Abstract
Objective
To evaluate the impact of methotrexate (MTX) dosage on clinical, functional and quality of life outcomes in patients with rheumatoid arthritis (RA) from two previous etanercept (ETN) trials after 24 months of treatment.
Methods
Patients with active RA in the ETN+MTX combination treatment arms of the Trial of Etanercept and Methotrexate with Radiographic Patient Outcomes (TEMPO) and COmbination of Methotrexate and ETanercept in Active Early Rheumatoid Arthritis (COMET) studies were pooled in this post hoc analysis and stratified by MTX dosage at 24 months, having MTX monotherapy groups as control: low dose, <10.0 mg/week; medium dose, 10.0–17.5 mg/week; and high dose, >17.5 mg/week. Data from these patient subgroups were included in descriptive summaries of demographic and disease characteristics at baseline. The following outcomes at 24 months were also evaluated for each subgroup: Disease Activity Score in 28 joints (DAS28) low disease activity (LDA) and remission; American College of Rheumatology 20%, 50% and 70% improvement criteria (ACR20, 50 and 70) responses; and changes from baseline in DAS28, Health Assessment Questionnaire Disease Index (HAQ-DI) and EuroQol 5-dimensions visual analogue scale (EQ-5D VAS).
Results
Baseline demographics were similar between the low, medium and high MTX dose groups in the ETN+MTX combination and MTX monotherapy arms, with the exception of disease duration (ETN+MTX low 5.5; medium 5.1; high 0.8 years vs MTX low 8.3; medium 4.7; high 0.8 years). Responses to ETN+MTX combination therapy at 24 months were consistently high across MTX dosage groups, with very similar rates of DAS28 LDA/remission and ACR20/50/70. Improvements in DAS28, HAQ-DI and EQ-5D VAS were also not dependent on MTX dosage in the combination treatment arm.
Conclusions
Patients with RA in the TEMPO and COMET trials who received ETN+MTX showed similar efficacy outcomes at 24 months, regardless of MTX dosage.
Trial registration numbers
NCT00195494 (COMET) and NCT00393471 (TEMPO).
Keywords: Rheumatoid Arthritis, DMARDs (biologic), DMARDs (synthetic), Anti-TNF
Key messages.
What is already known about this subject?
The anti-TNF agent etanercept (ETN) with methotrexate (MTX) is superior to ETN and MTX as monotherapy in rheumatoid arthritis (RA).
What does this study add?
The impact of MTX dose on clinical, functional and quality of life (QoL) outcomes in patients with RA when used in combination with ETN has not been investigated previously.
Responses to ETN+MTX combination therapy at 24 months were consistently high across MTX dosage groups, with very similar rates of DAS28 low disease activity/remission and ACR improvement criteria responses.
Improvements in DAS28, HAQ-DI and EQ-5D VAS were also not dependent on MTX dosage in the combination treatment arm.
How might this impact on clinical practice?
Lower doses of MTX in combination with ETN can be considered in patients with RA, while maintaining clinical efficacy and QoL.
Introduction
The development of biological therapies that target specific components of the immune system has revolutionised the treatment of rheumatoid arthritis (RA). In clinical trials, combination therapy with the anti-tumour necrosis factor (TNF) agent etanercept (ETN) with the synthetic disease-modifying antirheumatic drug (DMARD) methotrexate (MTX) has been shown to be superior to ETN and MTX as monotherapy.1–4 However, in the open-label ADd Enbrel Or REplace methotrexate (ADORE) study of patients with an inadequate response to MTX monotherapy, similar clinical responses were observed following ETN monotherapy or treatment with ETN+MTX.5
Combination therapy with ETN and MTX is recommended in patients with RA who fail to reach the treatment target, remission or low disease activity (LDA) with synthetic DMARDs, particularly when poor prognostic markers are present.6 7 For patients with previous intolerance to MTX, ETN monotherapy may be considered.8 When combined with ETN, MTX is usually administered in doses ranging from 7.5 to 25.0 mg/week.9 In patients who develop intolerable side effects to MTX during combination therapy, MTX may be discontinued or its dosage reduced. Moreover, patients who are in remission are sometimes not willing to take MTX due to the side-effect profile.10 11
Some European guidelines suggest reducing the dose of synthetic DMARDs when patients have achieved satisfactory disease control or sustained long-term remission.7 12 To date, comparable efficacy of ETN across different dosages of concomitant MTX has not been established in patients with early to established active RA. The purpose of the current post hoc analysis of patients’ data from two previous ETN trials was therefore to evaluate the impact of MTX dosage on clinical, functional and quality of life (QoL) outcomes in patients with RA after 24 months of treatment with ETN and different doses of MTX.
Methods
Data were pooled from two ETN clinical trials—COmbination of Methotrexate and ETanercept in Active Early Rheumatoid Arthritis (COMET) and Trial of Etanercept and Methotrexate with Radiographic Patient Outcomes (TEMPO).2 3 In both trials, a group of patients were randomised to receive double-blinded MTX monotherapy or a combination of ETN+MTX for 24 months consecutively. Patients who switched treatments after 12 months in the COMET trial or who received ETN monotherapy in the TEMPO trial were excluded from this analysis.
At baseline in both trials, patients in the MTX arm received 7.5 mg/week (3 capsules taken in 2 divided doses approximately 12 h apart).2 3 At the week 4 visit, if the patients had an inadequate response (defined as any painful or swollen joints), the patients’ oral dose was increased to 15 mg/week, taken in two divided doses approximately 12 h apart. At the week 8 visit, if the patients still had an inadequate response, the oral dose was increased to 20 mg/week. This titration schedule was true both for the MTX monotherapy arm and the combination therapy arm. Following dose titration, MTX treatment remained stable unless adverse events indicated dose reduction.
The study designs and eligibility and exclusion criteria were similar across the two trials, facilitating data pooling (table 1).
Table 1.
Eligibility and exclusion criteria
| COMET | TEMPO | |
|---|---|---|
| Eligibility Criteria | ||
| Age | ≥18 years | ≥18 years |
| Diagnosis | Active, adult-onset RA | Active, adult-onset RA |
| Disease duration | ≥3 months to <2 years | 6 months to 20 years |
| Disease activity | DAS28≥3.2, Westergren ESR≥28 mm/h, or CRP≥20 mg/L | ≥10 swollen and ≥12 painful joints and ≥1 of the following: Westergren ESR≥28 mm/h, CRP≥20 mg/L or morning stiffness for ≥45 min |
| Previous treatments | N/A | Less than satisfactory response to previous treatment with ≥1 DMARD other than MTX* |
| Exclusion Criteria | ||
| Previous treatments | MTX, ETN or other TNF antagonist | ETN or other TNF antagonist |
| Other DMARDs or corticosteroid injections in the 4 weeks before baseline† | Other DMARDs or corticosteroid injections in the 4 weeks before baseline | |
| Immunosuppressive drugs within 6 months of screening | ||
| Use of any investigational drug or biological within 3 months of screening | ||
*Patients previously treated with MTX could be enrolled, providing they had no investigator-defined clinically important toxic effects or lack of response and had not received MTX within 6 months of enrolment.
†Stable doses of oral corticosteroids (≤10 mg/day of prednisone or an equivalent agent) or a single non-steroidal anti-inflammatory drug were permitted if started ≥4 weeks before baseline and kept constant throughout the first 24 weeks of the study.
COMET, COmbination of Methotrexate and ETanercept in Active Early Rheumatoid Arthritis; CRP, C reactive protein; DAS28, Disease Activity Score in 28 joints; DMARD, disease-modifying antirheumatic drug; ESR, erythrocyte sedimentation rate; ETN, etanercept; MTX, methotrexate; N/A, not applicable; RA, rheumatoid arthritis; TEMPO, Trial of Etanercept and Methotrexate with Radiographic Patient Outcomes; TNF, tumour necrosis factor.
The main differences between the trials were the inclusion criteria for disease duration (up to 20 years in the TEMPO trial and <2 years in the COMET trial) and the assessment of baseline disease activity (table 1).
Post hoc analyses
Patients within the MTX arm and within the ETN+MTX arm in the pooled TEMPO (MTX, n=124; ETN+MTX, n=168) and COMET (MTX, n=94; ETN+MTX, n=108) studies were categorised into three groups according to their MTX dosage at 24 months: low dose, <10.0 mg MTX/week; medium dose, 10.0–17.5 mg MTX/week; or high dose, >17.5 mg MTX/week. Data from these subgroups of patients with known MTX doses at 24 months were included in descriptive summaries of baseline demographic and disease characteristics (table 2). Patients with an unknown MTX dose at 24 months were excluded from this post hoc analysis.
Table 2.
Patient demographics and characteristics
| Characteristic | ETN+MTX (n=276) |
MTX (n=218) |
||||
|---|---|---|---|---|---|---|
| Low MTX dose | Medium MTX dose | High MTX dose | Low MTX dose | Medium MTX dose | High MTX dose | |
| n=73 | n=155 | n=48 | n=39 | n=117 | n=62 | |
| Age, years | 51.1 (12.65) | 51.7 (12.74) | 51.7 (14.32) | 56.1 (12.72) | 50.7 (12.11) | 51.8 (13.18) |
| Female, n (%) | 55 (75) | 117 (75) | 33 (69) | 31 (79) | 94 (80) | 50 (81) |
| Weight, kg | 69.6 (15.53) | 70.2 (13.37) | 75.0 (17.70) | 67.6 (11.95) | 71.1 (16.26) | 70.4 (15.52) |
| Disease duration, years | 5.5 (5.55) | 5.1 (5.13) | 0.8 (1.48) | 8.3 (6.05) | 4.7 (4.91) | 0.8 (1.10) |
| Patient Global Assessment | 6.8 (2.15) | 7.1 (1.73) | 7.2 (1.82) | 6.8 (1.60) | 6.7 (1.83) | 6.4 (1.89) |
| DAS28 | 6.4 (1.05) | 6.8 (1.04) | 6.8 (0.99) | 6.5 (0.88) | 6.7 (0.99) | 6.3 (0.96) |
| HAQ-DI | 1.6 (0.67) | 1.8 (0.60) | 2.0 (0.57) | 1.6 (0.60) | 1.7 (0.68) | 1.7 (0.63) |
| EQ-5D VAS | 45.8 (24.85) | 40.9 (21.10) | 40.5 (22.77) | 41.8 (20.09) | 38.6 (21.32) | 48.8 (21.94) |
Data represent mean values (SD), unless otherwise specified.
DAS28, Disease Activity Score in 28 joints; EQ-5D VAS, EuroQol 5-dimensions visual analogue scale; ETN, etanercept; HAQ-DI, Health Assessment Questionnaire-Disability Index; MTX, methotrexate.
The primary end point was the proportion of patients achieving LDA or remission on the Disease Activity Score in 28 joints (DAS28) measure; >2.6 DAS28≤3.2 or DAS28≤2.6, respectively. DAS28 end points and the proportion of patients achieving American College of Rheumatology 20%, 50% and 70% improvement criteria (ACR 20/50/70) responses were assessed at 6, 12 and 24 months in each subgroup. Changes from baseline in DAS28, Health Assessment Questionnaire Disease Index (HAQ-DI) and EuroQol 5-Dimensions (EQ-5D) visual analogue scale (VAS) were also evaluated.
Statistical analyses
Continuous data were summarised using descriptive statistics at each time point. Categorical data were summarised in terms of frequency counts and associated percentages, calculated using non-missing data as the denominator. No data were imputed. Analyses for this post hoc study were purely descriptive.
Results
Patient demographics and characteristics
Patients in the ETN+MTX arm (n=276) had a mean age of 51.5 years, mean weight of 70.9 kg and mean disease duration of 4.5 years, and 74% were female (table 2).
Patients in the MTX arm (n=218) had a mean age of 52.0 years, mean weight of 70.3 kg and mean disease duration of 4.2 years, and 80% were female. Baseline demographics were similar between the low (<10.0 mg/week), medium (10.0–17.5 mg/week) and high (>17.5 mg/week) oral MTX dose groups in both treatment arms, with the exception of disease duration (table 2). Disease duration was numerically shorter in the high-dose MTX group of the ETN+MTX arm compared with the lower MTX dose groups, and was different in each MTX dose group of the MTX monotherapy arm. It is notable that patients enrolled in the COMET trial had a shorter mean disease duration (<1 year)2 than those enrolled in the TEMPO trial (>6 years)3 owing to different inclusion criteria (table 1). Clinical and patient-reported measures of disease activity at baseline were similar across low, medium and high MTX doses in the MTX monotherapy and ETN+MTX treatment arms (table 2).
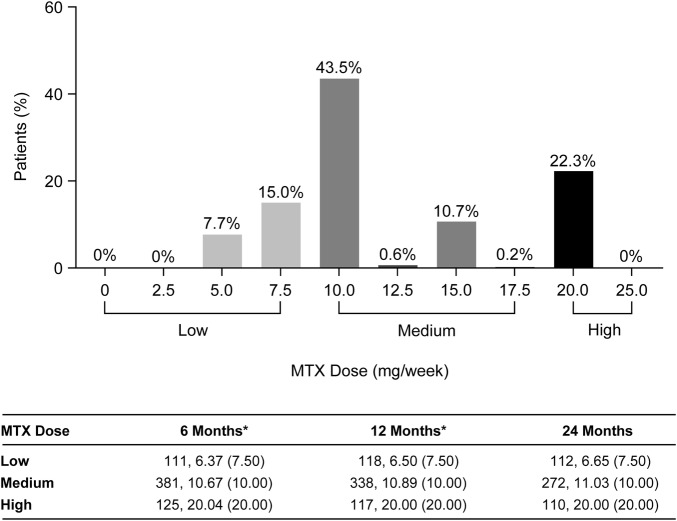
At 24 months, the majority of patients (55%) were receiving doses of 10.0–17.5 mg MTX/week (medium dose category) with a mean dose of 11.0 mg and a median dose of 10.0 mg (figure 1). Within each of the MTX dosing categories, the mean doses were similar and median doses were the same regardless of the time that the dose groups were defined. This indicates that following dose titration, the frequency of patients within each of the three MTX dosing categories was relatively stable throughout the study (figure 1). The mean cumulative MTX doses were similar and the median cumulative MTX doses were the same between the MTX monotherapy arm (MTX dose: mean=333 mg, median=260 mg) and the ETN+MTX combination therapy arm (MTX dose: mean=297 mg, median=260 mg).
Figure 1.
Frequency of methotrexate (MTX) doses at 24 months (plot) and summary of MTX doses across the MTX dosing categories (low, medium, high) based on data at 6, 12 and 24 months (table). Data represent n values, mean (median). *Patients with no MTX dose data at 24 months were excluded from the remainder of the post hoc summaries in this paper.
Efficacy outcomes
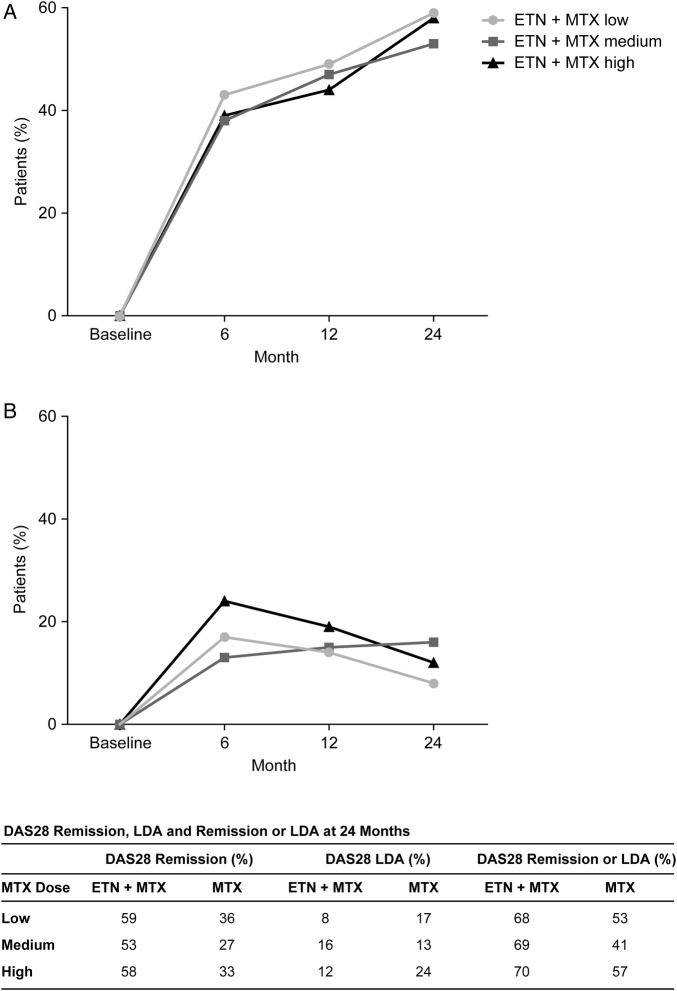
DAS28 responses to ETN+MTX combination therapy at 6, 12 and 24 months were consistently high across MTX dosage groups, with numerically similar rates of remission (figure 2). At 24 months, >60% of ETN+MTX patients achieved DAS28 remission or LDA; >50% had DAS28 remission and <20% had LDA (figure 2). The proportion of patients achieving DAS28 remission or LDA, or DAS28 remission only was numerically higher in patients receiving ETN+MTX combination therapy compared with those receiving MTX monotherapy at all time points (figure 2). Rates of DAS28 LDA did not appear to be related either to the MTX dose in the ETN+MTX arm or the MTX monotherapy arm of the study.
Figure 2.
Percentage of patients achieving (A) DAS28 remission and (B) DAS28 LDA at baseline and at 6, 12 and 24 months. DAS28 remission was defined as DAS28≤2.6 and LDA as >2.6 DAS28<3.2. DAS28, Disease Activity Score in 28 joints; ETN, etanercept; LDA, low disease activity; MTX, methotrexate.
In the ETN+MTX combination arm, improvements of 20%, 50% and 70% on the ACR scale were largely unaffected by the MTX dose at 6, 12 and 24 months (figure 3). Rates of ACR 20, 50 or 70 were paradoxically inversely related to the dose in patients receiving MTX monotherapy (figure 3). Patients receiving any dose of MTX monotherapy were also numerically less likely to achieve ACR 20, 50 or 70 compared with those receiving ETN+MTX combination therapy at 24 months.
Figure 3.
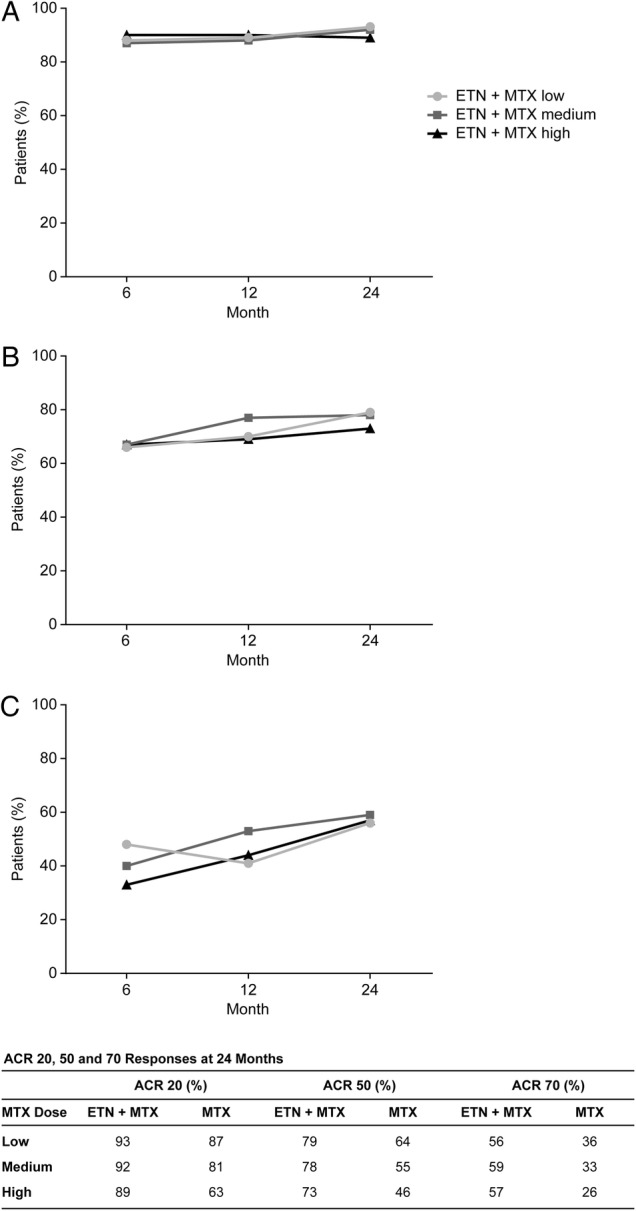
Percentage of patients achieving (A) ACR 20, (B) ACR 50 and (C) ACR 70 responses at 6, 12 and 24 months. ACR 20/50/70, American College of Rheumatology 20%, 50% and 70% improvement criteria; ETN, etanercept; MTX, methotrexate.
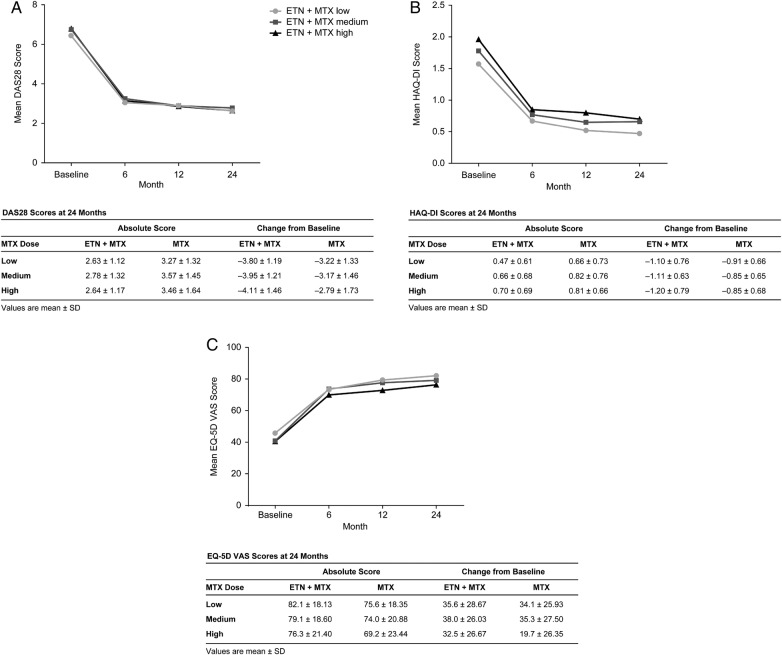
Improvements from baseline in DAS28, HAQ-DI and EQ-5D VAS were not dependent on MTX dosage in the ETN+MTX combination treatment arm (figure 4). Improvements from baseline to month 24 were of higher magnitude in those receiving ETN+MTX than in those receiving MTX monotherapy in all patient-reported outcome measures (figure 4).
Figure 4.
(A) DAS28, (B) HAQ-DI and (C) EQ-5D VAS scores at baseline and at 6, 12 and 24 months. Values are mean±SD. DAS28, Disease Activity Score in 28 joints; EQ-5D VAS, EuroQol 5-dimensions visual analogue scale; ETN, etanercept; HAQ-DI, Health Assessment Questionnaire Disability Index; MTX, methotrexate.
Discussion
In this subanalysis of two large randomised ETN studies, achievement of core clinical outcomes was independent of the dose of MTX used. This suggests that low doses of MTX can be considered in cases of MTX intolerance or for patients in remission who no longer wish to take high-dose MTX due to its adverse event profile.
While intensive MTX is recommended as the first-line therapy in the treatment of RA, MTX monotherapy achieves ACR/European League Against Rheumatism (EULAR)-defined satisfactory disease control in approximately 30% of patients only.7 13 14 The remaining ∼70% of patients will require more dynamic therapy, which often includes a biological DMARD in combination with MTX.7 13 Results from this study indicated that treatment with ETN+MTX resulted in greater improvement than treatment with MTX alone, further supporting the addition of ETN for improved outcomes in the treatment of RA.1–4 Clinical responses as measured by DAS28 LDA and remission rates, ACR 20/50/70 rates and change from baseline in DAS28 were similar for patients treated with ETN+MTX combination therapy, regardless of the MTX dose used. Similarly, HAQ-DI and EQ-5D VAS measures of QoL were maintained even at low MTX doses, when co-administered with ETN.
The historical literature suggests that MTX toxicity is dose-dependent and that low doses are the mainstay of RA therapy.15–18 However, optimal doses of MTX when used in combination therapy are not readily available. The recent blinded, controlled CONCERTO trial explored the risk–benefit profile of ascending doses of MTX in combination with adalimumab in early RA.14 The study reported a significant trend towards increased efficacy at MTX doses 2.5–20 mg/week when in combination with adalimumab, but similar clinical, radiographic and functional outcomes at MTX doses of 10 and 20 mg/week in combination with 40 mg/week adalimumab. Increased adalimumab efficacy could be attributed to reduced clearance and higher serum concentrations when administered with MTX >2.5 mg/week, with a plateau effect at MTX doses between 10 and 20 mg/week.14 19
Whereas MTX co-administration might alter the pharmacokinetic profile of adalimumab, no pharmacokinetic alternations to ETN were observed when administering doses of MTX 20 mg/week with ETN versus ETN alone.20 This could help to explain why increased efficacy of ETN+MTX over MTX monotherapy was not MTX dose-dependent in this study.
It has been demonstrated previously that the addition of ETN in patients with an inadequate response to MTX results in improvements that are a function of ETN alone and not of combination with MTX, whereas the effect in patients who were relatively MTX-naive may be additive.5 The patients included in this post hoc study were not known partial or inadequate MTX-responders and those from the COMET study received MTX only 4 weeks prior to baseline.2 3 It can therefore be postulated from this study that following induction with MTX, a maintenance dose of MTX<10 mg/week is sufficient to retain efficacy when used in combination with ETN.
A systematic literature review of MTX monotherapy recommended initial treatment with oral MTX up to 10–15 mg with escalation by 5 mg increments up to 20–30 mg/week if tolerated and response is inadequate.21 The findings presented in this post hoc analysis suggest that lower doses of MTX could be considered by physicians when using MTX in conjunction with ETN therapy. These findings may also provide important treatment options for patients with toxicity or tolerability issues with MTX, allowing low doses of MTX to be used without significant reductions in clinical outcomes or QoL. The Canadian Methotrexate and Etanercept Outcome (CAMEO) study concluded that withdrawing MTX after 6 months of ETN+MTX treatment in MTX inadequate responders was inferior to continuation with combination therapy.22 However, in a recent subanalysis of the COMET study, withdrawal of MTX from patients who had achieved disease remission with ETN+MTX at week 52 yielded similar rates of Boolean remission at week 104 compared with patients who remained on ETN+MTX.23 These data, together with ours, suggest that MTX dose-reduction or even withdrawal may be viable options for patients in remission, who would prefer to discontinue MTX treatment.
The retrospective nature of this study limited its power to determine the impact of lower MTX doses on clinical efficacy and required the retroactive allocation of patients into MTX dosing categories based on the dose at 24 months. Owing to the study designs, the MTX dose was reasonably stable over the duration of these studies, limiting the effect of this stratification; however, population selection at this time point may have biased the population towards treatment responders. The cumulative MTX dose was also similar in the monotherapy and combination therapy arms of the pooled analysis, which may have contributed to the observation of similar efficacy outcomes. Another limitation of this study is that MTX dosing in both the COMET and TEMPO studies was restricted to the oral route, even at high doses of MTX. The findings might therefore be different had subcutaneous dosing been permitted. It should also be acknowledged that pooling patient populations from COMET and TEMPO studies resulted in a diverse RA population—patients with early and late disease, with a higher proportion of patients previously exposed to MTX in the TEMPO cohort. It is possible that different conclusions would be reached if these analyses were conducted in the patient populations from both trials separately.24 However, the merging of two populations allowed us to describe the effect of the MTX concomitant dose under different conditions, thereby mimicking a real-life scenario.
Conclusion
In this post hoc analysis of the COMET and TEMPO trials, ETN+MTX showed similar efficacy outcomes regardless of the MTX dosage in patients with RA. Low-dose MTX (<10 mg/week) is sufficient to achieve efficacy outcomes similar to higher doses of MTX (10.0–17.5 mg/week, or >17.5 mg) when used in combination with ETN. In agreement with previous analyses,2 3 ETN+MTX combination therapy was more effective at improving clinical, functional and QoL outcomes in patients with RA than MTX monotherapy. These findings suggest that lower doses of MTX can be considered in patients who do not tolerate MTX, while maintaining clinical efficacy and QoL.
Acknowledgments
The authors wish to thank all patients who participated in the following trials and all investigators and medical staff of the participating centres: COMET (ClinicalTrials.gov Identifier: NCT00195494) and TEMPO (ClinicalTrials.gov Identifier: NCT00393471). Medical writing support was provided by Samantha Forster of Engage Scientific Solutions and was funded by Pfizer.
Footnotes
Funding: The COMET (NCT00195494) and TEMPO (NCT00393471) studies were sponsored by Wyeth Research, which was acquired by Pfizer in October 2009. This post-hoc analysis was sponsored by Pfizer.
Competing interests: GG and BK are full-time employees of Pfizer. FB is a full-time employee of Quanticate International, which was contracted by Pfizer to provide statistical input to the study and manuscript. UK was a former employee of Pfizer. TWJH has received lecture fees/consultancy fees from Merck, UCB, Bristol-Myers Squibb, Biotest AG, Pfizer, GSK, Novartis, Roche, Sanofi-Aventis, Abbott, Crescendo Bioscience, Nycomed, Boehringer Ingelheim, Takeda, Zydus and Eli Lilly.
Ethics approval: This is a post hoc analysis. Approval was obtained for the two parent studies. The COMET study was done in accordance with the ethical principles of the Declaration of Helsinki. The protocol and its amendments received independent ethics committee or institutional review board approval and regulatory review and approval before site initiation and recruitment of patients. The protocol for the TEMPO study was approved by appropriate local regulatory agencies and ethics committees for every participating centre. The trial was undertaken in accordance with the Declaration of Helsinki and was consistent with the principles of the International Conference on Harmonisation guidelines for Good Clinical Practice (1996 revision) in the European Community.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Emery P, Breedveld F, van der Heijde D et al. Two-year clinical and radiographic results with combination etanercept-methotrexate therapy versus monotherapy in early rheumatoid arthritis: a two-year, double-blind, randomized study. Arthritis Rheum 2010;62:674–82. 10.1002/art.27268 [DOI] [PubMed] [Google Scholar]
- 2.Emery P, Breedveld FC, Hall S et al. Comparison of methotrexate monotherapy with a Combination Of Methotrexate and Etanercept in Active, Early, Moderate to Severe Rheumatoid Arthritis (COMET): a randomised, double-blind, parallel treatment trial. Lancet 2008;372:375–82. 10.1016/S0140-6736(08)61000-4 [DOI] [PubMed] [Google Scholar]
- 3.Klareskog L, van der Heijde D, de Jager JP et al. Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomised controlled trial. Lancet 2004;363:675–81. 10.1016/S0140-6736(04)15640-7 [DOI] [PubMed] [Google Scholar]
- 4.van der Heijde D, Klareskog L, Rodriguez-Valverde V et al. Comparison of etanercept and methotrexate, alone and combined, in the treatment of rheumatoid arthritis: two-year clinical and radiographic results from the TEMPO study, a double-blind, randomized trial. Arthritis Rheum 2006;54:1063–74. 10.1002/art.21655 [DOI] [PubMed] [Google Scholar]
- 5.van Riel PL, Taggart AJ, Sany J et al. Efficacy and safety of combination etanercept and methotrexate versus etanercept alone in patients with rheumatoid arthritis with an inadequate response to methotrexate: the ADORE study. Ann Rheum Dis 2006;65:1478–83. 10.1136/ard.2005.043299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saag KG, Teng GG, Patkar NM et al. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum 2008;59:762–84. 10.1002/art.23721 [DOI] [PubMed] [Google Scholar]
- 7.Smolen JS, Landewé R, Breedveld FC et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs. Ann Rheum Dis 2010;69:964–75. 10.1136/ard.2009.126532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.European Medicines Agency. Enbrel: summary of product characteristics. Secondary Enbrel: summary of product characteristics. 2015. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000262/WC500027361.pdf (accessed 2 Mar 2015).
- 9.Cannon M. Methotrexate (Rheumatrex, Trexall). Secondary Methotrexate (Rheumatrex, Trexall) 2012. http://www.rheumatology.org/I-Am-A/Patient-Caregiver/Treatments/Methotrexate-Rheumatrex-Trexall (accessed 12 Apr 2016).
- 10.Salliot C, van der Heijde D. Long-term safety of methotrexate monotherapy in patients with rheumatoid arthritis: a systematic literature research. Ann Rheum Dis 2009;68:1100–4. 10.1136/ard.2008.093690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scott IC, Kingsley GH, Scott DL. Can we discontinue synthetic disease-modifying anti-rheumatic drugs in rheumatoid arthritis? Clin Exp Rheumatol 2013;31(4 Suppl 78):S4–8. 10.1136/annrheumdis-2013-203684 [DOI] [PubMed] [Google Scholar]
- 12.Deighton C, O'Mahony R, Tosh J et al. Management of rheumatoid arthritis: summary of NICE guidance. BMJ 2009;338:b702 10.1136/bmj.b702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh JA, Furst DE, Bharat A et al. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biological agents in the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken) 2012;64:625–39. 10.1002/acr.21641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burmester GR, Kivitz AJ, Kupper H et al. Efficacy and safety of ascending methotrexate dose in combination with adalimumab: the randomised CONCERTO trial. Ann Rheum Dis 2015;74:1037–44. 10.1136/annrheumdis-2013-204769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cronstein BN. Low-dose methotrexate: a mainstay in the treatment of rheumatoid arthritis. Pharmacol Rev 2005;57:163–72. 10.1124/pr.57.2.3 [DOI] [PubMed] [Google Scholar]
- 16.Furst DE, Koehnke R, Burmeister LF et al. Increasing methotrexate effect with increasing dose in the treatment of resistant rheumatoid arthritis. J Rheumatol 1989;16:313–20. [PubMed] [Google Scholar]
- 17.Mielants H, Veys EM, Van der Straeten C et al. The efficacy and toxicity of a constant low dose of methotrexate as a treatment for intractable rheumatoid arthritis: an open prospective study. J Rheumatol 1991;18:978–83. [PubMed] [Google Scholar]
- 18.Yamanaka H, Inoue E, Tanaka E et al. Influence of methotrexate dose on its efficacy and safety in rheumatoid arthritis patients: evidence based on the variety of prescribing approaches among practicing Japanese rheumatologists in a single institute-based large observational cohort (IORRA). Mod Rheumatol 2007;17: 98–105. 10.1007/s10165-006-0546-7 [DOI] [PubMed] [Google Scholar]
- 19.Abbott Laboratories. Package Insert HUMIRA (adalimumab). Secondary Package Insert HUMIRA (adalimumab) 2003. http://www.accessdata.fda.gov/drugsatfda_docs/label/2002/adalabb123102LB.htm (accessed 1 Jul 2014).
- 20.Zhou H, Mayer PR, Wajdula J et al. Unaltered etanercept pharmacokinetics with concurrent methotrexate in patients with rheumatoid arthritis. J Clin Pharmacol 2004;44:1235–43. 10.1177/0091270004268049 [DOI] [PubMed] [Google Scholar]
- 21.Visser K, Katchamart W, Loza E et al. Multinational evidence-based recommendations for the use of methotrexate in rheumatic disorders with a focus on rheumatoid arthritis: integrating systematic literature research and expert opinion of a broad international panel of rheumatologists in the 3E Initiative. Ann Rheum Dis 2009;68:1086–93. 10.1136/ard.2008.094474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pope JE, Haraoui B, Thorne JC et al. The Canadian Methotrexate and Etanercept Outcome study: a randomised trial of discontinuing versus continuing methotrexate after 6 months of etanercept and methotrexate therapy in rheumatoid arthritis. Ann Rheum Dis 2014;73:2144–51. 10.1136/annrheumdis-2013-203684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Emery P, Jones H, Marshall L, et al. Ann Rheum Dis. Continuation of etanercept monotherapy after achievement of remission with etanercept and methotrexate combination therapy: subanalysis from the COMET study. 2015;74(Supp 2):468. [DOI] [Google Scholar]
- 24.Fleischmann R, Koenig AS, Pedersen R et al. Treatment outcomes based on methotrexate dose range in patients with rheumatoid arthritis receiving etanercept+methotrexate versus methotrexate alone [Abstract]. Arthitis Rheum 2011;63:S178 10.1002/acr.20322 [DOI] [Google Scholar]