Abstract
Background
Disorders of lipid metabolism are very common. They play an important role in the pathogenesis of atherosclerosis and can be effectively treated by lifestyle changes and drugs.
Methods
This review is based on pertinent literature retrieved by a selective search.
Results
The main disorders of lipid metabolism are LDL-hypercholesterolemia, hypertriglyceridemia, mixed hyperlipoproteinemia, and low HDL cholesterol. The lipoprotein(a) level can also be elevated either in isolation or in combination with other disorders of lipid metabolism. According to the current European recommendations, an LDL-cholesterol target value should be defined on the basis of the overall cardiovascular risk. If this risk is very high, as in patients with documented atherosclerosis, the target value should be set at <70 mg/dL (<1.8 mmol/L). If the risk is lower, higher target values can be set: <100 mg/dL (<2.6 mmol/L) or <115 mg/dL (<3.0 mmol/L). Lifestyle changes are an effective treatment mainly for patients with hypertriglyceridemia and mixed disorders of lipid metabolism. Lowering the LDL-cholesterol concentration with statins is by far the most important type of pharmacotherapy. Patients who cannot tolerate statins or whose cholesterol level is not adequately lowered can be given ezetimibe instead. PCSK9 antibodies have been available since the autumn of 2015; they can apparently lower the LDL-cholesterol level by more than 50%, but no endpoint trials have yet been reported. At present, they should only be given to carefully selected patients. Fibrates and omega-3 fatty acids have been found to prevent cardiovascular events in monotherapy trials but yield no added benefit when given together with statins. The design of these trials was faulty, however, and the utility of such combinations in patients with mixed disorders of lipid metabolism or hypertriglyceridemia cannot yet be definitively assessed.
Conclusion
There is a causal relationship between hypercholesterolemia and the risk of vascular and cardiovascular events. A reduction of LDL cholesterol lessens the risk of cardiovascular events.
The intimate association between hypercholesterolemia and atherosclerosis has been known since Anitschkow published the results of his research in 1913 (1). However, the therapeutic potential of this connection has become fully apparent only in the past 20 years (2). Although the first successful work on lipid-lowering agents was carried out 50 years ago, the breakthrough came in 1994 with the 4S study, the first large-scale trial of statins (3). Reduction of LDL cholesterol with statins represents a potent approach to the primary and secondary prevention of cardiovascular disease (4). Statins are now the standard medication for every type of atherosclerosis and, in the presence of the corresponding risk factors, are also prescribed for primary prevention (5). Although the accumulated data are convincing, the decision whether an individual person will benefit from lipid lowering and which approach to follow has to be considered anew in every single case. The aim of this article is to help answer these questions and thus contribute to the avoidance of underuse, overuse, and inappropriate use of lipid-lowering medications.
Disorders of lipid metabolism
Familial hypercholesterolemia as model disease
To the present day, familial hypercholesterolemia—characterized by pronounced coronary heart disease (CHD) at an early age and very high LDL cholesterol levels—remains the most cogent, convincing proof of the close causal connection between elevated LDL cholesterol and atherosclerosis (6). Many recent studies have shown, however, that familial hypercholesterolemia is just the extreme expression of this relationship. The link between LDL cholesterol and atherosclerosis extends over the whole spectrum from genetically determined very low levels to extremely high concentrations (7).
From genetics to intervention
The genetic findings would have been of little importance without confirmation by intervention studies. Thus, studies on statins first demonstrated a virtually linear association between reduction in LDL cholesterol and the rate of cardiovascular events (2). A statin-induced lowering of LDL cholesterol by 1 mmol/L (ca. 39 mg/dL) leads to a 22% reduction in relative risk. A new study investigating the effect of ezetimibe has shown that this association also exists when the LDL reduction is brought about by a cholesterol adsorption inhibitor (IMPROVE-IT study) (8). An additional lowering of LDL cholesterol by 9% (from 69.5 mg/dL to 53.7 mg/dL) decreases the relative risk of a coronary event by 6.4% (absolute risk 32.7% versus 34.7%, number needed to treat [NNT] 350/year). Although the absolute effect is low, the study proves that a statin-independent lowering of LDL cholesterol can reduce the risk and it also shows that reduction of an already low LDL cholesterol level is reflected by a further decrease in risk. This has been confirmed by meta-analyses of statin studies (4, 9) and by data on new lipid-lowering agents (PCSK9 antibodies), which can induce an additional LDL cholesterol reduction of 50 to 60% and for which the approval studies indicate a greater than 50% decrease in risk (10, 11). However, these were not endpoint studies.
The IMPROVE-IT study also raises new questions. For example, it is not clear why neither cardiovascular mortality nor overall mortality was influenced at all. Moreover, the observed benefit was almost exclusively restricted to diabetics.
Altogether, the available data permit the conclusion that lowering of LDL cholesterol leads to a decrease in the rate of cardiovascular events. Whether this applies to all LDL cholesterol-lowering approaches remains open, but the answer is probably affirmative (at least for measures that lead to swifter elimination of LDL cholesterol from the plasma).
Changes in the concentrations of other lipids
While the data on LDL cholesterol all point in the same direction, the atherogenic potential of other lipid changes is harder to assess. Because hypertriglyceridemia almost always goes hand in hand with lowering of HDL cholesterol, it long remained unclear which factor—HDL cholesterol reduction or hypertriglyceridemia—was responsible for the increased cardiovascular risk. In the meantime, it has become clear that triglyceride-rich lipoproteins determine the risk of cardiovascular events (11– 14). HDL cholesterol levels are a good risk marker, but not a target parameter for treatment (15). A further lipidological factor with a causal connection to atherosclerosis is lipoprotein(a) (16), an LDL particle that contains apoprotein(a) as an additional protein. The function and the metabolism of this lipoprotein remain unknown (16, 17).
Discussion of the guideline target values
How the collected evidence can be translated into concrete recommendations for patient management is currently the subject of intensive debate. The national and international professional societies are in agreement that:
The focus should be on high-risk patients with proven atherosclerosis or multiple risk factors
Lifestyle modification measures are of great importance
Statin-based lowering of LDL cholesterol plays a pre-eminent part in medicinal treatment
There is no consensus on:
Whether defined target cholesterol concentrations should be attained or a certain statin dose should be recommended
What role should be taken by other lipid-lowering drugs (non-statins) in reducing LDL cholesterol or in treating other disorders of lipid metabolism
Whether and how patients with hypertriglyceridemia should be treated medicinally
It also has to be decided whether only the results of randomized controlled studies (RCTs) should be taken into account in formulating recommendations or whether the available evidence should be considered in its entirety. If only RCTs are considered, no target cholesterol levels can be laid down; one can merely define groups of patients who, generalizing somewhat, can be expected to benefit from intensive or less intensive treatment with statins, alone or in combination with ezetimibe. This is, in essence, the approach taken in the relevant German National Care Guideline (available in German at www.leitlinien.de/mdb/downloads/nvl/khk/archiv/khk-3aufl-vers1-kurz.pdf/view and in the US guideline (18). If, in contrast, all of the available evidence is taken into account, then target concentrations can be defined, e.g., LDL cholesterol <70 mg/dL in patients at very high risk of cardiovascular events. This is the approach preferred by the European societies and by the author of this article (19).
Therefore, in agreement with the European societies and a number of German societies, the author recommends the target cholesterol concentrations shown in Table 1 (19). Although these target values have never been tested directly in RCTs, they reflect scientifically established evidence. For example, data from studies using intravascular ultrasonography show that plaque regression can be demonstrated only at concentrations of LDL cholesterol <80 mg/dL (20). One should also not underestimate the value of fixed targets in communicating both with patients and with the physicians who will continue their treatment, and targets also promote compliance. A British study showed that a “treat-to-target” approach, compared with a fixed-dose scheme of treatment, achieved better compliance and was associated with a lower rate of cardiovascular events (21).
Practical clinical approach
The treatment strategy depends predominantly on the overall cardiovascular risk and the severity of the lipid metabolism disorder. Since these disorders are considered above all from the viewpoint of primary and secondary prevention of atherosclerosis, it is advisable to focus on patients who are at high or very high risk of cardiovascular events and lower their LDL cholesterol accordingly. Although an association between the extent of LDL cholesterol lowering and reduction in cardiovascular events is seen in persons with low as well as high absolute risk, the greatest effect is seen when maximal lowering of LDL cholesterol is achieved in a high-risk population (22). Thus, the NNT can fluctuate between 50 and several hundred, depending on the magnitude of the underlying risk (3, 23). It should be emphasized, however, that in primary prevention too, statin-based lowering of LDL cholesterol reduces not only overall mortality but also cardiovascular mortality and the rate of cardiovascular events (5).
Classification of lipid metabolism disorders
Table 2 shows when lipid and other laboratory parameters should be determined. The best way of classifying lipid metabolism disorders is descriptively (Table 3), based on the changes in concentration of the various types of lipids. LDL hypercholesterolemia is distinguished from mixed hyperlipoproteinemia, hypertriglyceridemia, and an isolated reduction in HDL cholesterol. All these disorders of lipid metabolism can be associated with elevated lipoprotein(a). The treatment of the individual lipid metabolism disorders is described below.
Table 2. Determination of laboratory parameters.
| Parameter | Measurement |
|---|---|
|
Lipid status (cholesterol, triglycerides, HDL cholesterol, LDL cholesterol) |
Depending on the clinical circumstances
|
| Lipoprotein(a) |
|
| Creatine kinase |
|
| Liver function |
|
*For example: menopause, major change in weight, comorbidity such as diabetes mellitus or atherosclerosis
Table 3. Descriptive classification of the dyslipoproteinemias.
| Cholesterol | Triglycerides | LDL cholesterol | HDL cholesterol | |
|---|---|---|---|---|
| LDL hypercholesterolemia | ↑ | – | ↑ | – |
| Hypertriglyceridemia | ↑ | ↑ | – | ↓ |
| Mixed hyperlipoproteinemia | ↑ | ↑ | ↑ | ↓ |
| Low HDL | – | – | – | ↓ |
| Lipoprotein(a) elevation | Isolated or in combination with other dyslipoproteinemias | |||
↑incrreased; ↓decreased; – unchanged
Exclusion of secondary lipid metabolism disorders
A number of diseases can have the consequence of secondary lipid metabolism disorders. Clinically, the most important of these are diabetes mellitus (hypertriglyceridemia or mixed hyperlipoproteinemia), hypothyroidism (LDL hypercholesterolemia), kidney diseases (hypertriglyceridemia, mixed hyperlipoproteinemia, lipoprotein(a) elevation), and cholestatic liver diseases (apparent LDL cholesterol elevation). Disorders of lipid metabolism are also observed in the context of other diseases (e.g., lymphoma, Cushing syndrome, and porphyria). When the lipid metabolism disorder is a secondary manifestation, treatment should be focused primarily on the underlying disease. Patients with diabetes mellitus or kidney disease often form exceptions to this rule, because optimal regulation or elimination of the underlying disease is not achieved and they show aspects of both primary and secondary lipid metabolism disorders.
Lifestyle modification
Lifestyle modification plays a significant role in the treatment of lipid metabolism disorders. Whatever measures are taken, however, the reduction of elevated LDL cholesterol concentrations rarely reaches 10% (24). The greatest effect is achieved by decreasing the intake of saturated fatty acids, i.e., particularly animal fats. The impact of orally consumed cholesterol is minor, so that the new recommendations in the USA dispense entirely with advising restriction of cholesterol intake. Lifestyle modification has a considerably greater effect in hypertriglyceridemia, alone or in combination with changes in the concentrations of other lipids. Strict limitation of alcohol consumption and reduction in the intake of rapidly resorbed carbohydrates sometimes lower the triglyceride level by >50% (25). Physical activity also improves the lipid profile.
Even though the effect on lipid concentrations is limited in some cases, modifications of lifestyle can have a favorable impact on the risk profile. For example, a Mediterranean diet with additional olive oil or nuts leads to a ca. 30% reduction of relative risk in high-risk patients (26, 27). Interestingly, eating nuts also leads to a lowering of LDL cholesterol, so it can be debated whether at least part of the risk reduction is determined by a favorable influence on the lipid profile (28).
LDL hypercholesterolemia
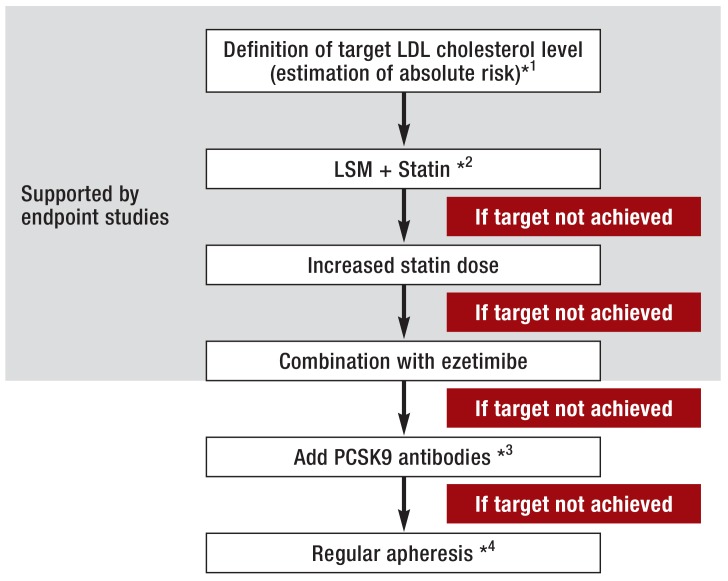
The European guidelines recommend that the target concentration for LDL cholesterol should depend on the overall risk (19) (Table 2, Figure). If this goal is not reached by lifestyle modification alone, administration of a statin represents the first step in medicinal treatment. If the target LDL cholesterol level has still not been attained after 4 to 6 weeks of treatment, the dose should be adjusted accordingly. In high-risk patients, lifestyle modification measures and statin treatment should be initiated simultaneously (19, 29). Based on the findings of the IMPROVE-IT study, ezetimibe should be added if the statin treatment alone fails to achieve the target LDL cholesterol concentration. Should the combination of a statin with ezetimibe still prove inadequate, PCSK9 antibodies (alirocumab and evolocumab, available since autumn 2015) can be given. As a last resort, patients with atherosclerosis and refractory LDL hypercholesterolemia can be treated with regular lipid apheresis.
Figure.
The author’s proposal for a treatment algorithm to achieve the target concentration of LDL cholesterol
*1Target LDL cholesterol level based on the absolute risk of cardiovascular disease
*2In patients at high or very high risk it may be best to start LSM and statin treatment simultaneously
*3Currently treatment with PCSK9 antibodies can be considered only in selected patients (very high risk and failure to get anywhere near the target level despite statin/ezetimibe treatment at highest possible intensity)
*4Currently treatment with apheresis can be considered only in selected patients (very high risk and failure to get anywhere near the target level despite medicinal treatment at highest possible intensity)
LSM, Lifestyle modification
Other statins (lovastatin, fluvastatin, pravastatin, rosuvastatin, pitavastatin) play a minor role in Germany. Fluvastatin and pravastatin have somewhat lower rates of adverse effects than atorvastatin and simvastatin, so they can be administered in patients who do not tolerate the latter (30). Rosuvastatin has a particularly strong LDL cholesterol-lowering effect, but patients in Germany have to pay part of the costs themselves.
A special situation is represented by acute coronary syndrome (ACS). Initial studies have shown that very early high-dose statin administration improves the prognosis of patients with ACS (31, 32). The most plausible explanation is direct improvement of endothelial function, independent of LDL cholesterol (33). Meanwhile, however, these results are being interpreted more cautiously (34). Nevertheless, most guidelines recommend starting with high-dose statin treatment in patients with ACS.
Mixed hyperlipoproteinemia
Owing to its close association with the metabolic syndrome, mixed hyperlipoproteinemia, in which the concentrations of both LDL cholesterol and triglycerides are raised, is the most frequently occurring disorder of lipid metabolism in diabetics (35). Here too, the primary treatment goal is regulation of the LDL cholesterol level. To this end a statin is prescribed, perhaps in combination with ezetimibe. With regard to the hypertriglyceridemia, modification of the patient’s lifestyle is the key measure. If this combination of lifestyle modification and statin treatment does not achieve the target concentrations or at least normalize the triglyceride level, combined medicinal treatment can be considered (25). In principle, a statin can be administered together with omega-3 fatty acids or fibrates, but both of these combinations have performed disappointingly in endpoint studies (Table 4) (36– 38). Because these studies were poorly designed, however, no definitive conclusion can be drawn: each of these two groups of substances reduced the cardiovascular risk in monotherapy studies (39, 40). In our center, therefore, after exhaustion of the LDL cholesterol-lowering options, patients with very high risk and a mixed lipid metabolism disorder are treated with statin + fibrate or statin + omega-3 fatty acids. In the absence of comparative studies, neither of these two treatments can be preferred to the other. It may be best to test both combinations and then continue with the one that is tolerated better and achieves a superior response.
Table 4. Endpoint studies with lipid-lowering medications.
| Medication | Monotherapy | Combination with statin | Comments |
|---|---|---|---|
| Statin | ++++ | NA |
|
| Ezetimibe | 0 | ++ |
|
| Bile acid binding agents | + | 0 |
|
| Niacin | + | – – |
|
| Fibrate | + | – |
|
| Omega-3 fatty acids | + | – – | |
| PCSK9 antibodies | 0 | 0 |
+, One positive endpoint study; ++, two positive endpoint studies; ++++, >3 positive endpoint studies; 0, not investigated in endpoint studies
–, One negative endpoint study; – –, two negative endpoint studies; NA, not applicable
Hypertriglyceridemia
In isolated hypertriglyceridemia the triglyceride concentration is often far above normal while LDL cholesterol is low. The total cholesterol level may be elevated. As in mixed hyperlipoproteinemia, isolated hypertriglyceridemia usually responds well to lifestyle modification. However, there is no way to predict whether a given patient will react particularly well or poorly. Because no convincing studies have been published, no consensus has been reached as to when medicinal treatment should be initiated. However, the threshold is lower in patients at high risk of atherosclerosis than when hypertriglyceridemia is discovered incidentally in an otherwise healthy person. There is general agreement that if the triglyceride level stays above 400 mg/dL (4.6 mmol/L) despite implementation of lifestyle modification measures, a fibrate can be given (25). The best options seem to be fenofibrate (good tolerability) or gemfibrozil (positive endpoint studies; should not be combined with statins) (40, e1). Alternatively omega-3 fatty acids can be given, in combination if indicated (25). Statins are generally of little use in isolated hypertriglyceridemia, because the LDL cholesterol is often already very low at the outset. Regardless of the LDL cholesterol concentration, patients with known atherosclerosis should receive a small dose of statin (e.g., 20 mg simvastatin or 10 mg atorvastatin daily).
Lipoprotein(a) elevation
The lipoprotein(a) concentration is largely genetically determined and therefore needs to be measured only once (with a second measurement for confirmation if required). Elevated lipoprotein(a) levels (>30 mg/dL or >75 nmol/L) have a causal connection with atherosclerosis—insofar as that can be derived from epidemiological and genetic data—but are not amenable to significant lowering by means of either lifestyle modification or administration of any currently available medications (16, 17). The focus is therefore on optimization of other risk factors, with particular importance attached to lowering of LDL cholesterol. The target level depends on the clinical circumstances and is set according to the patient’s overall risk. Patients with high lipoprotein(a) concentrations and progressive cardiovascular disease despite optimal regulation of all other risk factors can, in principle, be treated with regular lipid apheresis (17). It remains to be seen what role in the lowering of lipoprotein(a) will be played by new developments (17, e2).
Isolated HDL cholesterol reduction
An abnormally low level of HDL cholesterol is mostly found in conjunction with hypertriglyceridemia. Occasionally, however, a patient presents with a normal triglyceride concentration but low HDL cholesterol. Since the HDL cholesterol level is a marker for cardiovascular risk but is not causally linked with atherosclerosis, isolated raising of HDL cholesterol does not reduce the risk (15, e3). Modifications of lifestyle, particularly increased physical activity, play an important role in treatment, because they not only increase the HDL cholesterol level but also normalize the function of HDL cholesterol. Lowering of LDL cholesterol can reduce the overall risk.
New treatment approaches
A number of new approaches to the treatment of various disorders of lipid metabolism have been developed. Particularly significant among them are the proprotein convertase subtilisin/kexin type 9 (PCSK9) antibodies (e4, e5). These medications can lead to a 50 to 60% reduction in LDL cholesterol, even in patients previously treated with combined statin and ezetimibe (e6). PCSK9 antibodies have only a slight effect on the concentrations of triglycerides and HDL cholesterol. However, they lower the lipoprotein(a) level by up to 30% (e7). Until the endpoint studies are completed and published (probably at the end of 2016), PCSK9 antibodies should only be given to carefully selected patients, namely those with known atherosclerosis and pronounced LDL hypercholesterolemia who cannot be treated by other means because their levels are so high or they are intolerant to statins.
Table 1. The recommendations of the European Society of Cardiology/European Atherosclerosis Society for treatment of lipid disorders*.
| LDL cholesterol (primary target concentration) | |
|---|---|
|
Very high risk Known coronary heart disease or other manifestation of atherosclerosis, type 1 or type 2 diabetes with end-organ damage, chronic kidney failure, 10-year risk for fatal cardiovascular event ≥ 10% (SCORE) |
<70 mg/dl (<1.8 mmol/l) and/or ≥ 50% decrease from initial concentration |
|
High risk Clearly elevated risk factors such as familial hypercholesterolemia, severe hypertension, or 10-year risk ≥ 5 to <10% (score) |
<100 mg/dl (<2.6 mmol/l) |
|
Moderate risk 10-year risk ≥ 1to <5% (score) |
<115 mg/dl (<3.0 mmol/l) |
Key Messages.
Correct classification of the disorders of lipid metabolism is important. They are divided into LDL hypercholesterolemia, hypertriglyceridemia, mixed hyperlipoproteinemia, and isolated lowering of HDL cholesterol. Lipoprotein(a) elevation can occur in isolation or combined with other lipid metabolism disorders.
The necessity and intensity of treatment depend on the overall cardiovascular risk and the severity of the lipid metabolism disorder.
The primary outcome measure is the LDL cholesterol level. The European guidelines advise detemination of the target values according to the absolute risk; patients at high risk need a particularly pronounced lowering of LDL hypercholesterol.
Measures to modify the patient’s lifestyle are taken above all in hypertriglyceridemia and mixed hyperlipoproteinemia.
Statins play a predominant role in medicinal treatment due to the positive results of endpoint studies. The only combination treatment to show positive results in such studies is statin plus ezetimibe; combinations with fibrate and/or omega-3 fatty acids have so far shown no positive effects. PCSK9 antibodies can be given in selected patients. Lipid apheresis may be indicated in progressive atherosclerosis.
Acknowledgments
Translated from the original German by David Roseveare
Footnotes
Conflict of interest statement
Prof. Parhofer has received honoraria for lectures, payments for advisory board or Data Monitoring Committee (DMC) service, and/or research funding from the following companies: Abbott, Aegerion, Amgen, AstraZeneca, Boehringer-Ingelheim, Fresenius, Genzyme, Isis, Kaneka, Kowa, Lilly, MSD, Novartis, Pfizer, Regeneron, Roche, Sanofi, and Berlin-Chemie.
References
- 1.Anitschkow N, Chalatow S. Über experimentelle Cholesterinsteatose und ihre Bedeutung für die Entstehung einiger pathologischer Prozesse. Centralbl Allg Pathol Pathol Anatomie. 1913;14:1–9. [Google Scholar]
- 2.Holme I, Boman K, Brudi P, et al. Observed and predicted reduction of ischemic cardiovascular events in the Simvastatin and Ezetimibe in Aortic Stenosis trial. Am J Cardiol. 2010;105:1802–1808. doi: 10.1016/j.amjcard.2010.01.363. [DOI] [PubMed] [Google Scholar]
- 3.Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344:1383–1389. [PubMed] [Google Scholar]
- 4.Baigent C, Blackwell L, Emberson J, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–1681. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013;1 doi: 10.1002/14651858.CD004816.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watts GF, Gidding S, Wierzbicki AS, et al. Integrated guidance on the care of familial hypercholesterolaemia from the International FH Foundation. Int J Cardiol. 2014;171:309–325. doi: 10.1016/j.ijcard.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 7.Ference BA, Yoo W, Alesh I, et al. Effect of long-term exposure to lower low-density lipoprotein cholesterol beginning early in life on the risk of coronary heart disease: a Mendelian randomization analysis. J Am Coll Cardiol. 2012;60:2631–2639. doi: 10.1016/j.jacc.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 8.Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–2397. doi: 10.1056/NEJMoa1410489. [DOI] [PubMed] [Google Scholar]
- 9.Boekholdt SM, Hovingh GK, Mora S, et al. Very low levels of atherogenic lipoproteins and the risk for cardiovascular events: a meta-analysis of statin trials. J Am Coll Cardiol. 2014;64:485–494. doi: 10.1016/j.jacc.2014.02.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robinson JG, Farnier M, Krempf M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489–1499. doi: 10.1056/NEJMoa1501031. [DOI] [PubMed] [Google Scholar]
- 11.Sabatine MS, Giugliano RP, Wiviott SD, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1500–1509. doi: 10.1056/NEJMoa1500858. [DOI] [PubMed] [Google Scholar]
- 12.Jorgensen AB, Frikke-Schmidt R, Nordestgaard BG, Tybjaerg-Hansen A. Loss-of-function mutations in APOC3 and risk of ischemic vascular disease. N Engl J Med. 2014;371:32–41. doi: 10.1056/NEJMoa1308027. [DOI] [PubMed] [Google Scholar]
- 13.Jorgensen AB, Frikke-Schmidt R, West AS, Grande P, Nordestgaard BG, Tybjaerg-Hansen A. Genetically elevated non-fasting triglycerides and calculated remnant cholesterol as causal risk factors for myocardial infarction. Eur Heart J. 2013;34:1826–1833. doi: 10.1093/eurheartj/ehs431. [DOI] [PubMed] [Google Scholar]
- 14.Varbo A, Benn M, Tybjaerg-Hansen A, Jorgensen AB, Frikke-Schmidt R, Nordestgaard BG. Remnant cholesterol as a causal risk factor for ischemic heart disease. J Am Coll Cardiol. 2013;61:427–436. doi: 10.1016/j.jacc.2012.08.1026. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz GG, Olsson AG, Abt M, et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med. 2012;367:2089–2099. doi: 10.1056/NEJMoa1206797. [DOI] [PubMed] [Google Scholar]
- 16.Nordestgaard BG, Chapman MJ, Ray K, et al. Lipoprotein(a) as a cardiovascular risk factor: current status. Eur Heart J. 2010;31:2844–2853. doi: 10.1093/eurheartj/ehq386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parhofer KG. Lipoprotein(a): Medical treatment options for an elusive molecule. Curr Pharm Design. 2011;17:871–876. doi: 10.2174/138161211795428777. [DOI] [PubMed] [Google Scholar]
- 18.Goff DC, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:49–73. doi: 10.1161/01.cir.0000437741.48606.98. [DOI] [PubMed] [Google Scholar]
- 19.Catapano AL, Reiner Z, De Backer G, et al. ESC/EAS guidelines for the management of dyslipidaemias. The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS) Atherosclerosis. 2011;217:3–46. doi: 10.1016/j.atherosclerosis.2011.06.028. [DOI] [PubMed] [Google Scholar]
- 20.Nicholls SJ, Tuzcu EM, Sipahi I, et al. Statins, high-density lipoprotein cholesterol, and regression of coronary atherosclerosis. JAMA. 2007;297:499–508. doi: 10.1001/jama.297.5.499. [DOI] [PubMed] [Google Scholar]
- 21.Wei L, MacDonald TM, Watson AD, Murphy MJ. Effectiveness of two statin prescribing strategies with respect to adherence and cardiovascular outcomes: observational study. Pharmacoepidemiol Drug Saf. 2007;16:385–392. doi: 10.1002/pds.1297. [DOI] [PubMed] [Google Scholar]
- 22.Mihaylova B, Emberson J, Blackwell L, et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380:581–590. doi: 10.1016/S0140-6736(12)60367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ridker PM, MacFadyen JG, Fonseca FA, et al. Number needed to treat with rosuvastatin to prevent first cardiovascular events and death among men and women with low low-density lipoprotein cholesterol and elevated high-sensitivity C-reactive protein: justification for the use of statins in prevention: an intervention trial evaluating rosuvastatin (JUPITER) Circ Cardiovasc Qual Outcomes. 2009;2:616–623. doi: 10.1161/CIRCOUTCOMES.109.848473. [DOI] [PubMed] [Google Scholar]
- 24.Malhotra A, Shafiq N, Arora A, Singh M, Kumar R, Malhotra S. Dietary interventions (plant sterols, stanols, omega-3 fatty acids, soy protein and dietary fibers) for familial hypercholesterolaemia. Cochrane Database Syst Rev. 2014;6 doi: 10.1002/14651858.CD001918.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hegele RA, Ginsberg HN, Chapman MJ, et al. The polygenic nature of hypertriglyceridaemia: implications for definition, diagnosis, and management. Lancet Diabetes Endocrinol. 2014;2:655–666. doi: 10.1016/S2213-8587(13)70191-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Estruch R, Ros E, Salas-Salvado J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013;368:1279–1290. doi: 10.1056/NEJMc1806491. [DOI] [PubMed] [Google Scholar]
- 27.Bao Y, Han J, Hu FB, et al. Association of nut consumption with total and cause-specific mortality. N Engl J Med. 2001. 2013;369:2011–2020. doi: 10.1056/NEJMoa1307352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu L, Piotrowski K, Rau T, et al. Walnut-enriched diet reduces fasting non-HDL-cholesterol and apolipoprotein B in healthy Caucasian subjects: a randomized controlled cross-over clinical trial. Metabolism. 2014;63:382–391. doi: 10.1016/j.metabol.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 29.Roffi M, Patrono C, Collet JP, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC) Eur Heart J. 2016;14(37):267–315. doi: 10.1093/eurheartj/ehv320. [DOI] [PubMed] [Google Scholar]
- 30.Stroes ES, Thompson PD, Corsini A, et al. Statin-associated muscle symptoms: impact on statin therapy-European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur Heart J. 2015;36:1012–1022. doi: 10.1093/eurheartj/ehv043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–1504. doi: 10.1056/NEJMoa040583. [DOI] [PubMed] [Google Scholar]
- 32.Pasceri V, Patti G, Nusca A, Pristipino C, Richichi G, Di Sciascio G. Randomized trial of atorvastatin for reduction of myocardial damage during coronary intervention: results from the ARMYDA (Atorvastatin for Reduction of MYocardial Damage during Angioplasty) study. Circulation. 2004;110:674–678. doi: 10.1161/01.CIR.0000137828.06205.87. [DOI] [PubMed] [Google Scholar]
- 33.Sparrow CP, Burton CA, Hernandez M, et al. Simvastatin has anti-inflammatory and antiatherosclerotic activities independent of plasma cholesterol lowering. Arterioscler Thromb Vasc Biol. 2001;21:115–121. doi: 10.1161/01.atv.21.1.115. [DOI] [PubMed] [Google Scholar]
- 34.Vale N, Nordmann AJ, Schwartz GG, et al. Statins for acute coronary syndrome. Cochrane Database Syst Rev. 2014;9 doi: 10.1002/14651858.CD006870.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu L, Parhofer KG. Diabetic dyslipidemia. Metabolism. 2014;63:1469–1479. doi: 10.1016/j.metabol.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 36.Bosch J, Gerstein HC, Dagenais GR, et al. n-3 fatty acids and cardiovascular outcomes in patients with dysglycemia. N Engl J Med. 2012;367:309–318. doi: 10.1056/NEJMoa1203859. [DOI] [PubMed] [Google Scholar]
- 37.Ginsberg HN, Elam MB, Lovato LC, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563–1574. doi: 10.1056/NEJMoa1001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kromhout D, Giltay EJ, Geleijnse JM. n-3 fatty acids and cardiovascular events after myocardial infarction. N Engl J Med. 2010;363:2015–2026. doi: 10.1056/NEJMoa1003603. [DOI] [PubMed] [Google Scholar]
- 39.Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Lancet. 1999;354:447–455. [PubMed] [Google Scholar]
- 40.Rubins HB, Robins SJ, Collins D, et al. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group. N Engl J Med. 1999;341:410–418. doi: 10.1056/NEJM199908053410604. [DOI] [PubMed] [Google Scholar]
- e1.Frick MH, Elo O, Haapa K, et al. Helsinki Heart Study: primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia. Safety of treatment, changes in risk factors, and incidence of coronary heart disease. N Engl J Med. 1987;317:1237–1245. doi: 10.1056/NEJM198711123172001. [DOI] [PubMed] [Google Scholar]
- e2.Tsimikas S, Viney NJ, Hughes SG, et al. Antisense therapy targeting apolipoprotein(a): a randomised, double-blind, placebo-controlled phase 1 study. Lancet. 2015 doi: 10.1016/S0140-6736(15)61252-1. [DOI] [PubMed] [Google Scholar]
- e3.Briel M, Ferreira-Gonzalez I, You JJ, et al. Association between change in high density lipoprotein cholesterol and cardiovascular disease morbidity and mortality: systematic review and meta-regression analysis. BMJ. 2009;338 doi: 10.1136/bmj.b92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e4.Stein EA, Gipe D, Bergeron J, et al. Effect of a monoclonal antibody to PCSK9, REGN727/SAR236553, to reduce low-density lipoprotein cholesterol in patients with heterozygous familial hypercholesterolaemia on stable statin dose with or without ezetimibe therapy: a phase 2 randomised controlled trial. Lancet. 2012;380:29–36. doi: 10.1016/S0140-6736(12)60771-5. [DOI] [PubMed] [Google Scholar]
- e5.Stein EA, Honarpour N, Wasserman SM, Xu F, Scott R, Raal FJ. Effect of the proprotein convertase subtilisin/kexin 9 monoclonal antibody, AMG 145, in homozygous familial hypercholesterolemia. Circulation. 2013;128:2113–2120. doi: 10.1161/CIRCULATIONAHA.113.004678. [DOI] [PubMed] [Google Scholar]
- e6.Blom DJ, Hala T, Bolognese M, et al. A 52-week placebo-controlled trial of evolocumab in hyperlipidemia. N Engl J Med. 2014;370:1809–1819. doi: 10.1056/NEJMoa1316222. [DOI] [PubMed] [Google Scholar]
- e7.Raal FJ, Giugliano RP, Sabatine MS, et al. Reduction in lipoprotein(a) with PCSK9 monoclonal antibody evolocumab (AMG 145): a pooled analysis of more than 1,300 patients in 4 phase II trials. J Am Coll Cardiol. 2014;63:1278–1288. doi: 10.1016/j.jacc.2014.01.006. [DOI] [PubMed] [Google Scholar]
- e8.Baigent C, Landray MJ, Reith C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377:2181–2192. doi: 10.1016/S0140-6736(11)60739-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e9.Lipid Research Clinics Program. The Lipid Research Clinics Coronary Primary Prevention Trial results. I. Reduction in incidence of coronary heart disease. JAMA. 1984;251:351–364. doi: 10.1001/jama.1984.03340270029025. [DOI] [PubMed] [Google Scholar]
- e10.Bruckert E, Labreuche J, Amarenco P. Meta-analysis of the effect of nicotinic acid alone or in combination on cardiovascular events and atherosclerosis. Atherosclerosis. 2010;210:353–361. doi: 10.1016/j.atherosclerosis.2009.12.023. [DOI] [PubMed] [Google Scholar]
- e11.Canner PL, Berge KG, Wenger NK, et al. Fifteen year mortality in Coronary Drug Project patients: long-term benefit with niacin. J Am Coll Cardiol. 1986;8:1245–1255. doi: 10.1016/s0735-1097(86)80293-5. [DOI] [PubMed] [Google Scholar]
- e12.Boden WE, Probstfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–2267. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- e13.Landray MJ, Haynes R, Hopewell JC, et al. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med. 2014;371:203–212. doi: 10.1056/NEJMoa1300955. [DOI] [PubMed] [Google Scholar]
- e14.Jun M, Foote C, Lv J, et al. Effects of fibrates on cardiovascular outcomes: a systematic review and meta-analysis. Lancet. 2010;375:1875–1884. doi: 10.1016/S0140-6736(10)60656-3. [DOI] [PubMed] [Google Scholar]
- e15.Nordestgaard BG, Chapman MJ, Humphries SE, et al. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: consensus statement of the European Atherosclerosis Society. Eur Heart J. 2013;34:3478–3490. doi: 10.1093/eurheartj/eht273. [DOI] [PMC free article] [PubMed] [Google Scholar]