Abstract
Haploid mammalian embryonic stem cells (ESCs) hold great promise for functional genetic studies and assisted reproduction. Recently, rodent androgenetic haploid ESCs (AG-haESCs) were generated from androgenetic blastocysts and functioned like sperm to produce viable offspring via the intracytoplasmic AG-haESCs injection into oocytes. However, the efficiency of this reproduction was very low. Most pups were growth-retarded and died shortly after birth, which is not practical for producing knockout animals. Further investigation suggested a possible link between the low birthrate and aberrant expression of imprinted genes. Here, we report the high-frequency generation of healthy, fertile mice from H19-Igf2 imprinting-locus modified AG-haESCs, which maintained normal paternal imprinting and pluripotency. Moreover, it is feasible to perform further genetic manipulations in these AG-haESCs. Our study provides a reliable and efficient tool to rapidly produce gene-modified mouse models and will benefit reproductive medicine in the future.
Keywords: Haploid ES cells, AG-haESCs, imprinted gene, ICAHCI, gene-modified mice
Introduction
Recently, mammalian haploid embryonic stem cell (ESC) lines were successfully established in mouse, rat and monkey [1–4]. They displayed unique potential for functional genetic studies and hold great promise for assisted reproduction [5, 6]. Moreover, androgenetic haploid ESCs (AG-haESCs) were isolated from androgenetic rodent blastocysts and functioned like sperm to produce viable, fertile progeny after intracytoplasmic injection into mature oocytes [3, 7, 8], providing a new approach for rapidly producing genetically modified mice. However, the efficiency was extremely low, and many of these semi-cloned (SC) pups were growth-retarded and died shortly after birth, most likely due to instable paternal imprinting in these AG-haESCs.
Genomic imprinting has an essential role in mammalian development [9–11]. Among the ~150 reported murine imprinted genes, the H19-Igf2 locus was first identified and shown to be essential for normal fetal growth [12, 13]. Furthermore, viable bi-maternal mice were produced from reconstructed eggs containing fully grown oocytes and non-growing oocytes that harbored a deletion in the H19-Igf2 locus [14]. Interestingly, abnormal H19 imprinting was observed in the prolonged cultured AG-haESCs and growth-retarded newborns mentioned above [7, 8]. We therefore asked whether genetic modification of the H19-Igf2 locus in AG-haESCs could yield fertile transgenic mice at a high frequency.
Results
Genetic modification of the H19-Igf2 locus in AG-haESCs
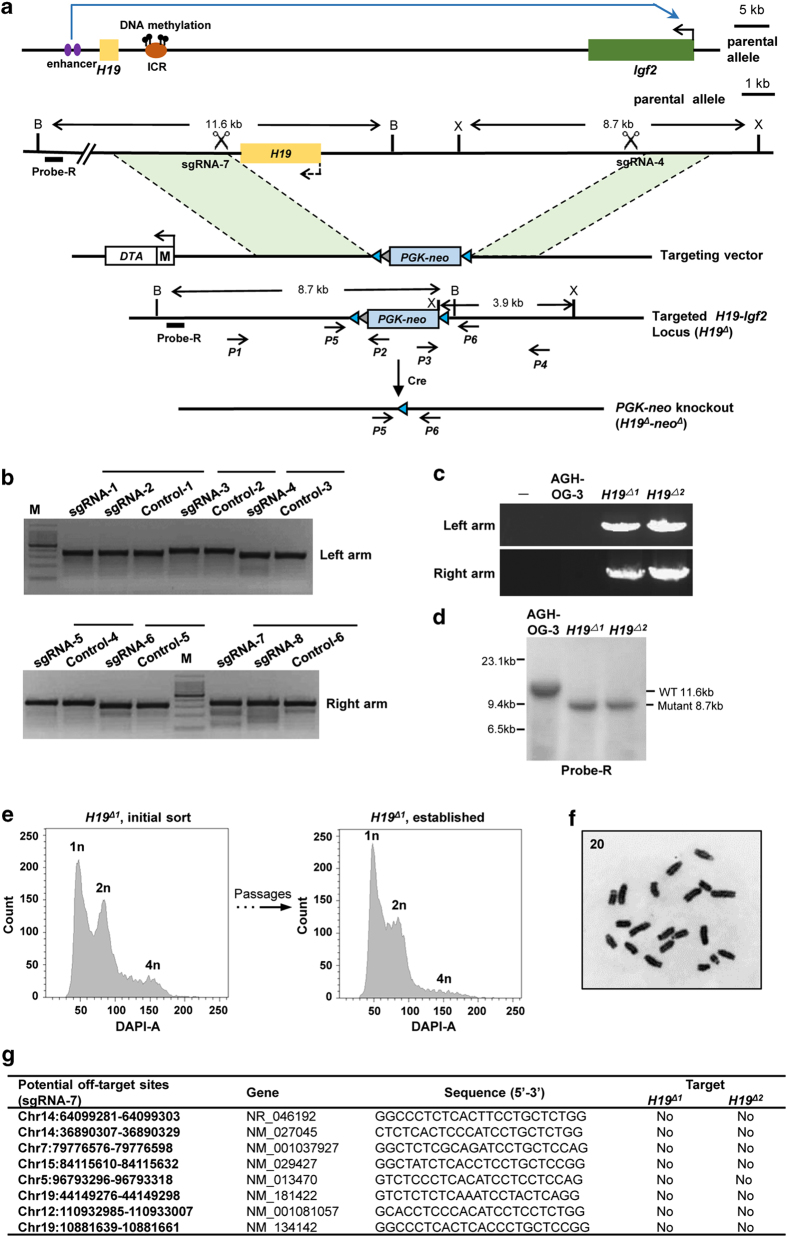
The mouse H19 gene produces a 2.3 kb long non-coding RNA exclusively expressed from the maternal allele and physically linked to the Igf2 gene on chromosome 7. They are reciprocally imprinted. The imprinting control region (ICR) within the H19-Igf2 locus is essential for transcriptional insulation of the maternal Igf2 allele [15]. To disrupt H19 gene expression in AG-haESCs, we used clustered regularly interspaced short palindromic repeat (CRISPR)-Cas9 enhanced homologous recombination [16, 17] to knock out the 13 kb region of H19 that includes the transcription unit and the ICR (Figure 1a). We designed four single guide RNAs (sgRNAs) targeting different sites up- or downstream of the region and investigated the specificity of these sgRNAs via the Surveyor assay [18]. Cas9/sgRNA-4 and Cas9/sgRNA-7 transfection efficiently cleaved at the target loci and were used for gene targeting (Figure 1b). We co-transfected the pCCI-H19, Cas9-sgRNA-4 and Cas9-sgRNA-7 plasmids into AGH-OG-3 AG-haESCs harboring the Oct4 promoter-driven eGFP (Oct4-eGFP) transgene. The positive clones were examined by PCR (Figure 1c) and were further validated by Southern blot analysis (Figure 1d). Among the 96 picked colonies, 29 positive clones harbored the desired H19-ICR deletion (H19 Δ ). We randomly chose two clones containing considerable haploid cells for further experiments. The two AG-haESC lines, referred to as H19Δ1 and H19Δ2, were established through consecutive passages and multiple rounds of fluorescence-activated cell sorting (FACS) for haploid cells (Figure 1e). Karyotyping analysis showed that these cells contained a haploid set of 20 chromosomes (Figure 1f). Comparative genomic hybridization (CGH) analysis confirmed the genome integrity of the two haploid cell lines (Supplementary Table S1). Analyses of potential off-target regions showed that none of the eight predicted sites were mutated by CRISPR/Cas9 in the two-cell clones (Figure 1g).
Figure 1.
Genetic modification of the H19-Igf2 locus in AG-haESCs. (a) Schematic representation of CRISPR-Cas9 assisted homologous recombination to target the H19-Igf2 locus in AG-haESCs (H19ΔAG-haESCs). The PGK-neo cassette was deleted by Cre (H19Δ-neoΔAG-haESCs). (b) Surveyor assay for Cas9-mediated cleavage up- and downstream of the H19 locus in AG-haESCs. (c) Validation of gene targeting in H19ΔAG-haESCs by PCR. (d) Confirmation of gene targeting in H19ΔAG-haESCs by Southern blot. (e) Establishment of the H19Δ1AG-haESC line after FACS enrichment for haploid cells. (f) Karyotype of H19Δ1 AG-haESCs showing normal haploidy. (g) Identification of the potential off-targets of CRISPR-Cas9 in H19ΔAG-haESCs.
Characteristics of H19Δ AG-haESCs
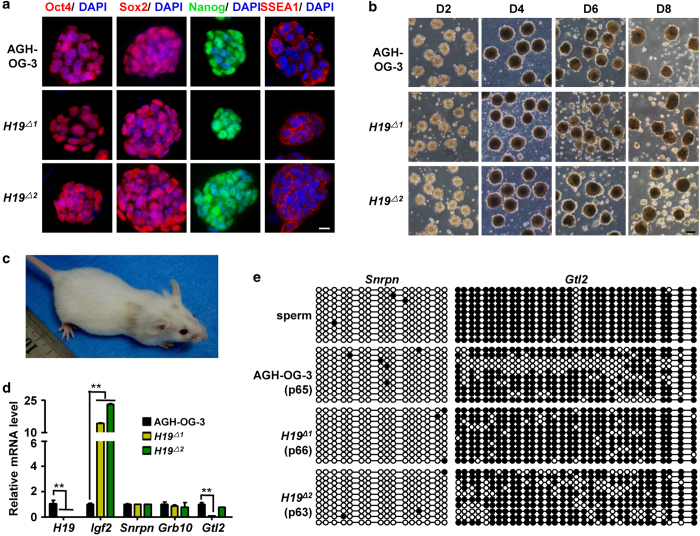
Cultured H19Δ AG-haESCs exhibited dome-shaped colony morphology similar to diploid mouse ESCs and expressed the pluripotency genes Oct4, Sox2, Nanog and SSEA1 (Figure 2a). These cells formed embryoid bodies when cultured in suspension (Figure 2b). The pluripotency was further tested by injection of the H19ΔAG-haESCs into diploid ICR blastocysts derived from mice with white coats. One chimeric mouse was obtained and survived to adulthood without obvious defects (Figure 2c). These studies provided evidence for the pluripotency of H19Δ AG-haESCs.
Figure 2.
Characteristics of H19ΔAG-haESCs. (a) Immunofluorescence staining of AGH-OG-3 and H19ΔAG-haESCs. Scale bar, 20 μM. (b) Different days of embryoid bodies (EB) formation of AGH-OG-3 and H19ΔAG-haESCs. Scale bar, 200 μM. (c) Adult chimeric mouse produced by microinjection of haploid H19Δ1AG-haESC into diploid blastocysts. (d) Expression of imprinted genes measured by quantitative reverse transcription PCR (RT-qPCR). AGH-OG-3 AG-haESCs were used as control. Error bars, ±s.d. n=3. **P<0.01. (e) Methylation analysis of the Snrpn and Gtl2 DMRs in H19ΔAG-haESCs. Sperm DNA was used as control. Open circles represent unmethylated CpG sites, whereas filled circles represent methylated CpG sites.
We then analyzed whether the H19-ICR deletion affected the imprinting status of AG-haESCs. We first examined the expression of H19, Igf2, Snrpn (maternally imprinted), Grb10 (paternally imprinted) and Gtl2 (paternally imprinted) in AGH-OG-3 and the two H19Δhaploid cell lines. As expected, the expression of H19 was nearly undetectable in the two H19Δ cell lines, whereas the Igf2 level was significantly upregulated (Figure 2d). Snrpn and Grb10 expression in H19ΔAG-haESCs was comparable to expression in AGH-OG-3 AG-haESCs (Figure 2d). Interestingly, the expression level of Gtl2 in the two H19Δ cell lines was varied. Gtl2 was expressed at low levels in H19Δ1AG-haESCs, whereas its expression in H19Δ2AG-haESCs was similar to AGH-OG-3 cells (Figure 2d). This demonstrated the intraclonal heterogeneity of Gtl2 expression in AGH-OG-3 cells. To further assess the DNA methylation profile of the imprinted genes, we performed bisulfite sequencing to analyze the ICRs of the Snrpn and Gtl2 loci. H19Δ1AG-haESCs showed normal methylation patterns similar to sperm in the ICRs of Snrpn and Gtl2, whereas late-passage (p65) AGH-OG-3 cells displayed abnormal methylation in the Gtl2 ICR. H19Δ2AG-haESCs harbored an abnormal methylation of the Gtl2 ICR similar to AGH-OG-3 cells (Figure 2e). The normal Gtl2 ICR methylation in H19Δ1AG-haESCs indicated that some late-passage AGH-OG-3 cells maintained normal imprinting of the Gtl2 ICR. The methylation status of the Snrpn and Gtl2 ICRs in H19Δ1 and H19Δ2AG-haESCs was consistent with the expression of Snrpn and Gtl2 in these cells. These results indicated that H19-ICR deletion in AG-haESCs had a negligible impact on the imprinting status of genes except for the H19-Igf2 locus.
H19Δ AG-haESCs efficiently support the generation of healthy SC mice
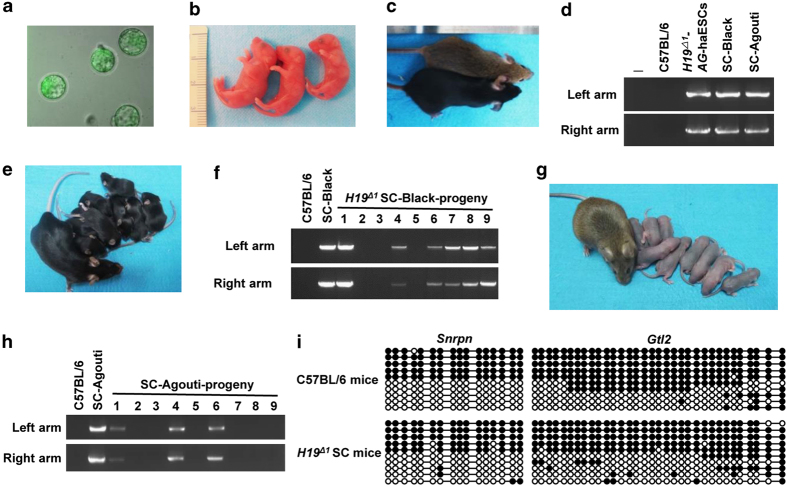
We were interested in testing whether H19-ICR deletion in AG-haESCs increased the efficiency of producing offspring. We injected FACS-sorted G0- or G1- phase H19ΔAG-haESCs into pre-activated oocytes via intracytoplasmic AG-haESCs injection (ICAHCI). H19ΔAG-haESCs contributed to embryos, as judged by Oct4-eGFP expression in developed blastocysts (Figure 3a). We transferred 80 two-cell embryos from the H19Δ1AG-haESC line into two pseudopregnant ICR mice and obtained three female full-term pups. All pups had normal body size and did not exhibit obvious developmental retardation after birth (Figure 3b). Two pups grew to adulthood with black (SC-Black) and agouti (SC-Agouti) fur, respectively (Figure 3c and d). Because H19Δ1AG-haESCs had a C57BL/6 background, the black or agouti coat colors of SC mice depended on the oocytes used for ICAHCI, which were derived from CD-1 or B6D2F1 (C57BL/6×DBA/2) mice, respectively. The SC mice delivered healthy progeny with litter sizes of 8–10 when mated with C57BL/6 males (Figure 3e and g). We obtained three SC-Black litters and two SC-Agouti litters. Approximately half of these carried the H19-ICR deletion, consistent with the expected Mendelian ratio (Figure 3f and h). The rate of SC mice born was ~4% of H19Δ1AG-haESCs ICAHCI (Table 1) compared with the ~2.2% efficiency of generating SC mice from early passage AGH-OG-3 (passage 17) AG-haESCs ICAHCI and 0% from late-passage AGH-OG-3 cells (passage 22) [7]. Many of the AGH-OG-3 AG-haESC derived pups were growth-retarded and died within 1 h of birth [7]. However, no growth-retarded pups were produced from H19Δ1AG-haESCs ICAHCI, even though the cells were from a very late passage (more than passage 56). The growth retardation of SC mice might be due to the loss of methylation in the H19 ICR and the consequent lower expression of Igf2. In SC mice generated by H19Δ1AG-haESCs ICAHCI, H19-ICR was deleted, leading to the normal expression of Igf2. The methylation status of two other imprinted genes, Snrpn and Gtl2, was normal in SC mice (Figure 3i). These results demonstrated that H19ΔAG-haESCs could function like sperm to produce normal mice and transmit their genetic material to progeny. Thus, modification of the H19-Igf2 locus was sufficient to generate healthy and fertile SC mice at a high efficiency.
Figure 3.
Generation of ICAHCI offspring by H19Δ1AG-haESCs. (a) Blastocysts generated by injection of H19Δ1AG-haESCs into oocytes. The H19Δ1AG-haESCs carried the Oct4-eGFP transgene. (b) Three SC pups from ICAHCI using H19Δ1AG-haESCs. (c) Adult SC mice derived from ICAHCI using H19Δ1AG-haESCs. (d) PCR analysis of H19 deletion in SC mice. (e) Adult H19Δ1SC-Black mouse and its progeny. (f) PCR analysis of H19 deletion in the progeny of H19Δ1SC-Black mice. The primer pairs P1–P2 and P3–P4 were used. (g) Adult H19Δ1SC-Agouti mouse and its progeny. (h) PCR analysis of the H19 deletion in the progeny of SC-Agouti mice. (i) Methylation analysis of imprinting in H19Δ1SC pups. C57BL/6 mice DNA was used as control. Open circles represent unmethylated CpG sites, whereas filled circles represent methylated CpG sites.
Table 1. Developmental efficiencies of ICAHCI embryos.
| Donor AG-haESCs | Passage number | Embryo stage | Number of transferred embryos | No. of normal pups (% of transferred embryos) | No. of pups surviving to adulthood (% of transferred embryos) | No. of Growth-retarded pups (% of transferred embryos) |
|---|---|---|---|---|---|---|
| OG-3a | p22 | Two-cell embryo | 102 | 0 | 0 | 3 (2.9) |
| H19Δ1 | >p56 | Two-cell embryo | 80 | 3 (3.8) | 2 (2.5) | 0 |
| H19Δ1-neoΔ1 | — | Two-cell embryo | 50 | 1 (2) | ND | 0 |
| Round spermatids | — | Two-cell embryo | 61 | 5 (8.2) | 5 (8.2) | 0 |
Abbreviations: AG-haESCs, androgenetic haploid embryonic stem cells; ICAHCI, intracytoplasmic AG-haESCs injection; ND, not determined.
The data were from Yang et al.7
Generation of genetically modified mice using H19Δ AG-haESCs
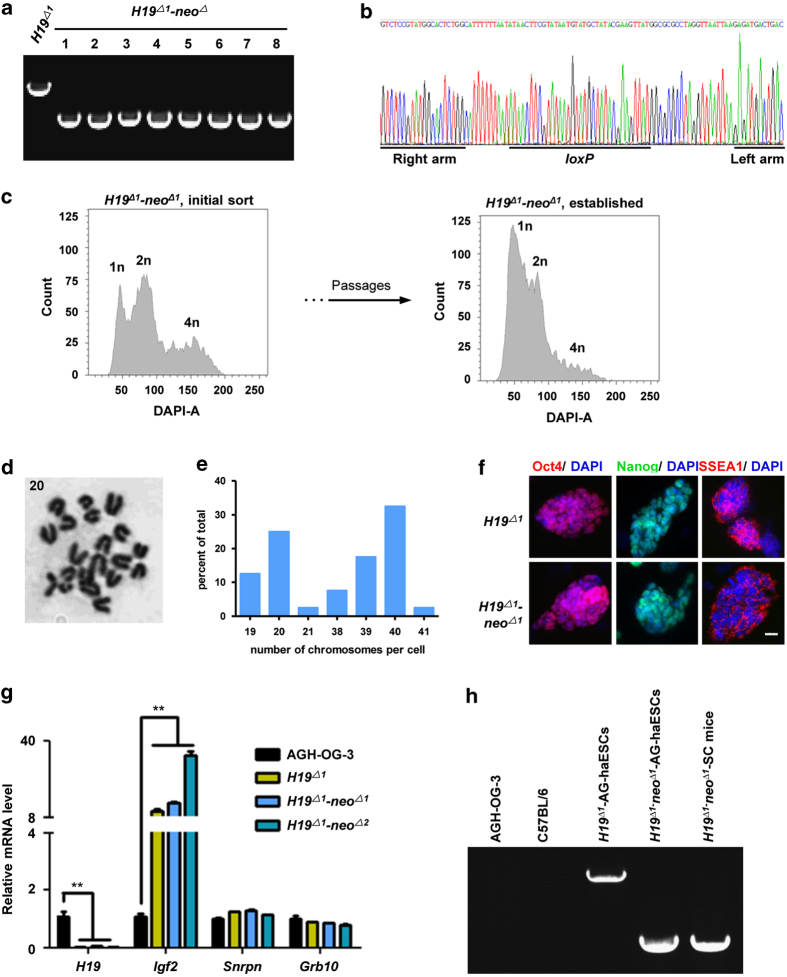
To further analyze the feasibility of producing genetically modified mouse models from H19ΔAG-haESCs, we deleted a loxP flanked PGK-neo cassette by transient expression of Cre in the H19Δ1AG-haESC line (Figure 1a). Among the 96 picked clones, the PGK-neo cassette was correctly deleted in most clones, as analyzed by PCR (Figure 4a). The remaining loxP site in the H19-Igf2 locus was further confirmed by DNA sequencing (Figure 4b). We then randomly chose 24 correctly deleted clones (H19Δ1-neoΔ) and performed FACS to determine the haploid subpopulation in these clones. The haploid ESC lines were established through consecutive passages, followed by two rounds of FACS for haploid cells (Figure 4c). The H19Δ1-neoΔ AG-haESCs had normal haploid karyotypes (Figure 4d and e), expressed pluripotency markers (Figure 4f), and imprinted genes comparable to H19ΔAG-haESCs (Figure 4g). After injecting the sorted G0- or G1- phase H19Δ1-neoΔ1AG-haESCs into the pre-activated oocytes, we successfully obtained 50 two-cell stage embryos and transplanted them into pseudopregnant females. One full-term live pup derived from the H19Δ1-neoΔ1AG-haESC line was obtained (Table 1). Genotype analysis confirmed PGK-neo cassette deletion in the SC animal (Figure 4h). Our results demonstrated that H19ΔAG-haESCs can be used to rapidly generate gene-modified mice.
Figure 4.
Generation of ICAHCI offspring by H19Δ1-neoΔAG-haESCs. (a) Subclones of PGK-neo knockouts in H19Δ1AG-haESCs by PCR. The primer pairs P5–P6 were used. (b) DNA sequence of PCR products amplified from the H19 gene of H19Δ1-neoΔAG-haESCs. (c) Establishment of the H19Δ1-neoΔ1AG-haESC line after FACS enrichment for haploid cells. (d) Chromosome counting in H19Δ1-neoΔ1AG-haESCs showing normal haploidy. (e) The distribution of absolute chromosome numbers of H19Δ1-neoΔ1AG-haESCs under cell culture conditions. (f) Immunofluorescence staining of H19Δand H19Δ1-neoΔ1AG-haESCs. Scale bar, 20 μM. (g) Expression of imprinted genes measured by quantitative reverse transcription PCR (RT-qPCR). AGH-OG-3 AG-haESCs were used as control. Error bars, ±s.d. n=3. **P<0.01. (h) PCR analysis of PGK-neo knockout in H19Δ1-neoΔ1SC mice.
Discussion
Although we do not know why the paternally imprinted gene H19 easily lost methylation in AG-haESCs, successful restoration of Igf2 expression by H19-ICR deletion faithfully improved the efficiency to generate viable SC mice. Other imprinted genes, such as Grb10, Dlk1 and Gtl2, also affect embryonic growth [11]. Moreover, Dlk1-Dio3 ICR deletion significantly increased the efficiency of generating parthenogenetic mice [14, 19]. It is tempting to investigate whether additional modification of these imprinted genes in AG-haESCs would increase the efficiency of producing SC mice, although the expression level and methylation status of these genes in AG-haESCs were comparable to those in sperm [7, 8]. During the submission of this work, an independent study [20] reported the highly efficient generation of healthy and fertile SC pups from AG-haESCs carrying deletions in the H19-DMR alone or in addition with Gtl2-DMR, confirming our findings. Gtl2-DMR deletion ensured the full silencing of the paternally imprinted gene Gtl2 in AG-haESCs, consistent with the normal imprinting status of Gtl2 in H19Δ1AG-haESCs.
Modification of the H19-Igf2 locus in AG-haESCs faithfully improved the efficiency of generating healthy SC mice via the ICAHCI technology. Our work provides another approach for quickly generating gene-modified mice in addition to the VelociMouse [21] and CRISPR-Cas9 [22] technologies. The H19ΔAG-haESCs have ‘sperm-like’ activity and can replace sperm in reproduction to overcome their limitations. Sperm are not able to propagate and are thus difficult to genetically modify in vitro. In addition, many established methods and resources for genetic studies of diploid ES cells are immediately applicable to H19ΔAG-haESCs. This work provides inspiration for possible human AG-haESC research and may benefit male infertility in the future, similar to the contribution of spermatogonial stem cells [23].
Materials and methods
Mice
All mouse experimental protocols were approved by the Institutional Animal Care and Use Committee at Peking Union Medical College & Chinese Academy of Medical Sciences. And all animal care and experimental methods were carried out in accordance with the institutional ethical guidelines for animal experiments. Male mice of C57BL/6 were used for sperm collection. B6D2F1 (C57BL/6×DBA/2) and CD-1 female mice were used to provide oocytes for micromanipulation. Female mice of ICR were used to provide blastocysts for microinjection and were also used as pseudopregnant mice. C57BL/6 mice were used for animal mating.
AG-haESCs
Androgenetic haploid ES cell line AGH-OG-3 was obtained from Dr Jinsong Li’s lab in Shanghai (China). The cells were cultured in ESC medium supplemented with 15% fetal bovine serum, 1 000 U ml−1 leukemia inhibitory factor, 3 μM CHIR99021 and 1 μM PD0325901.
DNA content analysis
The dissociated cells were incubated with 10 μg ml−1 Hoechst 33342 at 37 °C for 30 min. Then the haploid 1n peak was purified by flow-cytometry (BD FACSAria III, San Jose, CA, USA). Flow-cytometric data were analyzed using the BD FACSDiva software.
Vector construction
For pCCI-H19 construction, the left and right homologous arms were amplified from bacterial artificial chromosome (RP23-209022). Eag I and Pac I restriction sites were added to the amplified left homologous arm, and Sma I and Xho I sites were added to the right arm. Eag I and Pac I digested left arm was cloned into pCCI18 backbone (digested with Not I and Pac I). The products were then digested with Xho I and ligated with Sma I and Xho I digested right arm.
Construction of CRISPR plasmids
The pX330 plasmid was bought from Addgene, Cambridge, MA, USA. The sgRNA-1, -2, -3, -4, -5, -6, -7 and -8 oligos were synthesized, annealed and ligated to the pX330 plasmid that was digested with Bbs I (New England Biolabs, Ipswitch, MA, USA). The sequences of designed sgRNAs were listed in Supplementary Table S2.
Surveyor assay
The AG-haESCs were transfected with CRISPR-sgRNA plus pPB-puro plasmids and selected by puromycin for 36 h. Genomic DNA from wild-type and transfected AG-haESCs was extracted. PCR products were denatured, annealed and treated with T7EN1 (New England Biolabs). The primers used for PCR were listed in Supplementary Table S2.
Gene targeting in AGH-OG-3 AG-haESCs
The pCCI-H19, Cas9-sgRNA-4 and Cas9-sgRNA-7 plasmids were co-transfected into AGH-OG-3 AG-haESCs. The cells were selected by G418 (150 μg/ml) for 7 days. Colonies were picked and analyzed by PCR and flow cytometer sorting. The CAGGS-Cre plasmid was electroporated into H19Δ1AG-haESCs to delete the loxP flanked PGK-neo cassette.
Southern blotting
Wild-type and H19ΔAG-haESC genomic DNA was digested using BglII restriction enzyme. The digested DNA was subsequently separated on a 1% agarose gel for 5 h, and then transferred to a nylon membrane and hybridized with α-32P random primer-labeled probes.
Prediction of potential off-targets
The potential off-target regions were predicted using the online tool, http://crispr.mit.edu/. The regions with score >0.5 and mismatch ⩽4 were considered as the potential off-target sites. They were amplified using High-Fidelity DNA Polymerase (New England Biolabs) and sequenced.
Intracytoplasmic injection
ICAHCI was described as previously [8]. In brief, mature oocytes were collected from the oviduct of super-ovulated female B6D2F1 and CD-1 mice and were pre-activated by 10 mM SrCl2 in calcium-free CZB medium for 30 min before microinjection. G0- or G1-phase purified AG-haESCs were collected and injected into oocytes separately. When the constructed embryos developed to the 2-cell stage in KSOM-AA medium (Sigma, St Louis, MO, USA), they were transferred to the oviduct of pseudopregnant ICR mice at 0.5 d.p.c.
Comparative genomic hybridization analysis
The genomic DNA of wild-type and H19Δ AG-haESCs was extracted using the Wizard Genomic DNA Purification Kit (Promega, Madison, WI, USA) and sent to the CapitalBio Corporation (Changping District, Beijing, China) for CGH analysis. The SurePrint G3 Mouse CGH 4×180 K microarrays (Agilent, Santa Clara, CA, USA) were used.
Karyotype analysis
Exponentially growing ES cells were incubated with 0.2 μg ml−1 colcemid (Sigma) for 2–3 h at 37 °C. After trypsinization, the collected cells were incubated in 0.075 M KCl hypotonic solution for 15 min at 37 °C. Hypotonic solution-treated ES cells were fixed in fresh ice cold 3:1 methanol/acetic acid at room temperature and dropped onto the precleaned slides. The chromosome spreads were stained with Giemsa solution (10% v/v Giemsa to 7.0 pH phosphate buffer) for 5 min and washed in ddH2O. More than 30 metaphase spreads were analyzed.
Immunostaining
ES cells on coverslips were fixed in 4% paraformaldehyde/ phosphate buffer saline (PBS) for 15 min at room temperature. The fixed cells were then permeabilized with 0.5% Triton X-100 for 10 min and were blocked in 3% bovine serum albumin for 1 h at room temperature. The cells were incubated overnight at 4 °C with primary antibodies, anti-oct3/4 (sc-5279; Santa Cruz, Dallas, TX, USA), anti-sox2 (sc-17320; Santa Cruz), anti-nanog (AB5731; Millipore, Billerica, MA, USA), and anti-SSEA1 (sc-21702; Santa Cruz). The cells were treated with the secondary antibodies for 1 h at room temperature. The nuclei were dyed with 4′,6-diamidino-2-phenylindole (DAPI). Images were acquired by the fluorescent microscope (Zeiss, Oberkochen, Germany).
Quantitative reverse transcription PCR
Total RNA was extracted from AG-haESCs using Trizol reagent (Invitrogen, Waltham, MA, USA). 1.0 μg of total RNA was reverse transcribed using the PrimeScript II 1st strand cDNA synthesis kit (TaKaRa, Dalian, China). The real-time PCR reaction was performed using SYBR Premix Ex Taq II (TaKaRa) and run on Roche 480 Light Cycler. The amount of GAPDH expression was used to normalize all values.
Bisulphite sequencing
Genomic DNA of ESCs, sperm and mouse tail was treated with the EpiTect Bisulphite (Qiagen, Hilden, Germany) for bisulphite conversion according to the manufacturer’s instructions. Differentially methylated regions (DMRs) of Snrpn and Gtl2 were amplified. The PCR products were cloned into pMD18-T vectors (TakaRa) and sequenced for unmethylated C to T conversion. Bisulphite primers are presented in Supplementary Table S2.
Embryoid-body formation
Embryoid bodies were formed from wild-type and H19ΔAG-haESCs. Cultured ES cells were dissociated with trypsin and sedimentated for 30 min at 37 °C. 1.5×106 cells were transferred to low attachment 90-mm-diameter bacteriological grade Petri dishes in differentiating medium containing high-glucose Dulbecco's modified Eagle's medium (Gibco, Waltham, MA, USA), 15% fetal bovine serum, 2 mM GlutaMax, 1% non-essential amino acids, and 100 μM β-mercaptoethanol. embryoid bodies were replaced with fresh differentiation medium every other day.
Statistical analysis
The Student’s t-test was used to analyze significant differences. P<0.05 was considered significant. The data analyses were performed using Prism GraphPad software.
Accession codes
CGH data are deposited at the Gene Expression Omnibus under accession number GSE67563.
Acknowledgments
We thank X Wang and L Cheng for technical assistance; Dr Jingsong Li (Chinese Academy of Sciences) for providing AGH-OG-3 haploid ES cell line; Dr Chunsheng Han (Chinese Academy of Sciences) for mouse sperm DNA samples. This work was supported by grants from the National Key Basic Research Program of China (2011CB965203, 2013CB967002 to YH) and the National Natural Science Foundation of China (91231111 to YH).
Footnotes
(Supplementary information is linked to the online version of the paper on the Cell Discovery website.)
The authors declare competing financial interests.
References
- Leeb M , Wutz A . Derivation of haploid embryonic stem cells from mouse embryos. Nature 2011; 479: 131–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elling U , Taubenschmid J , Wirnsberger G et al. Forward and reverse genetics through derivation of haploid mouse embryonic stem cells. Cell Stem Cell 2011; 9: 563–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W , Li X , Li T et al. Genetic modification and screening in rat using haploid embryonic stem cells. Cell Stem Cell 2014; 14: 404–414. [DOI] [PubMed] [Google Scholar]
- Yang H , Liu Z , Ma Y et al. Generation of haploid embryonic stem cells from Macaca fascicularis monkey parthenotes. Cell Res 2013; 23: 1187–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wutz A . Haploid mouse embryonic stem cells: rapid genetic screening and germline transmission. Annu Rev Cell Dev Biol 2014; 30: 705–722. [DOI] [PubMed] [Google Scholar]
- Elling U , Penninger JM . Genome wide functional genetics in haploid cells. FEBS Lett 2014; 588: 2415–2421. [DOI] [PubMed] [Google Scholar]
- Yang H , Shi L , Wang BA et al. Generation of genetically modified mice by oocyte injection of androgenetic haploid embryonic stem cells. Cell 2012; 149: 605–617. [DOI] [PubMed] [Google Scholar]
- Li W , Shuai L , Wan H et al. Androgenetic haploid embryonic stem cells produce live transgenic mice. Nature 2012; 490: 407–411. [DOI] [PubMed] [Google Scholar]
- Fedoriw A , Mugford J , Magnuson T . Genomic imprinting and epigenetic control of development. Cold Spring Harb Perspect Biol 2012; 4: a008136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton SC , Surani MA , Norris ML . Role of paternal and maternal genomes in mouse development. Nature 1984; 311: 374–376. [DOI] [PubMed] [Google Scholar]
- Plasschaert RN , Bartolomei MS . Genomic imprinting in development, growth, behavior and stem cells. Development 2014; 141: 1805–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- xBartolomei MS , Zemel S , Tilghman SM . Parental imprinting of the mouse H19 gene. Nature 1991; 351: 153–155. [DOI] [PubMed] [Google Scholar]
- DeChiara TM , Robertson EJ , Efstratiadis A . Parental imprinting of the mouse insulin-like growth factor II gene. Cell 1991; 64: 849–859. [DOI] [PubMed] [Google Scholar]
- Kono T , Obata Y , Wu Q et al. Birth of parthenogenetic mice that can develop to adulthood. Nature 2004; 428: 860–864. [DOI] [PubMed] [Google Scholar]
- Kaffer CR , Grinberg A , Pfeifer K . Regulatory mechanisms at the mouse Igf2/H19 locus. Mol Cell Biol 2001; 21: 8189–8196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong L , Ran FA , Cox D et al. Multiplex genome engineering using CRISPR/Cas systems. Science 2013; 339: 819–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mali P , Yang L , Esvelt KM et al. RNA-guided human genome engineering via Cas9. Science 2013; 339: 823–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guschin DY , Waite AJ , Katibah GE et al. A rapid and general assay for monitoring endogenous gene modification. Methods Mol Biol 2010; 649: 247–256. [DOI] [PubMed] [Google Scholar]
- Kawahara M , Wu Q , Takahashi N et al. High-frequency generation of viable mice from engineered bi-maternal embryos. Nat Biotechnol 2007; 25: 1045–1050. [DOI] [PubMed] [Google Scholar]
- Zhong C , Yin Q , Xie Z et al. CRISPR-Cas9-Mediated Genetic Screening in Mice with Haploid Embryonic Stem Cells Carrying a Guide RNA Library. Cell Stem Cell 2015; 17: 221–232. [DOI] [PubMed] [Google Scholar]
- Dechiara TM , Poueymirou WT , Auerbach W et al. VelociMouse: fully ES cell-derived F0-generation mice obtained from the injection of ES cells into eight-cell-stage embryos. Methods Mol Biol 2009; 530: 311–324. [DOI] [PubMed] [Google Scholar]
- Wang H , Yang H , Shivalila CS et al. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell 2013; 153: 910–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota H , Brinster RL . Technology insight: In vitro culture of spermatogonial stem cells and their potential therapeutic uses. Nat Clin Pract Endocrinol Metab 2006; 2: 99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.