Abstract
Background
Loss of muscle mass, particularly in old age, can restrict mobility and physical function. Sleep is thought to play a key role in the maintenance of muscle mass; sleep disturbances have a prevalence of 6–30% in Germany. In this study, based on data from the Berlin Aging Study II (BASE-II), we analyze the relationship between sleep efficiency and quality on the one hand, and muscle mass and muscle function on the other.
Methods
We analyzed cross-sectional data from 1196 subjects (52.5% women; 68 ± 4 years). Sleep behavior was assessed with questions from the Pittsburgh Sleep Quality Index; appendicular lean mass (ALM) with dual x-ray absorptiometry; and muscle function with a measure of grip strength and with questionnaires about physical activity and impairment of physical activities. Low muscle mass was determined from the ALM corrected by the body-mass index (BMI), i.e., from the ratio ALM/BMI.
Results
19.1% of the women and 13.4% of the men reported poor sleep quality. Men whose ALM/BMI ratio was below the cutoff value for low muscle mass more frequently reported very poor sleep efficiency (9.1%, versus 4.8% in women; p<0.002). The adjusted odds ratio for low muscle mass was 2.8 for men with poor sleep quality (95% confidence interval: [1.1; 6.7]) and 4.3 for men with poor sleep efficiency [1.2; 15.1]. In women, there was no statistically significant association between sleep quality and efficiency on the one hand and ALM/BMI values below cutoff on the other, but poor sleep quality was found to be associated with reduced grip strength (16.25 kg ± 2.33 kg versus 15.67 kg ± 2.38 kg; p = 0.009) and low appendicular lean mass (ALM: 16.25 kg ± 2.33 kg versus 15.67 kg ± 2.38 kg; p = 0.016).
Conclusion
These findings support the hypothesis of a link between sleep and muscle mass. The dependence of muscle mass on sleep behavior needs to be investigated in longitudinal studies.
The prevalence of sleep-related complaints among both hospital patients and those who visit their primary care physician is reported as 6 to 30%. Problems in falling asleep and sleeping through the night are particularly frequent in patients over 65 years old (1, 2, e1). Sleep quality, sleep quantity, and sleep architecture change with increasing age:
Decreased efficiency, quality, and total duration of sleep
Decreased duration of rapid eye movement (REM) sleep and deep sleep
Increased sleep latency and time spent awake during the night
Increased light sleep and phases of wakefulness
Increased sleep and drowsiness during the day.
These largely physiological changes in sleep patterns do not necessarily constitute illness and are subject to individual variation. Insufficient exercise, absence of cognitive stimulation, external factors (e.g., noise, unfamiliar surroundings, wrong room temperature), mental stress (such as depression or anxiety disorder), and somatic factors (e.g., nycturia, dementia, sleep apnea, cardiovascular disease, and pain) all have a negative impact on the sleep of older people in particular (e2, e3, 6).
Sleep is associated with biological and mental regeneration processes. Insufficient or non-restorative sleep may lead to inability to concentrate, cognitive deterioration, reduced physical performance capacity, restricted social interactions, decreased quality of life, or a higher risk of falling (7– 10, e3, e4). Daytime tiredness and concentration difficulties can contribute to a decrease in physical activity leading to a reduction in muscle mass (11).
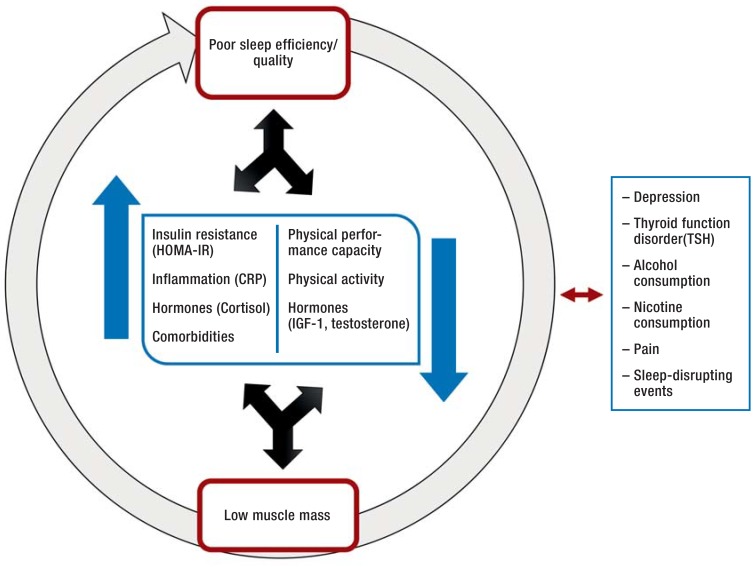
Furthermore, metabolic, hormonal, and immunological changes are observed in persons with decreased quality and quantity of sleep (12). The anabolic hormones IGF-1 and testosterone, which play important roles in protein synthesis and thus in maintenance of muscle mass, are down-regulated by sleep deficit (12). On the one hand, the insulin resistance associated with lack of sleep is thought to be a major risk factor for the loss of muscle mass and muscle function in old age (13). On the other, reduced muscle mass itself favors insulin resistance, because the muscles are the principal site for both the action and the storage of glucose. Sleep and muscle mass thus interact (Figure 1). Particularly at advanced age, loss of muscle function can lead to premature restriction of daily activities and ultimately to the early need for care. On the assumption that sleep and muscle mass are interconnected, we analyzed data from the Berlin Aging Study II (BASE-II) on sleep quality and sleep efficiency in community-dwelling elderly and investigated possible associations with muscle mass, grip strength, and physical activity.
Figure 1.
Possible associations between sleep quality/efficiency and muscle mass (modified from [16]) HOMA-IR, homeostasis model for insulin resistance; CRP, C-reactive protein; IGF-1, insulin-like growth factor-1; TSH, thyroid-stimulating hormone
Methods
Cross-sectional data on sleep behavior (Pittsburgh Sleep Quality Index), appendicular lean mass (ALM), and muscle function (measurement of hand grip strength and questionnaire on physical activity) from 1196 BASE-II participants (52.5% of them women; mean age 68 ± 4 years) were available for analysis. BASE-II is an epidemiological cohort study with the primary aim of investigating mechanisms of pathogenesis in the context of the aging process (14). Low muscle mass was determined on the basis of the cut-off values for body mass index (BMI)–corrected muscle mass (ALM/BMI), as defined in 2014 by the US Foundation for the National Institutes of Health (FNIH) Sarcopenia Project (15– 17). The methods are described in detail in the eBox.
eBox. Methods.
-
Study population
We analyzed cross-sectional data from the Berlin Aging Study II (BASE-II), an epidemiological study that set out to identify factors associated with pathogenesis. In a multidisciplinary approach, the BASE-II survey obtained (bio)medical, genetic, socioeconomic, and psychological data in a group of 60- to 84-year-old people living independently in their own homes and in a control group of young adults (20 to 36 years) (14). The participants were a convenience sample recruited via advertisements in local newspapers and on public transport. For our purposes, data on sleep behavior, muscle mass, and body mass index (BMI) were available from 1196 older people (>60 years). For a subgroup of 269 probands (62.2% men; 68 ± 4 years) we also had access to information on sleep-disrupting events, including pain, excessive warmth, cold, and noise, and on consumption of sleep-inducing medications. The study was approved by the ethics committee of Charité – University Medicine Berlin (project number EA2/029/09).
-
Sleep behavior
Sleep behavior was characterized on the basis of responses to questions from the Pittsburgh Sleep Quality Index (PSQI) (18, 19). The PSQI records the participants’ subjective assessment of their sleep quality (very good, fairly good, fairly bad, very bad), habitual sleeping times, sleep duration, time taken to fall asleep, and getting-up time. Sleep efficiency was defined as the proportion of the time spent in bed at night (from going to bed to getting up) during which the proband actually slept. Over 85% of participants assessed their sleep efficiency as poor (e5, e6, 20). The PSQI questions about sleep quality, sleep efficiency, sleep latency, and sleep duration were answered by all the probands we investigated. A subgroup of 269 probands received the complete PSQI survey including questions on sleep-disturbing factors (pain, noise, cold, excessive warmth, nocturnal coughing, frequent waking, breathing difficulties), consumption of sleep-inducing drugs, and drowsiness during the daytime.
-
Physical activity
We used the Rapid Assessment of Physical Activity (RAPA) questionnaire to determine physical activity (21). This instrument records how many times per week and for how long (more/less than 20/30 min per day) the responder carries out light, moderate, or heavy physical activity. If two or more questions were answered with “yes,” the highest possible group was selected. To establish any limitations of physical activity, the probands were also questioned about difficulties experienced on moderate physical exertion, climbing several steps, crossing more than one street, lifting and carrying, or bending and kneeling. The possible answers were no difficulty, slight difficulty, and serious difficulty. Muscle strength was assessed by measuring maximal isometric hand grip strength with a Smedley Dynamometer (Scandidact, Dänemark).
-
Body composition
Body composition was determined by means of dual X-ray absorptiometry (DXA), using the Hologic QDR DiscoveryTM device for measurement and the software APEX, version 3.0.1, for analysis. This technique separates fat mass from lean mass. Muscle mass in kilograms was calculated as appendicular lean mass (ALM), i.e., the sum of the lean mass of the arms and legs (without bone mass). To establish the BMI-corrected lean mass, the ratio of ALM and BMI (ALM/BMI) was determined. ALM/BMI <0.512 in women and ALM/BMI < 0.789 in men was defined as low muscle mass, which according to the US Foundation for the National Institutes of Health (FNIH) (15, 22) may be associated with an elevated risk of functional restrictions (reduced grip strength or walking speed). Because these cut-off values are not validated for younger persons, the DXA data were used only for the older group of BASE-II probands.
-
Clinical chemistry
Blood samples obtained after the participant had fasted for at least 8 h were subjected to comprehensive laboratory examination. Glucose concentration was determined by photometry, HbA1c by ion-exchange high-performance liquid chromatography (HPLC). Thyroid-stimulating hormone (TSH) and testosterone were measured by electrochemiluminescence immunoassay (ECLIA). Insulin resistance was calculated using the fasting glucose and insulin levels in the homeostasis model of insulin resistance (HOMA-IR) as fasting glucose (mg/dL) × fasting insulin (mU/mL)/405. The concentration of C-reactive protein (CRP) was determined in serum samples by means of an immunoassay.
Alcohol consumption, smoking behavior, and comorbidities
-
Alcohol consumption (amount and frequency) and smoking habits (current/ever/never) were recorded as part of the medical history. The presence of type-2 diabetes was ascertained by means of laboratory tests and, in probands without a known diagnosis of type-2 diabetes, by administering an oral glucose tolerance test (oGTT) according to the specifications of the German Diabetes Society (Deutsche Diabetesgesellschaft, DDG) (fasting blood sugar >126 mg/dL; 2-h levels >200 mg/dL or HbA1c level >6.5%) (23). The prevalence of other illnesses, such as depression, coronary heart disease, chronic obstructive pulmonary disease (COPD), or malignant diseases, was assessed using a medical history checklist. In the analyses, these and other diseases were accounted for by using a morbidity index that was based on categories of the Charlson Comorbidity Index and has been described in earlier publications by our group (24, 25). Furthermore, a history of depression, which may have a negative impact on both sleep and muscle mass, was included in the calculations.
Statistical analysis
For statistical evaluation we used the software IBM SPSS Version 21 (IBM Corp., Armonk, NY). First, a descriptive data analysis was carried out (Table 1). The Kolmogorov–Smirnov test was used to establish whether the involved variables were distributed normally. For further exploratory analysis we used parametric tests (t-test) for normally distributed variables and non-parametric tests (Mann–Whithney U-test) for non-normally distributed variables. P values <0.05 were rated as showing a significant difference. In the case of multiple comparisons of the same group of probands (Table 2), the primary endpoints sleep quality and sleep efficiency were subjected to Bonferroni correction, whereupon p values <0.025% were rated as significant. The results of the Mann–Whithney U-test are depicted in the boxplot (Fgure 2) with regard to the mean comparison of BMI-corrected muscle mass for the outcome criteria sleep quality and sleep efficiency. The chi-square test was used to determine group differences in frequency distributions.
Binary logistic regression models were then calculated to establish the odds ratio for probands with poor sleep efficiency (sleep efficiency <85%) or bad sleep quality (fairly bad or very bad) to display low muscle mass (below the sex-specific ALM/BMI of the FNIH). The models were adjusted for age, height, weight, physical activity, comorbidities, depression, alcohol consumption, smoking status, TSH, testosterone, HOMA-IR, and CRP (Table 3) (Model 3). Finally, models 1 to 3 were calculated with grip strength as dependent variable (linear regression models).
Results
Complete cross-sectional data on sleep efficiency and sleep quality, ALM, and BMI from 1196 study participants were available (52.5% of them women; mean age 68 ± 4 years). The BMI was needed to calculate ALM/BMI. The clinical and sleep characteristics of the male and female probands are shown in Table 1.
Table 1. Characteristics of the male and female study participants.
| Women (n = 628) | men (n = 568) | p | ||
|---|---|---|---|---|
| Age (years) | 68 ± 4 | 69 ± 4 | 0.005 | |
| BMI (kg/m2) | 26.4 ± 4.6 | 27.1 ± 3.6 | <0.001 | |
| Sleep | ||||
| Time to fall asleep (min) | 21 ± 25 | 15 ± 18 | <0.001 | |
| Getting-up time (time±SD in min) | 07:18 ± 59 | 07:18 ± 63 | n. s. | |
| Time in bed (h) | 8.15 ± 0.97 | 8.11 ± 1.07 | n. s. | |
| Time asleep (h) | 6.9 ± 1 | 7.1 ± 1 | <0.001 | |
| Sleep quality (n [%]) | Very good | 136 (21.7) | 163 (28.7) | 0.005 |
| Fairly good | 372 (59.2) | 329 (57.9) | ||
| Fairly bad | 111 (17.7) | 67 (11.8) | ||
| Very bad | 9 (1.4) | 9 (1.6) | ||
| Sleep efficiency (n [%]) | Very good | 386 (61.5) | 412 (72.5) | 0.002 |
| Fairly good | 119 (18.9) | 85 (15.0) | ||
| Fairly poor | 66 (10.5) | 44 (7.7) | ||
| Very poor | 57 (9.1) | 27 (4.8) | ||
| No regular consumption of sleep-inducing drugs (n [%])* | 86 (87.8) | 154 (95.7) | 0.025 | |
| Lifestyle/comorbidities (n [%]) | ||||
| Regular smoking | 50 (8.0) | 66 (11.6) | <0.001 | |
| Regular alcohol consumption | 560 (89.2) | 522 (91.9) | n. s. | |
| Depression | 85 (14.4) | 49 (9.4) | 0.006 | |
| Cardiac insufficiency | 16 (2.5) | 15 (2.6) | n. s. | |
| Coronary heart disease | 17 (2.7) | 31 (5.5) | n. s. | |
| Diabetes mellitus | 55 (9.4) | 88 (17.6) | <0.001 | |
| COPD | 36 (5.7) | 26 (4.6) | n. s. | |
| Arterial hypertension | 270 (43.0) | 276 (48.6) | n. s. | |
| Muscle mass and physical performance capacity | ||||
| ALM (kg) | 16.14 ± 2.35 | 23.93 ± 2.88 | <0.001 | |
| ALM/BMI | 0.62 ± 0.10 | 0.89 ± 0.12 | <0.001 | |
| Maximum hand grip strength (kg) | 26.81 ± 4.94 | 41.87 ± 6.79 | <0.001 | |
| Problems with moderate physical activity (n [%]) | 150 (24.7) | 83 (15.8) | <0.001 | |
| Problems climbing stairs (n [%]) | 205 (33.7) | 131 (25.0) | 0.006 | |
| Problems crossing the street (n [%]) | 59 (9.7) | 34 (6.5) | n. s. | |
| Laboratory parameters | ||||
| TSH (mU/L) | 2.1 ± 1.1 | 1.7 ± 0.58 | <0.001 | |
| CRP (mg/L) | 2.06 ± 2.82 | 1.83 ± 2.40 | n. s. | |
| HOMA-IR | 2.15 ± 1.85 | 2.96 ± 5.59 | <0.001 | |
| Testosterone (ng/mL) | 0.24 ± 0.31 | 5.08 ± 2.14 | <0.001 | |
SD, standard deviation; COPD, chronic obstructive pulmonary disease; ALM, appendicular lean mass; BMI, body mass index; ALM/BMI, BMI-corrected muscle mass; TSH, thyroid-stimulating hormone; CRP, C-reactive protein; HOMA-IR, homeostasis model of insulin resistance; n. s., not significant
*Data available from 269 probands; results expressed as mean ± standard deviation or absolute value and percentage
Sleep characteristics
Overall, 16.4% of the study participants (19.1% of the women and 13.4% of the men) subjectively described their sleep quality as fairly bad or very bad, while 16.2% (19.6% of the women and 12.5% of the men) stated poor sleep efficiency (<85%). Thus, more women than men reported poor sleep efficiency and bad sleep quality. The self-reported mean duration of sleep was statistically significantly lower in women than in men (6.9 ± 1 h versus 7.1 ± 1 h; p<0.001), and women took longer to fall asleep (21 ± 25 min versus 15 ± 18 min; p<0.001).
Association between cut-off values for body mass index–corrected muscle mass and sleep
The clinical characteristics and sleep characteristics of the male and female probands by ALM/BMI cut-off value are shown in Table 2. Altogether, 77 women (12.3%) and 106 men (18.7%) were below the sex-specific ALM/BMI cut-off values.
Table 2. Characteristics of the male and female study participants below and above the cut-off values for body mass index-corrected muscle mass.
| Women (n = 628) | Men (n = 568) | ||||||
|---|---|---|---|---|---|---|---|
| ALM/BMI >0.512 (n = 551) | ALM/BMI <0.512 (n = 77) | p | ALM/BMI >0.789 (n = 462) | ALM/BMI <0.789 (n = 106) | p | ||
| Age (years) | 68 ± 4 | 69 ± 4 | 0.050 | 69 ± 4 | 69 ± 4 | n. s. | |
| BMI (kg/m2) | 25.7 ± 4.1 | 31.2 ± 4.8 | 0.001 | 26.4 ± 2.9 | 30.2 ± 4.5 | 0.001 | |
| Time to fall asleep (min) | 22 ± 26 | 17 ± 18 | n. s. | 14 ± 15 | 20 ± 28 | n. s. | |
| Getting-up time (time±SD in min) | 07:18 ± 58 | 07:17 ± 60 | n. s. | 07:17 ± 60 | 07:19 ± 75 | n. s. | |
| Time in bed (h) | 8.16 ± 0.95 | 8.03 ± 1.12 | n. s. | 8.07 ± 1.05 | 8.27 ± 1.16 | n. s. | |
| Time asleep (h) | 7 ± 1 | 7 ± 1 | n. s. | 7 ± 1 | 7 ± 1 | n. s. | |
| Sleep quality (n [%]) | Very good | 121 (22.0) | 15 (19.5) | n. s. | 131 (28.4) | 32 (30.2) | 0.001 |
| Fairly good | 323 (58.6) | 49 (63.6) | 282 (61.0) | 47 (44.3) | |||
| Fairly bad | 99 (18.0) | 12 (15.6) | 43 (9.3) | 24 (22.6) | |||
| Very bad | 8 (1.5) | 1 (1.3) | 6 (1.3) | 3 (2.8) | |||
| Sleep efficiency (n [%]) | Very good | 340 (61.7) | 46 (59.7) | n. s. | 341 (73.8) | 71 (67.0) | 0.017 |
| Fairly good | 105 (19.1) | 14 (18.2) | 66 (14.3) | 19 (17.9) | |||
| Fairly poor | 54 (9.8) | 12 (15.6) | 38 (8.2) | 6 (5.7) | |||
| Very poor | 52 (9.4) | 5 (6.5) | 17 (3.7) | 10 (9.4) | |||
| No regular consumption of sleep-inducing drugs (n [%])* | 67 (84.8) | 19 (100.0) | n. s. | 121 (96.0) | 33 (94.3) | n. s. | |
| Lifestyle (n [%]) | |||||||
| Regular smoking | 46 (8.3) | 4 (5.2) | n. s. | 59 (12.8) | 7 (6.6) | 0.024 | |
| Regular alcohol consumption | 494 (89.7) | 66 (85.7) | n. s. | 424 (91.8) | 99 (92.5) | n. s. | |
| Physical performance capacity | |||||||
| Maximum hand grip strength (kg) | 27.1 ± 4.9 | 24.7 ± 4.8 | <0.001 | 42.6 ± 4.8 | 38.8 ± 6.6 | <0.001 | |
| Problems with moderate physical activity (n [%]) | 119 (22.3) | 31 (41.7) | <0.001 | 60 (14) | 23 (24.5) | <0.001 | |
| Problems climbing stairs (n [%]) | 165 (31) | 40 (54.5) | <0.001 | 92 (21.4) | 39 (41.6) | <0.001 | |
| Problems crossing the street (n [%]) | 41 (7.7) | 18 (24) | <0.001 | 23 (5.3) | 11 (11.7) | 0.026 | |
| Laboratory parameters | |||||||
| TSH (mU/L) | 2 ± 1 | 2 ± 4 | n. s. | 2.2 | 3.6 | n. s. | |
| CRP (mg/L) | 1.9 ± 2.8 | 3.2 ± 2.6 | <0.001 | 1.7 ± 2.5 | 2.3 ± 2.1 | <0.001 | |
| HOMA-IR | 2.0 ± 1.9 | 3.0 ± 1.9 | <0.001 | 2.5 ± 3.4 | 4.8 ± 10.5 | <0.001 | |
| Testosterone (ng/mL) | 0.24 ± 0.32 | 0.19 ± 0.14 | n. s. | 5.23 ± 2.12 | 4.20 ± 2.04 | <0.001 | |
SD, standard deviation; ALM, appendicular lean mass; BMI, body mass index; ALM/BMI, BMI-corrected muscle mass; TSH, thyroid-stimulating hormone; CRP, C-reactive protein; HOMA-IR, homeostasis model of insulin resistance; n. s., not significant
*Data available from 269 probands; results expressed as mean ± standard deviation or absolute value and percentage
Comparison of the sexes (Table 2) showed that a significantly higher proportion of men had low BMI-corrected muscle mass (< ALM/BMI cut-off value). Individual sleep characteristics such as sleep latency, getting-up time, time in bed, and sleep duration were not associated with low muscle mass (< ALM/BMI cut-off value) in men or women. Both men and women with low muscle mass more frequently reported deleterious effects on physical activity, including problems with moderate activity, climbing stairs, and crossing the street. Moreover, probands of both sexes had lower maximal hand grip strength. Particularly the homeostasis model assessment of insulin resistance (HOMA-IR) as a surrogate marker for insulin resistance and C-reactive protein (CRP) as a marker for inflammation were elevated in men and women whose muscle mass was below the ALM/BMI cut-off value (Table 2).
In addition, male study participants with low muscle mass displayed significantly poorer sleep efficiency and sleep quality even after Bonferroni correction. Furthermore, the anabolic hormone testosterone was significantly reduced in men with low muscle mass. These findings were not observed in women.
Association of sleep quality/efficiency with muscle mass, grip strength, and physical performance capacity
We also investigated possible connections of sleep quality and sleep efficiency with ALM, ALM/BMI, grip strength, and physical performance capacity (eTable).
eTable. Characteristics of the male and female study participants by sleep quality and sleep efficiency.
| Women (n = 628) | Men (n = 568) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sleep efficiency >85% | Sleep efficiency <85% | p | Sleep quality good | Sleep quality bad | p | Sleep efficiency >85% | Sleep efficiency <85% | p | Sleep quality good | Sleep quality bad | p | |
| Age (years) | 68 ± 4 | 69 ± 3 | 0.006 | 68 ± 4 | 69 ± 3 | n. s. | 69 ± 4 | 69 ± 4 | n. s. | 69 ± 4 | 68 ± 4 | n. s. |
| BMI (kg/m2) | 26.4 ± 4.6 | 25.6 ± 3.82 | n. s. | 26.6 ± 4.68 | 25.27 ± 3.93 | 0.003 | 27.08 ± 3.6 | 27.27 ± 3.58 | n. s. | 27.1 ± 3.58 | 27.02 ± 3.71 | n. s. |
| ALM (kg) | 16.2 ± 2.36 | 15.6 ± 2.16 | 0.077 | 16.25 ± 2.33 | 15.67 ± 2.38 | 0.016 | 23.97 ± 2.87 | 23.13 ± 2.97 | n. s. | 24.09 ± 2.86 | 22.89 ± 2.82 | <0.001 |
| ALM/BMI | 0.62 ± 0.1 | 0.62 ± 0.09 | n. s. | 0.62 ± 0.1 | 0.63 ± 0.1 | n. s. | 0.89 ± 0.12 | 0.86 ± 0.13 | 0.017 | 0.9 ± 0.12 | 0.86 ± 0.11 | 0.004 |
| Maximum hand grip strength (kg) | 26.96 ± 5 | 25.33 ± 4.13 | 0.014 | 27.06 ± 4.91 | 25.76 ± 4.97 | 0.009 | 41.93 ± 6.81 | 40.56 ± 6.18 | n. s. | 42.11 ± 6.76 | 40.26 ± 6.78 | 0.083 |
| Problems with moderate physical activity (n [%]) | 136 (24.6) | 14 (25) | n. s. | 121 (24.6) | 29 (24.8) | n. s. | 77 (15.4) | 6 (26) | n. s. | 65 (14.3) | 18 (25.8) | 0.036 |
| CRP (mg/L) | 2 ± 2.69 | 2.59 ± 3.89 | n. s. | 2.06 ± 2.77 | 2.06 ± 3.04 | n. s. | 1.82 ± 2.27 | 2.03 ± 4.31 | n. s. | 1.86 ± 2.49 | 1.69 ± 1.68 | n. s. |
| Testosterone (ng/mL) | 0.24 ± 0.26 | 0.26 ± 0.62 | n. s. | 0.23 ± 0.26 | 0.26 ± 0.46 | n. s. | 5.01 ± 2.14 | 4.93 ± 2.14 | n. s. | 5.03 ± 2.10 | 5.40 ± 2.36 | n. s. |
| TSH (mU/L) | 2 ± 2 | 2 ± 2 | n. s. | 2 ± 1 | 2 ± 3 | n. s. | 2 ± 3 | 2 ± 1 | n. s. | 2 ± 3 | 2 ± 3 | n. s. |
| HOMA-IR | 2.1 ± 1.9 | 2.3 ± 1.5 | n. s. | 2.2 ± 2.0 | 2.0 ± 1.1 | n. s. | 3.0 ± 5.7 | 3.1 ± 2.8 | n. s. | 2.9 ± 5.6 | 3.5 ± 5.7 | n. s. |
Results expressed as mean ± standard deviation or absolute numbers and percentages
ALM, appendicular lean mass; BMI, body mass index; ALM/BMI, BMI-corrected muscle mass; TSH, thyroid-stimulating hormone; CRP, C-reactive protein; HOMA-IR, homeostasis model of insulin resistance; n. s., not significant
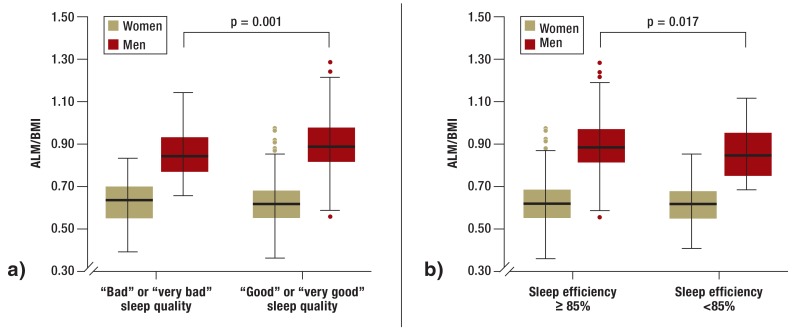
Men with subjectively assessed bad sleep quality or poor sleep efficiency had both significantly lower ALM and lower BMI-corrected muscle mass (Figure 2). Grip strength, however, was not statistically significantly reduced in men with either worse sleep quality or poorer sleep efficiency. Self-reported difficulties in performing moderate physical activities were the only parameter to occur more frequently in probands with poor sleep efficiency.
Figure 2.
Dependency of muscle mass (ALM/BMI) on sleep quality (a) and sleep efficiency (b) in men and women
ALM, appendicular lean mass; BMI, body mass index; ALM/BMI, BMI-corrected muscle mass; sleep quality, self-reported quality of sleep; sleep efficiency, percentage of time in bed spent asleep
Women with subjectively bad sleep quality or poor sleep efficiency did not exhibit lower BMI-corrected muscle mass (Figure 2). Nevertheless, in those with bad sleep quality the non-BMI-corrected ALM was statistically significantly reduced. In contrast to the male probands, however, poor sleep efficiency and bad sleep quality were statistically significantly associated with grip strength (eTable).
Subgroup analysis
In the subgroup analysis of 259 study participants (62.6% of them men; mean age 68 ± 4 years) who answered supplementary questions on factors that disturbed their sleep (e.g., pain, excessive warmth, cold, noise, and nycturia) and on their consumption of sleep-inducing drugs, the following factors were stated to disrupt or interrupt sleep at least once each week: pain 19.7%, excessive warmth 24.3%, cold 11.2%, coughing 15.8%, and breathing problems 6.7%. More women than men reported pain (27.5% versus 14.9%; p = 0.016) and consumption of sleep-inducing drugs (12.2% versus 4.3%; p = 0.025). The other reasons for interruption of sleep were independent of sex.
Study participants with low muscle mass (< ALM/BMI cut-off value) and probands over the ALM/BMI cut-off value had comparable frequencies of sleep-disrupting events such as nycturia and pain and showed similar impairments of physical activity and grip strength (data not shown).
Consumption of sleep-inducing drugs was also associated neither with grip strength nor with muscle mass. Among women, however, self-reported physical performance capacity was greatly reduced in those who took such medications. A statistically significant difference was found with regard to the ability to carry out moderately strenuous activities (p = 0.032; data not shown).
Regression analysis
Finally, regression models were calculated to determine the odds ratio (OR) of bad sleep quality (fairly bad or very bad) and poor sleep efficiency (<85%) with regard to ALM/BMI cut-off values. The results in the male study participants are shown in Table 3. In men, the model with the most parameters (model 3: adjusted for age, height, weight, physical activity, comorbidities, depression, alcohol consumption, smoking status, thyroid-stimulating hormone (TSH), testosterone, HOMA-IR, and CRP; R2 according to Nagelkerke = 0.581) revealed an OR of 2.8% with a 95% confidence interval of [1.1; 6.7] for bad sleep quality and OR 4.3 [1.2; 15.1] for poor sleep efficiency. In contrast, these two sleep characteristics showed no statistically significant connection in women.
Table 3. Relative risk of low muscle mass for men with poor sleep efficiency and bad sleep quality.
| Model 1 OR, 95% CI | p | Model 2 OR, 95% CI | p | Model 3 OR, 95% CI*3 | p | |
|---|---|---|---|---|---|---|
| Poor sleep efficiency*1 | 3.6 [1.2; 10.1] | 0.021 | 3.7 [1.1; 12.1] | 0.031 | 4.3 [1.2; 15.1] | 0.021 |
| Bad sleep quality*2 | 2.6 [1.3; 5.4] | 0.007 | 2.4 [1.1; 5.2] | 0.034 | 2.8 [1.1; 6.7] | 0.021 |
CI, confidence interval; OR, odds ratio; TSH, thyroid-stimulating hormone; HOMA-IR, homeostasis model of insulin resistance; CRP, C-reactive protein
*1sleep efficiency <85%; *2subjective sleep quality fairly bad or very bad; *3R 2 (according to Nagelkerke) = 0.581
Model 1: Age, height, weight
Model 2: Model 1 + physical activity + comorbidities (morbidity index) + lifestyle factors (alcohol consumption and smoking status)
Model 3: Model 2 + depression, laboratory parameters (TSH, testosterone, HOMA-IR, and CRP)
Models 1 to 3 were also calculated for grip strength (data not shown). In model 1 (p = 0.003) and model 2 (p = 0.045) poor sleep efficiency had a significant effect on grip strength in women. This was not the case in model 3 (p = 0.084). Bad sleep quality and grip strength were not significantly associated with one another in any of the models.
Discussion
We analyzed associations between muscle mass and sleep in a group of 1196 older people from BASE-II, all living independently in their own homes. The OR of being below the ALM/BMI cut-off value in model 3 was 3.64 [1.43; 9.31] in men with bad sleep quality and 4.23 [1.20; 14.88] in men with poor sleep efficiency.
Bad sleep quality and ALM were also associated in women, but ALM/BMI and ALM/BMI cut-off value were not associated with either sleep quality or sleep efficiency. However, hand grip strength, as a measure of muscle strength, was statistically significantly reduced in women with poor sleep efficiency or bad sleep quality. Results relating to grip strength were not seen in regression model 3.
Calculation of the regression models incorporated factors (Table 3, Figure 1) that can exert an influence on both sleep behavior and muscle mass (26). Muscle mass decreases with increasing age and depends on height and weight (model 1). Advanced age and high BMI also affect sleep behavior. Physical activity is among the positive influences on muscle mass/function and sleep, while lifestyle factors such as alcohol consumption and smoking, together with comorbidities, have negative effects (model 2). Model 3 included monitoring of metabolic (insulin resistance; HOMA-IR) and hormonal parameters (testosterone and TSH) (27).
Owing to the cross-sectional design of our study, the differences in results between men and women cannot be adequately explained. Insulin resistance, which is particularly likely to develop in men with reduced duration of sleep, may play an important role, as may other metabolic and hormonal changes (28, 29). Furthermore, there are indications that BMI can increase due to reduced sleep duration. The increase in BMI is a monotonic trend in men (elevated BMI with decreased sleep duration), whereas in women there is a U-shaped curve (29). The fact that our results also relate to BMI-corrected muscle mass may have contributed to the differences between men and women. The results for hand grip strength, which were positively associated with BMI, could also have been influenced (30, 31). Men had a significantly higher BMI than women (Table 1).
However, this analysis of older people with few comorbidities who were living independently at home, based on the standardized Pittsburgh Sleep Quality Index (PSQI) questionnaire, shows that poor sleep efficiency and/or bad sleep quality are often reported in advanced age (bad sleep quality 16.4%, poor sleep efficiency 16.2%). A subgroup analysis of 269 probands from whom supplementary data on consumption of sleep-inducing drugs and events that disturb sleep were obtained (using questions from the PSQI questionnaire) revealed, furthermore, that sleep-disrupting events, waking phases during the night, and regular consumption of sleep-inducing drugs represent serious problems. This is particularly important with regard to the principal results of the study: bad sleep quality and poor sleep efficiency are associated with reduced muscle mass and reduced grip strength.
The results of our analysis are in agreement with current working hypotheses. Study participants with reduced sleeping time or inefficient sleep, especially with regard to sleep apnea, show hormonal or metabolic changes (12, 28, 32, 33, e7–e10). Hormones like testosterone and IGF-1 are down-regulated and cortisol is up-regulated (33, e10). Especially IGF-1 is a central factor in protein synthesis in muscle. IGF-1 disinhibits secretion of growth hormone and promotes insulin resistance. This is thought to be an important mediator for the loss of muscle mass and muscle strength and for the development of functional impairments in old age (34, 35). This analysis also found increased levels of HOMA-IR as marker of insulin resistance in probands below the ALM/BMI cut-off value. The inflammation parameter CRP, which we also found to be elevated in study participants below the ALM/BMI cut-off value, is also discussed as a factor in muscle atrophy (36).
Participants in studies of the effects of sleep deprivation have shown reduction in muscle mass and atrophy of the musculature (37, 38).
Analyses by our group have demonstrated that the ALM/BMI cut-off value can also be used in connection with frailty and physical performance capacity in the BASE-II cohort (22). Correction for height and weight, as performed by McLean et al. and others, better reflects limitations of physical performance capacity and grip strength and is thus a more suitable marker for clinically relevant low muscle mass (15, 16, 39). Nevertheless, it must be realized that the use of fixed cut-off values for ALM/BMI may distort the results in individual cases, because different phenotypes—in respect of height, weight, and muscle mass—can yield identical BMI scores. Persons who have identical BMI but differ greatly in height have widely discrepant BMI-corrected muscle mass results. Correspondingly, we also investigated associations with muscle mass and grip strength independently of these fixed cut-off values.
Reduced muscle mass plays a crucial role in advanced age and is associated not only with the performance of routine daily activities but also with geriatric syndromes such as frailty or falling. Muscle mass is therefore a central topic in geriatrics (22, 40).
To what extent sleep affects maintenance of muscle mass, whether muscle mass influences sleep or a positive influence on sleep behavior contributes to maintenance of muscle mass, cannot be clarified by this cross-sectional study. Longitudinal investigations are warranted to answer this question.
Limitations
Our results are subject to limitations. The data on sleep quality and efficiency, obtained using a questionnaire, are self-reported and thus subjective. With regard to sleep quality, the study participants were restricted to the following choices:
Very bad
Fairly bad
Fairly good
Very good.
The lack of an intermediate category between “fairly bad” and “fairly good” means that a proband’s actual sleep quality may be over- or underestimated. The same is true for the questions about physical activity and physical performance capacity. Physical activity was analyzed solely by questionnaire, not by objective means such as actigraphy. Data on sleep-disrupting events and consumption of sleep-inducing drugs were available only from a subgroup of 269 probands, so it was not possible to evaluate the PSQI cut-off values for the whole study population. However, inclusion of these sleep-disrupting events does not change the results. Finally, the BASE-II cohort is not a random sample of the general population: BASE-II participants’ health status and health awareness were higher, on average, compared to the general population (14). Diseases such as chronic obstructive pulmonary disease (COPD) and coronary heart disease are under-represented, so the results from this study collective cannot simply be extrapolated to the total population. We would expect a cohort with more comorbidities to feature a higher prevalence of functional restrictions than in our study, together with a tendency towards strengthening of the associations we have described here.
Key Messages.
The prevalence of bad sleep quality and reduced sleep efficiency in older people (>60 years) is high. Women are affected more strongly than men.
Bad sleep quality and low sleep efficiency in older people are associated not only with functional restrictions such as lessening of grip strength and reduction of physical activity, but also with muscle mass and function.
Non-restorative sleep, just like reduced muscle mass, is associated with metabolic (insulin resistance) and hormonal changes.
Acknowledgments
Translated from the original German by David Roseveare.
Footnotes
Conflict of interest statement
Dr. Norman has received study support (third-party funding) from Nutricia, a specialized health-care branch of the food manufacturer Danone.
The remaining authors declare that they have no conflict of interest.
References
- 1.Foley DJ, Monjan AA, Brown SL, Simonsick EM, Wallace RB, Blazer DG. Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep. 1995;18:425–432. doi: 10.1093/sleep/18.6.425. [DOI] [PubMed] [Google Scholar]
- 2.Ohayon MM, Zulley J. Correlates of global sleep dissatisfaction in the German population. Sleep. 2001;24:780–787. [PubMed] [Google Scholar]
- 3.Münch M. Der Schlaf und die innere Uhr im Alter. Therapeutische Umschau. 2014;71:657–662. doi: 10.1024/0040-5930/a000606. [DOI] [PubMed] [Google Scholar]
- 4.Dijk DJ, Duffy JF, Riel E, Shanahan TL, Czeisler CA. Ageing and the circadian and homeostatic regulation of human sleep during forced desynchrony of rest, melatonin and temperature rhythms. J Physiol. 1999;516:611–627. doi: 10.1111/j.1469-7793.1999.0611v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miles LE, Dement WC. Sleep and aging. Sleep. 1980;3:1–220. [PubMed] [Google Scholar]
- 6.Bliwise DL, Foley DJ, Vitiello MV, Ansari FP, Ancoli-Israel S, Walsh JK. Nocturia and disturbed sleep in the elderly. Sleep Med. 2009;10:540–548. doi: 10.1016/j.sleep.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Birbaumer N, Schmidt RF, editors. Biologische Psychologie. Berlin, Heidelberg: Springer Medizin Verlag; 2010. Zirkadiane Periodik, Schlaf und Traum; pp. 535–569. [Google Scholar]
- 8.Kripke DF, Garfinkel L, Wingard DL, Klauber MR, Marler MR. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry. 2002;59:131–136. doi: 10.1001/archpsyc.59.2.131. [DOI] [PubMed] [Google Scholar]
- 9.Ancoli-Israel S, Cooke JR. Prevalence and comorbidity of insomnia and effect on functioning in elderly populations. J Am Geriatr Soc. 2005;53:264–271. doi: 10.1111/j.1532-5415.2005.53392.x. [DOI] [PubMed] [Google Scholar]
- 10.Spira AP, Covinsky K, Rebok GW, et al. Poor sleep quality and functional decline in older women. J Am Geriatr Soc. 2012;60:1092–1098. doi: 10.1111/j.1532-5415.2012.03968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naylor E, Penev PD, Orbeta L, et al. Daily social and physical activity increases slow-wave sleep and daytime neuropsychological performance in the elderly. Sleep. 2000;23:87–95. [PubMed] [Google Scholar]
- 12.Dattilo M, Antunes HK, Medeiros A, et al. Sleep and muscle recovery: endocrinological and molecular basis for a new and promising hypothesis. Med Hypotheses. 2011;77:220–222. doi: 10.1016/j.mehy.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 13.Kalyani RR, Corriere M, Ferrucci L. Age-related and disease-related muscle loss: the effect of diabetes, obesity, and other diseases. Lancet Diabetes Endocrinol. 2014;2:819–829. doi: 10.1016/S2213-8587(14)70034-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bertram L, Böckenhoff A, Demuth I, et al. Cohort Profile: the Berlin Aging Study II (BASE-II) Int J Epidemiol. 2014;43:703–712. doi: 10.1093/ije/dyt018. [DOI] [PubMed] [Google Scholar]
- 15.McLean RR, Shardell MD, Alley DE, et al. Criteria for clinically relevant weakness and low lean mass and their longitudinal association with incident mobility impairment and mortality: the Foundation for the National Institutes of Health (FNIH) Sarcopenia Project. J Gerontol A Biol Sci Med Sci. 2014;69:576–583. doi: 10.1093/gerona/glu012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cawthon PM, Peters KW, Shardell MD, et al. Cutpoints for low appendicular lean mass that identify older adults with clinically significant weakness. J Gerontol A Biol Sci Med Sci. 2014;69:567–575. doi: 10.1093/gerona/glu023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alley DE, Shardell MD, Peters KW, et al. Grip strength cutpoints for the identification of clinically relevant weakness. J Gerontol A Biol Sci Med Sci. 2014;69:559–566. doi: 10.1093/gerona/glu011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buysse DJ, Reynolds CF, Monk TH, Hoch CC, Yeager AL, Kupfer DJ. Quantification of subjective sleep quality in healthy elderly men and women using the Pittsburgh Sleep Quality Index (PSQI) Sleep. 1991;14:331–338. [PubMed] [Google Scholar]
- 19.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 20.Müller T, Paterok B, editors. 2nd edition. Vol. 88. Göttingen: Hogrefe Verlag; 2010. Schlaftraining: Ein Therapiemanual zur Behandlung von Schlafstörungen. [Google Scholar]
- 21.Topolski TD, LoGerfo J, Patrick DL, Williams B, Walwick J, Patrick MB. Peer reviewed: the Rapid Assessment of Physical Activity (RAPA) among older adults. Prev Chronic Dis. 2006;3 [PMC free article] [PubMed] [Google Scholar]
- 22.Spira D, Buchmann N, Nikolov J, et al. Association of low lean mass with frailty and physical performance: a comparison between two operational definitions of sarcopenia—data from the Berlin aging study II (BASE-II) J Gerontol A Biol Sci Med Sci. 2015;70:779–784. doi: 10.1093/gerona/glu246. [DOI] [PubMed] [Google Scholar]
- 23.Kellerer M, Siegel E. Praxisempfehlungen der Deutschen Diabetes Gesellschaft. Diabetologie und Stoffwechsel. 2013;8:103–240. [Google Scholar]
- 24.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 25.Gerstorf D, Hülür G, Drewelies J, et al. Secular changes in late-life cognition and well-being: towards a long bright future with a short brisk ending? Psychol Aging. 2015;30:301–310. doi: 10.1037/pag0000016. [DOI] [PubMed] [Google Scholar]
- 26.Narici MV, Maffulli N. Sarcopenia: characteristics, mechanisms and functional significance. Br Med Bull. 2010;95:139–159. doi: 10.1093/bmb/ldq008. [DOI] [PubMed] [Google Scholar]
- 27.Auyeung TW, Kwok T, Leung J, et al. Sleep duration and disturbances were associated with testosterone level, muscle mass, and muscle strength—a cross-sectional study in 1274 older men. J Am Med Dir Assoc. 2015;16:630.e1–630e6. doi: 10.1016/j.jamda.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 28.Knutson KL, Spiegel K, Penev P, van Cauter E. The metabolic consequences of sleep deprivation. Sleep Med Rev. 2007;11:163–178. doi: 10.1016/j.smrv.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kripke DF, Garfinkel L, Wingard DL, Klauber MR, Marler MR. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry. 2002;59:131–136. doi: 10.1001/archpsyc.59.2.131. [DOI] [PubMed] [Google Scholar]
- 30.Gale CR, Martyn CN, Cooper C, Sayer AA. Grip strength, body composition, and mortality. Int J Epidemiol. 2007;36:228–235. doi: 10.1093/ije/dyl224. [DOI] [PubMed] [Google Scholar]
- 31.Pieterse S, Manandhar M, Ismail S. The association between nutritional status and handgrip strength in older Rwandan refugees. Eur J Clin Nutr. 2002;56:933–939. doi: 10.1038/sj.ejcn.1601443. [DOI] [PubMed] [Google Scholar]
- 32.Yu JH, Yun CH, Ahn JH, et al. Evening chronotype is associated with metabolic disorders and body composition in middle-aged adults. J Clin Endocrinol Metab. 2015;100:1494–1502. doi: 10.1210/jc.2014-3754. [DOI] [PubMed] [Google Scholar]
- 33.von Treuer K, Norman TR, Armstrong SM. Overnight human plasma melatonin, cortisol, prolactin, TSH, under conditions of normal sleep, sleep deprivation, and sleep recovery. J Pineal Res. 1996;20:7–14. doi: 10.1111/j.1600-079x.1996.tb00232.x. [DOI] [PubMed] [Google Scholar]
- 34.Bassil MS, Gougeon R. Muscle protein anabolism in type 2 diabetes. Curr Opin Clin Nutr Metab Care. 2013;16:83–88. doi: 10.1097/MCO.0b013e32835a88ee. [DOI] [PubMed] [Google Scholar]
- 35.Morley JE. Diabetes, sarcopenia, and frailty. Clin Geriatr Med. 2008;24:455–469. doi: 10.1016/j.cger.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 36.Lang CH, Frost RA, Vary TC. Regulation of muscle protein synthesis during sepsis and inflammation. Am J Physiol Endocrinol Metab. 2007;293:E453–E459. doi: 10.1152/ajpendo.00204.2007. [DOI] [PubMed] [Google Scholar]
- 37.Nedeltcheva AV, Kilkus JM, Imperial J, Schoeller DA, Penev PD. Insufficient sleep undermines dietary efforts to reduce adiposity. Ann Intern Med. 2010;153:435–441. doi: 10.1059/0003-4819-153-7-201010050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kant GJ, Genser SG, Thorne DR, Pfalser JL, Mougey EH. Effects of 72 hour sleep deprivation on urinary cortisol and indices of metabolism. Sleep. 1984;7:142–146. doi: 10.1093/sleep/7.2.142. [DOI] [PubMed] [Google Scholar]
- 39.Cawthon PM, Peters KW, Shardell MD, et al. Cutpoints for low appendicular lean mass that identify older adults with clinically significant weakness. J Gerontol A Biol Sci Med. 2014;69:567–575. doi: 10.1093/gerona/glu023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Landi F, Liperoti R, Russo A, et al. Sarcopenia as a risk factor for falls in elderly individuals: results from the ilSIRENTE study. Clin Nutr. 2012;31:652–658. doi: 10.1016/j.clnu.2012.02.007. [DOI] [PubMed] [Google Scholar]
- e1.Schlack R, Hapke U, Maske U, Busch M, Cohrs S. Häufigkeit und Verteilung von Schlafproblemen und Insomnie in der deutschen Erwachsenenbevölkerung. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2013;56:740–748. doi: 10.1007/s00103-013-1689-2. [DOI] [PubMed] [Google Scholar]
- e2.Mazza M, Della Marca G, De Risio S, Mennuni GF, Mazza S. Sleep disorders in the elderly. Clin Ter. 2004;155:391–394. [PubMed] [Google Scholar]
- e3.Cooke JR, Ancoli-Israel S. Normal and abnormal sleep in the elderly. In: Vinken PJ, Bruyn GW, editors. Handbook of clinical neurology. Vol. 98. Amsterdam: Elsevier B. V.; 2011. pp. 653–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e4.Goel N, Rao H, Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Semin Neurol. 2009;29:320–339. doi: 10.1055/s-0029-1237117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e5.Staedt J, Gudlowski Y, Hauser M, editors. Rat und Hilfe für Betroffene und Angehörige. Vol. 103. Stuttgart W: Kohlhammer; 2009. Schlafstörungen im Alter. [Google Scholar]
- e6.Hähnlein V, Rimpel J, editors. Ein integratives Lehrbuch. Vol. 314. Stuttgart: Klett-Cotta; 2008. Systemische Psychosomatik. [Google Scholar]
- e7.Tufik S, Andersen ML, Bittencourt LR, Mello MT. Paradoxical sleep deprivation: neurochemical, hormonal and behavioral alterations. Evidence from 30 years of research. An Acad Bras de Cienc. 2009;81:521–538. doi: 10.1590/s0001-37652009000300016. [DOI] [PubMed] [Google Scholar]
- e8.Spiegel K, Leproult R, van Cauter E, editors. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- e9.Vardar-Yagli N, Saglam M, Savci S, et al. Impact of sleep quality on functional capacity, peripheral muscle strength and quality of life in patients with chronic obstructive pulmonary disease. Expert Rev of Respir Med. 2015;9:233–239. doi: 10.1586/17476348.2015.1009041. [DOI] [PubMed] [Google Scholar]
- e10.Everson CA, Crowley WR. Reductions in circulating anabolic hormones induced by sustained sleep deprivation in rats. Am J Physiol Endocrinol Metab. 2004;286:E1060–E1070. doi: 10.1152/ajpendo.00553.2003. [DOI] [PubMed] [Google Scholar]