOFA score | 0.829 | <0.001 | 0.713 | 0.944 |
| MFI of PD-L1 on monocytes + SOFA score | 0.799 | <0.001 | 0.682 | 0.917 |
AUC area under the curve, MFI mean of fluorescence intensities, PD-L1 programmed cell death receptor ligand-1, SAPS II simplified acute physiology score II, SOFA sepsis-related organ failure assessment
Fig. 3.
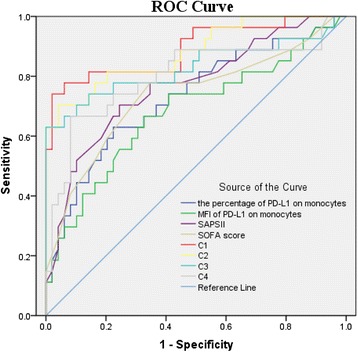
Receive operating characteristic (ROC) curve for predicting 28-day mortality in septic shock patients. AUCs: the percentage of PD-L1 on monocytes (dark blue line), 0.729; MFI of PD-L1 on monocytes (green line), 0.681; SAPS II (pink line), 0.768; SOFA score (brown line), 0.736; the percentage of PD-L1 on monocytes in combination with SAPS II (C1, red line), 0.891; MFI of PD-L1 on monocytes in combination with SAPS II (C2, yellow line), 0.881; the percentage of PD-L1 on monocytes in combination with SOFA score (C3, pale blue line), 0.829; and MFI of PD-L1 on monocytes in combination with SOFA score (C4, gray line), 0.799. MFI mean of fluorescence intensities, PD-L1 programmed cell death receptor ligand-1, SAPS II simplified acute physiology score II, SOFA sepsis-related organ failure assessment
Fig. 4.
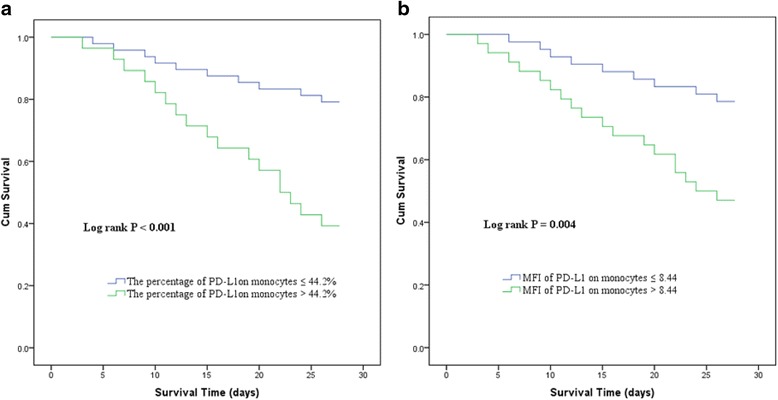
Survival curves of patients with septic shock according to the percentage of monocytes expressing PD-L1 (a) and MFI of PD-L1 on monocytes (b). MFI mean of fluorescence intensities, PD-L1 programmed cell death receptor ligand-1
Using a cutoff value of 44.2 % (for the percentage of monocytes expressing PD-L1) for predicting 28-day mortality in patients with septic shock, the sensitivity was 68.0 %, specificity was 77.6 %, the positive predictive value (PPV) was 65.7 %, and the negative predictive value (NPV) was 79.2 %. Using a cutoff value of 8.44 (for MFI of PD-L1 on monocytes) for predicting 28-day mortality in patients with septic shock, the sensitivity was 66.7 %, specificity was 67.3 %, the PPV was 62.9 %, and the NPV was 78.6 %.
PD-L1 expression as an independent predictor of 28-day mortality in septic shock patients
Univariate and multivariate logistic regression were used to identify PD-1-related molecules associated with 28-day mortality for patients with septic shock. Multivariate logistic regression analysis showed that only PD-L1 expression on monocytes was independently associated with 28-day mortality. The detailed data are presented in Table 4.
Table 4.
Logistic regression analysis of independent factors for 28-day mortality in patients with septic shock
| Variable | B | SE | Wald | P value | Odds ratio | 95 % confidence interval for EXP(B) | |
|---|---|---|---|---|---|---|---|
| Lower limit | Upper limit | ||||||
| Percentage of monocytes expressing PD-L1 | 1.943 | 0.745 | 6.811 | 0.009 | 6.981 | 1.622 | 30.041 |
| Lymphocytes | 0.088 | 0.794 | 0.012 | 0.912 | 1.092 | 0.230 | 5.173 |
| SOFA score | 1.550 | 0.687 | 5.091 | 0.024 | 4.713 | 1.226 | 18.117 |
| SAPS II | 1.558 | 0.723 | 4.646 | 0.031 | 4.749 | 1.152 | 19.584 |
| Number of comorbidities | 1.887 | 0.726 | 6.761 | 0.009 | 6.598 | 1.591 | 27.352 |
| Constant | −4.133 | 0.918 | 20.256 | 0.000 | 0.016 | ||
PD-L1 programmed cell death receptor ligand-1, SAPS II simplified acute physiology score II, SOFA sepsis-related organ failure assessment
Combination of PD-L1 expression with SOFA score or SAPS II in septic shock patients
We further explored the prognostic significance of a combination of independent predictors and conventional clinical risk parameters in septic shock patients. Interestingly, a combination of monocyte PD-L1 expression enhanced the ability of the SOFA score or SAPS II to predict 28-day mortality in patients with septic shock. The prognostic value of PD-L1 expression on monocytes in combination with SAPS II for predicting 28-day mortality was significantly higher than each parameter alone. The detailed data are presented in Table 3 and Fig. 3.
Discussion
The present study demonstrated that, among PD-1-related molecules, only monocyte PD-L1 expression after 3–4 days of sepsis was valuable for the risk stratification of septic patients. Monocyte PD-L1 expression was also an independent predictor of mortality in septic shock patients. Additionally, our study demonstrated that monocyte PD-L1 expression combined with clinical risk parameter (i.e., SAPS II) could enhance the ability to predict 28-day mortality in patients with septic shock. To the best of our knowledge, this is the largest number of patients with sepsis for which PD-1-related molecules were explored, and our findings may be useful for septic patient stratification and prognosis evaluation.
Sepsis is a complex pathophysiological process. It is widely accepted that although there is a predominance of the hyperinflammatory phase after sepsis initiation, sepsis then rapidly develops a state of immunosuppression [17, 18]. Because of the application of antibiotics and other aggressive treatments, many patients may survive the initial proinflammatory stage, but eventually die later in a state of immunosuppression [18, 19]. PD-1 and its ligand PD-L1 are thought to play major roles in immunosuppressive mechanisms. Blockade of the PD-1/PD-L1 inhibitory pathway has been successfully used in cancer and septic animals, and may have similar beneficial effects in septic shock patients [20–23]. Since sepsis-induced immunosuppression may play an important role in mortality during sepsis, there is interest in identifying patients with sepsis who may benefit from anti-PD-1 or anti-PD-L1 antibody therapy. Our finding may be useful for subsequent studies of PD-1/PD-L1 blockade in sepsis.
Although previous studies showed that increased PD-1 expression on T cells and monocyte PD-L1 expression were associated with increased occurrence of mortality and nosocomial infections [24, 25], we observed that there were no differences in PD-1 expression on T cells between septic patients and septic shock patients, and also that it was not independently associated with 28-day mortality in patients with septic shock. Therefore, PD-1 expression on T cells may be not a reliable “danger signal” for immunosuppression in septic patients. Interestingly, we observed that monocyte PD-L1 expression was valuable for the risk stratification of septic patients, and that it was an independent predictor of mortality. In that sense, monocyte PD-L1 expression may be useful for patient stratification in clinical trials attempting to boost the immune system in septic patients. Patients with high levels of monocytes expressing PD-L1 should be considered as immunosuppressed, which should be taken into consideration regarding potentially deleterious effects.
Previous studies have demonstrated that anti-PD-1 and anti-PD-L1 antibodies could inhibit lymphocyte apoptosis, reverse immune dysfunction, attenuate organ dysfunction, and improve survival in a murine model of sepsis [21, 25, 26]. In that sense, PD-1 and PD-L1 were ideal targets to restore immune status in sepsis. The reasons may be due to the fact that PD-L1 plays a major role in the PD-1/PD-L1 pathway by exerting inhibitory effects, while PD-1 is regarded as an auxiliary part of that process. Previous mechanistic studies of PD-L1 provided several lines of evidence in support of this point: PD-L1 blockage altered plasma cytokine levels, but PD-1 blockage did not [20, 25]. Our study showed that PD-L1 expression on monocytes was independently associated with 28-day mortality in patients with septic shock, but PD-1 on T cells was not; more studies are still needed to certify this conclusion.
Another intriguing finding of our study was that a combination of monocyte PD-L1 expression with SAPS II or SOFA score significantly enhanced the accuracy of predicting 28-day mortality in patients with septic shock. The optimized information provided by the use of combined markers was clearly illustrated in our study. Conventional clinical risk parameters indicated the severity of disease. The levels of co-inhibitory receptor molecules indicated the degree of immunosuppression that is characteristic of an inefficient clearance of invasive microbial pathogens. Combination of monocyte PD-L1 expression and conventional clinical risk parameters may be optimal candidates for predicting the prognosis of septic shock. Of particular importance, we demonstrated that monocyte PD-L1 expression combined with SAPS II was the best factor for predicting 28-day mortality in septic shock patients. These results suggested that monocyte PD-L1 expression may enhance the ability of clinical markers to evaluate the prognosis of septic shock patients.
Some limitations need to be considered in our study. First, the sample size was relatively small, and it was a single-center study. The current study findings should be confirmed by a multicenter study. Second, dynamic changes in the levels of monocyte PD-L1 expression remain targets for future studies. Third, because of the differences in the brand of flow cytometer used in different institutions, the cutoff values of PD-L1 on monocytes to predict poor outcome may be different. Therefore, similar to monocyte HLA-DR measurement [27], it is necessary to establish a standard protocol in different institutions. Previous studies have demonstrated that low levels of monocyte HLA-DR expression were associated with impairment of monocytic cellular functions [28, 29]. Correlation of PD-L1 values with HLA-DR expression may provide more useful information.
Conclusions
In conclusion, among PD-1-related molecules only monocyte PD-L1 expression after 3–4 days of sepsis is associated with risk stratification and mortality in septic patients. Furthermore, measurement of monocyte PD-L1 expression was a promising independent prognostic marker for septic shock patients.
Acknowledgements
The study was conducted thanks to the helpful contributions of the ED staff and Biochemistry Laboratory staff.
Abbreviations
- AUC
area under the curve
- ED
emergency department
- EDTA
ethylenediamine tetraacetic acid
- FSC
forward scatter
- HLA-DR
human leukocyte antigen-DR
- MAP
mean arterial pressure
- MFI
mean of fluorescence intensities
- NPV
negative predictive value
- PD-1
programmed cell death receptor-1
- PD-L1
programmed cell death receptor ligand-1
- PPV
positive predictive value
- ROC
receiver operating characteristic curve
- SAPS II
simplified acute physiology score II
- SBP
systolic blood pressure
- SIRS
system inflammatory response syndrome
- SOFA
Sepsis-related organ failure assessment
- SSC
side scatter
- USI
urinary system infection
- WBC
white blood cell
Footnotes
Competing interests
The authors declare they have no competing interests.
Authors’ contributions
CS-L conceived and designed the study, and was involved in drafting the manuscript and provided research funding. YF, HY, LZ, and ZJ collected data and critically revised the manuscript. RS collected and analyzed data, performed statistical analysis, and drafted and critically revised the manuscript. All authors gave final approval for manuscript publication and agree to be accountable for all aspects of this work.
Authors’ information
C-SL is a specialist of critical disease and the chief of the Emergency Department of Beijing Chao-yang Hospital. RS, YF, HY, LZ, and ZJ are graduate students of Emergency Medicine who are conducting a study of sepsis.
References
- 1.Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 2.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Intensive Care Med. 2013;39(2):165–228. doi: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Torgersen C, Moser P, Luckner G, Mayr V, Jochberger S, Hasibeder WR, et al. Macroscopic postmortem findings in 235 surgical intensive care patients with sepsis. Anesth Analg. 2009;108:1841–1847. doi: 10.1213/ane.0b013e318195e11d. [DOI] [PubMed] [Google Scholar]
- 4.Boomer JS, To K, Chang KC, Takasu O, Osborne DF, Walton AH, et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA. 2011;306:2594–2605. doi: 10.1001/jama.2011.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Monneret G, Lepape A, Voirin N, Bohe J, Venet F, Debard AL, et al. Persisting low monocyte human leukocyte antigen-DR expression predicts mortality in septic shock. Intensive Care Med. 2006;32:1175–1183. doi: 10.1007/s00134-006-0204-8. [DOI] [PubMed] [Google Scholar]
- 6.Hotchkiss RS, Tinsley KW, Swanson PE, Schmieg RE, Jr, Hui JJ, Chang KC, et al. Sepsis-induced apoptosis causes progressive profound depletion of B and CD4+ T lymphocytes in humans. J Immunol. 2001;166:6952–6963. doi: 10.4049/jimmunol.166.11.6952. [DOI] [PubMed] [Google Scholar]
- 7.Venet F, Chung CS, Kherouf H, Geeraert A, Malcus C, Poitevin F, et al. Increased circulating regulatory T cells (CD4(+)CD25(+)CD127(–)) contribute to lymphocyte anergy in septic shock patients. Intensive Care Med. 2009;35:678–686. doi: 10.1007/s00134-008-1337-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang X, Venet F, Wang YL, Lepape A, Yuan Z, Chen Y, et al. PD-1 expression by macrophages plays a pathologic role in altering microbial clearance and the innate inflammatory response to sepsis. Proc Natl Acad Sci U S A. 2009;106:6303–6308. doi: 10.1073/pnas.0809422106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Landelle C, Lepape A, Voirin N, Tognet E, Venet F, Bohé J, et al. Low monocyte human leukocyte antigen-DR is independently associated with nosocomial infections after septic shock. Intensive Care Med. 2010;36:1859–1866. doi: 10.1007/s00134-010-1962-x. [DOI] [PubMed] [Google Scholar]
- 10.Agata Y, Kawasaki A, Nishimura H, Ishida Y, Tsubata T, Yagita H, et al. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol. 1996;8:765–772. doi: 10.1093/intimm/8.5.765. [DOI] [PubMed] [Google Scholar]
- 11.Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. 2007;8:239–245. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- 12.Okazaki T, Honjo T. PD-1 and PD-1 ligands: from discovery to clinical application. Int Immunol. 2007;19:813–824. doi: 10.1093/intimm/dxm057. [DOI] [PubMed] [Google Scholar]
- 13.Nurieva RI, Liu X, Dong C. Yin-Yang of costimulation: crucial controls of immune tolerance and function. Immunol Rev. 2009;229:88–100. doi: 10.1111/j.1600-065X.2009.00769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hotchkiss RS, Monneret G, Payen D. Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. Lancet Infect Dis. 2013;13(3):260–268. doi: 10.1016/S1473-3099(13)70001-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. International Sepsis Definitions Conference: 2001 SCCM/ESICM/ACCP/ATS/SIS International sepsis definitions conference. Intensive Care Med. 2003;29(4):530–538. doi: 10.1007/s00134-003-1662-x. [DOI] [PubMed] [Google Scholar]
- 16.Calandra T, Cohen J. The international sepsis forum consensus conference on definitions of infection in the intensive care unit. Crit Care Med. 2005;33:1538–1548. doi: 10.1097/01.CCM.0000168253.91200.83. [DOI] [PubMed] [Google Scholar]
- 17.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 18.Munford RS, Pugin J. Normal responses to injury prevent systemic inflammation and can be immunosuppressive. Am J Respir Crit Care Med. 2001;163:316–321. doi: 10.1164/ajrccm.163.2.2007102. [DOI] [PubMed] [Google Scholar]
- 19.Monneret G, Venet F, Pachot A, Lepape A. Monitoring immune dysfunctions in the septic patient: a new skin for the old ceremony. Mol Med. 2008;14:64–78. doi: 10.2119/2007-00102.Monneret. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, Zhou Y, Lou J, Li J, Bo L, Zhu K, et al. PD-L1 blockade improves survival in experimental sepsis by inhibiting lymphocyte apoptosis and reversing monocyte dysfunction. Crit Care. 2010;14(6):R220. doi: 10.1186/cc9354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brahmamdam P, Inoue S, Unsinger J, Chang KC, McDunn JE, Hotchkiss RS. Delayed administration of anti-PD-1 antibody reverses immune dysfunction and improves survival during sepsis. J Leukoc Biol. 2010;88(2):233–240. doi: 10.1189/jlb.0110037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guignant C, Lepape A, Huang X, Kherouf H, Denis L, Poitevin F, et al. Programmed death-1 levels correlate with increased mortality, nosocomial infection and immune dysfunctions in septic shock patients. Crit Care. 2011;15(2):R99. doi: 10.1186/cc10112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Y, Li J, Lou J, Zhou Y, Bo L, Zhu J, et al. Upregulation of programmed death-1 on T cells and programmed death ligand-1 on monocytes in septic shock patients. Crit Care. 2011;15(1):R70. doi: 10.1186/cc10059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu W, Bao R, Fan X, Tao T, Zhu J, Wang J, et al. PD-L1 blockade attenuated sepsis-induced liver injury in a mouse cecal ligation and puncture model. Mediators Inflamm. 2013;2013:361501. doi: 10.1155/2013/361501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Docke WD, Hoflich C, Davis KA, Rottgers K, Meisel C, Kiefer P, et al. Monitoring temporary immunodepression by flow cytometric measurement of monocytic HLA-DR expression: a multicenter standardized study. Clin Chem. 2005;2005(51):2341–2347. doi: 10.1373/clinchem.2005.052639. [DOI] [PubMed] [Google Scholar]
- 28.Astiz M, Saha D, Lustbader D, Lin R, Rackow E. Monocyte response to bacterial toxins, expression of cell surface receptors, and release of anti-inflammatory cytokines during sepsis. J Lab Clin Med. 1996;128:594–600. doi: 10.1016/S0022-2143(96)90132-8. [DOI] [PubMed] [Google Scholar]
- 29.Manjuck J, Saha DC, Astiz M, Eales LJ, Rackow EC. Decreased response to recall antigens is associated with depressed co-stimulatory receptor expression in septic critically ill patients. J Lab Clin Med. 2000;135:153–160. doi: 10.1067/mlc.2000.104306. [DOI] [PubMed] [Google Scholar]


