Abstract
In a series of recent experiments, we found that if rats are presented with two temporal cues, each signifying that reward will be delivered after a different duration elapses (e.g., tone-10 seconds / light-20 seconds), they will behave as if they have computed a weighted average of these respective durations. In the current article, we argue that this effect, referred to as “temporal averaging”, can be understood within the context of Bayesian Decision Theory. Specifically, we propose and provide preliminary data showing that, when averaging, rats weight different durations based on the relative variability of the information their respective cues provide.
Keywords: Interval timing, time perception, temporal averaging, cue integration, multisensory integration, cue combination, maximum likelihood estimation, Bayesian
Introduction
Time is central to our experience of the world. For example, both basic functions, such as effectively executing motor movements [1,2], and higher-level processes, such as perceiving causality [3–5], require an accurate representation of the passage of time. Interval timing refers to the perception of time in the seconds-to-minutes range [6]. Recently, interval timing studies have begun to investigate how behavior is affected when conflicting temporal information is present within the environment [7**–10]. Results from these experiments often resemble findings from studies investigating Bayesian Decision Theory. Here, we review and attempt to integrate these two areas of research.
Temporal Averaging
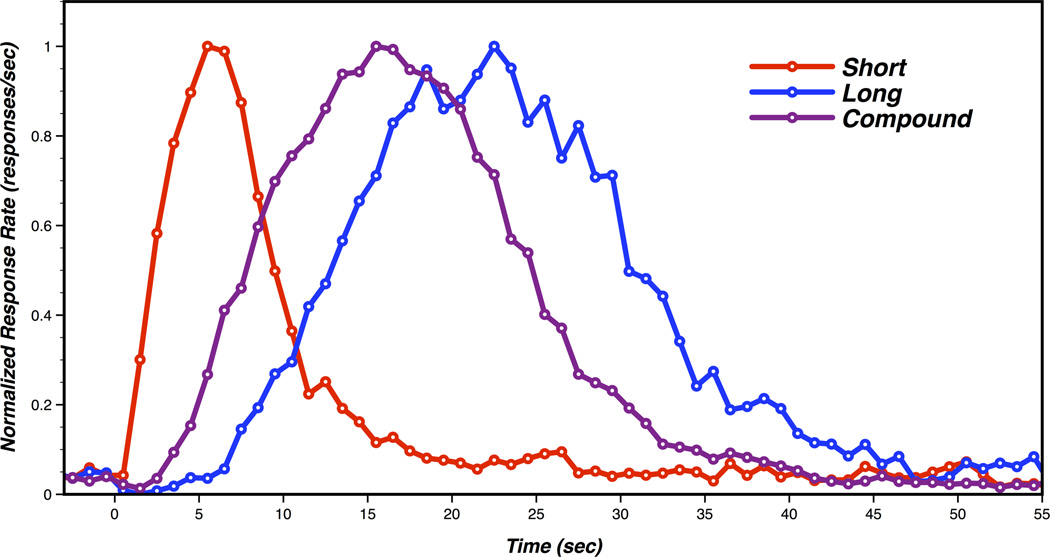
Interval timing is often examined in animals using the peak interval (PI) procedure [11–14]. In a PI task, the presentation of a stimulus (e.g., tone) indicates that reward can be earned for making an operant response after a fixed “criterion duration” has elapsed (e.g., 10 seconds). Probe trials are also included, during which the stimulus is presented for 3–4 times the length of the criterion duration and no reinforcement is provided. When plotted as a function of time, average response rate during probe trials typically resembles a Gaussian distribution, with a mode centered over the time at which reward is usually earned (i.e., “peak time”) (for examples, see Figure 1).
Figure 1.
Probe trial data from a temporal averaging experiment adapted with permission from Kurti et al. [7]. Short and long refer to trials where the short and long cues were presented in isolation. Compound refers to responding during trials in which both the short and long cues were presented simultaneously.
The spreads of these response distributions reflect variability in temporal estimates, and grow in direct proportion to the duration being timed [15]. For example, if the criterion duration were doubled, both the peak time and spread of the original distribution would increase by a factor of two. Consequently, when the spread of a response distribution is divided by its peak time, the resulting value, referred to as the coefficient of variation (CV), will remain constant across different durations. This pattern of variability is observed in a variety of timing tasks and is referred to as the scalar property of interval timing [16,17].
Recently, we conducted a PI study in which rats were trained to associate two cues (tone and light) with two different durations (e.g., 10 and 20 seconds, respectively) [8]. After the rats were well trained, we added “compound” probe trials in which both the tone and light were presented simultaneously. We included these trials to assess how rats would respond when presented with conflicting temporal information regarding when reward would become available. When cues were presented in isolation, responses were centered over their respective criterion durations. However, during compound trials, rats produced scalar response distributions that fell in between the two criterion times. In other words, rats appeared to time a single temporal expectation that reflected a combination of the two component durations. Therefore, this effect was referred to as “temporal averaging”.
Further work showed that compound peak times usually fall closer to the long duration, suggesting rats time a weighted average during compound trials with more weight being given to the long cue (see Figure 1) [7**,9,15,18*]. This increased weight has been attributed to the fact that experimenters often assign the long duration’s cue with a higher reinforcement probability (ratio of rewarded trials to probe trials) to equate response rates for the two cues, as response rates tend to decrease with increasing duration. In support of this, several studies have shown that compound peak times can be predicted well by computing an average of the component peak times when each is weighted by its respective reinforcement probability [7**,9,15].
Averaging behavior and Bayesian Decision Theory
Averaging behavior has also been observed during investigations of spatial navigation [19–22]. For example, if pigeons are presented with multiple landmarks that provide discrepant information regarding the location of a hidden goal, they will navigate based on an average of self-to-goal vectors derived from each landmark [23]. Importantly, landmarks that have been closer to the goal in the past will be weighted more heavily, presumably because closer landmarks provide more reliable spatial information. We will refer to this effect as “spatial averaging”.
Recently, Cheng et al. [24] proposed that spatial averaging can be explained using Bayesian Decision Theory (BDT); a prominent framework used to study how information is integrated in order to optimize behavior. According to BDT, when multiple sources of information (e.g., A and B) can be used to inform a judgment, observers should compute a weighted average of estimates derived from each source, as given in the equation below:
| (1) |
A and B refer to estimates from each source; WA and WB correspond to the weights given to each estimate; and IAB refers to the integrated estimate. More reliable information should be weighted more heavily during this averaging process. Mathematically, the “reliability” of a given source is defined as the inverse variance of the information it provides, and weights are computed as follows:
| (2) |
| (3) |
BDT can be applied to spatial averaging by assuming A and B represent navigation vectors derived from two landmarks [24]. If the closer of the two landmarks provides more reliable (i.e., less variable) spatial information, its corresponding vector would be weighted more heavily, as seen in the data [23]. To the best of our knowledge, the relationship between spatial averaging and BDT has not been formally tested. However, this integration process has been extensively evaluated during studies investigating a phenomenon referred to as “cue integration” [for reviews, see 25**–28].
An example of cue integration that is particularly relevant to the topic of spatial averaging comes from Alias and Burr [29], who asked human subjects to judge the spatial location of briefly presented auditory and/or visual cues. The experimenters blurred the visual stimulus to different degrees on different trials in order to vary its reliability. During single-modality trials, cues were presented individually. This allowed Bayesian weights to be computed for each cue-type and blur-level based on variance in participants’ judgments. During multi-modality trials, both cues were presented simultaneously and in different spatial positions. During these trials, participants appeared to compute an average of the two cue-locations (i.e., “integrate” both locations into a single estimate), and the weights given to each cue matched closely with the Bayesian predictions.
Studies using similar tasks have shown that cue integration occurs within [30–33] and across [34–38] a variety of modalities, and generalizes to a variety of judgments such as shape [33,37], size [38], slant [30,32,34], and height [36]. Collectively, this area of research suggests that cue integration is a general computational framework that observers use to combine information from different sources.
Temporal Averaging and Bayesian Decision Theory
Given the similarities between temporal averaging, spatial averaging, and cue integration, we sought to re-evaluate our past temporal averaging data within a Bayesian framework. As described above, rats weight the longer duration more heavily during compound trials. From a Bayesian standpoint, this suggests the long duration’s cue provides more reliable information than the short. If so, responses should be less variable when the long cue is presented in isolation, relative to the short cue. This poses a potential problem, as absolute variability in temporal estimates increases with increasing duration. However, across several of our experiments, CVs for the short cue have been significantly larger than those of both the long and compound cue [7**–9]. In other words, relative, as opposed to absolute, variability in responding has been higher for the short cue than the long.
These CV differences likely relate to the fact that we typically assign the short and long cues with low and high reinforcement probabilities, respectively, in order to generate equivalent response rates for both stimuli. Specifically, during PI tasks, a cue’s reinforcement probability is known to impact the amount of behavioral variation it elicits. For example, using a PI procedure, Roberts [39] showed that decreasing the reinforcement probability of a 40s cue caused spreads to increase [for similar results see 40,41]. This effect is not specific to interval timing behavior, as recent studies have begun to establish that reinforcement probability is inversely related to variation in various forms of operant behavior [42–44]. Many have suggested that, with lower reinforcement probabilities, increases in response variation allow subjects to discover better strategies for obtaining reward [45,46]. However, Stahlman, Young, and Blaisdell [47] recently documented this effect during a Pavlovian task (i.e., where no response strategy is required) calling this explanation into question. This finding suggests that, as a general rule, variation in conditioned behavior may increase as a cue’s predictiveness of reward decreases. If so, manipulations of reinforcement probability would have an interesting parallel to manipulations of reliability during cue integration studies. Specifically, during cue integration studies, decreasing a cue’s reliability typically entails corrupting its appearance [e.g., 35,36,38]. In other words, the ability to perceive an event (i.e., the cue) is altered. Manipulating reinforcement probability may have a similar effect with the primary difference being that the ability to predict, rather than perceive, an event (i.e., reward) is altered.
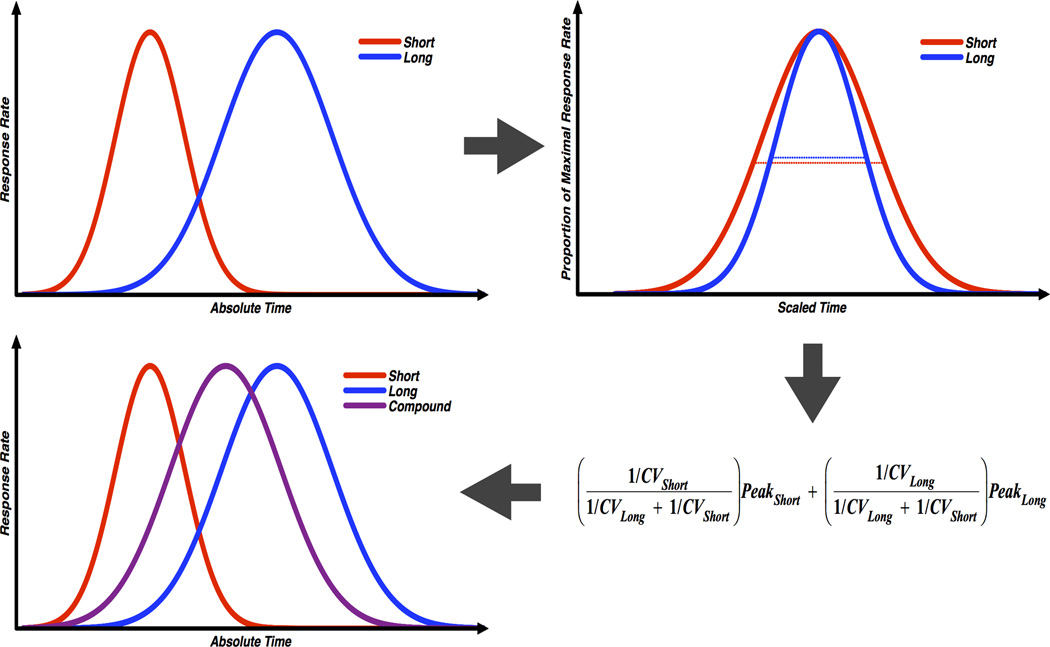
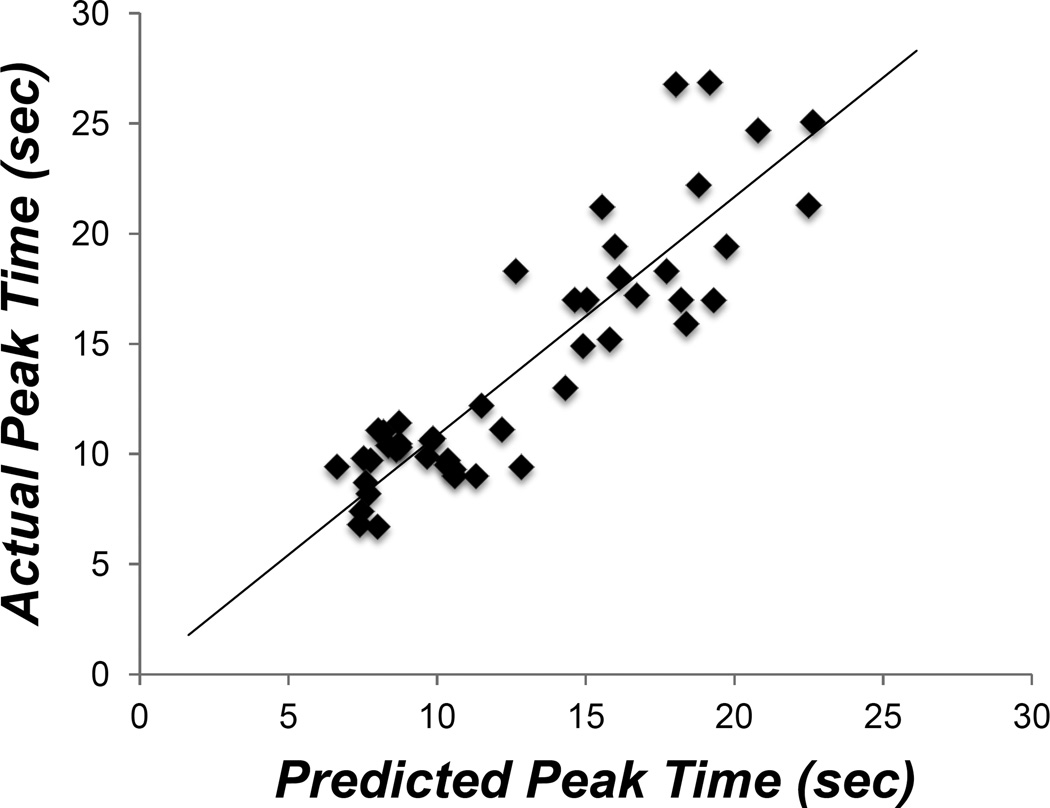
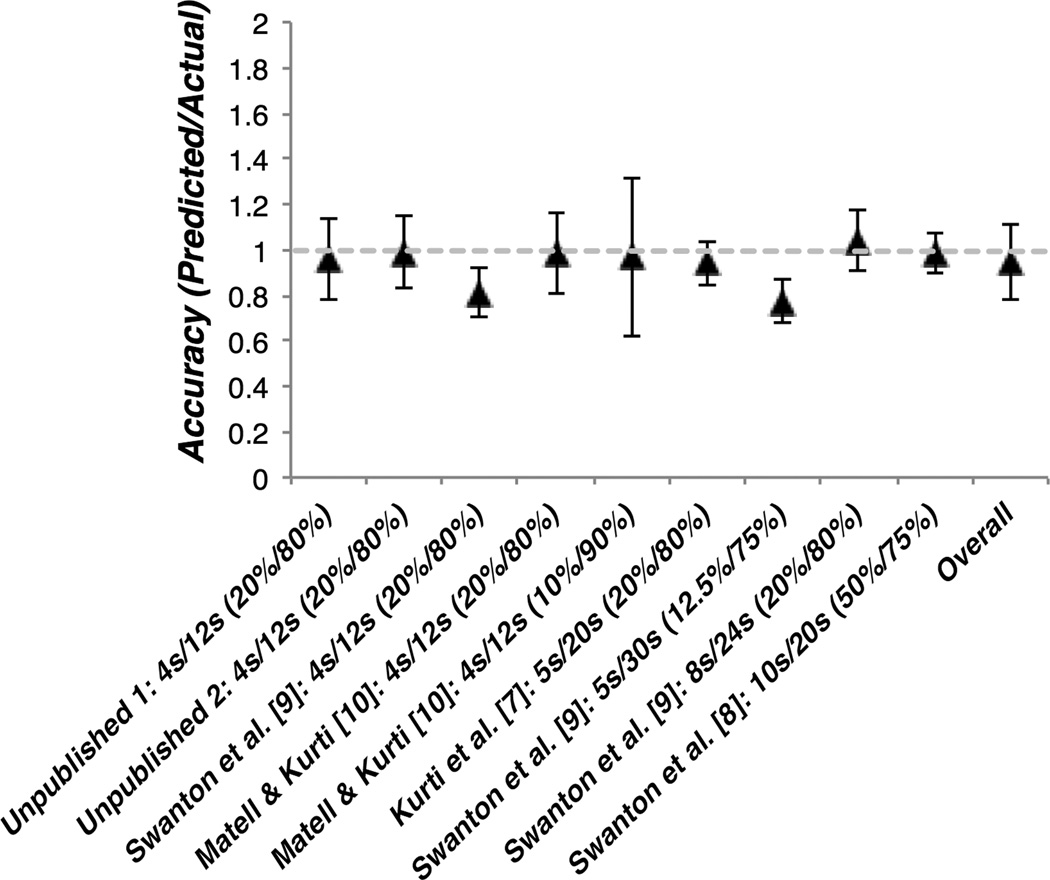
To evaluate whether BDT can be used to explain temporal averaging behavior, we reanalyzed all of our prior compounding data in which temporal averaging was observed. We computed Bayesian weights for each rat, with component cue CVs serving as a measure of variation, and used these to compute a weighted average of the component peak times (for further description, see Figure 2). Predicted and actual compound peak times are plotted against one another in Figure 3, and the accuracy of the Bayesian predictions for each study are plotted in Figure 4. Both figures show that temporal averaging behavior is well accounted for by BDT.
Figure 2.
Schematic of Bayesian weighting during compound trials. Top left: Hypothetical short and long probe trial responding. Top right: Data from the same short and long distributions. However, for each cue, the proportion of maximal response rate across time is expressed as a proportion of the peak time (100% corresponds to the maximums of either function). Plotting the data in this way shows that relative variability in short cue responding is higher than the long cue, despite the fact that the opposite is true in terms of absolute variability. Bottom right: The equation used to compute predicted compound peak times based on the relative variation (CV) in responding to the short and long cue. Bottom left: Same as top left; however, predicted compound responding is now plotted. Note that increased relative variation for the short cue causes the compound function to fall closer to the long duration.
Figure 3.
Scatterplot of actual compound peak times from rats in all prior temporal averaging experiments and predicted compound peak times under BDT based on relative variation in component cue responding. A line of best fit with a constrained intercept of zero is also plotted (slope=1.08, R=.891).
Figure 4.
Average accuracy of Bayesian predictions for rats in each study and across all rats. Accuracy was computed for each rat by dividing the predicted compound peak time by the actual compound peak time. Values of 1 represent perfect accuracy, whereas values above and below 1 correspond to over and under-prediction, respectively. The criterion durations and reinforcement probabilities of the short and long cues are included next to each study citation.
Conclusions and Implications
Establishing a connection between temporal averaging and cue integration could have important implications. For example, the neural mechanisms that mediate interval timing are currently unclear. However, if temporal averaging is indeed a form of cue integration, it may be possible to constrain neural models of interval timing using well-established neurophysiological theories of cue integration.
For example, according to the “probabilistic population coding” hypothesis of cue integration [48–50], the cortex represents stimuli as probability distributions, via collections of neurons with tuning curves that differ in regard to their preferred stimulus value. When multiple stimuli are presented simultaneously, Ma et al. [48] showed that cue integration can easily occur by linear combination of the distributions produced by each stimulus (i.e., by multiplying the two distributions element-wise). If temporal averaging and cue integration are related, this would imply the existence of neurons with “temporal tuning curves” (i.e., neurons that respond differentially when exposed to different durations). This conclusion has been reached based on behavioral data before [51,52] and is supported by evidence from electrophysiology studies. For example, Merchant et al. [53**] recently observed neurons in the medial premotor cortex with Gaussian tuning curves that responded preferentially to specific durations, regardless of the modality of the stimulus being timed. Similarly, studies using PI tasks or comparable designs have documented neurons that exhibit Gaussian-like or ramping firing profiles during elapsing intervals that appear to be tuned to the programmed criterion duration [54–58**].
However, the variability of these firing profiles grows in proportion to the duration being timed [57]. Consequently, while multiplication of the tuning curve activity associated with a short and long duration across time during a compound trial would lead to an intermediate peak, it can be shown that the compound peak time would fall closer to the short duration, which is inconsistent with the data. For such a mechanism to work, there would have to be neurons that were tuned according to the relative variability of their preferred duration. It is possible that such neurons exist. However, given the predominance of scalar variability across interval timing tasks [16], this possibility seems unlikely.
An alternative is that there are temporally tuned neurons that are responsive to the duration associated with a cue, but do not generate timed behavior directly. For example, upon presenting a temporal cue, neurons representing its associated duration could become responsive and activate a downstream timing process that generates temporally controlled behavior. If two cues were presented simultaneously, the population activity associated with their respective durations could be combined, causing the downstream timing process to time an intermediate interval. This proposal is similar to the conceptual explanation of temporal averaging that we have relied on in the past. Specifically, we have proposed that, at the start of a compound trial, the temporal memories associated with the short and long cues are retrieved and integrated into an averaged temporal expectation, which is then timed in an otherwise normal manner [15]. In support of this proposal, Mita et al. [59] found neurons in the presupplementary motor area that fired transiently at the onset of a temporal cue. Importantly, the nature of these responses differed as a function of the duration associated with the cue being presented.
Finally, pursuing the relationship between cue integration and temporal averaging might help explain instances where temporal averaging is not observed. Specifically, while not discussed in detail here for sake of space, temporal averaging is usually only observed when the tone and light are associated with the short and long durations, respectively. Counterbalanced groups (light-short/tone-long) show only partial signs of averaging during compound trials, as responses overlap considerably with the light’s associated duration [7**,9,60, for further discussion see 61]. It is unclear why this “modality effect” occurs. However, several cue integration studies have reported similar effects, in which cues presented in certain modalities are weighed more heavily than expected by BDT [35,62–65]. Authors have accounted for this effect by proposing subjects have a prior probability distribution that creates a tendency to favor information from one modality over another [35]. Models of interval timing that incorporate Bayesian priors are beginning to be developed [66*–70]. Therefore, it would be interesting to see if these models can be used to account for this effect.
Highlights.
When presented with multiple temporal cues, rats will time an average of their respective durations
Non-temporal averaging behavior has been observed during cue integration studies
Cue integration findings have been interpreted using Bayesian Decision Theory
We evaluate temporal averaging using this Bayesian perspective
Acknowledgments
Research addressed in this article was supported by grants from the National Institute of Health, DA29809 to MSM, and T32-NS007421 to BJD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
The authors have no conflicts of interest to disclose.
References and Recommended Reading
- 1.Laje R, Cheng K, Buonomano DV. Learning of temporal motor patterns: an analysis of continuous versus reset timing. Front Integr Neurosci. 2011;5:61. doi: 10.3389/fnint.2011.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buonomano DV, Laje R. Population clocks: Motor timing with neural dynamics. Trends Cogn Sci. 2010;14:520–527. doi: 10.1016/j.tics.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heron J, Hanson JV, Whitaker D. Effect before cause: Supramodal recalibration of sensorimotor timing. PLoS ONE. 2009;4:e7681. doi: 10.1371/journal.pone.0007681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stetson C, Cui X, Montague PR, Eagleman DM. Motor-sensory recalibration leads to an illusory reversal of action and sensation. Neuron. 2006;51:651–659. doi: 10.1016/j.neuron.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 5.Greville WJ, Buehner MJ. Temporal predictability facilitates causal learning. J Exp Psychol Gen. 2010;139:756–771. doi: 10.1037/a0020976. [DOI] [PubMed] [Google Scholar]
- 6.Gibbon J, Church RM, Meck WH. Scalar timing in memory. Ann NY Acad Sci. 1984;423:52–77. doi: 10.1111/j.1749-6632.1984.tb23417.x. [DOI] [PubMed] [Google Scholar]
- 7. Kurti AN, Swanton DS, Matell MS. The potential link between temporal averaging and drug-taking behavior. In: Arstila V, Lloyd D, editors. Subjective Time: The Philosophy, Psychology, and Neuroscience of Temporality. MIT Press; 2013. pp. 599–620. ** This chapter provides a review of past temporal averaging data, including a meta-analysis and discussion of the ability to predict compound peak times with a reinforcement probability weighted average. In addition, it demonstrates that rats are capable of averaging different durations associated with distinct proprioceptive cues. This contrasts with prior investigations in which durations have been associated with different cross-modal cues (specifically, auditory and visual stimuli). As such, it shows that temporal averaging may be a general response to being presented with conflicting temporal information.
- 8.Swanton DN, Gooch CM, Matell MS. Averaging of temporal memories by rats. J Exp Psychol Anim Behav Process. 2009;35:434–439. doi: 10.1037/a0014021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swanton DN, Matell MS. Stimulus compounding in interval timing: the modality-duration relationship of the anchor durations results in qualitatively different response patterns to the compound cue. J Exp Psychol Anim Behav Process. 2011;37:94–107. doi: 10.1037/a0020200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matell MS, Kurti AN. Reinforcement probability modulates temporal memory selection and integration processes. Acta Psychol. 2014;147:80–91. doi: 10.1016/j.actpsy.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agostino PV, Cheng R-K, Williams CL, West AE, Meck WH. Acquisition of response thresholds for timed performance is regulated by a calcium-responsive transcription factor, CaRF. Genes Brain Behav. 2013;12:633–644. doi: 10.1111/gbb.12059. [DOI] [PubMed] [Google Scholar]
- 12.Balci F, Gallistel CR, Allen BD, Frank KM, Gibson JM, Brunner D. Acquisition of peak responding: What is learned? Behav Processes. 2009;80:67–75. doi: 10.1016/j.beproc.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cordes S, Gallistel CR. Intact interval timing in circadian CLOCK mutants. Brain Res. 2008;1227:120–127. doi: 10.1016/j.brainres.2008.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurti AN, Matell MS. Nucleus accumbens dopamine modulates response rate but not response timing in an interval timing task. Behav Neurosci. 2011;125:215–225. doi: 10.1037/a0022892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matell MS, Henning AM. Temporal memory averaging and post-encoding alterations in temporal expectation. Behav Process. 2013;95:31–39. doi: 10.1016/j.beproc.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gibbon J. Ubiquity of scalar timing with a Poisson clock. J Math Psychol. 1992;36:283–293. [Google Scholar]
- 17.Gibbon J. Scalar expectancy theory and Weber’s law in animal timing. Psychol Rev. 1977;84:279–325. [Google Scholar]
- 18. Matell MS. Searching for the holy grail: Temporally informative firing patterns in the rat. Adv Exp Med Biol. 2014;829:209–234. doi: 10.1007/978-1-4939-1782-2_12. * This article provides a review of findings from electrophysiological studies of interval timing. In addition, the author discusses how the observation of temporal averaging can be used to inform both current and future neural models of interval timing.
- 19.Cheng K. Some psychophysics of the pigeon’s use of landmarks. J Comp Physiol [A] 1988;162:815–826. doi: 10.1007/BF00610970. [DOI] [PubMed] [Google Scholar]
- 20.Cheng K. The determination of direction in landmark-based spatial search in pigeons: A further test of the vector sum model. Anim Learn Behav. 1994;22:291–301. [Google Scholar]
- 21.Gibson B, McGowan F. Rats average entire vectors when navigating toward a hidden goal: a test of the vector sum model in rodents. Behav Process. 2014;102:18–24. doi: 10.1016/j.beproc.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 22.Spetch ML, Mondloch MV. Control of pigeons’ spatial search by graphic landmarks in a touch-screen task. J Exp Psychol Anim Behav Process. 1993;19:353–372. [Google Scholar]
- 23.Cheng K. The vector sum model of pigeon landmark use. J Exp Psychol Anim Behav Process. 1989;15:366–375. [Google Scholar]
- 24.Cheng K, Shettleworth SJ, Huttenlocher J, Rieser JJ. Bayesian integration of spatial information. Psychol Bull. 2007;133:625–637. doi: 10.1037/0033-2909.133.4.625. [DOI] [PubMed] [Google Scholar]
- 25. Fetsch CR, DeAngelis GC, Angelaki DE. Bridging the gap between theories of sensory cue integration and the physiology of multisensory neurons. Nat Rev Neurosci. 2013;14:492–442. doi: 10.1038/nrn3503. ** The article is accessible to all readers and provides an excellent overview of behavioral, neurophysiological, and computational work on cue integration.
- 26.Ernst MO, Bülthoff HH. Merging the senses into a robust percept. Trends Cogn Sci. 2004;8:162–169. doi: 10.1016/j.tics.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 27.Alais D, Newell FN, Mamassian P. Multisensory processing in review: from physiology to behaviour. Seeing Perceiving. 2010;23:3–38. doi: 10.1163/187847510X488603. [DOI] [PubMed] [Google Scholar]
- 28.Seilheimer RL, Rosenberg A, Angelaki DE. Models and processes of multisensory cue combination. Curr Opin Neurobiol. 2014;25:38–46. doi: 10.1016/j.conb.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alais D, Burr D. The ventriloquist effect results from near-optimal bimodal integration. Curr Biol. 2004;14:257–262. doi: 10.1016/j.cub.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 30.Backus BT, Banks MS, van Ee R, Crowell JA. Horizontal and vertical disparity, eye position, and stereoscopic slant perception. Vision Res. 1999;39:1143–1170. doi: 10.1016/s0042-6989(98)00139-4. [DOI] [PubMed] [Google Scholar]
- 31.Blake A, Bülthoff HH, Sheinberg D. Shape from texture: Ideal observers and human psychophysics. Vision Res. 1993;33:1723–1737. doi: 10.1016/0042-6989(93)90037-w. [DOI] [PubMed] [Google Scholar]
- 32.Girshick AR, Banks MS. Probabilistic combination of slant information: Weighted averaging and robustness as optimal percepts. J Vis. 2009;9:1–20. doi: 10.1167/9.9.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnston EB, Cumming BG, Landy MS. Integration of stereopsis and motion shape cues. Vis Res. 1994;34:2259–2275. doi: 10.1016/0042-6989(94)90106-6. [DOI] [PubMed] [Google Scholar]
- 34.Banks MS, Bülthoff HH, Ernst MO. Touch can change visual slant perception. Nat Neurosci. 2000;3:69–73. doi: 10.1038/71140. [DOI] [PubMed] [Google Scholar]
- 35.Battaglia PW, Jacobs RA, Aslin RN. Bayesian integration of visual and auditory signals for spatial localization. J Opt Soc Am A. 2003;20:1391–1397. doi: 10.1364/josaa.20.001391. [DOI] [PubMed] [Google Scholar]
- 36.Ernst MO, Banks MS. Humans integrate visual and haptic information in a statistically optimal fashion. Nature. 2002;415:429–433. doi: 10.1038/415429a. [DOI] [PubMed] [Google Scholar]
- 37.Helbig HB, Ernst MO. Optimal integration of shape information from vision and touch. Exp Brain Res. 2007;179:595–606. doi: 10.1007/s00221-006-0814-y. [DOI] [PubMed] [Google Scholar]
- 38.Hillis JM, Ernst MO, Banks MS. Combining sensory information: Mandatory fusion within, but not between, senses. Sci. HW Wilson - AST. 2002;298:1627–1630. doi: 10.1126/science.1075396. [DOI] [PubMed] [Google Scholar]
- 39.Roberts S. Isolation of an internal clock. J Exp Psychol Anim Behav Process. 1981;7:242–268. [PubMed] [Google Scholar]
- 40.Kaiser DH. The proportion of fixed interval trials to probe trials affects acquisition of the peak procedure fixed interval timing task. Behav Process. 2008;77:100–108. doi: 10.1016/j.beproc.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaiser DH. Fewer peak trials per session facilitate acquisition of peak responding despite elimination of response rate differences. Behav Process. 2009;80:12–19. doi: 10.1016/j.beproc.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 42.Gharib A, Gade C, Roberts S. Control of variation by reward probability. J Exp Psychol Anim Behav Process. 2004;30:271–282. doi: 10.1037/0097-7403.30.4.271. [DOI] [PubMed] [Google Scholar]
- 43.Stahlman WD, Blaisdell AP. Reward probability and the variability of foraging behavior in rats. Int J Comp Psychol. 2011;24:168–176. [Google Scholar]
- 44.Stahlman WD, Roberts S, Blaisdell AP. Effect of reward probability on spatial and temporal variation. J Exp Psychol Anim Behav Process. 2010;36:77–91. doi: 10.1037/a0015971. [DOI] [PubMed] [Google Scholar]
- 45.Gharib A, Derby S, Roberts S. Timing and the control of variation. J Exp Psychol Anim Behav Process. 2001;27:165–178. [PubMed] [Google Scholar]
- 46.Roberts S, Gharib A. Variation of bar-press duration: Where do new responses come from? Behav Process. 2006;72:215–223. doi: 10.1016/j.beproc.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 47.Stahlman WD, Young ME, Blaisdell AP. Response variability in pigeons in a Pavlovian task. Learn Behav. 2010;38:111–118. doi: 10.3758/LB.38.2.111. [DOI] [PubMed] [Google Scholar]
- 48.Ma WJ, Beck JM, Latham PE, Pouget A. Bayesian inference with probabilistic population codes. Nat Neurosci. 2006;9:1432–1438. doi: 10.1038/nn1790. [DOI] [PubMed] [Google Scholar]
- 49.Beck J, Ma WJ, Latham PE, Pouget A. Probabilistic population codes and the exponential family of distributions. Prog Brain Res. 2007;165:509–519. doi: 10.1016/S0079-6123(06)65032-2. [DOI] [PubMed] [Google Scholar]
- 50.Ma WJ, Pouget A. A neural implementation of optimal cue integration. In: Trommershauser J, Konrad K, Michael L, editors. Sensory Cue Integration. Oxford University Press; 2011. pp. 393–405. [Google Scholar]
- 51.Bartolo R, Merchant H. Learning and generalization of time production in humans: Rules of transfer across modalities and interval durations. Exp Brain Res. 2009;197:91–100. doi: 10.1007/s00221-009-1895-1. [DOI] [PubMed] [Google Scholar]
- 52.Heron J, Aaen-Stockdale C, Hotchkiss J, Roach NW, McGraw PV, Whitaker D. Duration channels mediate human time perception. Proc Biol Sci. 2012;279:690–698. doi: 10.1098/rspb.2011.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Merchant H, Pérez O, Zarco W, Gámez J. Interval tuning in the primate medial premotor cortex as a general timing mechanism. J Neurosci. 2013;33:9082–9096. doi: 10.1523/JNEUROSCI.5513-12.2013. **During a timing task, the authors observed neurons in the medial premotor cortex that showed Gaussian firing profiles that peaked around the duration being timed. In other words, these appeared to be "interval tuned" neurons with preferred durations. Therefore, this data provides support for the notion of temporal tuning curves.
- 54.Matell MS, Meck WH, Nicolelis MAL. Interval timing and the encoding of signal duration by ensembles of cortical and striatal neurons. Behav Neurosci. 2003;117:760–773. doi: 10.1037/0735-7044.117.4.760. [DOI] [PubMed] [Google Scholar]
- 55.Matell MS, Shea-Brown E, Gooch C, Wilson AG, Rinzel J. A heterogeneous population code for elapsed time in rat medial agranular cortex. Behav Neurosci. 2011;125:54–73. doi: 10.1037/a0021954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Parker KL, Chen KH, Kingyon JR, Cavanagh JF, Narayanan NS. D1-dependent 4 Hz oscillations and ramping activity in rodent medial frontal cortex during interval timing. J Neurosci. 2014;34:16774–16783. doi: 10.1523/JNEUROSCI.2772-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xu M, Zhang S, Dan Y, Poo M. Representation of interval timing by temporally scalable firing patterns in rat prefrontal cortex. Proc Natl Acad Sci U. S. A. 2014;111:480–485. doi: 10.1073/pnas.1321314111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mello GBM, Soares S, Paton JJ. A scalable population code for time in the striatum. Curr Biol. 2015;25:1113–1122. doi: 10.1016/j.cub.2015.02.036. **Using a fixed-interval task, the authors observed neurons in the striatum that fired at specific times into an elapsing interval. When the criterion duration was changed, the time at which these neurons fired scaled with the new interval (e.g., firing after 4 and 8 seconds into a 12 and 24 second interval, respectively). Thus, these neurons’ "temporal response fields" coded relative duration. This contrasts with many studies that have found neurons that are sensitive to absolute duration [e.g., 55].
- 59.Mita A, Mushiake H, Shima K, Matsuzaka Y, Tanji J. Interval time coding by neurons in the presupplementary and supplementary motor areas. Nat Neurosci. 2009;12:502–507. doi: 10.1038/nn.2272. [DOI] [PubMed] [Google Scholar]
- 60.Delamater AR, Nicolas DM. Temporal averaging across stimuli signaling the same or different reinforcing outcomes in the peak procedure. Int J Comp Psychol. 2015;28:1–17. [PMC free article] [PubMed] [Google Scholar]
- 61.De Corte BJ, Matell MS. Temporal averaging across multiple response options: Insight into the mechanisms underlying integration. Anim Cogn. 2015 doi: 10.1007/s10071-015-0935-4. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pick HL, Warren DH, Hay JC. Sensory conflict in judgments of spatial direction. Percept Psychophys. 1969;6:203–205. [Google Scholar]
- 63.Power RP. The dominance of touch by vision: Sometimes incomplete. Perception. 1980;9:457–466. doi: 10.1068/p090457. [DOI] [PubMed] [Google Scholar]
- 64.Rock I, Victor J. Vision and touch: An experimentally created conflict between the two senses. Science. 1964;143:594–596. doi: 10.1126/science.143.3606.594. [DOI] [PubMed] [Google Scholar]
- 65.Knudsen EI, Brainard MS. Creating a unified representation of visual and auditory space in the brain. Annu Rev Neurosci. 1995;18:19–43. doi: 10.1146/annurev.ne.18.030195.000315. [DOI] [PubMed] [Google Scholar]
- 66. Shi Z, Church RM, Meck WH. Bayesian optimization of time perception. Trends Cogn Sci. 2013;17:556–564. doi: 10.1016/j.tics.2013.09.009. * This article shows how past information-processing models of interval timing can be integrated with broader Bayesian decision-making theories. Unlike the current article, this paper discusses how duration information garnered from prior experience can be used to optimize ongoing temporal estimates.
- 67.Gu B-M, Jurkowski AJ, Malapani C, Lake JI, Meck WH. Bayesian models of interval timing and the migration of temporal memories as a function of Parkinson’s disease and dopamine-related error processing. In: Vatakis A, Allman M, editors. Time distortions in mind: Temporal processing in clinical populations. Brill; 2014. pp. 284–329. [Google Scholar]
- 68.Jazayeri M, Shadlen MN. Temporal context calibrates interval timing. Nat Neurosci. 2010;13:1020–1026. doi: 10.1038/nn.2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Acerbi L, Wolpert DM, Vijayakumar S. Internal representations of temporal statistics and feedback calibrate motor-sensory interval timing. PLoS Comput Biol. 2012;8:e1002771. doi: 10.1371/journal.pcbi.1002771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jazayeri M, Shadlen MN. A neural mechanism for sensing and reproducing a time interval. Curr Biol. 2015;25:2599–2609. doi: 10.1016/j.cub.2015.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]