Abstract
Global losses of biodiversity have galvanised efforts to understand how changes to communities affect ecological processes, including transmission of infectious pathogens. Here, we review recent research on diversity–disease relationships and identify future priorities. Growing evidence from experimental, observational and modelling studies indicates that biodiversity changes alter infection for a range of pathogens and through diverse mechanisms. Drawing upon lessons from the community ecology of free-living organisms, we illustrate how recent advances from biodiversity research generally can provide necessary theoretical foundations, inform experimental designs, and guide future research at the interface between infectious disease risk and changing ecological communities. Dilution effects are expected when ecological communities are nested and interactions between the pathogen and the most competent host group(s) persist or increase as biodiversity declines. To move beyond polarising debates about the generality of diversity effects and develop a predictive framework, we emphasise the need to identify how the effects of diversity vary with temporal and spatial scale, to explore how realistic patterns of community assembly affect transmission, and to use experimental studies to consider mechanisms beyond simple changes in host richness, including shifts in trophic structure, functional diversity and symbiont composition.
Keywords: Amplification effect, biodiversity loss, biodiversity–ecosystem function, community ecology, dilution effect, disease ecology, symbiont
Introduction
The idea that the diversity of an ecological community can influence the transmission and dynamics of pathogens traces back over 50 years. In 1958, pioneering ecologist Charles S. Elton observed that ‘outbreaks [of infectious diseases] most often happen on cultivated or planted land…that is, in habitats and communities very much simplified by man’ (p. 147). However, a qualitative understanding of the role of diversity in pathogen transmission pre-dates Elton; for centuries, farmers have recognised that disease reduction in crops is an important benefit of intercropping (Vandermeer 1989) and crop rotation (Curl 1963). When diversity suppresses the density of individual species, the transmission of many infectious agents is inhibited (Mitchell et al. 2002; Begon 2008; Johnson et al. 2012b; Joseph et al. 2013; Lacroix et al. 2014). This basic heuristic works well for the simplest disease systems consisting of one host and one pathogen species (Dobson et al. 2006), but in systems with multiple hosts or multiple pathogens, the role of diversity becomes both more complicated and more interesting.
At the most fundamental level, persistence of a parasite often requires a minimum threshold of host diversity, such that many infections cannot occur if their host(s) are not present or sufficiently abundant. Thus, systems with more host species offer a greater number of available niches for symbionts to exploit, often leading to a positive correlation between host and parasite richness (Lafferty 2012; Kamiya et al. 2014). However, parasite richness is not equivalent to disease risk and in fact can be inversely related to disease incidence and severity (Johnson et al. 2013a; Rottstock et al. 2014). Recent emphasis has been on evaluating how changes in the diversity of free-living species affect the capacity of established pathogens to spread among suitable hosts (i.e. transmission), particularly for those that cause pathology in humans and species of economic or conservation importance (Ezenwa et al. 2006; Allan et al. 2009; Myers et al. 2013; Becker et al. 2014). The richness and abundance of alternate hosts, infection ‘decoys’, predators and even other symbionts have tremendous potential to suppress or enhance parasite transmission (Fig. 1). When the net effect of these mechanisms leads to an overall decrease in disease risk with increases in community diversity, this is termed a ‘dilution effect’; the opposite pattern, when increases in diversity enhance the risk of infection within a system, is called an ‘amplification effect’ (Keesing et al. 2006).
Figure 1.
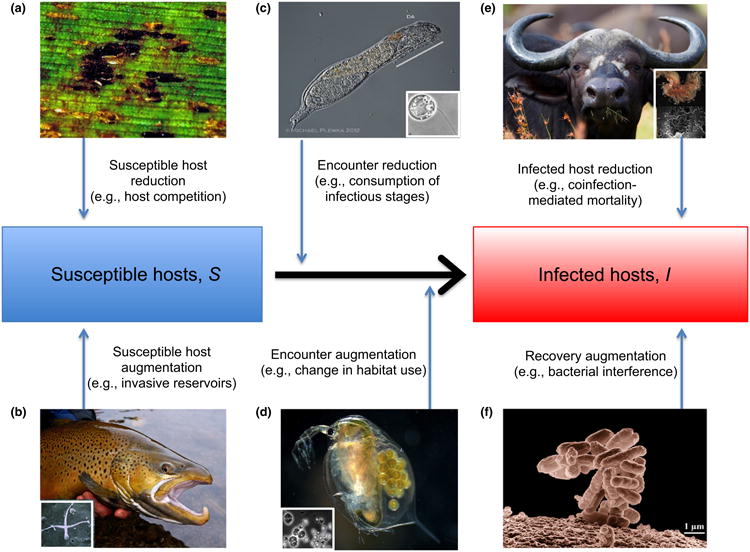
Mechanisms through which diversity can alter pathogen transmission or disease risk (sensu Keesing et al. 2006). (a) Decreases or (b) increases in the density of susceptible hosts. Higher plant diversity reduces host availability for fungal pathogens (Mitchell et al. 2002), whereas invasive brown trout provide a reservoir for Myxobolus cerebralis, the cause of whirling disease (Vincent 1996); (c) Decreases or (d) increases in the encounter rate between suitable hosts and parasites. Consumption of chytrid zoospores by predators reduced infection in amphibians (c) (Schmeller et al. 2014), whereas fish increased infections in Daphnia magna by altering their habitat use (d) (Decaestecker et al. 2002); Changes in the rates at which infected hosts die (e) or recover (f). In (e), coinfection by nematodes and bacteria increased mortality in African buffalo, likely lowering transmission (Ezenwa & Jolles 2015); in (f), healthy faecal bacteria reduced pathogenic infections in humans (Costello et al. 2012).
As links between diversity and infection have become more apparent, two important questions have emerged. First, how often do dilution and amplification effects occur? And second, what features are shared among systems that exhibit these phenomena? With rising interest in addressing these questions, however, new complexities and sources of disagreement have emerged. Recently there has been a polarising debate over whether diversity losses will generally increase pathogen transmission or whether responses will be idiosyncratic and highly variable among systems (Keesing et al. 2010; Ostfeld & Keesing 2012, 2013; Randolph & Dobson 2012; Lafferty & Wood 2013; Salkeld et al. 2013; Wood & Lafferty 2013). A more productive approach may be to delineate under what combinations of host, parasite and environmental conditions changes in diversity are likely to either increase or decrease disease risk. With this in mind, we here (1) review recent advances and sources of confusion related to the diversity–disease linkage, (2) draw upon lessons from community ecology to anchor the topic firmly in the broader ecological literature and (3) identify future research directions and testable hypotheses in diversity–disease research. Using the framework of community ecology as a foundation, we explore linkages between disease and established theories related to biodiversity and ecosystem function (BEF), biotic invasions, community assembly and scale-dependency. Rather than weighing evidence for and against the dilution effect, we highlight key research directions necessary to transform diversity–disease research into a more predictive framework.
The Nexus Between Biodiversity and Disease
Explorations of the relationship between diversity and animal disease in the 1990s and early 2000s centred around Lyme disease (LD) in the northeastern United States. Prior research on LD and similar vector-borne zoonoses had focused on specific reservoir hosts (those that maintain and amplify pathogens), but generally neglected the broader host community. A more inclusive focus, quantifying the effects of various vertebrate hosts on tick abundance and infection prevalence, revealed strong interspecific differences and suggested that LD risk varied with host community composition (e.g. Schmidt & Ostfeld 2001; LoGiudice et al. 2003). One key innovation was the emphasis on hosts that can inhibit host-to-vector transmission (‘dilution hosts’), thereby reducing vector infection prevalence and subsequent vector-to-host transmission. Simple models parameterised with field data (Schmidt & Ostfeld 2001; Ostfeld & LoGiudice 2003) suggested that the risk of contracting bacterial infection for humans was lower in forest ecosystems containing a high natural diversity of vertebrate hosts (Schmidt & Ostfeld 2001; Ostfeld & LoGiudice 2003), many of which are epidemiological ‘dead ends’ for the bacterium that causes LD (Borrelia burgdorferi) (Ostfeld & Keesing 2000a). Recent research at both small and large spatial scales has supported these predictions (Turney et al. 2014; Werden et al. 2014).
The LD research leading to the dilution effect concept was largely inductive, relying on empirical observations from one disease system to build more general theory. This research led to the development of specific criteria by which one would expect high host diversity to reduce risk of exposure to vector-borne diseases, namely: (1) generalised feeding by the vector, (2) differences between hosts in quality for pathogens and vectors and (3) a tendency for highly susceptible hosts to dominate in low-diversity communities (Ostfeld & Keesing 2000b). Research on comparable vector-borne diseases, including West Nile fever (a viral infection transmitted by mosquitoes) and Chagas disease (a protozoan infection transmitted by reduviid bugs), generally found support for all three criteria, generating patterns consistent with the dilution effect hypothesis (Ezenwa et al. 2006; Allan et al. 2009; Koenig et al. 2010; Gottdenker et al. 2012; Johnson et al. 2012a) (but see Loss et al. 2009; Salkeld et al. 2013).
Although the dilution effect was initially formulated for vector-borne diseases, later research broadened the criteria and explored their application to disease systems with other transmission modes. Any disease system in which (1) host species vary in competence (i.e. their ability to support and transmit infection) and (2) encounters between highly susceptible hosts and infectious stages tend to persist or predominate in low-diversity communities has the potential to exhibit a dilution effect. Subsequent studies have examined the effects of changes in host diversity for directly transmitted zoonoses (e.g. hantaviruses) (reviewed by Khalil et al. 2014), parasites with complex life cycles (reviewed by Johnson & Thieltges 2010) and other pathogens with free-living infectious stages (e.g. amphibian chytridiomycosis) (Searle et al. 2011; Becker et al. 2014; Venesky et al. 2014). A concurrent extension of this research has investigated the diversity of system components beyond the host community, including predators, competitors and coinfecting symbionts. Reductions in predator diversity have been correlated with increased prevalence of Sin Nombre hantavirus in deer mice (Peromyscus maniculatus) (Dizney & Ruedas 2009; Orrock et al. 2011) and higher infectious disease levels in coral reefs (Raymundo et al. 2009; see also Rohr et al. 2015). The experimental exclusion of large herbivores in East African savanna ecosystems led to a doubling in the density of their competitors, rodents and their associated fleas (McCauley et al. 2008; Keesing & Young 2014; Young et al. 2014; but see Borer et al. 2009). In addition to the effects of predators and competitors, changes in symbiont or vector richness also have the potential to alter the risk of pathogenic disease. High parasite diversity reduced both total and per capita infection of amphibians with the virulent parasite Ribeiroia ondatrae through an intrahost dilution effect (Johnson & Hoverman 2012; Johnson et al. 2013a).
The inductive approach was later complemented by a deductive one, in which general theory was used to derive predictions that could be tested in specific systems (Dobson 2004; Rudolf & Antonovics 2005; Keesing et al. 2006). Keesing et al. (2006) formalised definitions for the dilution effect and its corollary, the amplification effect. These authors also provided a set of specific mechanisms to help focus subsequent research, particularly given that multiple, potentially opposing mechanisms may operate simultaneously. Begon (2008) distinguished between the ability of a diverse host assemblage to regulate the abundance of reservoir hosts (susceptible host regulation, sensu Keesing et al. 2006) vs. the assemblage's ability to disrupt pathogen transmission between hosts independent of changes in density (encounter reduction, sensu Keesing et al. 2006), suggesting that little evidence existed for the latter pathway. In a large-scale manipulation of plant species diversity, for instance Mitchell et al. (2002) found that the effect of plant (host) species richness on naturally colonising viral disease severity was indirect: diverse communities suppressed the abundance of reservoir hosts rather than suppressing transmission directly. Subsequent experimental studies manipulating host diversity and density independently, however, have found clear evidence for both pathways. For example Johnson et al. (2008, 2013a,b) found significant, independent effects of host density and diversity on infection and disease in amphibians caused by R. ondatrae. Venesky et al. (2014) manipulated both total density and species diversity of tadpoles exposed to Batrachochytrium dendrobatidis and found that diversity but not density-affected B. dendrobatidis abundance (see also Searle et al. 2011; Becker et al. 2014). By linking experimental and field-based approaches, both of these sets of studies further demonstrated that, whereas multiple disease outcomes were possible in experimental systems, increases in amphibian host diversity were much more likely to decrease rather than increase pathogen transmission and host pathology under natural conditions.
Debates about the generality of the dilution effect, the conditions in which dilution and amplification effects are likely, and the scale at which such effects manifest have recently emerged (e.g. Randolph & Dobson 2012; Ostfeld 2013; Ostfeld & Keesing 2013; Wood & Lafferty 2013). In a critique of the dilution effect applied to vector-borne infections, Randolph & Dobson (2012) argued that dilution effects may occur in some simple systems but are much less likely in the complex environments typical of many zoonoses and vector-borne diseases. They speculated that increases in host species richness might enhance the abundance of vectors, thereby amplifying transmission potential, and re-emphasised prior arguments (e.g. LoGiudice et al. 2003) concerning the importance of changes in host species composition – rather than species richness per se – in controlling patterns of infection. To apply a more quantitative approach to this question, Salkeld et al. (2013) conducted a meta-analysis of 13 published and unpublished studies that included information on the relationship between host diversity and varying metrics of infection for zoonotic diseases (primarily WNV, LD, hantaviruses and plague). The overall effect of diversity on infection was negative yet variable, from which the authors suggested that diseases respond idiosyncratically to changes in biodiversity (see also Young et al. 2013). More recently, however, Civitello et al. (2015) conducted a meta-analysis including zoonoses and other diseases (N = 202 effect sizes from 61 parasite species) and found consistent evidence for dilution effects in diverse host communities, independent of host or parasite type.
In a review of LD and the factors affecting transmission, Wood & Lafferty (2013) emphasised the potential for complex interactions among land use change, host species composition and zoonotic disease risk. In particular, the authors speculated that spatial scale might mediate the form of the diversity–disease relationship; while vertebrate diversity might inhibit transmission at fine scales (e.g. within forest patches), at broader spatial scales (e.g. the transition from urban to forested areas), some minimum amount of wildlife diversity is necessary to allow establishment of B. burgdorferi, which requires a tick vector and one or more suitable vertebrate hosts. This minimum diversity threshold appears to be quite low, however, and more recent studies at scales ranging from small islands in the St. Lawrence River (Werden et al. 2014) to the eastern half of the United States (Turney et al. 2014) indicate linear decreases in LD risk or incidence with increasing host diversity.
Taken together, this overview of recent research helps to illustrate the rapidly growing interest in diversity–disease relationships. Since its original description, the dilution effect has generated tremendous empirical and theoretical interest (e.g. see reviews by Ostfeld & Keesing (2012) and Cardinale et al. (2012). An additional 90 studies published between 2012 and April 2014 have assessed diversity effects on diseases of humans (both zoonotic [n = 16 studies] and non-zoonotic [n = 43 studies]), wildlife or livestock [n = 19] and plants [n = 12]) (see Appendix S1 in Supporting Information). These studies, which collectively assess how the diversity of host communities, parasite communities and the host microbiome affect fungal, bacterial, viral and helminth parasites, find broad support for a negative effect of diversity on disease. Interestingly, changes in microbial diversity have also recently been linked to some diseases not generally considered to have infectious aetiologies (e.g. cystic fibrosis, auto-immune disorders, see Appendix S1). Nonetheless, emerging debates and seemingly contradictory interpretations – even of similar data sets – emphasise the urgent need to address misconceptions, provide clarifying terminology, and identify future research directions to help synthesise this growing field. We propose that a closer consideration of research antecedents to diversity–disease investigations within the broader field of community ecology will help to anchor the field and offer insights into its theoretical foundations and necessary next steps.
Linking Community Ecology and Disease Ecology
Community diversity as an extension of population genetic diversity
Exploration of the role of diversity in disease dynamics has many antecedents in population biology and community ecology. From a conceptual standpoint, the dilution effect represents a relatively simple extension of the Red Queen hypothesis to host communities. The Red Queen hypothesis is often invoked as an explanation for the pervasiveness of sexual reproduction; by providing an opportunity for genetic recombination, sex helps protect hosts from ever-adapting parasites and pathogens. Correspondingly, species that can reproduce sexually or asexually tend to favour sexual reproduction in the face of higher parasite exposure (Lively 2010). Clay et al. (2008) proposed extending this premise from populations of individual hosts composed of multiple genotypes to communities of host species varying in susceptibility to infection. Thus, just as increases in genotypic diversity within a host population can reduce infection success in a single-host–single parasite system (Lively 2010), increases in host species richness – and by extension total allelic diversity – can alter infection of multihost parasites (e.g. ‘Red Queen Communities’, Clay et al. 2008). The parallels between these Red Queen arguments and the dilution effect are striking (Ostfeld & Keesing 2012). Trade-offs in the ability of pathogens to invade one host genotype against the ability to invade others are analogous to trade-offs for pathogens infecting one host species against the ability to infect others. Diverse genotypes in a host population might suppress prevalence of infection within that population similarly to diverse species suppressing prevalence within a host community.
Biodiversity, ecosystem function and disease
Beyond genetics, diverse ecological communities can affect both species interactions and the interplay between biotic and abiotic components of ecosystems, as exemplified by nearly two decades of research into the relationship between BEF (Cardinale et al. 2012). Through this extensive body of research, gradients in species richness have been linked to changes in decomposition, primary production, carbon sequestration and the risk of species invasions. The hypothesis of biotic resistance, for instance highlights the capacity for more diverse native communities to resist invasions by non-native species, often through competition, predation or allelopathy (Levine et al. 2004; Kimbro et al. 2013). Similarly, diverse plant communities often inhibit abundance of or damage by herbivores, thereby increasing agricultural and biofuel crops, in accordance with the associational resistance hypothesis (Barbosa et al. 2009; Letourneau et al. 2011). In parallel, free-living species from diverse trophic levels and functional groups can directly or indirectly influence the invasion of pathogens or their subsequent transmission. Potential mechanisms linking diversity and disease outcomes are diverse (Fig. 1), and can involve diversity-mediated changes to hosts (e.g. in their density, behaviour, or physiology; see Appendix S1) or to parasites (e.g. consumption of infectious agents, interactions among coinfecting microorganisms). For instance van Elsas et al. (2012) showed that soil microbial diversity inhibited invasion by the bacterium Escherichia coli O157 : H7, which is pathogenic to humans. Importantly, however, lessons from community ecology emphasise that whether such effects occur – and their direction – will be sensitive to the scale under consideration.
Scale and its importance for diversity–disease relationships
The relationship between native biodiversity and species invasions is often strikingly scale-dependent (Shea & Chesson 2002; Fridley et al. 2007); at the local scales within which species interact, resource competition and predation can lead to a negative correlation between native and invasive species richness. In contrast, however, the richness of native and invasive species are more likely to correlate positively at regional scales due to parallel responses to resource gradients or disturbance regimes (Levine et al. 2004; Kimbro et al. 2013). This ‘invasion paradox’ helps to illustrate the essential importance of scale and its influence on underlying ecological processes, which may involve species interactions at local scales but be dominated by factors affecting colonisation, extinction and historical legacy at larger scales (Fridley et al. 2007; Araújo & Rozenfeld 2014).
Correspondingly, the relationship between biodiversity and infection risk will often depend strongly on spatial scale. For instance the mechanisms underlying the dilution effect involve local scales in which community diversity inhibits a pathogen from establishing and transmitting between susceptible hosts (Keesing et al. 2006). However, host biodiversity often correlates positively with overall parasite richness or the presence of a particular infection (i.e. the ‘diversity begets diversity’ hypothesis; (Hechinger & Lafferty 2005; Dunn et al. 2010; Wood & Lafferty 2013; Kamiya et al. 2014). This observation is believed to emerge from the habitat heterogeneity hypothesis: higher host diversity facilitates an increase in the number/types of infections that can be supported (Lafferty 2012), thereby leading to higher parasite richness, particularly for specialist parasites or those that require multiple hosts to complete their life cycles (Dunn et al. 2010; Kamiya et al. 2014).
Rather than contradicting each other, these lines of inquiry are complementary; they emphasise both different responses (parasite diversity as opposed to infection or disease risk) and different ecological processes (colonisation among communities as opposed to transmission within communities) (Box 1, Morand et al. 2014). Thus, exploring how biodiversity affects whether a specific pathogen can establish is a very different question from how changes in local diversity affect the capacity of an established pathogen to transmit (Hechinger & Lafferty 2005; Johnson et al. 2013a). For instance biogeographic analyses indicate that overall parasite diversity and the number of infectious human pathogens is greater in the tropics (Guernier et al. 2004); however, this view from 30 000 feet tells us little about how well a pathogen will spread within particular patches of a tropical forest (i.e. what controls local transmission?). Reconciling these scale-dependent relationships leads to at least two important take-home messages. First, studies should identify their scale of focus (spatial extent, grain and ecological scale) and whether the inferred interaction involves transmission or colonisation. Higher host diversity could function to promote parasite colonisation (and thus parasite diversity) while nonetheless inhibiting transmission of each pathogen species (Fig. 2; Box 1). Because parasites, vectors and hosts differ in mobility and range size, definitions of scale should carefully consider the biology of the specific disease system (Box 1).
Box 1. Empirical insights into the diversity–disease ‘paradox’.
Recent work on biodiversity and disease has emphasised two, seemingly paradoxical perspectives: increases in host diversity can correlate positively with overall parasite diversity (‘diversity begets diversity’ hypothesis; Hechinger & Lafferty 2005; Dunn et al. 2010), whereas biodiversity losses can promote pathogen transmission (‘dilution effect’, Keesing et al. 2006, 2010). Reconciliation of these perspectives requires explicit consideration of the response variables involved and the scale over which interactions occur (Hechinger & Lafferty 2005). Parasite richness is not equivalent to disease risk, which is often a function of the prevalence or abundance of an especially virulent pathogen. Perhaps more importantly, while the diversity begets diversity hypothesis focuses on parasite colonisation, the dilution effect is often applied to changes in transmission of an established parasite. In part, this is a difference in scale: regions higher in overall diversity (e.g. lower latitudes) support a richer parasite fauna, including a greater number of pathogenic species (Dunn et al. 2010). Within a region or community, however, local changes in transmission may nonetheless be influenced by patterns of community diversity.
A major challenge to evaluating the merits of this explanation involves the rarity of data to simultaneously examine the relationship between host diversity and both parasite richness and metrics of disease risk. However, recent studies are beginning to offer insights into this issue. For instance in a long-term, large-scale manipulation involving 82 experimental plots in Germany, Rottstock et al. (2014) evaluated how plant host richness (1–60 species) and functional group diversity (1–4 groups) affected infection by obligate fungal pathogens. As predicted by the diversity begets diversity hypothesis, pathogen diversity increased log-linearly with host diversity. Importantly, however, both the overall percentage of infected plants within a plot and the severity of infection decreased with host diversity. The causal mechanisms were twofold: a reduction in the amount of cover of individual host plant species at high diversity owing to greater resource competition and increased ‘barriers to infection’ with greater plant species heterogeneity.
Similarly, in a survey of amphibian communities in California, Johnson et al. (2013a) reported that trematode parasite richness correlated positively with host richness; however, among wetlands that supported the most pathogenic trematode, R. ondatrae, higher amphibian host richness reduced transmission success between snail and amphibian hosts by 78.4%, leading to corresponding decreases in pathology. This result, which was supported by laboratory and mesocosm experiments, stemmed from the non-random assembly of host communities; the most competent hosts tended to be both the most widespread and the most abundant, with progressive decreases in community competence as richness increased. Thus, in more diverse assemblages, a higher fraction of trematode cercariae were lost when attempting to penetrate or persist within less-competent hosts.
Figure 2.
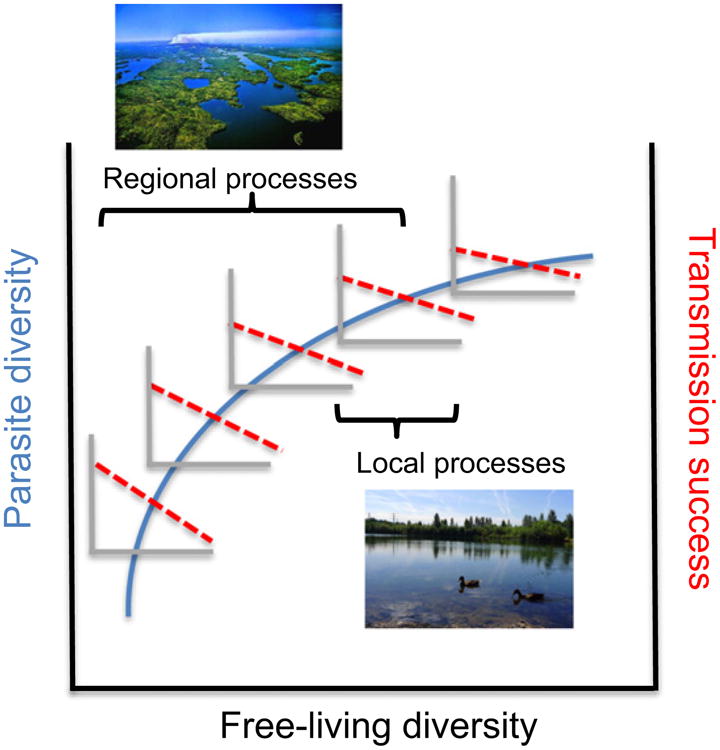
Diversity could have scale-dependent and even opposing effects on parasite colonisation, a regional process determining parasite diversity (shown in blue on the left y-axis), and pathogen transmission (shown red dashed lines on the right y-axis), a local scale process involving the capacity of a virulent pathogen to spread among hosts. Here, the positive effect of free-living richness on parasite richness begins to saturate with overall increases in diversity as a hypothetical community assembles in an increasingly substitutive (rather than additive) manner. Concurrently, the negative relationship between diversity and local pathogen transmission (i.e. a dilution effect) is strongest at low to intermediate levels of free-living richness, after which additional increases in richness have more modest effects on transmission. However, because parasites, vectors and hosts differ in mobility and range size, studies need to carefully consider the ‘ecological scale’ of the specific disease system under study.
Second, researchers should be mindful of the distinction between parasite diversity (i.e. the richness of parasite groups) and disease risk (i.e. the abundance or prevalence of a virulent infection). The dilution effect hypothesis has always been focused on the transmission and resultant abundance or prevalence of particular disease-causing infections. Parasite diversity and disease risk may often respond differently to changes in host diversity. For instance Rottstock et al. (2014) showed that, within a single study, experimental increases in plant diversity led to higher parasite richness but lower infection prevalence and pathology (Box 1). In some cases, changes in parasite diversity within communities or individual hosts also have the potential to affect disease. For instance if concurrent infections exacerbate host damage or inhibit immune responses, higher parasite diversity can lead to more severe pathology (Ezenwa & Jolles 2015). In others cases, higher parasite or symbiont richness could lower disease risk, as can occur if antagonistic interactions among symbionts reduce the abundance or virulence of pathogenic species (Fig. 1) (Johnson & Hoverman 2012; Junemann et al. 2012).
Assembly theory and the links among species richness, abundance and community composition
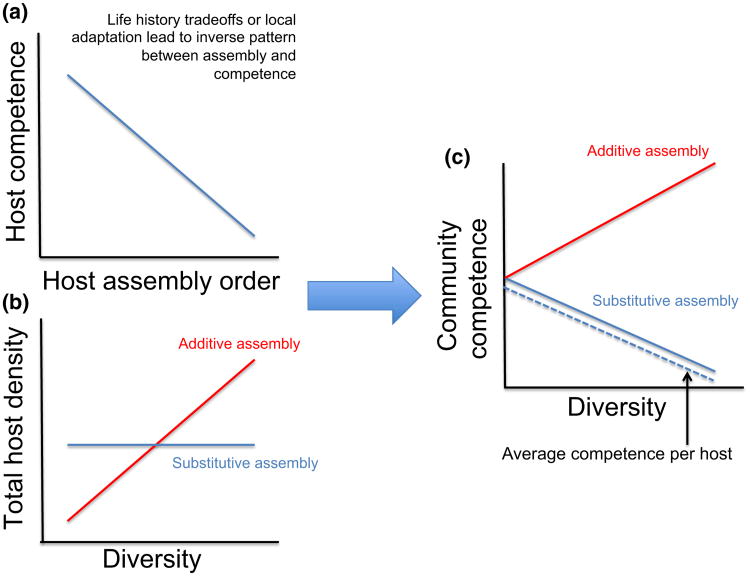
Community ecology also provides insights into the relationship between species richness, composition and abundance. Debates related to the diversity–disease relationship often focus on whether observed changes in infection result from shifts in host species richness per se or in community composition (LoGiudice et al. 2003; Ostfeld & LoGiudice 2003; Randolph & Dobson 2012; Salkeld et al. 2013). However, an essential question is whether there is a predictable relationship between the richness of a community and the identities or functional traits of its species (Ostfeld & Keesing 2000a; Schmidt & Ostfeld 2001; LoGiudice et al. 2003). If the order in which species assemble is deterministic (rather than stochastic) and correlates positively with their susceptibility, then more diverse communities will support a higher fraction of low-competence hosts, leading to a dilution effect, all else being equal (Ostfeld & LoGiudice 2003; Joseph et al. 2013) (Fig. 3). This scenario may occur when host species undergo life history trade-offs between infection susceptibility and either colonisation ability or resistance to extirpation, or when pathogens locally adapt to the most frequently encountered host species (Hantsch et al. 2013; Huang et al. 2013; Johnson et al. 2013b). For instance Lacroix et al. (2014) found that decreases in grassland community diversity were associated with an increased prevalence of barley and cereal yellow dwarf viruses owing to progressive dominance by the most competent hosts in species-poor assemblages.
Figure 3.
Contrasting the effects of additive and substitutive assembly on the relationship between diversity and community competence. Even if host species assembly order is inversely related to the species' competence (a), whether changes in diversity lead to a reduction in infection risk depends on how communities assemble. If communities assemble additively, then the total density (or biomass) of hosts will increase with species richness; substitutive assembly assumes a fixed carrying capacity for the community, such that increases in diversity are associated with replacement of established individuals to maintain a constant total density (b). This leads to very different patterns in the total competence of the community (the product of each species' abundance multiplied by its competence), which can strongly influence transmission and disease risk (depending on whether transmission is density- or frequency-dependent), even if the average competence per host (dashed line) decreases in both scenarios (c).
A second essential question relates to how the abundance or suitability of hosts and vectors changes with species richness. Stated another way, how does the community competence – which is the sum of each host species' competence multiplied by its abundance – change with diversity? If the overall abundance or biomass in a community increases along a richness gradient – even if the proportion of highly competent hosts decreases – high-diversity systems may nonetheless support more infection (amplification effect). For instance in a simulation-based study, Mihaljevic et al. (2014) showed that the effects of host diversity on the transmission ability of a generalist pathogen (i.e. community R0) depended on both its transmission mode (density- vs. frequency-dependent) and how community abundance changed with richness (additive, substitutive or saturating) (Fig. 3). Thus far, however, empirical data on the relationship between abundance and richness remain surprisingly scarce, making it difficult to infer which patterns of transmission are most likely to occur. Finally, while these examples focus on the diversity and abundance of host species, a pressing priority is to move beyond host diversity to consider the assembly of non-host species (e.g. predators, competitors, symbionts), which can directly or indirectly affect transmission through changes in host behaviour, host physiology or their probability of encountering infectious stages (Fig. 1) (Ostfeld & Holt 2004; Johnson et al. 2010; Schmeller et al. 2014; Rohr et al. 2015). For instance most diversity–disease studies address dynamics of a single pathogen or disease without considering diversity effects on other pathogens or symbionts (Myers et al. 2013). Incorporation of a more inclusive set of focal symbionts, analogous to the consideration of multiple ecosystem functions in the BEF literature, would provide information that is more broadly applicable to both theory and applications to health policy and management.
The Future of Diversity–Disease Research: Toward a Predictive Framework
Given the parallels between diversity–disease research and previous work in community ecology (Clay et al. 2008; Cardinale et al. 2012; van Elsas et al. 2012; Becker et al. 2014), a timely opportunity exists to direct momentum beyond polarising debates and towards a more inclusive study of the community ecology of disease. This effort requires developing a more mechanistic approach to identifying the conditions under which biodiversity affects community composition and the capacity of pathogens to spread or cause illness (Dobson 2004; Rigaud et al. 2010). Addressing this challenge is not a trivial undertaking. Because the response (disease risk) is an interaction shaped by multiple host and parasite species, progress demands detailed information on host life history and competence, parasite virulence and transmission dynamics, the density of individual species and the overall community and the assembly/disassembly patterns for free-living as well as symbiont species. For many systems, even those with public health significance, basic questions about the form of transmission remain unanswered, much less the manner in which transmission changes with species composition and diversity (Bonds et al. 2012). Correcting this deficiency requires a focused effort to enhance both the conceptual and empirical foundations of disease ecology. Below we highlight key steps forward as a function of observational, experimental and modelling approaches.
Observational field studies
Defining disease and diversity
It is important to select metrics of biodiversity and disease on the basis of specific, a priori hypotheses about functional relationships or relevance to policy and management. Disease risk has been variously measured using the density of infected intermediate hosts or vectors, infection prevalence in hosts or vectors, pathogen shedding rates, cases of disease, impacts on host populations and transmission rates (Keesing et al. 2006). Diversity might differentially affect these metrics, and the choice of a response should be made with a clear model of system function and the scale of the process under consideration. We recommend that studies identify whether they examine the effects of diversity on parasite invasion in previously uninfected populations or on parasite prevalence and abundance within endemic areas (Box 1). Similarly, specific components of diversity might affect pathogen transmission differently. Depending on the system, one might expect the richness or evenness of different groups (e.g. hosts, competitors and non-hosts) or trophic levels (e.g. predators, other parasites and microbes) to have a greater influence on disease processes. Researchers assessing biodiversity and ecosystem functioning have expanded their focus from richness and identities of species to that of functional groups and traits (Naeem et al. 2009; Bello et al. 2010), which are defined relative to the ecosystem function of interest and offer a more mechanistic link to performance (Webb et al. 2010). For pathogen transmission, relevant host traits include competence (ability to acquire and sustain infection), infectivity (capacity to release or transmit infectious stages), tolerance (ability to tolerate infection) and relative abundance (Streicker et al. 2013). Non-host species could be assigned to functional or trait groups based on their impact on transmission or on the availability of suitable hosts.
Detecting dynamic effects
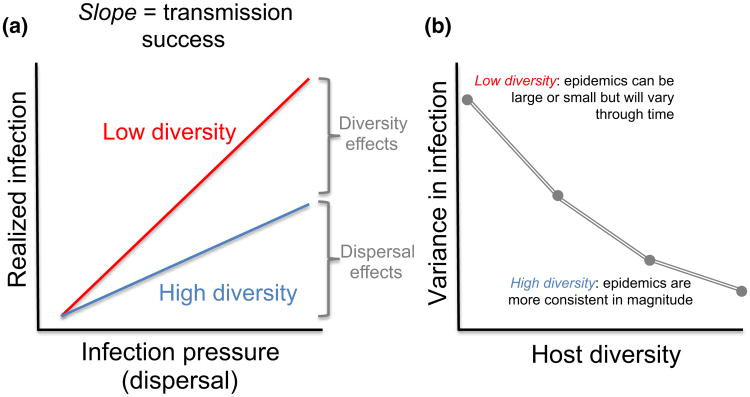
Observational studies of disease are often plagued by the challenges of inferring dynamic processes from static patterns, which can be especially problematic for disease systems that exhibit marked intra and interannual variation (Altizer et al. 2006). Currently, many field studies test for a bivariate, often linear relationship between disease risk and host diversity among sites. This approach is almost certainly overly simplistic. For instance while many infections are highly dynamic over short time periods, local species richness is likely to change much more slowly. This indicates that an ecologically ‘slow’ variable like richness is unlikely to be the primary driver of short-term infection dynamics, which are responding to measures of ‘infection pressure’ associated with vector abundance, host density, climate and the production of infectious stages. Consequently, the effects of diversity may often manifest as a moderator, interacting with infection pressure to determine observed infection either currently (Fig. 4) or at future time steps. For instance Hamer et al. (2011) consistently found that the best predictor of WNV infection in Culex mosquitoes was the interaction between bird diversity (or richness) and the community force of infection, a measure that incorporated bird competence and vector feeding preferences (see also Johnson et al. 2013b). Similarly, results of modelling studies suggest that some of the strongest effects of host richness may be on the variance of disease metrics, with the presence and severity of epidemics predicted to fluctuate more widely in low diversity communities (Mihaljevic et al. 2014) (Fig. 4). This finding also parallels results from BEF research showing progressive decreases in the variance of particular response with increases in community richness (Cardinale et al. 2012).
Figure 4.
Hypothesised relationships among diversity, infection pressure and infection variance in a community. (a) Host infection is expected to be a function of both infection pressure (e.g. the density of infected vectors, reservoirs or infectious propagules) and the diversity of hosts; the interaction between these terms reflects pathogen transmission success, which here is shown to weaken at higher host diversity. Host diversity thus acts as a ‘niche-based’ filter upon dispersal pressure, which will also be affected by climate, resource availability and community structure. (b) Modelling studies suggest that host diversity will strongly affect the variance in infection or transmission (Mihaljevic et al. 2014). Regardless of transmission mode or assembly pattern, species-poor communities have higher variance in epidemic size (over time or space), whereas diverse communities exhibit lower variance, emphasising the importance of collecting sufficient data to explore infection responses and their temporal or spatial variance along diversity gradients.
Experimental studies
Because biodiversity tends to vary non-randomly across the landscape (e.g. in response to resource availability or colonisation opportunities), isolating the influence of host diversity on disease processes relative to concurrently changing (confounding) factors remains a major hurdle. Thus, correlational field studies will often be limited in their capacity for causal inference and in identifying the mechanisms linking observed diversity–disease relationships. The number and scale of most experimental studies of the diversity–disease relationship conducted to date remain small, particularly for animal disease systems (Box 2). While such experiments have provided insights about the epidemiological processes linking free-living richness and parasite transmission, including the broad importance of both susceptible host regulation and encounter reduction (Fig. 1), the next generation of experimental studies needs to move beyond testing whether or not dilution/amplification effects can occur and toward the use of realistic manipulations that evaluate both the strength and scale of diversity effects on disease dynamics in complex communities (Mitchell et al. 2002; Johnson et al. 2013a; Rottstock et al. 2014; Venesky et al. 2014; Rohr et al. 2015). Incorporating insights from experimental studies of BEF will be especially valuable (Cardinale et al. 2012). While initially the subject of considerable controversy, the BEF field has made progress in identifying how and when biodiversity changes are likely to influence ecosystem responses, in large part through an emphasis on rigorous experimentation followed by meta-analytic syntheses (Cardinale et al. 2011, 2012).
Box 2. Experimental studies of the diversity–disease relationship.
Experimental Approaches
Experimental studies examining the effects of species richness on infection are essential for understanding the mechanisms underlying diversity–disease relationships. By altering community richness and composition, independent of other factors that covary in nature, experiments allow an explicit assessment of how species composition affects responses such as host density, parasite transmission and infection-mediated pathology. We consulted published reviews (Cardinale et al. 2012; Ostfeld & Keesing 2012) and conducted Web of Science searches for experimental studies on the diversity–disease relationship. To be included, studies had to (1) directly manipulate richness as an independent variable (i.e. rather than manipulating other metrics of diversity [e.g. evenness], using indirect manipulations that influenced richness, or studying a natural experiment) and (2) have treatments that included more than two species (i.e. rather than simply monospecific vs. heterospecific) (following Cardinale et al. 2011), although this excluded some studies included in other analyses (e.g. Civitello et al. 2015). Studies that varied the genetic strain of hosts (rather than species richness) were similarly excluded.
From this search, we identified 21 studies published between 1999 and April of 2014. Because many studies included more than one parasite or more than one experiment, this included 89 total manipulations, for which we present the relative frequencies on focal parasite (A), focal hosts (B), experimental venue (C) and type of diversity manipulated (D). Of these, most involved foliar fungal pathogens of plants or helminth and fungal infections in amphibians, and ranged from small-scale alterations within cages, soil microcosms and aquaria to larger scale studies in outdoor mesocosms and grassland plots. Studies involving zoonotic infections were especially rare relative to their frequency among field-based studies of dilution effects (Appendix S1). By comparison, Cardinale et al. (2012) identified > 500 diversity manipulations among nearly 200 publications in their meta-analysis of ecosystem function, emphasising the relative rarity of experimental studies on dilution and amplification effects.
Animal vs. Plant Experiments
Owing to their origin within large-scale biodiversity projects (e.g. Jena Experiment, Cedar Creek, BIOTREE), plant diversity–disease studies often included large numbers of species (1–60) and multiple (randomised) permutations of plant community composition. Because many of these infections involve host specialists, increases in richness tend to decrease the density of suitable hosts leading to a corresponding decrease in disease severity by naturally colonising parasites (Knops et al. 1999; Mitchell et al. 2002) (but see Rottstock et al. 2014). In contrast, manipulations of animal infections tend to have a narrower range of richness treatments (1–6), smaller spatial scales, and often focus on particular host or parasite species (e.g. a focal host). However, animal disease experiments were more likely to manipulate infection directly and often contrast density vs. composition effects (e.g. by comparing additive vs. substitutive designs) (Johnson et al. 2008, 2012b; Thieltges et al. 2008; Searle et al. 2011; Venesky et al. 2014) (Fig. 4). At least in some cases, such experiments have also considered the influence of realistic vs. randomised assemblage structure (Johnson & Hoverman 2012) (Fig. 5) or attempted to partition the additive and non-additive contributions of diversity (Becker et al. 2014).
Functional Diversity
The majority of identified studies investigated the influence of variation in the richness of host species that differed in competence (including non-competent or ‘decoy’ hosts) on transmission success. Experiments involving other trophic levels (e.g. predators) often varied only the presence rather than the richness of predators and therefore did not meet our search criteria. This was also true of many microbiome studies, for which hosts tended to be experimentally enhanced or diminished in their microfauna (e.g. via antibiotic treatment). Thus far, manipulations involving the diversity of multiple trophic groups (e.g. hosts, predators and symbionts) are especially rare (Thieltges et al. 2008; Johnson et al. 2013a).
Structuring experimental communities
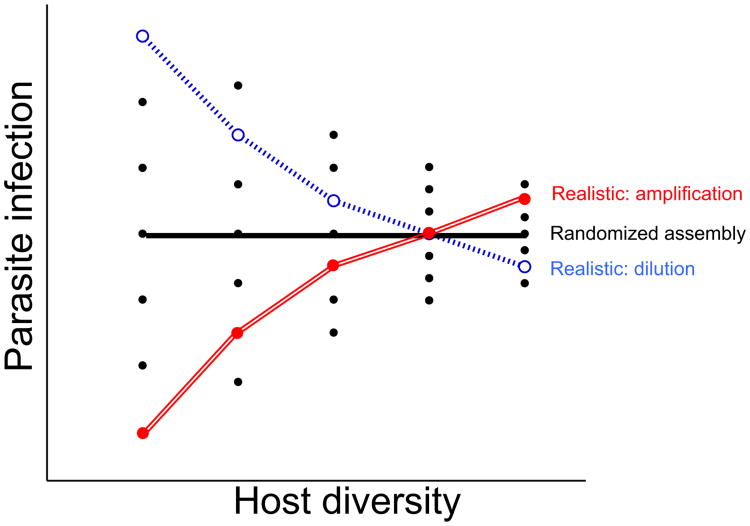
Because both host density and host diversity can influence pathogen transmission, experiments that use additive designs, in which total community abundance increases with the number of species, vs. substitutive designs, in which total abundance is constant across richness levels, will often yield strikingly different outcomes (Mihaljevic et al. 2014). While potentially having similar effects on mean competence (averaged among hosts), these designs differ in their influence on total community abundance and community competence (summed among all host individuals) (Fig. 3). Ideally, both designs should be contrasted in parallel to help differentiate between density- vs. diversity-mediated effects on transmission (Johnson et al. 2012b, 2013b). However, even with a relatively small number of host species, including all species combinations quickly becomes intractable. Recognising that host community assembly is often non-random, one way around this challenge is to focus on realistic assemblages observed at field sites (Mihaljevic et al. 2014). Contrasting a subset of randomly selected vs. realistic compositions nested within each level of richness can further help identify whether effects of diversity depend on the identity of a particular host species (e.g. ‘sampling effect’) or are an emergent (non-additive) property of richness (e.g. ‘complementarity’) (Bracken et al. 2008; Becker et al. 2014; Venesky et al. 2014) (Box 2), which is a particularly exciting future research direction.
While focus thus far has primarily been on additive processes, BEF research has illustrated the widespread potential for non-additive mechanisms, in which the outcomes in multi-species assemblages cannot be directly predicted from species' responses in isolation owing to factors such as interspecific inhibition or niche partitioning (Johnson & Hoverman 2012; Venesky et al. 2014). For instance Becker et al. (2014) found that experimental increases in amphibian host richness reduced infection by B. dendrobatidis both overall and for many species individually, which the authors attributed to greater habitat partitioning between aquatic and terrestrial species (i.e. complementarity). Other, non-additive mechanisms in disease systems could include selective feeding behaviour by vectors (i.e. feeding preferences changing with host community composition), non-random infections by parasite free-living stages or competition-mediated changes in host susceptibility. If, for instance the competence of host species changes in the presence of other species (e.g. due to competition), simply knowing what species are present and in what abundance may not be enough to quantify community competence.
Functional diversity
Non-host species – including both predators and other symbionts – can also affect parasite transmission. Predators can alter the availability of susceptible hosts (Ostfeld & Holt 2004; Borer et al. 2009), the abundance of parasites or their infective stages (Orlofske et al. 2012) and even the behavioural or physiological interactions between hosts and parasites (Decaestecker et al. 2002; Rohr et al. 2015). Given that predators are often particularly vulnerable to species loss (Estes et al. 2011), understanding how changes in diversity affect predator-mediated effects on disease risk change is an important priority. For instance Rohr et al. (2015) combined field research, modelling and experiments to help illustrate what forms of predation reduced parasite transmission; while intraguild predators that consumed both parasites and hosts had no net effect on infection, non-intraguild predators that reduced the availability of infectious parasites led to a reduction in infection. Concurrently, a growing emphasis on coinfection research has illustrated the potential for other parasites and commensal microbes to alter the infection success or persistence of pathogenic species, raising intriguing questions about the ‘hidden role’ of parasite and microbial interactions in affecting the diversity–disease relationship (Appendix S1). For instance inflammatory bowel disease caused by Clostridium difficile often follows antibiotic therapy, which reduces microbial diversity within the host intestinal tract. Song et al. (2013) found that high microbial diversity in human patients resulting from transplantation of faecal microbiota reduced the severity of diarrhoea and colitis associated with infection with C. difficile. Microbial diversity per se rather than the presence of particular bacterial taxa appeared responsible for resolving symptoms associated with C. difficile infection, although the mechanisms by which the microbial community suppress the pathogen are not clear.
Modelling studies
Previous models outline simple scenarios under which dilution and amplification are expected. For instance when transmission is frequency-dependent, dilution effects are predicted to occur whenever transmission within species is greater than that between different species; when transmission is density-dependent and the host community assembles additively (rather than substitutively), amplification will result (Dobson 2004; Rudolf & Antonovics 2005) (Fig. 3). Recent extensions of these models to incorporate host traits suggest that dilution effects are expected when host competence and extirpation risk correlate negatively, but amplification effects are increasingly expected as the correlation between competence and extirpation risk weakens (Joseph et al. 2013). These efforts lay the foundation for future modelling approaches to explore disease systems: (1) that have characteristics of both frequency- and density-dependent transmission; (2) in which community assembly is neither entirely additive nor substitutive (i.e. saturating) (Mihaljevic et al. 2014); (3) in which patterns of community disassembly vary depending on what drives biodiversity loss (e.g. habitat destruction vs. direct exploitation) and (4) for which host specificity of parasites varies as a function of host diversity over either ecological or evolutionary time. Ideally, future models should explore the simultaneous influence of specific mechanisms leading to both dilution and amplification and what factors mediate their net outcome. For instance higher host diversity might increase the number of feeding opportunities for vectors and thus amplify vector abundance, even while it dilutes infection prevalence (Ostfeld & Keesing 2000a; Swei et al. 2011) or deflects vectors from biting humans. Linking of modelling and empirical efforts would be enhanced through careful attention to the response variables under consideration; many empirical dilution studies focus on observed levels of infection or disease in a focal host group such as humans or hosts of conservation concern, while modelling studies tend to emphasise a parasite's reproductive ratio (R0) – something nearly impossible to measure in nature. Two important frontiers therefore involve exploring how diversity influences community-wide transmission, and whether the consequences of diversity changes vary depending on whether a single host or a community is the focus.
Collectively, recent findings from field-based, experimental and modelling studies have helped identify commonalities and offer guidance for future directions to advance the field. Development of a predictive framework depends on appropriate and operational definitions of disease and diversity for a focal system, characterisation of functional traits of community members relevant to disease risk, determination of how those species respond to drivers of biodiversity loss, characterisation of transmission mode(s) and consideration of dynamical changes in both diversity and disease risk. A dilution effect would be expected if:
members of the community differ substantially in their impact on maintenance and transmission of the focal pathogen(s). This condition is common to many pathogens, regardless of taxonomic affiliation or transmission mode;
the species or groups of species most responsible for pathogen maintenance/transmission (‘amplifying’ species) tend to persist as biodiversity is lost (nested community structure). Thus, the community structure is ‘nested’ and linked to a key epidemiological trait (host species competence), such that the most competent species are common across assemblages; and
- species that are more likely to be present or abundant in diverse communities – whether they are hosts, predators, competitors or other symbionts – reduce one or more of the following:
- the abundance of amplifying species;
- the susceptibility of amplifying species;
- the tolerance of amplifying species;
- encounters between amplifying species and pathogen;
- encounters between amplifying species and vectors;
- overall competence of the host community;
- abundance of the pathogen; (h) the abundance of vectors.
Evidence suggests these conditions exist in a wide variety of ecological systems, leading to observations of dilution effects across micro- and macroparasites, aquatic and terrestrial systems and different types of host organisms (Supporting Information, Ostfeld & Keesing 2012). Based on theory, these conditions might be more likely when transmission is frequency-dependent, community assembly is substitutive or saturating (rather than strictly additive), and when parasites exhibit local adaptation to common hosts or hosts undergo life history trade-offs between colonisation and resistance (Dobson 2004; Rudolf & Antonovics 2005; Joseph et al. 2013; Mihaljevic et al. 2014).
Conclusions
Since Elton's prescient yet anecdotal suggestions of a link between biodiversity and crop diseases more than 50 years ago, tremendous progress has been made both in understanding how changes in community composition affect pathogen transmission and in identifying systems in which this occurs (Appendix S1). Based on an emerging body of both field surveys and mechanistic experiments involving multihost infections, there is now clear empirical evidence indicating that biodiversity loss is associated with increased transmission or disease severity for a wide range of important pathogens of plants, wildlife and humans (Keesing et al. 2006; Johnson & Thieltges 2010; Cardinale et al. 2012; Ostfeld & Keesing 2012; Civitello et al. 2015). These effects often stem from diversity-mediated changes in the availability of susceptible hosts or the likelihood that they encounter infectious stages (Fig. 1). Identifying the links among host traits (e.g. competence), their relative abundance and patterns of community assembly/disassembly hold perhaps the greatest promise for understanding how species loss will affect transmission (Huang et al. 2013; Johnson et al. 2013b; Lacroix et al. 2014), although at present this information is lacking even for many of the most well-studied disease systems.
Concurrently, however, these efforts have exposed the need for more intensive and rigorous investigations into the diversity–disease relationship with the goal of a broader-level of synthesis (Box 3). Of particular importance is extending beyond simple correlations between diversity and disease risk to incorporate more process-based effects that can determine underlying mechanisms. Testing the influence of diversity on transmission requires corresponding information on spatial or temporal variation in infection pressure, which will be influenced by concurrent changes in climate, resource availability and biota. Because community diversity and composition also vary in response to environmental gradients and historical legacy (Petermann et al. 2010), isolating the specific effects of diversity on different pathogens will be greatly aided by experimental manipulations. Collectively, these points highlight a shortage of vital empirical data: despite growing interest in disease ecology generally and the influence of diversity specifically, we still lack the essential information necessary to test how often and in what disease systems the conditions outlined here as a predictive framework for identifying ‘diversity-dependent’ transmission apply. Indeed, arguments over whether diversity effects on disease are predictable or idiosyncratic largely reflect the depth of this knowledge gap and the need for additional information to identify generalities, which we hope will catalyse more fundamental integration at the interface between community ecology and disease.
Box 3. A prospectus for future work on diversity-disease relationships.
Future work on diversity–disease relationships has the potential to benefit from valuable lessons learned in community ecology, particularly from debates about the relationship between biodiversity and ecosystem function (BEF). In particular, future investigations should:
- Delineate key distinctions regarding response and predictor variables. Similar to the identification of specific ecosystem functions (e.g. primary production, nutrient cycling rate) responding to the variable biodiversity, disease-related response variables need to be specified, for instance:
- Between parasite diversity and disease risk (see Box 1 and main text);
- Between the influence of diversity of a single group (i.e. hosts) or the community at large on infection in sensitive (focal) hosts or among all host species
- Between the effects of diversity on parasite transmission and abundance of a single parasite vs. the entire parasite community (see Box 1 and main text);
- In selecting or comparing forms of diversity as the predictor, including host richness, functional diversity, Shannon diversity and genetic diversity
- Gather more empirical data in the field and laboratory. Conclusions regarding the generality of dilution and amplification effects are necessarily provisional until more research is done. For example we recommend:
- In natural communities, identifying the relationships among assembly order, host competence and community abundance (e.g. additivity vs. substitutivity) along natural gradients in species richness;
- In experimental communities, performing experiments at more realistic scales (e.g. field manipulations, particularly for animal systems) and contrasting random vs. realistic species assemblages;
- With long-term research, identifying and assessing the relative magnitude of diversity-mediated mechanisms in affecting transmission and disease outcomes over multiple temporal and spatial scales (i.e. dynamics), particularly relative to other, concurrent forms of environmental change.
- Explore new conceptual areas. More opportunities exist for applying more general community ecology concepts to disease ecology, including the following recommendations:
- Expand from purely additive vs. purely substitutive community structures to consider less extreme and more realistic patterns;
- Explore the influence of multiple components of diversity on pathogen transmission, including functional diversity (e.g. predators, non-reservoir hosts) and diversity of other symbionts (e.g. coinfections, microbiomes);
- Compare the degree to which effects of diversity depend on the identity of a particular host species (e.g. ‘sampling effect’) or are an emergent property of richness (e.g. ‘complementarity’), using lessons learned from BEF research as a guide;
- Explore how the relationships between host diversity, parasite diversity and disease risk vary among systems and across nested, hierarchical scales.
Supplementary Material
Figure 5.
Hypothetical effects of random vs. realistic community structures on pathogen infection success and disease risk. Because the selection of a particular focal host group and of particular community permutations will influence the perceived diversity–disease relationship, it is important to consider the influence of realistic changes in community structure on total infection. If communities assemble randomly and there are no non-additive effects (e.g. complementarity), then diversity will have no relationship with average parasite infection (solid black line). If, however, if the order in which species assemble is deterministic and negatively related to community competence (i.e. competent species are replaced or ‘diluted’ by less competent hosts at higher diversity), then experiments designed based on ‘realistic’ patterns of community structure will show dilution effects (blue line). If assembly is deterministic but positively related to total community competence, amplification effects are predicted (red line).
Acknowledgments
For comments and feedback helpful in developing or improving the manuscript, we thank B. Hoye, M. Joseph, J. Mihaljevic, D. Preston, Y. Springer, C. Wood and discussions with participants of the ‘Linking Biodiversity and Ecosystem Services’ at the Socio-Environmental Synthesis Center (SESYNC). We further acknowledge Eric Seabloom, Jon Chase and three anonymous reviewers for valuable suggestions in the revision process. The authors were supported through grants from NSF (DEB-1149308, DEB-1456527, DEB-1354332, EF-0813035) and NIH (R01GM109499).
Footnotes
Supporting Information: Additional Supporting Information may be downloaded via the online version of this article at Wiley Online Library (www.ecologyletters.com).
Authorship: PTJJ developed an outline and all authors contributed to writing and revising the manuscript.
References
- Allan BF, Langerhans RB, Ryberg WA, Landesman WJ, Griffin NW, Katz RS, et al. Ecological correlates of risk and incidence of West Nile virus in the United States. Oecologia. 2009;158:699–708. doi: 10.1007/s00442-008-1169-9. [DOI] [PubMed] [Google Scholar]
- Altizer S, Dobson A, Hosseini P, Hudson P, Pascual M, Rohani P. Seasonality and the dynamics of infectious diseases. Ecol Lett. 2006;9:467–484. doi: 10.1111/j.1461-0248.2005.00879.x. [DOI] [PubMed] [Google Scholar]
- Araújo MB, Rozenfeld A. The geographic scaling of biotic interactions. Ecography. 2014;37:406–415. [Google Scholar]
- Barbosa P, Hines J, Kaplan I, Martinson H, Szczepaniec A, Szendrei Z. Associational resistance and associational susceptibility: having right or wrong neighbors. Annu Rev Ecol Evol Syst. 2009;40:1–20. [Google Scholar]
- Becker CG, Rodriguez D, Toledo LF, Longo AV, Lambertini C, Correa DT, et al. Partitioning the net effect of host diversity on an emerging amphibian pathogen. Proc R Soc Lond B. 2014;281:20141796. doi: 10.1098/rspb.2014.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begon M. Effects of host diversity on disease dynamics. In: Ostfeld RS, Keesing F, Eviner VT, editors. Infectious Disease Ecology: Effects of Ecosystems on Disease and of Disease on Ecosystems. Princeton University Press; Princeton, NJ: 2008. pp. 12–29. [Google Scholar]
- Bello F, Lavorel S, Díaz S, Harrington R, Cornelissen JC, Bardgett R, et al. Towards an assessment of multiple ecosystem processes and services via functional traits. Biodivers Conserv. 2010;19:2873–2893. [Google Scholar]
- Bonds MH, Dobson AP, Keenan DC. Disease ecology, biodiversity, and the latitudinal gradient in income. PLoS Biol. 2012;10:e1001456. doi: 10.1371/journal.pbio.1001456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borer ET, Mitchell CE, Power AG, Seabloom EW. Consumers indirectly increase infection risk in grassland food webs. Proc Natl Acad Sci USA. 2009;106:503–506. doi: 10.1073/pnas.0808778106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracken MES, Friberg SE, Gonzalez-Dorantes CA, Williams SL. Functional consequences of realistic biodiversity changes in a marine ecosystem. Proc Natl Acad Sci USA. 2008;105:924–928. doi: 10.1073/pnas.0704103105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinale BJ, Matulich KL, Hooper DU, Byrnes JE, Duffy E, Gamfeldt L, et al. The functional role of producer diversity in ecosystems. Am J Bot. 2011;98:572–592. doi: 10.3732/ajb.1000364. [DOI] [PubMed] [Google Scholar]
- Cardinale BJ, Duffy JE, Gonzalez A, Hooper DU, Perrings C, Venail P, et al. Biodiversity loss and its impact on humanity. Nature. 2012;486:59–67. doi: 10.1038/nature11148. [DOI] [PubMed] [Google Scholar]
- Civitello DJ, Cohen J, Fatima H, Halstead NT, Liriano J, McMahon TA, et al. Biodiversity inhibits natural enemies: broad evidence for the dilution effect. Proc Natl Acad Sci USA. 2015;112:8667–8671. doi: 10.1073/pnas.1506279112. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay K, Reinhart K, Rudgers J, Tintjer T, Koslow J, Flory SL. Red Queen Communities. In: Ostfeld RS, Felicia K, Eviner VT, editors. Infectious Disease Ecology: Effects of Ecosystems on Disease and of Disease on Ecosystems. Princeton University Press; Princeton University Press, Princeton: 2008. pp. 145–178. [Google Scholar]
- Costello EK, Stagaman K, Dethlefsen L, Bohannan BJM, Relman DA. The application of ecological theory toward an understanding of the human microbiome. Science. 2012;336:1255–1262. doi: 10.1126/science.1224203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curl EA. Control of plant diseases by crop rotation. Bot Rev. 1963;29:413–479. [Google Scholar]
- Decaestecker E, De Meester L, Ebert D. In deep trouble: habitat selection constrained by multiple enemies in zooplankton. Proc Natl Acad Sci USA. 2002;99:5481–5485. doi: 10.1073/pnas.082543099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dizney LJ, Ruedas LA. Increased host species diversity and decreased prevalence of Sin Nombre virus. Emerg Infect Dis. 2009;15:1012–1018. doi: 10.3201/eid1507.081083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson A. Population dynamics of pathogens with multiple host species. Am Nat. 2004;164:S64–S78. doi: 10.1086/424681. [DOI] [PubMed] [Google Scholar]
- Dobson A, Cattadori I, Holt RD, Ostfeld RS, Keesing F, Krichbaum K, et al. Sacred cows and sympathetic squirrels: the importance of biological diversity to human health. PLoS Med. 2006;3:714–718. doi: 10.1371/journal.pmed.0030231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn RR, Davies TJ, Harris NC, Gavin MC. Global drivers of human pathogen richness and prevalence. Proc R Soc Lond B. 2010;277:2587–2595. doi: 10.1098/rspb.2010.0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Elsas JD, Chiurazzi M, Mallon CA, Elhottova D, Kristufek V, Salles JF. Microbial diversity determines the invasion of soil by a bacterial pathogen. Proc Natl Acad Sci USA. 2012;109:1159–1164. doi: 10.1073/pnas.1109326109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elton CS. The Ecology of Invasions by Animals and Plants. University of Chicago Press; Chicago, IL: 1958. [Google Scholar]
- Estes JA, Terborgh J, Brashares JS, Power ME, Berger J, Bond WJ, et al. Trophic downgrading of planet Earth. Science. 2011;333:301–306. doi: 10.1126/science.1205106. [DOI] [PubMed] [Google Scholar]
- Ezenwa VO, Jolles AE. Opposite effects of anthelmintic treatment on microbial infection at individual versus population scales. Science. 2015;347:175–177. doi: 10.1126/science.1261714. [DOI] [PubMed] [Google Scholar]
- Ezenwa VO, Godsey MS, King RJ, Guptill SC. Avian diversity and West Nile virus: testing associations between biodiversity and infectious disease risk. Proc R Soc Lond B. 2006;273:109–117. doi: 10.1098/rspb.2005.3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridley JD, Stachowicz JJ, Naeem S, Sax DF, Seabloom EW, Smith MD, et al. The invasion paradox: reconciling pattern and process in species invasions. Ecology. 2007;88:3–17. doi: 10.1890/0012-9658(2007)88[3:tiprpa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Gottdenker NL, Chaves LF, Calzada JE, Saldana A, Carroll CR. Host life history strategy, species diversity, and habitat influence Trypanosoma cruzi vector infection in changing landscapes. PLoS Negl Trop Dis. 2012;6:e1884. doi: 10.1371/journal.pntd.0001884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guernier V, Hochberg ME, Guegan JF. Ecology drives the worldwide distribution of human diseases. PLoS Biol. 2004;2:740–746. doi: 10.1371/journal.pbio.0020141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer GL, Chaves LF, Anderson TK, Kitron UD, Brawn JD, Ruiz MO, et al. Fine-scale variation in vector host use and force of infection drive localized patterns of West Nile virus transmission. PLoS ONE. 2011;6:e23767. doi: 10.1371/journal.pone.0023767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantsch L, Braun U, Scherer-Lorenzen M, Bruelheide H. Species richness and species identity effects on occurrence of foliar fungal pathogens in a tree diversity experiment. Ecosphere. 2013;4 Article 81. [Google Scholar]
- Hechinger RF, Lafferty KD. Host diversity begets parasite diversity: bird final hosts and trematodes in snail intermediate hosts. Proc R Soc Lond B. 2005;272:1059–1066. doi: 10.1098/rspb.2005.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ZYX, de Boer WF, van Langevelde F, Olson V, Blackburn TM, Prins HHT. Species' life-history traits explain interspecific variation in reservoir competence: a possible mechanism underlying the dilution effect. PLoS ONE. 2013;8:e54341. doi: 10.1371/journal.pone.0054341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PTJ, Hoverman JT. Parasite diversity and coinfection determine pathogen infection success and host fitness. Proc Natl Acad Sci USA. 2012;109:9006–9011. doi: 10.1073/pnas.1201790109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PTJ, Thieltges DW. Diversity, decoys and the dilution effect: how ecological communities affect disease risk. J Exp Biol. 2010;213:961–970. doi: 10.1242/jeb.037721. [DOI] [PubMed] [Google Scholar]
- Johnson PTJ, Hartson RB, Larson DJ, Sutherland DR. Diversity and disease: community structure drives parasite transmission and host fitness. Ecol Lett. 2008;11:1017–1026. doi: 10.1111/j.1461-0248.2008.01212.x. [DOI] [PubMed] [Google Scholar]
- Johnson PTJ, Dobson A, Lafferty KD, Marcogliese DJ, Memmott J, Orlofske SA, et al. When parasites become prey: ecological and epidemiological significance of eating parasites. Trends Ecol Evol. 2010;25:362–371. doi: 10.1016/j.tree.2010.01.005. [DOI] [PubMed] [Google Scholar]
- Johnson BJ, Munafo K, Shappell L, Tsipoura N, Robson M, Ehrenfeld J, et al. The roles of mosquito and bird communities on the prevalence of West Nile virus in urban wetland and residential habitats. Urban Ecosyst. 2012a;15:513–531. doi: 10.1007/s11252-012-0248-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PTJ, Preston DL, Hoverman JT, Henderson JS, Paull SH, Richgels KLD, et al. Species diversity reduces parasite infection through cross-generational effects on host abundance. Ecology. 2012b;93:56–64. doi: 10.1890/11-0636.1. [DOI] [PubMed] [Google Scholar]
- Johnson PTJ, Preston DL, Hoverman JT, LaFonte BE. Host and parasite diversity jointly control disease risk in complex communities. Proc Natl Acad Sci USA. 2013a;110:16916–16921. doi: 10.1073/pnas.1310557110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PTJ, Preston DL, Hoverman JT, Richgels KLD. Biodiversity decreases disease through predictable changes in host community competence. Nature. 2013b;494:230–233. doi: 10.1038/nature11883. [DOI] [PubMed] [Google Scholar]
- Joseph MB, Mihaljevic JR, Orlofske SA, Paull SH. Does life history mediate changing disease risk when communities disassemble? Ecol Lett. 2013;16:1405–1412. doi: 10.1111/ele.12180. [DOI] [PubMed] [Google Scholar]
- Junemann S, Prior K, Szczepanowski R, Harks I, Ehmke B, Goesmann A, et al. Bacterial community shift in treated periodontitis patients revealed by ion torrent 16S rRNA gene amplicon sequencing. PLoS ONE. 2012;7:e41606. doi: 10.1371/journal.pone.0041606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya T, O'Dwyer K, Nakagawa S, Poulin R. Host diversity drives parasite diversity: meta-analytical insights into patterns and causal mechanisms. Ecography. 2014;37:689–697. [Google Scholar]
- Keesing F, Young TP. Cascading consequences of the loss of large mammals in an African savanna. Bioscience. 2014;64:487–495. [Google Scholar]
- Keesing F, Holt RD, Ostfeld RS. Effects of species diversity on disease risk. Ecol Lett. 2006;9:485–498. doi: 10.1111/j.1461-0248.2006.00885.x. [DOI] [PubMed] [Google Scholar]
- Keesing F, Belden LK, Daszak P, Dobson A, Harvell CD, Holt RD, et al. Impacts of biodiversity on the emergence and transmission of infectious diseases. Nature. 2010;468:647–652. doi: 10.1038/nature09575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil H, Hornfeldt B, Evander M, Magnusson M, Olsson G, Ecke F. Dynamics and drivers of hantavirus prevalence in rodent populations. Vector-Borne Zoonotic Dis. 2014;14:537–551. doi: 10.1089/vbz.2013.1562. [DOI] [PubMed] [Google Scholar]
- Kimbro DL, Cheng BS, Grosholz ED. Biotic resistance in marine environments. Ecol Lett. 2013;16:821–833. doi: 10.1111/ele.12106. [DOI] [PubMed] [Google Scholar]
- Knops JMH, Tilman D, Haddad NM, Naeem S, Mitchell CE, Haarstad J, et al. Effects of plant species richness on invasion dynamics, disease outbreaks, insect abundances and diversity. Ecol Lett. 1999;2:286–293. doi: 10.1046/j.1461-0248.1999.00083.x. [DOI] [PubMed] [Google Scholar]
- Koenig WD, Hochachka WM, Zuckerberg B, Dickinson JL. Ecological determinants of American crow mortality due to West Nile virus during its North American sweep. Oecologia. 2010;163:903–909. doi: 10.1007/s00442-010-1627-z. [DOI] [PubMed] [Google Scholar]
- Lacroix C, Jolles A, Seabloom EW, Power AG, Mitchell CE, Borer ET. Non-random biodiversity loss underlies predictable increases in viral disease prevalence. J R Soc Interface. 2014;11:20130947. doi: 10.1098/rsif.2013.0947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafferty KD. Biodiversity loss decreases parasite diversity: theory and patterns. Philos T R Soc B. 2012;367:2814–2827. doi: 10.1098/rstb.2012.0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafferty KD, Wood CL. It's a myth that protection against disease is a strong and general service of biodiversity conservation: response to Ostfeld and Keesing. Trends Ecol Evol. 2013;28:503–504. doi: 10.1016/j.tree.2013.06.012. [DOI] [PubMed] [Google Scholar]
- Letourneau DK, Armbrecht I, Rivera BS, Lerma JM, Carmona EJ, Daza MC, et al. Does plant diversity benefit agroecosystems? A synthetic review. Ecol Appl. 2011;21:9–21. doi: 10.1890/09-2026.1. [DOI] [PubMed] [Google Scholar]
- Levine JM, Adler PB, Yelenik SG. A meta-analysis of biotic resistance to exotic plant invasions. Ecol Lett. 2004;7:975–989. [Google Scholar]
- Lively CM. The effect of host genetic diversity on disease spread. Am Nat. 2010;175:E149–E152. doi: 10.1086/652430. [DOI] [PubMed] [Google Scholar]
- LoGiudice K, Ostfeld RS, Schmidt KA, Keesing F. The ecology of infectious disease: effects of host diversity and community composition on Lyme disease risk. Proc Natl Acad Sci USA. 2003;100:567–571. doi: 10.1073/pnas.0233733100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loss SR, Hamer GL, Walker ED, Ruiz MO, Goldberg TL, Kitron UD, et al. Avian host community structure and prevalence of West Nile virus in Chicago, Illinois. Oecologia. 2009;159:415–424. doi: 10.1007/s00442-008-1224-6. [DOI] [PubMed] [Google Scholar]
- McCauley DJ, Keesing F, Young T, Dittmar K. Effects of the removal of large herbivores on fleas of small mammals. J Vector Ecol. 2008;33:263–268. doi: 10.3376/1081-1710-33.2.263. [DOI] [PubMed] [Google Scholar]
- Mihaljevic JR, Joseph MB, Orlofske SA, Paull SH. The scaling of host density with richness affects the direction, shape, and detectability of diversity-disease relationships. PLoS ONE. 2014;9:e97812. doi: 10.1371/journal.pone.0097812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell CE, Tilman D, Groth JV. Effects of grassland plant species diversity, abundance, and composition on foliar fungal disease. Ecology. 2002;83:1713–1726. [Google Scholar]
- Morand S, Jittapalapong S, Suputtamongkol Y, Abdullah MT, Huan TB. Infectious diseases and their outbreaks in Asia-Pacific: biodiversity and its regulation loss matter. PLoS ONE. 2014;9:e90032. doi: 10.1371/journal.pone.0090032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers SS, Gaffikin L, Golden CD, Ostfeld RS, Redford KH, Ricketts TH, et al. Human health impacts of ecosystem alteration. Proc Natl Acad Sci USA. 2013;110:18753–18760. doi: 10.1073/pnas.1218656110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeem S, Bunkder DE, Hector A, Loreau M, Perrings C. Biodiversity, Ecosystem Functioning, and Human Wellbeing: An Ecological and Economic Perspective. Oxford University Press; Oxford: 2009. [Google Scholar]
- Orlofske SA, Jadin RC, Preston DL, Johnson PTJ. Parasite transmission in complex communities: predators and alternative hosts alter pathogenic infections in amphibians. Ecology. 2012;93:1247–1253. doi: 10.1890/11-1901.1. [DOI] [PubMed] [Google Scholar]
- Orrock JL, Allan BF, Drost CA. Biogeographic and ecological regulation of disease: prevalence of Sin Nombre virus in island mice Is related to island area, precipitation, and predator richness. Am Nat. 2011;177:691–697. doi: 10.1086/659632. [DOI] [PubMed] [Google Scholar]
- Ostfeld RS. A Candide response to Panglossian accusations by Randolph and Dobson: biodiversity buffers disease. Parasitology. 2013;140:1196–1198. doi: 10.1017/S0031182013000541. [DOI] [PubMed] [Google Scholar]
- Ostfeld RS, Holt RD. Are predators good for your health? Evaluating evidence for top-down regulation of zoonotic disease reservoirs. Front Ecol Environ. 2004;2:13–20. [Google Scholar]
- Ostfeld RS, Keesing F. Biodiversity and disease risk: the case of Lyme disease. Conserv Biol. 2000a;14:722–728. [Google Scholar]
- Ostfeld RS, Keesing F. The function of biodiversity in the ecology of vector-borne zoonotic diseases. Canadian Journal of Zoology-Revue Canadienne De Zoologie. 2000b;78:2061–2078. [Google Scholar]
- Ostfeld RS, Keesing F. Effects of host diversity on infectious disease. Annu Rev Ecol Evol Syst. 2012;43(43):157–182. [Google Scholar]
- Ostfeld RS, Keesing F. Straw men don't get Lyme disease: response to Wood and Lafferty. Trends Ecol Evol. 2013;28:502–503. doi: 10.1016/j.tree.2013.05.009. [DOI] [PubMed] [Google Scholar]
- Ostfeld RS, LoGiudice K. Community disassembly, biodiversity loss, and the erosion of an ecosystem service. Ecology. 2003;84:1421–1427. [Google Scholar]
- Petermann JS, Fergus AJF, Roscher C, Turnbull LA, Weigelt A, Schmid B. Biology, chance, or history? The predictable reassembly of temperate grassland communities. Ecology. 2010;91:408–421. doi: 10.1890/08-2304.1. [DOI] [PubMed] [Google Scholar]
- Randolph SE, Dobson ADM. Pangloss revisited: a critique of the dilution effect and the biodiversity-buffers-disease paradigm. Parasitology. 2012;139:847–863. doi: 10.1017/S0031182012000200. [DOI] [PubMed] [Google Scholar]
- Raymundo LJ, Halford AR, Maypa AP, Kerr AM. Functionally diverse reef-fish communities ameliorate coral disease. Proc Natl Acad Sci USA. 2009;106:17067–17070. doi: 10.1073/pnas.0900365106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigaud T, Perrot-Minnot MJ, Brown MJF. Parasite and host assemblages: embracing the reality will improve our knowledge of parasite transmission and virulence. Proc R Soc Lond B. 2010;277:3693–3702. doi: 10.1098/rspb.2010.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohr JR, Civitello DJ, Crumrine PW, Halstead NT, Miller AD, Schotthoefer AM, et al. Predator diversity, intraguild predation, and indirect effects drive parasite transmission. Proc Natl Acad Sci USA. 2015;112:3008–3013. doi: 10.1073/pnas.1415971112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottstock T, Joshi J, Kummer V, Fischer M. Higher plant diversity promotes higher diversity of fungal pathogens, while it decreases pathogen infection per plant. Ecology. 2014;95:1907–1917. doi: 10.1890/13-2317.1. [DOI] [PubMed] [Google Scholar]
- Rudolf VHW, Antonovics J. Species coexistence and pathogens with frequency-dependent transmission. Am Nat. 2005;166:112–118. doi: 10.1086/430674. [DOI] [PubMed] [Google Scholar]
- Salkeld DJ, Padgett KA, Jones JH. A meta-analysis suggesting that the relationship between biodiversity and risk of zoonotic pathogen transmission is idiosyncratic. Ecol Lett. 2013;16:679–686. doi: 10.1111/ele.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmeller DS, Blooi M, Martel A, Garner TWJ, Fisher MC, Azemar F, et al. Microscopic aquatic predators strongly affect infection dynamics of a globally emerged pathogen. Curr Biol. 2014;24:176–180. doi: 10.1016/j.cub.2013.11.032. [DOI] [PubMed] [Google Scholar]
- Schmidt KA, Ostfeld RS. Biodiversity and the dilution effect in disease ecology. Ecology. 2001;82:609–619. [Google Scholar]
- Searle CL, Biga LM, Spatafora JW, Blaustein AR. A dilution effect in the emerging amphibian pathogen Batrachochytrium dendrobatidis. Proc Natl Acad Sci USA. 2011;108:16322–16326. doi: 10.1073/pnas.1108490108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea K, Chesson P. Community ecology theory as a framework for biological invasions. Trends Ecol Evol. 2002;17:170–176. [Google Scholar]
- Song Y, et al. Microbiota dynamics in patients treated with fecal microbiota transplantation for recurrent Clostridium difficile infection. PLoS ONE. 2013;8:e81330. doi: 10.1371/journal.pone.0081330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streicker DG, Fenton A, Pedersen AB. Differential sources of host species heterogeneity influence the transmission and control of multihost parasites. Ecol Lett. 2013;16:975–984. doi: 10.1111/ele.12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swei A, Ostfeld RS, Lane RS, Briggs CJ. Impact of the experimental removal of lizards on Lyme disease risk. Proc R Soc Lond B. 2011;278:2970–2978. doi: 10.1098/rspb.2010.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thieltges DW, Bordalo MD, Hernandez AC, Prinz K, Jensen KT. Ambient fauna impairs parasite transmission in a marine parasite-host system. Parasitology. 2008;135:1111–1116. doi: 10.1017/S0031182008004526. [DOI] [PubMed] [Google Scholar]
- Turney S, Gonzalez A, Millien V. The negative relationship between mammal host diversity and Lyme disease incidence strengthens through time. Ecology. 2014;95:3244–3250. [Google Scholar]
- Vandermeer J. The Ecology of Intercropping. Cambridge University Press; Cambridge: 1989. [Google Scholar]
- Venesky MD, Liu X, Sauer EL, Rohr JR. Linking manipulative experiments to field data to test the dilution effect. J Anim Ecol. 2014;83:557–565. doi: 10.1111/1365-2656.12159. [DOI] [PubMed] [Google Scholar]
- Vincent ER. Whirling disease and wild trout: the Montana experience. Fisheries. 1996;21:32–33. [Google Scholar]
- Webb CT, Hoeting JA, Ames GM, Pyne MI, Poff NL. A structured and dynamic framework to advance traits-based theory and prediction in ecology. Ecol Lett. 2010;13:267–283. doi: 10.1111/j.1461-0248.2010.01444.x. [DOI] [PubMed] [Google Scholar]
- Werden L, Barker IK, Bowman J, Gonzales EK, Leighton PA, Lindsay LR, et al. Geography, deer, and host biodiversity shape the pattern of Lyme disease emergence in the Thousand Islands Archipelago of Ontario Canada. PLoS ONE. 2014;9:e85640. doi: 10.1371/journal.pone.0085640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood CL, Lafferty KD. Biodiversity and disease: a synthesis of ecological perspectives on Lyme disease transmission. Trends Ecol Evol. 2013;28:239–247. doi: 10.1016/j.tree.2012.10.011. [DOI] [PubMed] [Google Scholar]
- Young H, Griffin RH, Wood CL, Nunn CL. Does habitat disturbance increase infectious disease risk for primates? Ecol Lett. 2013;16:656–663. doi: 10.1111/ele.12094. [DOI] [PubMed] [Google Scholar]
- Young HS, Dirzo R, Helgen KM, McCauley DJ, Billeter SA, Kosoy MY, et al. Declines in large wildlife increase landscape-level prevalence of rodent-borne disease in Africa. Proc Natl Acad Sci USA. 2014;111:7036–7041. doi: 10.1073/pnas.1404958111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.