Abstract
Objective
To evaluate the prognostic significance of right ventricular function assessed by echocardiography after start or escalation of targeted therapy in patients with pulmonary arterial hypertension.
Methods
Study design: longitudinal study. Setting: tertiary referral centre for pulmonary hypertension. Patients: 81 consecutive patients with pulmonary arterial hypertension (33 naive and 48 prevalent). Interventions: right heart catheterisation and echocardiography performed prior to starting or escalating targeted therapy and repeated in 55 patients after 4–12 months of therapy. Main outcome measure: survival after follow-up examinations.
Results
11 patients died and 7 were lost to follow-up during the first year; 8 patients underwent first follow-up evaluation beyond 1 year. 55 patients were re-evaluated after therapy; during the subsequent follow-up period of 25 months, 9 patients died, 7 worsened from WHO I/II to III/IV and 15 remained in WHO III/IV despite therapy. A baseline tricuspid annular plane systolic excursion (TAPSE) ≥15 mm was associated with a lower risk of death (HR=0.32; 95% CI 0.12 to 0.83, p=0.012). Attaining a TAPSE≥15 mm after therapy was associated with a significantly lower risk of death or clinical worsening (HR=0.2; 95% CI 0.1 to 0.6, p=0.002) and a lower risk of death which approached statistical significance (HR=0.3; 95% CI 0.2 to 1.1, p=0.075). Per cent changes in TAPSE were loosely related to changes in pulmonary vascular resistances after therapy (R=0.37).
Conclusions
In patients with pulmonary arterial hypertension, the evaluation of right ventricular function by TAPSE after targeted therapy is useful to predict subsequent prognosis, regardless of the haemodynamic effects of therapy.
Keywords: Cardiac imaging and diagnostics
Key questions.
What is already known about this subject?
Right ventricular function evaluated by cardiac magnetic resonance or by radionuclide angiography at baseline and after targeted therapy is a crucial determinant of mortality and morbidity in patients with pulmonary arterial hypertension.
What does this study add?
The study shows that a readily available measure of right ventricular function obtained at echocardiography has a prognostic role in the follow-up of patients with pulmonary arterial hypertension, similar to what has been demonstrated with more complex imaging techniques.
How might this impact on clinical practice?
These results may prove very useful to clinicians given that echocardiography is a less expensive and more readily available imaging technique than cardiac magnetic resonance and radionuclide angiography.
Introduction
Haemodynamic parameters assessing right ventricular (RV) function are important determinants of morbidity and mortality in patients with pulmonary arterial hypertension (PAH).1–3 In recent years, imaging of RV structure and function by cardiac magnetic resonance (CMR) proved to be helpful to stratify the prognosis of such patients.4–6 CMR is in fact considered accurate and reproducible in the assessment of size, morphology and function of this cardiac chamber.7 Importantly, it has been shown that changes in RV ejection fraction in incident patients receiving targeted therapy predict long-term outcome, whereas changes in pulmonary vascular resistance (PVR) do not, since RV dysfunction may progress despite treatment-induced decrease in PVR.8 Similar results were observed in a study which used radionuclide angiography to measure RV ejection fraction at baseline and 4–6 months after start of targeted therapy; the study also confirmed that changes in RV ejection fraction after therapy are not a function of PVR changes.9
On the basis of these premises, we aimed at evaluating the prognostic role of echocardiography in the follow-up of patients with PAH. Several echocardiographic parameters have been associated with poor prognosis in patients with PAH, in particular the readily available measure of longitudinal excursion of the tricuspid annular plane (tricuspid annular plane systolic excursion, TAPSE).10–18 However, no data have been published showing the usefulness of repeated echocardiographic evaluations during the follow-up of such patients. Therefore, we retrospectively evaluated a cohort of consecutive incident and prevalent patients with PAH who were hospitalised to perform right heart catheterisation and tailor medical therapy. The values of TAPSE and of the ratio of TAPSE to Doppler estimate of pulmonary artery systolic pressure (PASP) after start or escalation of PAH-specific therapy were correlated with the clinical events during follow-up.
Methods
Patients
The study population includes 81 patients affected by PAH, diagnosed according to guidelines recommendations,7 aged between 18 and 75 years, admitted to the Cardiology Division of Policlinico S. Matteo Hospital, Pavia, from 2000 to 2012. Incident patients were hospitalised to complete the diagnostic workup, to perform right heart catheterisation and to start targeted therapy; prevalent patients were hospitalised because their clinical conditions were unsatisfactory, in order to perform right heart catheterisation and thus escalate targeted therapy. WHO functional class assessment, 6 min walking test and echocardiography were performed within 24 h from the haemodynamic evaluation. The investigation conforms with the principles outlined in the Declaration of Helsinki. Patients signed an informed consent to participate in the study approved by the Institutional Review Board of Fondazione IRCCS Policlinico S. Matteo for longitudinal, non-pharmacological, non-sponsored studies, which complies with the Italian legislation (Codex on the Privacy, D. Lgs. 30 giugno 2003, n. 196). Despite the retrospective design of the study, all patient data, including the WHO functional class, were included in the database at the time of the visit, not ‘a posteriori’.
Echocardiographic examination
A standard M-mode, two-dimensional (2D) and Doppler study was performed using commercially available equipments (GE Healthcare). The examination included PASP, TAPSE (measured by M-mode tracing with 2D-echo guidance) and the ratio of TAPSE to PASP, an index of in vivo RV length–force relationship of prognostic usefulness in heart failure.19 PASP was estimated on the basis of the peak tricuspid regurgitation velocity, taking into account right atrial pressure evaluated on the basis of the inferior vena cava diameter and its respiratory variation. Data on the intraobserver and interobserver agreement of the most relevant echocardiographic indicators of RV function in our laboratory have been published previously.16
Six-minute walk test
A non-encouraged test was performed according to the American Thoracic Society guidelines.20
Right heart catheterisation
Right heart catheterisation was performed using a balloon-tipped catheter. The following haemodynamic parameters were measured or calculated: pulmonary capillary wedge pressure; systolic, diastolic and mean pulmonary artery pressure; right atrial pressure; cardiac output, calculated by thermodilution in most cases or by the Fick method in case of severe tricuspid regurgitation (whenever the Fick method was used at baseline, the same method was used at the follow-up examination); cardiac index, obtained by dividing the cardiac output by the body surface area; PVR, calculated as (mean pulmonary artery pressure−pulmonary capillary wedge pressure)/cardiac output.
Statistical analysis
Descriptive statistics were used to summarise data gathered in the study. For categorical variables, absolute and relative (%) frequencies were computed; for continuous variables, means and SDs or medians and IQR were reported. The inverse Kaplan-Meier method was used to compute the median follow-up time. Event rates were reported per 100 persons/year. Cumulative survival was calculated on the basis of Kaplan-Meier estimates; the relative risk of having an event (HR) and its 95% CI were computed using a Cox model. The primary end point of survival analysis was the combination of (1) death or (2) worsening of functional status (deterioration by at least one WHO class or (3) persistence of WHO class III or IV despite therapy start/escalation; the secondary end point was all-cause death. Survival analysis was performed in all patients after baseline evaluation and in the subgroup of patients having a follow-up evaluation after the control evaluation. No data have been inputed for censored patients. In order to simplify the clinical interpretation of the statistical results, for the purpose of this analysis all parameters were categorised. TAPSE was categorised as >15 or ≤15 mm and PVR as ≥8 or <8 wood units (WU) according to data published in the literature.8 In the absence of published data on TAPSE/PASP in patients with PAH, this parameter was categorised according to its median value. To assess whether the response to therapy at the follow-up visit was associated with better prognosis, patients were considered to have had a successful response in TAPSE if TAPSE increased above (or did not decrease below) 15 mm and in PVR if PVR decreased below (or did not increase above) 8 WU. Patients were considered to have had a successful response in TAPSE/PASP if TAPSE/PASP increased above (or did not decrease below) its median value.
The correlation between changes in TAPSE and changes in PVR was assessed with the Pearson R (and 95% CI) or the Fisher's exact test on the continuous and categorical scales, respectively. Bivariable models were also fitted to account for the combined effect of TAPSE and PVR favourable responses at 12 months on the combined end point. Stata V.13.1 (StataCorp, College Station, Texas, USA) was used for computation. A two-sided p<0.05 was retained for statistical significance.
Results
Patient characteristics and follow-up
The baseline clinical, haemodynamic and echocardiographic characteristics of the total study population are summarised in table 1. Aetiology was as follows: idiopathic in 60%, associated with connective tissue disease in 24%, associated with HIV disease in 10%, associated with portal hypertension in 4%, related to use of anorexigen drugs in 2% of patients. All patients were in sinus rhythm. In the entire cohort, the majority of patients were prevalent patients. Incident and prevalent patients had substantially similar values of TAPSE values at baseline, as 69% of patients with baseline TAPSE>15 mm was prevalent and 55% of patients with baseline TAPSE≤15 was also prevalent. The median follow-up after baseline evaluation was 36 months (25–75th 31–56 months). Eleven patients died, one underwent lung transplantation and was censored from analysis, six were lost to follow-up, eight were not rehospitalised during the first year. The clinical, haemodynamic and echocardiographic characteristics of the 55 patients who repeated right heart catheterisation and echocardiography within 1 year (median interval time 10 months, minimum 4, maximum 12 months) are shown in table 2. After treatment, mean pulmonary artery pressure, PVR and cardiac index improved, whereas right atrial pressure did not change significantly; TAPSE improved and, importantly, this improvement was associated with a significant reduction in the presence and degree of tricuspid regurgitation; the improvement in TAPSE/PASP was of borderline statistical significance.
Table 1.
Total study population (n=81)
| Baseline | |
|---|---|
| Age (years) | 51±16 |
| Males (n) | 30 |
| WHO II/III/IV (%) | 43/51/6 |
| BNP (pg/mL) median, 25–75th | 204, 67–361 |
| 6-MWD (m) | 354±127 |
| Naive patients (%) | 41 |
| HR (bpm) | 79±15 |
| Systolic BP (mm Hg) | 124±22 |
| mPAP (mm Hg) | 50.6±13.1 |
| CI (L/min/m2) | 2.4±0.6 |
| PVR (dyn/s cm5) | 836±412 |
| RAP (mm Hg) | 8.5±6.2 |
| TAPSE (mm) | 15.3±4.2 |
| RVEDD (mm) | 36.5±7.3 |
| RVEDA (cm2) | 29.5±9.0 |
| RVFAC (%) | 27±12 |
| RV wall thickness (mm) | 6.4±1.6 |
| Moderate/severe tricuspid regurgitation (%) | 59 |
| TAPSE/PASP | 0.21±0.10 |
6-MWD, 6 min walk distance; BNP, brain natriuretic peptide; BP, blood pressure; CI, cardiac index; HR, heart rate; mPAP, mean pulmonary artery pressure; PASP, Doppler estimate of systolic pulmonary artery pressure; PVR, pulmonary vascular resistance; RAP, right atrial pressure; RVEDA, right ventricular end diastolic area; RVEDD, right ventricular end diastolic diameter; RVFAC, right ventricular fractional area change; TAPSE, tricuspid annular plane systolic excursion.
Table 2.
Follow-up study population (n=55)
| Baseline | After therapy | p Value | |
|---|---|---|---|
| Age (years) | 49±15 | ||
| Incident/prevalent (n) | 25/30 | ||
| Males (n) | 19 | ||
| WHO II/III/IV (%) | 51/45/4 | 60/38/2 | 0.0001 |
| BNP (pg/mL) median, 25–75th | 308, 73–423 | 85, 25–186 | 0.0004 |
| 6-MWD (m) | 368±136 | 400±130 | 0.0644 |
| Naive patients (%) | 45 | 0 | |
| Monotherapy (%) | 40 | 40 | |
| Double combination therapy (%) | 15 | 20 | |
| Triple combination therapy (%) | 0 | 20 | |
| HR (bpm) | 77±15 | 74±13 | 0.1802 |
| Systolic BP (mm Hg) | 123±24 | 125±17 | 0.3782 |
| mPAP (mm Hg) | 50.1±11.9 | 46.7±13.5 | 0.0448 |
| CI (L/min/m2) | 2.4±0.5 | 2.7±0.8 | 0.0008 |
| PVR (dyn/s cm5) | 820±377 | 678±338 | 0.0079 |
| RAP (mm Hg) | 8.2±5.6 | 8.0±4.7 | 0.7192 |
| TAPSE (mm) | 15.5±3.9 | 16.8±4.1 | 0.0296 |
| RVEDD (mm) | 36.1±8.8 | 36.1±6.3 | 0.8346 |
| RVEDA (cm2) | 29.3±8.7 | 28.1±8.1 | 0.2700 |
| RVFAC (%) | 25±9 | 27±8 | 0.1539 |
| RV wall thickness (mm) | 6.1±1.4 | 5.9±1.5 | 0.4797 |
| Moderate/severe tricuspid regurgitation (%) | 57 | 32 | 0.0001 |
| TAPSE/PASP | 0.21±0.10 | 0.24±0.11 | 0.0794 |
6-MWD, 6 min walk distance; BNP, brain natriuretic peptide; BP, blood pressure; CI, cardiac index; HR, heart rate; mPAP, mean pulmonary artery pressure; PASP, Doppler estimate of systolic pulmonary artery pressure; PVR, pulmonary vascular resistance; RAP, right atrial pressure; RVEDA, right ventricular end diastolic area; RVEDD, right ventricular end diastolic diameter; RVFAC, right ventricular fractional area change; TAPSE, tricuspid annular plane systolic excursion.
Per cent changes in TAPSE between baseline and follow-up were significantly though loosely related to changes in PVR (R=0.37, 95% CI 0.11 to 0.58, p=0.007; figure 1). The median follow-up period after the control examinations was 25 months (25–75th 16–46 months). During subsequent follow-up, 9 patients died, 7 deteriorated by one WHO class and 15 remained in WHO class III/IV despite start or escalation of targeted therapy.
Figure 1.
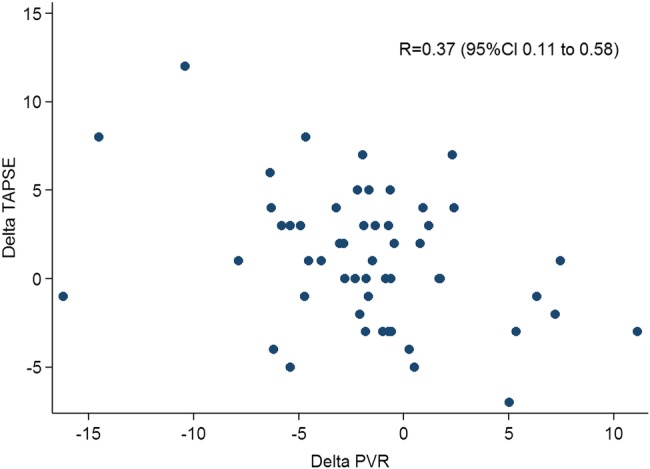
Changes in tricuspid annular plane systolic excursion (TAPSE) versus changes in pulmonary vascular resistance (PVR). The figure shows a statistically significant but loose association between changes in TAPSE and changes in PVR after therapy.
Prognostic importance of TAPSE and TAPSE/PASP at baseline
Low TAPSE at baseline (≤15 mm) was associated with a higher risk of death (HR=3.17; 95% CI 1.18 to 8.49, p=0.012; figure 2). High PVR at baseline (≥8 WU) was not associated with a higher risk of death (HR=0.80; 95% CI 0.35 to 1.82, p=0.594). A TAPSE/PASP value below the median value of 0.19 mm/mm Hg was not significantly associated with a higher risk of death (HR=1.92; 95% CI 0.83 to 4.55, p=0.118).
Figure 2.
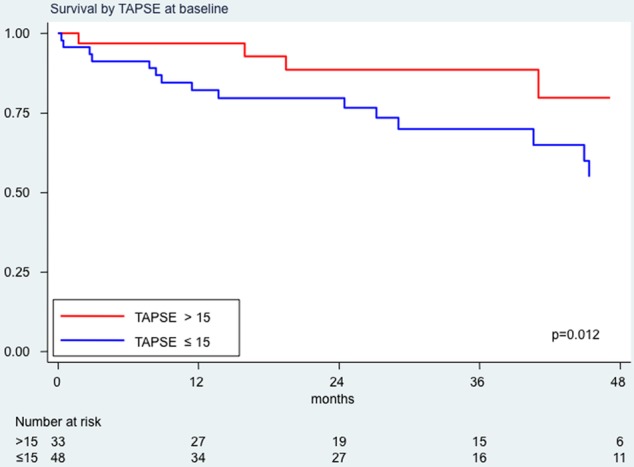
Survival analysis by tricuspid annular plane systolic excursion (TAPSE) values at baseline. In red, survival curve for patients with TAPSE values at baseline >15 mm; in blue, survival curve for patients with baseline TAPSE values ≤15 mm.
Prognostic importance of TAPSE and TAPSE/PASP at follow-up evaluation
A TAPSE value >15 mm at the follow-up echocardiographic evaluation was associated with a lower risk of death which approached statistical significance (HR=0.3; 95% CI 0.2 to 1.1, p=0.075; figure 3) and a significantly lower risk of death or clinical worsening (HR=0.2; 95% CI 0.1 to 0.6, p=0.002; figure 4). Of interest, the group of patients with TAPSE>15 mm (n=30) and the group of patients with TAPSE≤15 mm (n=25) had a substantially similar proportion of patients with monotherapy or double combination or triple combination therapy (respectively 43%, 40% and 17% in the former group and 28%, 47% and 25% in the latter); similar age (respectively 50 and 47 years) and similar aetiology (respectively 50% and 48% of idiopathic patients with PAH, and 17% and 28% of patients with connective tissue disease).
Figure 3.
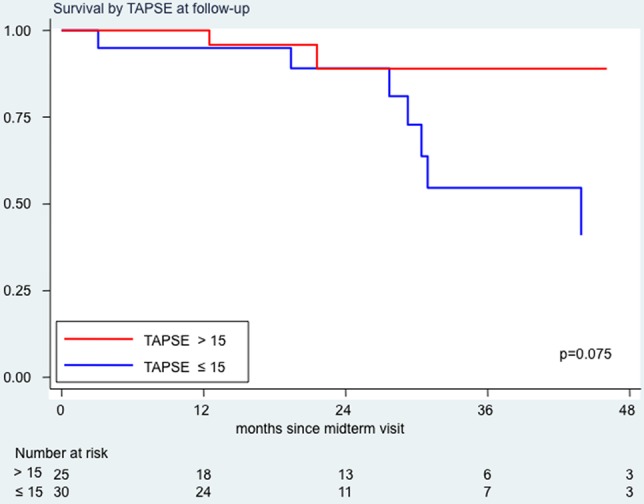
Survival analysis by tricuspid annular plane systolic excursion (TAPSE) values at follow-up examination. In red, survival curve for patients with TAPSE values at follow-up >15 mm; in blue, survival curve for patients with follow-up TAPSE values ≤15 mm.
Figure 4.
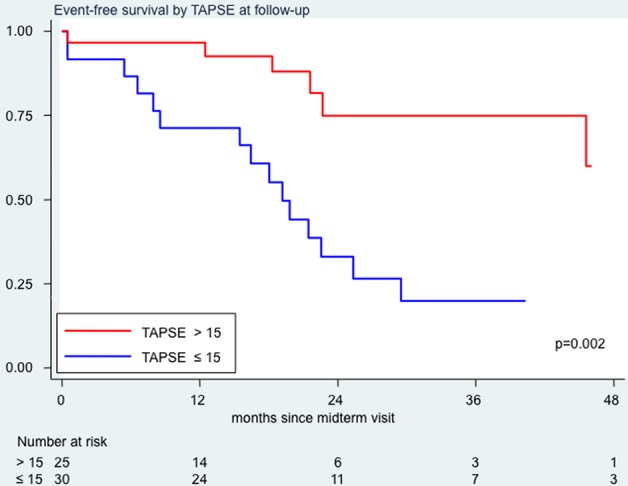
Event-free survival analysis by tricuspid annular plane systolic excursion (TAPSE) values at follow-up examination. In red, survival curve for patients with TAPSE values at follow-up >15 mm; in blue, survival curve for patients with TAPSE values at follow-up ≤15 mm.
A PVR value <8 WU at follow-up right heart catheterisation was neither associated with a lower risk of death (HR=0.68; 95% CI 0.20 to 2.30, p=0.524) nor with a lower risk of death or clinical worsening (HR=0.62; 95% CI 0.26 to 1.53, p=0.289).
Succeeding in obtaining TAPSE/PASP values >0.19 mm/mm Hg at the follow-up echocardiographic evaluation was associated with a lower risk of death (HR=0.3; 95% CI 0.7 to 1.0, p=0.038) and a lower risk of death or clinical worsening (HR=0.3; 95% CI 0.1 to 0.7, p=0.009).
Relative changes in TAPSE and in PVR were not associated with outcome.
In a post hoc bivariable analysis considering both the changes in TAPSE and the changes in PVR after therapy, having TAPSE values ≥15 mm and PVR values <8 WU at the follow-up evaluation was not associated with a better outcome than having only TAPSE values ≥15 mm (p=0.511). Furthermore, the association of reduced PVR values without improvement in TAPSE at follow-up was associated with the numerically highest risk of events, although this did not reach statistical significance (HR 2.32; 95% CI 0.72 to 7.44, p=0.159 vs no change in PVR and no change in TAPSE).
Discussion
The main finding of the present study is that in a population of incident and prevalent patients with PAH starting or escalating targeted therapy, attaining a TAPSE value above 15 mm after therapy predicts a better long-term outcome. A deteriorating or persistently low TAPSE predicts poorer prognosis even if it is associated with a reduction in PVR. Changes in TAPSE under targeted therapy are loosely related to changes in PVR.
Prognostic role of RV function in patients with PAH
RV function is known to be a crucial determinant of mortality and morbidity in patients with PAH. However, few studies have so far evaluated the effects of targeted treatment on changes in RV function and its relationship with prognosis. Initial studies using CMR to assess the structure and function of the right ventricle included small populations of patients and failed to demonstrate a clear relationship between changes in RV function and prognosis.21–23 Recently, in 76 patients with incident PAH who survived at least 1 year to undergo a repeated right heart catheterisation and CMR after beginning of targeted therapy, it was found that changes in RV ejection fraction predicted long-term outcome whereas changes in PVR did not; PVR decreased in most patients after therapy but 25% of those patients with decreased PVR showed a deterioration of RV ejection fraction and a poor prognosis.8 Similarly, a study using radionuclide angiography of the right ventricle showed that patients with a stable or increased RV ejection fraction at 3–6 months had a significantly lower cardiovascular mortality.9 The take-home message of these two studies is that RV dysfunction may progress in patients with incident PAH despite treatment-induced decrease in PVR and is associated with high risk despite the haemodynamic changes which would indicate therapy efficacy.
Echocardiography is not as accurate as CMR in the evaluation of RV structure and function; however, its clinical usefulness resides in being a more widespread and simpler technique than CMR and radionuclide ventriculography. This study evaluates for the first time the prognostic relevance of two readily available variables such as TAPSE and TAPSE/PASP in the follow-up of patients with PAH undergoing targeted therapy. TAPSE is known to be as a robust index of RV function and the TAPSE/PASP ratio is a recently suggested index of in vivo RV length–force relationship, of demonstrated prognostic usefulness in heart failure.19 The study shows that TAPSE is associated with survival and can track changes in patients' clinical conditions. TAPSE/PASP seems a less powerful prognostic indicator; in fact, it was not significantly associated with survival at baseline, although it was associated with survival after therapy. However, further exploration of this measurement as a prognostic marker is justified, since the optimal dichotomous threshold of TAPSE/PASP in pulmonary hypertension is still not known; the median value of 0.19 mm/mm Hg observed in the present series is much lower than the optimal value reported for prognostic stratification in patients with heart failure (0.36 mm/mm Hg), in contrast with the observation that the optimal prognostic cut-offs for TAPSE in heart failure and in pulmonary hypertension are quite similar (respectively at 14 and at 15 mm).16 24 Interestingly, the absolute values of TAPSE at follow-up were found to be prognostically useful, not the relative changes after therapy; this is similar to what has already been reported for the 6 min walk test in patients with PAH.25 The reason is that despite changes induced by therapies, absolute values may remain below the high-risk threshold. Furthermore, the demonstration of the usefulness of follow-up reassessment of TAPSE was obtained in a mixed population of incident and prevalent patients, starting or escalating targeted therapy; therefore, the results of this study might apply to the majority of patients with PAH followed up in pulmonary hypertension centres. In agreement with previous data, no strong correlation was observed between changes in PVR and changes in TAPSE after therapy. The right ventricle is known to adapt its structure and function to the pulmonary circulation overload and, in fact, impressive reverse RV remodelling changes have been observed in patients undergoing either lung transplantation or pulmonary endoarterectomy.26–28 The mild-to-moderate reverse remodelling effects exerted by targeted therapies could, as a first hypothesis, be related to the relatively modest effect of pharmacological therapy on RV afterload.29 In addition, we still do not know how much haemodynamic improvement can be considered relevant to induce functional or structural changes in the right ventricle. This is partly the consequence of the fact that the assessment of RV afterload is often imprecise in routine examinations; in fact, we commonly use PVR changes to monitor drug effects, although a precise pathophysiological description of RV overload should include peripheral resistance, arterial compliance and the proximal pulmonary artery impedance. Finally, the absence of reverse remodelling might be the consequence of an insufficient haemodynamic response to medical treatments, as well as of genetic differences in the adaptation of the right ventricle to pressure overload.30 The poor relationship observed between changes in TAPSE and changes in PVR reinforces the suggestion that repeated evaluations of RV function are necessary in the follow-up of such patients to monitor the response to therapy.
Limitations
First of all, this is a single-centre retrospective analysis of response to treatment of naive patients or of patients with unsatisfactory conditions in whom a haemodynamic and echocardiographic follow-up could be performed within 12 months after the start or escalation of targeted therapy. The study therefore included a relatively low number of patients. However, the sample size was sufficient to answer the predefined objective of the study, that is, to investigate the prognostic significance of PVR and TAPSE values during the follow-up. The number of events also allowed us to fit a bivariable Cox model on the significance of changes in TAPSE and in PVR. The combined end point included death and WHO functional class evaluation; persistence of WHO functional class III or IV after 3 months on therapy is in fact known to be strongly associated with poor survival in patients with PAH.3
We acknowledge that larger studies are necessary to confirm the prognostic significance of changes in echocardiographic markers of RV function, including both TAPSE and the TAPSE/PASP ratio, the degree of tricuspid regurgitation and the diastolic compression of the left ventricle, the strain indices of RV function and to test the hypothesis that deterioration or lack of improvement in RV function despite PVR reduction identifies patients at the highest risk of hard events. Furthermore, it has to be clarified whether the right ventricle of incident patients responds better to PAH-specific therapy than the right ventricle of prevalent patients. Finally, multicentre studies are definitely needed to define which technique is most useful to assess changes in RV function and whether changes in RV function are more informative than changes in clinical or functional parameters or changes in biomarkers.
Conclusions
After start or escalation of targeted therapy, a simple reassessment of RV function using TAPSE provides prognostic information substantially unrelated to the haemodynamic effects of therapy on PVR. Imaging of RV function with echocardiography can be a clinically useful tool in the follow-up of patients affected by PAH.
Footnotes
Contributors: SG was involved in conception and design of the study, data collection, interpretation of the data, drafting the manuscript, final approval of the manuscript submitted. SP was involved in data collection, statistical analysis, interpretation of the data, drafting the manuscript, critical revision of the manuscript, final approval of the manuscript submitted. CK was involved in database management, statistical analysis, interpretation of the data, critical revision of the manuscript, final approval of the manuscript submitted. EG, LS, CR, AT and SS were involved in data collection, interpretation of the data, critical revision of the manuscript, final approval of the manuscript submitted.
LOV was involved in interpretation of the data, critical revision of the manuscript, final approval of the manuscript submitted.
Competing interests: None declared.
Ethics approval: Fondazione IRCCS Policlinico S. Matteo, Pavia, Italy.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.D'Alonzo GE, Barst RJ, Ayres SM et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med 1991;115:343–9. [DOI] [PubMed] [Google Scholar]
- 2.McLaughlin VV, Shillington A, Rich S. Survival in primary pulmonary hypertension: the impact of epoprostenol therapy. Circulation 2002;106:1477–82. 10.1161/01.CIR.0000029100.82385.58 [DOI] [PubMed] [Google Scholar]
- 3.Sitbon O, Humbert M, Nunes H et al. Long-term intravenous epoprostenol infusion in primary pulmonary hypertension: prognostic factors and survival. J Am Coll Cardiol 2002;40:780–8. 10.1016/S0735-1097(02)02012-0 [DOI] [PubMed] [Google Scholar]
- 4.van Wolferen SA, Marcus JT, Boonstra A et al. Prognostic value of right ventricular mass, volume, and function in idiopathic pulmonary arterial hypertension. Eur Heart J 2007;28:1250–7. 10.1093/eurheartj/ehl477 [DOI] [PubMed] [Google Scholar]
- 5.McLure LE, Peacock AJ. Cardiac magnetic resonance imaging for the assessment of the heart and pulmonary circulation in pulmonary hypertension. Eur Respir J 2009;33:1454–66. 10.1183/09031936.00139907 [DOI] [PubMed] [Google Scholar]
- 6.Trip P, Kind T, van de Veerdonk MC et al. Accurate assessment of load-independent right ventricular systolic function in patients with pulmonary hypertension. J Heart Lung Transplant 2013;32:50–5. 10.1016/j.healun.2012.09.022 [DOI] [PubMed] [Google Scholar]
- 7.Galiè N, Humbert M, Vachiery JL et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS) Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016;37:67–119. 10.1093/eurheartj/ehv317 [DOI] [PubMed] [Google Scholar]
- 8.van de Veerdonk MC, Kind T, Marcus JT et al. Progressive right ventricular dysfunction in patients with pulmonary arterial hypertension responding to therapy. J Am Coll Cardiol 2011;58:2511–19. 10.1016/j.jacc.2011.06.068 [DOI] [PubMed] [Google Scholar]
- 9.Courand PY, Pina-Jomir G, Khouatra C et al. Prognostic value of right ventricular ejection fraction in pulmonary arterial hypertension. Eur Respir J 2015;45:139–49. 10.1183/09031936.00158014 [DOI] [PubMed] [Google Scholar]
- 10.Yeo TC, Dujardin KS, Tei C et al. Value of a Doppler-derived index combining systolic and diastolic time intervals in predicting outcome in primary pulmonary hypertension. Am J Cardiol 1998;81:157–61. [DOI] [PubMed] [Google Scholar]
- 11.Hinderliter Al, Willis PW IV, Long W et al. , for the PPH Study Group. Frequency and prognostic significance of pericardial effusion in primary pulmonary hypertension. Am J Cardiol 1999;84:481–4. 10.1016/S0002-9149(99)00342-2 [DOI] [PubMed] [Google Scholar]
- 12.Sebbag I, Rudski LG, Therrien J et al. Effect of chronic infusion of epoprostenol on echocardiographic right ventricular myocardial performance index and its relation to clinical outcome in patients with primary pulmonary hypertension. Am J Cardiol 2001;88:1060–3. 10.1016/S0002-9149(01)01995-6 [DOI] [PubMed] [Google Scholar]
- 13.Bustamante-Labarta M, Perrone S, De La Fuente RL et al. Right atrial size and tricuspid regurgitation severity predict mortality or transplantation in primary pulmonary hypertension. J Am Soc Echocardiogr 2002;15:1160–4. 10.1067/mje.2002.123962 [DOI] [PubMed] [Google Scholar]
- 14.Raymond RJ, Hinderliter AL, Willis PW et al. Echocardiographic predictors of adverse outcome in primary pulmonary hypertension. J Am Coll Cardiol 2002;39:1214–19. 10.1016/S0735-1097(02)01744-8 [DOI] [PubMed] [Google Scholar]
- 15.Forfia PR, Fisher MR, Mathai SC et al. Tricuspid annular displacement predicts survival in pulmonary hypertension. Am J Respir Crit Care Med 2006;174:1034–41. 10.1164/rccm.200604-547OC [DOI] [PubMed] [Google Scholar]
- 16.Ghio S, Klersy C, Magrini G et al. Prognostic relevance of the echocardiographic assessment of right ventricular function in patients with idiopathic pulmonary arterial hypertension. Int J Cardiol 2010;140:272–8. 10.1016/j.ijcard.2008.11.051 [DOI] [PubMed] [Google Scholar]
- 17.Ghio S, Pazzano AS, Klersy C et al. Clinical and prognostic relevance of echocardiographic evaluation of right ventricular geometry in patients with idiopathic pulmonary arterial hypertension. Am J Cardiol 2011;107:628–32. 10.1016/j.amjcard.2010.10.027 [DOI] [PubMed] [Google Scholar]
- 18.Mathai SC, Sibley CT, Forfia PR et al. Tricuspid annular plane systolic excursion is a robust outcome measure in systemic sclerosis-associated pulmonary arterial hypertension. J Rheumatol 2011;38:2410–18. 10.3899/jrheum.110512 [DOI] [PubMed] [Google Scholar]
- 19.Guazzi M, Bandera F, Pelissero G et al. Tricuspid annular plane systolic excursion and pulmonary arterial systolic pressure relationship in heart failure: an index of right ventricular contractile function and prognosis. Am J Physiol Heart Circ Physiol 2013;305:H1373–81. 10.1152/ajpheart.00157.2013 [DOI] [PubMed] [Google Scholar]
- 20.ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002;166:111–17. [DOI] [PubMed] [Google Scholar]
- 21.Roeleveld RJ, Vonk-Noordegraaf A, Marcus JT et al. Effects of epoprostenol on right ventricular hypertrophy and dilatation in pulmonary hypertension. Chest 2004;125:572–9. 10.1378/chest.125.2.572 [DOI] [PubMed] [Google Scholar]
- 22.Chin KM, Kingman M, de Lemos JA et al. Changes in right ventricular structure and function assessed using cardiac magnetic resonance imaging in bosentan-treated patients with pulmonary arterial hypertension. Am J Cardiol 2008;101:1669–72. 10.1016/j.amjcard.2008.01.055 [DOI] [PubMed] [Google Scholar]
- 23.Wilkins MR, Paul GA, Strange JW et al. Sildenafil versus Endothelin Receptor Antagonist for Pulmonary Hypertension (SERAPH) study. Am J Respir Crit Care Med 2005;171:1292–7. 10.1164/rccm.200410-1411OC [DOI] [PubMed] [Google Scholar]
- 24.Ghio S, Recusani F, Klersy C et al. Prognostic usefulness of the tricuspid annular plane systolic excursion in patients with congestive heart failure secondary to idiopathic or ischemic dilated cardiomyopathy. Am J Cardiol 2000;85:837–42. 10.1016/S0002-9149(99)00877-2 [DOI] [PubMed] [Google Scholar]
- 25.Savarese G, Paolillo S, Costanzo P et al. Do changes of 6-minute walk distance predict clinical events in patients with pulmonary arterial hypertension? A meta-analysis of 22 randomized trials. J Am Coll Cardiol 2012;60:1192–201. 10.1016/j.jacc.2012.01.083 [DOI] [PubMed] [Google Scholar]
- 26.Bristow MR, Zisman LS, Lowes BD et al. The pressure-overloaded right ventricle in pulmonary hypertension. Chest 1998;114(1 Suppl):101S–6S. 10.1378/chest.114.1_Supplement.101S [DOI] [PubMed] [Google Scholar]
- 27.Kasimir MT, Seebacher G, Jaksch P et al. Reverse cardiac remodelling in patients with primary pulmonary hypertension after isolated lung transplantation. Eur J Cardiothorac Surg 2004;26:776–81. 10.1016/j.ejcts.2004.05.057 [DOI] [PubMed] [Google Scholar]
- 28.D'Armini AM, Zanotti G, Ghio S et al. Reverse right ventricular remodeling after pulmonary endarterectomy. J Thorac Cardiovasc Surg 2007;133:162–8. 10.1016/j.jtcvs.2006.08.059. [DOI] [PubMed] [Google Scholar]
- 29.Galiè N, Manes A, Negro L et al. A meta-analysis of randomized controlled trials in pulmonary arterial hypertension. Eur Heart J 2009;30:394–403. 10.1093/eurheartj/ehp022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Voelkel NF, Quaife RA, Leinwand LA et al. National Heart, Lung, and Blood Institute Working Group on Cellular and Molecular Mechanisms of Right Heart Failure. Right ventricular function and failure: report of a National Heart, Lung, and Blood Institute working group on cellular and molecular mechanisms of right heart failure. Circulation 2006;114:1883–91. 10.1161/CIRCULATIONAHA.106.632208 [DOI] [PubMed] [Google Scholar]


