Abstract
Background
Adverse events occurring after vaccination are routinely reported to the Vaccine Adverse Event Reporting System (VAERS). We studied serious adverse events (SAEs) of a neurologic nature reported after receipt of influenza A (H1N1) 2009 monovalent vaccine during the 2009–10 influenza season. Investigators in the Clinical Immunization Safety Assessment (CISA) Network sought to characterize these SAEs and to assess their possible causal relationship to vaccination.
Methods
Centers for Disease Control and Prevention (CDC) and Food and Drug Administration (FDA) physicians reviewed all SAE reports (as defined by the Code of Federal Regulations, 21CFR§314.80) after receipt of H1N1 vaccine reported to VAERS between October 1st 2009 and March 31st 2010. Non-fatal SAE reports with neurologic presentation were referred to CISA investigators, who requested and reviewed additional medical records and clinical information as available. CISA investigators assessed the causal relationship between vaccination and the event using modified WHO criteria as defined.
Results
212 VAERS reports of non-fatal serious neurological events were referred for CISA review. Case reports were equally distributed by gender (50.9% female) with an age range of 6 months to 83 years (median 38 years). The most frequent diagnoses reviewed were: Guillain-Barré Syndrome (37.3%), seizures (10.8%), cranial neuropathy (5.7%), and acute disseminated encephalomyelitis (3.8%). Causality assessment resulted in classification of 72 events as “possibly” related (33%), 108 as “unlikely” related (51%), and 20 as “unrelated” (9%) to H1N1 vaccination; none were classified as “probable” or “definite” and 12 were unclassifiable (6%).
Conclusion
The absence of a specific test to indicate whether a vaccine component contributes to the pathogenesis of an event occurring within a biologically plausible time period makes assessing causality difficult. The development of standardized protocols for providers to use in evaluation of adverse events following immunization, and rapid identification and follow-up of VAERS reports could improve causality assessment.
Keywords: Adverse event following immunization, H1N1 vaccine, Causality assessment
Introduction
In 2009, a novel influenza A virus emerged in Mexico and the United States [1–2] and spread rapidly worldwide [1]. Within 6 months, monovalent H1N1 vaccines (“H1N1 vaccine”) were developed, manufactured and licensed in the same manner as seasonal influenza vaccine, and recommended for general use in the United States (U.S.). However, heightened media attention increased public concerns about potential vaccine side effects [3–4]. H1N1 vaccine safety was monitored by several systems [5] including the Vaccine Adverse Event Reporting System (VAERS). VAERS is the U.S. national spontaneous vaccine safety surveillance system established in 1990 and co-sponsored by the Centers for Disease Control and Prevention (CDC) and the Food and Drug Administration (FDA) [6]. As a spontaneous reporting system, VAERS is subject to several limitations: variability in reporting, lack of data validation and completeness, and lack of denominator data on the number of vaccine doses administered [7].
Following widespread use of the H1N1 vaccine in the United States during the fall of 2009, members of the Clinical Consult Case Review (CCCR) working group, one component of the Clinical Immunization Safety Assessment (CISA) network [8], convened to discuss vaccine safety questions derived from VAERS reports. Because of the reported increase in Guillain-Barré Syndrome (GBS) case reports following immunization with the 1976 swine flu vaccine [9], assessing any possible relationship between the 2009 H1N1 vaccine and GBS was a key focus. In consultation with neurological experts, CISA investigators assigned every GBS case report a level of diagnostic certainty defined by the Brighton Collaboration [10](Table 1), an international scientifically independent network of researchers dedicated to providing high quality vaccine safety information. In addition to GBS, the Brighton Collaboration has developed standardized definitions for other adverse events following immunization (AEFI) to help in the collection and assessment of vaccine safety information [10].
Table 1.
Description of Brighton Criteria Definitions for GBS levels of diagnostic certainty
| Brighton Level 1 |
|
| Brighton Level 2 |
|
| Brighton Level 3 |
|
The Brighton Collaboration. https://brightoncollaboration.org/internet/en/index.html.
VAERS is not designed to assess a causal relationship between any vaccine and adverse event, thus CISA investigators applied a CISA-modified set of causality criteria to assess the possible causal relationship of the H1N1 vaccine to these events (Table 2). Many adverse health outcomes occur at all ages for which no specific cause can be identified and some will occur by chance following any intervention, such as receipt of vaccines [11]. Causality criteria have been developed by the World Health Organization (WHO) (Table 2) and used globally, predominantly in developing countries [12]. The WHO criteria are based primarily on the temporal relationship between vaccination and onset of the adverse event, and have thus been questioned for not adequately accounting for other more likely potential causes of the adverse event or considering the evidence supporting such an association [13]. For example, the WHO causality assessment of “possible” stipulates that the AEFI “could also be explained by a concurrent disease or other drugs or chemicals”. Thus, a patient with both a concurrent infection and an antecedent vaccination could meet the “possible” criteria even though the concurrent infection represented a more likely cause. For purposes of causality assessment in this study, we applied CISA modified WHO criteria which require that other more likely possible causes had been excluded before a case can be classified as possibly related to vaccination[14]. The revised criteria also explicitly incorporated the strength of the existing evidence for a causal relationship between the vaccine and adverse event of interest. We summarize the causality assessment of cases of serious neurological adverse events reported to VAERS following H1N1 vaccination based on our modified WHO criteria.
Table 2.
WHO causality assessment criteriaa compared with CISA investigator modified criteriab used in this report
| CISA Modified Criteria | Original WHO criteria | ||
|---|---|---|---|
| Definite | The report documents that the vaccine was given before the onset of the signs and symptoms and that the timing of onset was consistent with a known mechanism or published literature; there is substantial existing evidence in the medical literature establishing a causal relationship between vaccine(s) and the event, and other known causes of the event had been excluded. | Very Likely /Certain | Clinical event with plausible time relationship to vaccine administration, and which cannot be explained by concurrent disease or other drugs or chemicals |
| Probable | The report documents that the vaccine was given before the onset of symptoms and that the temporal relationship was consistent with a biologic mechanism or published literature; there is some evidence in the medical literature for a causal relationship between vaccine(s) and the event, and other known causes of the event had been excluded or were unlikely. | Probable | Clinical event with a reasonable time relationship to vaccine administration, and is unlikely to be attributed to concurrent disease or other drugs or chemicals |
| Possible | The report documents that the vaccine was given before the onset of symptoms; the medical literature does not establish or refute a causal relationship between vaccine(s) and the event, and known causes that are more likely associated with event had been excluded. | Possible | Clinical event with a reasonable time relationship to vaccine administration, but which could also be explained by concurrent disease or other drugs or chemicals |
| Unlikely | The report documents that the vaccine was given before the onset of symptoms; the medical literature does not establish or refute a causal relationship between vaccine(s) and the event, and there were other known causes of the clinical event that were more likely and/or had not been excluded. | Unlikely | Clinical event whose time relationship to vaccine administration makes a causal connection improbable, but which could plausibly be explained by underlying disease or other drugs or chemicals |
| Unrelated | The onset of the event was prior to vaccine administration; or there is substantial evidence in the medical literature that the vaccine does not cause the event; or there was a co-existing disease/condition, drug, or vaccine that caused the event; or the temporal relationship between vaccination and the event was not consistent with the biological onset of clinical event. | Unrelated | Clinical event with an incompatible time relationship to vaccine administration, and which could be explained by underlying disease or other drugs or chemicals. |
Collet JP, MacDonald N, Cashman N, et al. Monitoring signals for vaccine safety: the assessment of individual adverse event reports by an expert advisory committee. Advisory Committee on Causality Assessment. Bull World Health Organ. 2000;78(2):178–185.
Rosenberg M, Sparks R, McMahon A, Iskander J, Campbell JD, Edwards KM. Serious adverse events rarely reported after trivalent inactivated influenza vaccine (TIV) in children 6–23 months of age. Vaccine 2009 Jul 9;27(32):4278–83.
Methods
Identification and Presentation of Case Reports
All serious VAERS reports after receipt of H1N1 vaccines received between October 1st 2009 and March 31st 2010 were reviewed by CDC and/or FDA physicians. VAERS reports were classified as “serious” if the VAERS report indicated hospitalization, prolongation of a hospitalization, permanent disability, or were considered by the person filing the report to be life-threatening (ref: Code of Federal Regulations, 21CFR§314.80) [15]. Non-fatal serious reports of neurologic adverse events (i.e., involving weakness, sensory loss, or loss of consciousness) were reviewed by CDC physicians who confirmed that cases were classified correctly. Those which met criteria to be serious events were referred to CISA. However, deaths and military case reports were excluded as they were separately investigated by state health departments in collaboration with the CDC or the Department of Defense, respectively.
Each VAERS report was reviewed by one CISA site, whose investigator(s) also requested additional medical records and communicated with reporting physicians to request additional clinical information, if needed. Investigators presented the case reports and additional information in a structured format to the CCCR team during weekly scheduled conference calls. After initial review, the investigator(s) at each site contacted the provider listed on the VAERS report to learn the outcome of each neurological event.
Causality Assessment and Case Report Follow Up
The CCCR Working Group assessed causality using a modified version of the causality guidelines developed by the World Health Organization (WHO) in 2000 [12] (Table 2). Causality was judged on four criteria: 1) was the vaccine administered prior to the onset of the event; 2) was the time between vaccination and the event compatible with a known mechanism for the adverse event; 3) was there an association between the vaccine and the AEFI reported in the literature; 4) were there other possible causes for the AEFI (e.g., infectious illness). Unlike the WHO criteria, the modified criteria used here addressed the epidemiologic evidence of an association. In addition, these criteria expanded upon the definitions of temporal relationship between the AEFI and vaccine administration and more specifically addressed potential other causes (Table 2).
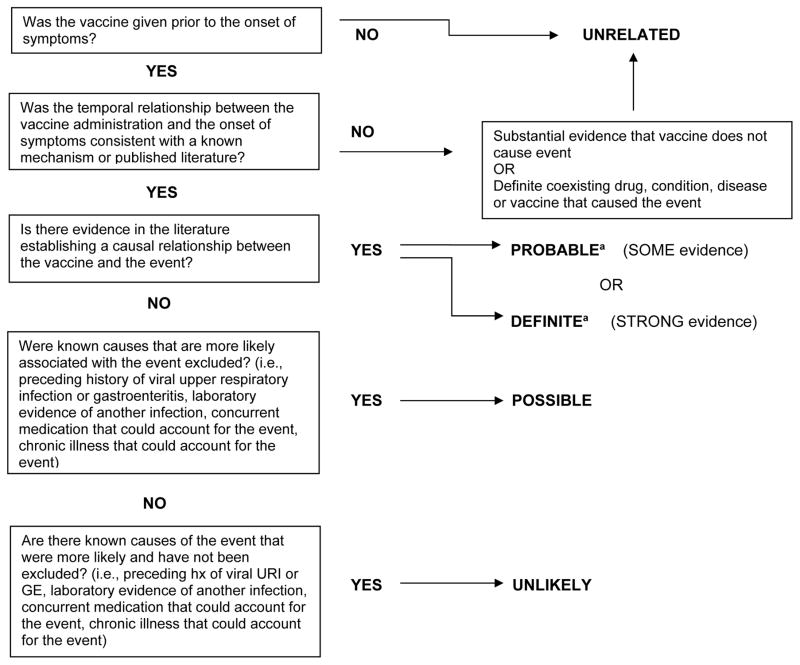
Because the 2009 H1N1 antigen had not been used previously in a vaccine, and only limited post-licensure safety data for the H1N1 vaccine were available in the literature at the time of this review, and because adverse events such as Guillain-Barre had been associated with some seasonal influenza vaccines but not the majority, we did not utilize the “definite” or “probable” categories in the modified WHO causation criteria. For a causal assessment of “possible” using these modified criteria, known causes that were more likely associated with the event needed to have been excluded. Therefore, if an upper respiratory or gastrointestinal illness was reported in the 4 weeks preceding GBS symptom onset, both associated with GBS in the literature [16], the case would not be causally assessed as “possibly” related to vaccine (and therefore would be classified as either “unlikely” or “unrelated”) since the preceding illness represented a “more likely” cause of the adverse event [17–18] (Figure 1). In reports with laboratory evidence of a co-existent infection, the working group did not assess these cases as causally “unrelated” to vaccine, but rather “unlikely”, since it was not certain that the co-existent infection caused the adverse event.
Figure 1.
Flow diagram depicting Modified WHO causality assessment
aBecause the H1N1 vaccine contained a novel strain, at the time of our review the evidence in the literature neither supported or refuted any causal association between this specific vaccine and the adverse events reviewed. Therefore, causal assessments of definite or probable were not applied.
Results
Characteristics of reported cases
During the time period from October 1, 2009 to March 31, 2010, 212 non-fatal serious neurological case reports following immunization with H1N1 vaccine were referred to CISA. Case reports were equally distributed by gender, with 50.9% female. Ages ranged from 6 months to 83 years of age (median age of 38 years, mean of 36.14 years). Fifty-five (25.9%) case reports documented receipt of at least one other vaccine in the 4 weeks prior to the onset of symptoms. Most subjects (75%) received inactivated influenza vaccine, 10% received live-attenuated influenza vaccine, and 15% of case reports did not include the vaccine type.
Neurological Adverse Events
The largest category of neurologic adverse events reviewed was GBS (n=79, 37.3%) (Table 3). Of these, the CCCR determined that 75 met Brighton diagnostic criteria [10] (Table 1). In total, 16 met criteria for level 1 (the highest level of diagnostic certainty), 57 met level 2 criteria, and 1 met level 3 criteria. One additional case report met Brighton criteria for Fisher variant GBS. Four reports of GBS were physician diagnosed but did not meet Brighton criteria based on available clinical information. A broad range of other neurological AEFI was reviewed including seizures, cranial neuropathy, and acute disseminated encephalomyelitis (ADEM) (Table 3). Final diagnoses were determined by CCCR physicians with the assistance of neurologists at CISA sites.
Table 3.
Neurological diagnoses in 212 H1N1 case reports reviewed by CISA
| Diagnosis | No. of case reports in this group (n = 212) | % of total case reports reviewed |
|---|---|---|
| Guillain-Barré Syndrome (GBS) 75 met Brighton Collaborationa diagnostic criteria, 4 did not but were physician diagnosed. |
79 | 37.3 |
| Seizure | 23 | 10.8 |
| Cranial Neuropathy | 12 | 5.7 |
| Demyelinating disorder of unclear etiology | 9 | 4.2 |
| Acute Demyelinating Encephalomyelitis (ADEM) | 8 | 3.8 |
| Weakness of unclear etiology | 8 | 3.8 |
| Cerebrovascular Disease | 7 | 3.3 |
| Encephalitis | 7 | 3.3 |
| Parasthesias | 7 | 3.3 |
| Psychogenic | 7 | 3.3 |
| Demyelinating disorder of known etiology | 7 | 3.3 |
| Sensory Neuropathy | 5 | 2.4 |
| Meningitis | 4 | 1.9 |
| Movement Disorder | 3 | 1.4 |
| Syncope | 2 | 0.9 |
| Miscellaneousb | 24 | 11.3 |
Brighton Collaboration[10]
Acute Vestibular Neuritis, Acute Vestibular Neuritis, Memory loss, Migraine, Speech impairment, Hearing loss, Hypertensive encephalopathy, Brachial plexopathy, Possible Corticobasilar Degeneration Syndrome, Myastenia Gravis exacerbation, Pain syndrome, Diabetic Radiculopathy, Degenerative Disc Disease of the Spine, Dystonia, Cerebellitis, Diffuse body numbness/pain, Brain tumor, Myalgias, Nonspecific disease, Transverse Myelitis vs Neoplasm, Spinal stenosis, Fluctuating neurological symptoms, , ADEM vs transverse myelitis vs tumor, Idiopathic Thrombocytopenic Purpura
Clinical Course and Concurrent Infectious Findings
The interval from vaccine administration to the onset of symptoms ranged from 11 days before to 146 days following immunization, with a median onset of 7 days post immunization. We were able to contact the initial reporter to determine resolution or progression of the reported SAE for 137 cases (64.6%). At the time of last contact, 3 were reported to have had no improvement or worsening of initial symptoms, 44 had complete recovery and 89 had partial recovery. However, these outcome data were often obtained within weeks after the initial report as CCCR investigators were frequently unable to contact providers at later dates. In 20 reports, there was some laboratory evidence of a concurrent infectious process at the time of the event (Table 4).
Table 4.
H1N1 case reports submitted to CISA with documented concurrent infections
| Diagnosis | Infectious etiology | Testing |
|---|---|---|
| Encephalitis | Influenza, type A | Nasopharyngeal rapid test positive |
| Acute Demyelinating Encephalomyelitis | Parainfluenza | PCR nasopharyngeal positive |
| Acute Demyelinating Encephalomyelitis | Parainfluenza | Positive culture nasal wash |
| Seizure | H1N1 influenza, wild type | Nasal swab culture positive |
| Demyelinating Disorder of Unclear Etiology | Hepatitis B | Previously diagnosed |
| Demyelinating Disorder of Unclear Etiology | Mycoplasma | Serum IgM positive |
| Seizure | Group A Streptococcus | Positive culture |
| Bell’s Palsy | Herpes Simplex Virus | Unknown test |
| Encephalitis | Mycoplasma and Enterovirus | IgM positive |
| Transverse Myelitis | Mycoplasma | Serum Mycoplasma IgM and IgG positive |
| GBS, Brighton level 2 a | Group A Streptococcus | Rapid antigen detection test positive |
| GBS, Brighton level 2 a | Pneumococcus | Positive blood culture |
| GBS, Brighton level 2 a | Mycoplasma | “Serum Reactive” IgM |
| GBS, Brighton level 2 a | Borrelia burgdorferi | Positive antibody screen and western blot |
| GBS, Brighton level 2 a | Epstein Barr Virus | EBV IgM positive serum |
| GBS, Brighton level 2 a | Enterovirus | Positive PCR in CSF |
| GBS, Brighton level 1 a | Cytomegalovirus | Serum IgM and IgG positive |
| GBS, Brighton level 1 a | Cytomegalovirus | CSF and serum DNA positive |
| GBS, physician diagnosed | Human Immunodeficiency Virus | Previously diagnosed |
Brighton Collaboration [10]
Causality Assessment
Causality assessment by the CCCR resulted in 72 case reports with “possible,” 108 with “unlikely,” and 20 with “unrelated” association with the H1N1 vaccine. Diagnoses with causal assessments of “possible” included GBS, Bell’s Palsy, sensory neuropathy, ADEM, demyelinating neurological disorder of unclear etiology, febrile seizure, afebrile seizure, weakness of unclear etiology, parasthesias, ataxia, transverse myelitis, optic neuritis, myasthenia gravis exacerbation, cerebrovascular accident, and sixth cranial nerve palsy (Table 5). Using the modified WHO criteria, reports were not classified as definitely or probably causally related to the vaccine because there was limited evidence to support or refute an association with the H1N1 vaccine (Figure 1). In 12 case reports, there was either insufficient information or no clear diagnosis (e.g., tumor versus transverse myelitis) for the group to assign causality; therefore, these were categorized as “unclassifiable”.
Table 5.
H1N1 Case reports submitted to CISA with causal assessments of “possible” according to the modified WHO criteria
| Diagnosis | Number of case reports | Mean age (years) | Mean interval to symptom onset (days) | Vaccine type (TIVa, LAIVb, unknown) | |
|---|---|---|---|---|---|
| GBS | Level 1c | 11 | 51.5 | 17.7 | 7 TIV, 1 LAIV, 3 unknown |
| Level 2c | 30 | 53.2 | 11.9 | 27 TIV, 3 unknown | |
| Physician diagnosedd | 2 | 17.5 | 13.0 | 1 LAIV, 1 unknown | |
| Cranial neuropathy | Bell’s Palsy | 4 | 38 | 9 | 2 TIV, 1 LAIV, 1 unknown |
| Optic neuritis | 1 | 46 | 14 | TIV | |
| Sixth nerve palsy | 1 | 15 | 13 | TIV | |
| Seizure | Prior history, febrile | 2 | 14.5 | 4.5 | TIV |
| Prior history, afebrile | 1 | 16 | 8 | TIV | |
| No prior history, afebrile | 3 | 2.0 | 5.3 | 1 TIV, 1 unknown | |
| Other | ADEM | 4 | 20.7 | 19.8 | 2 TIV, 1 LAIV, 1 unknown |
| Transverse Myelitis | 1 | 16 | 30 | TIV | |
| Sensory neuropathy | 3 | 42.0 | 6.7 | 2 TIV, 1 unknown | |
| Demyelinating disorders of unclear etiology | 3 | 27 | 16.3 | 2 TIV, 1 LAIV | |
| Parasthesias | 2 | 44.5 | 16.5 | 2 TIV | |
| Ataxia | 1 | 2 | 22 | LAIV | |
| Acute infarct of the basal ganglia | 1 | 6 | 2 | TIV | |
| Myasthenia gravis exacerbation | 1 | 65 | 14 | TIV | |
| Weakness, unclear etiology | 1 | 37 | 1 | TIV |
Trivalent inactivated influenza vaccine,
Live-attenuated influenza vaccine,
Brighton Collaboration [10],
GBS diagnosed by neurologist, but did not meet Brighton Collaboration diagnostic criteria based on available data.
Discussion
Pre-licensure (phase 3) clinical trials of vaccines are usually only able to identify adverse events that occur at rates of 1:10,000 or greater [19]. Therefore post-licensure monitoring for vaccine safety is crucial for detecting rare serious AEFI [19–20]. A total of 105,211,620 doses of inactivated H1N1 vaccine and 21,755,200 doses of live attenuated H1N1 vaccine were distributed in the U.S. as of April 28, 2010 [5]. Because of the GBS association seen previously with swine-origin influenza vaccine [9], we conducted a critical examination of serious non-fatal neurological AEFI reported to VAERS following immunization with 2009 H1N1 vaccine. Of several vaccine safety monitoring systems in place, VAERS is the spontaneous reporting system used by manufacturers, providers and the general public in the U.S. to report any event following vaccination whether or not the reporter believes the vaccine caused the event. The CCCR applied modified WHO causality criteria to these VAERS reports to determine the feasibility of such an exercise and its utility in vaccine safety monitoring following a concentrated public immunization campaign during this influenza pandemic. The purpose of this manuscript is to describe the structure and results of this systematic approach, to identify challenges encountered in attempting to assess causality, and to propose future improvements for this process.
For causality assessment, multiple criteria must be addressed [11, 21]. First, consideration should be given to the likelihood that the event could have occurred by chance alone through assessment of the background rate of disease [22–23]. Temporal association with vaccination is often the trigger for concern, but does not define a causal relationship [22]. For example, speculation existed about hepatitis B vaccination causing or triggering relapses of multiple sclerosis (MS) based on case reports. The rate of MS in the United States is estimated at 109 cases per 100,000 persons[24], and the onset or exacerbation of disease after vaccination appears to be coincidental as the increasingly large body of epidemiological evidence reveals no increased rate of MS onset or relapse after receipt of any licensed vaccine [25–27]. The modified WHO criteria used in this review addressed the epidemiologic evidence for causal assessment, thus taking into account whether an event occurred at a greater rate than the background incidence.
Second, there should be biological plausibility for the AEFI to be caused by the vaccine. The timing of the AEFI in relationship to vaccine administration is critical. One case report we reviewed reported onset of symptoms 11 days prior to the vaccine administration, which clearly illustrated that it did not result from the vaccine. Further, the event should occur within a defined window of risk following vaccine administration and for many AEFIs this defined risk window is not established. The CCCR working group assigned a risk interval for onset of GBS symptoms, and all demyelinating disorders, of 8 weeks following receipt of vaccine based on the available evidence [9, 18]. Also, the pathophysiologic mechanism by which the vaccine causes disease must make biological sense. For many of the neurological events reviewed, a pathophysiological mechanism could not be established.
Finally, causality assessment is difficult when assessing individual reports of AEFI, particularly if no prior association of the event with the vaccine of interest has been established through previous population-based studies. Because the epidemiological evidence did not support or refute a causal association between the H1N1 vaccine and the AEFI at the time of our review, we were limited to a maximum causal assessment of “possible”. As with any new vaccine, evidence of safety accumulates over time due to post-licensure safety monitoring. Since we began our review, a number of studies have been published evaluating the causal relationship between the H1N1 vaccine and GBS or other AEFI with various methods throughout the world [23, 28–30]; some report a slight increased risk of GBS associated with H1N1 vaccine (reporting rates of verified GBS reports within the 42 day window were 0.42 and 1.75 per million H1N1 vaccinations for age <25 yrs and age ≥25 yrs) [29], and some report no increased risk [23, 30]. Thus, these manuscripts support the 2003 consensus by the Institute of Medicine for seasonal influenza vaccine, that the “evidence is inadequate to accept or reject a causal relationship” rather than favoring a causal association as seen with the 1976 swine-origin vaccine [31]. However, because the causal association remains unclear, and a relationship between GBS and influenza vaccine is biologically plausible, continued post-marketing surveillance and research are warranted to further investigate this relationship. Of these reports evaluating H1N1 vaccine and GBS, only one applied standardized case definitions for GBS and took into consideration other causes for the event [30]. As questions of vaccine safety have international impact, the routine use of standardized case definitions, such the Brighton criteria, are beneficial. Also a standardized causality assessment instrument would also be helpful.
Although the modifications in the WHO criteria more thoroughly address components of causality assessment for individual reports of AEFI, CISA is working on a more detailed algorithmic approach for assessing causality in individual cases to illustrate the complexity of the multiple steps and factors that are necessary to consider when assessing causality.
The limitations of VAERS made the causality assessment challenging for multiple reasons. First, individuals can report to VAERS at any point after the AEFI. This is problematic because in many instances it was not possible to ask providers to obtain specimens, imaging or other testing to evaluate for other causes of the event in a timely manner. In 20 case reports, evidence of another infection was identified during the review of all available information (Table 4). However, minimal if any laboratory evaluation was available in the overwhelming majority of VAERS reports reviewed even though several infectious agents have been associated with the reported neurological events. Furthermore, for one third of case reports reviewed we were not able to obtain more detailed history or follow-up information despite the majority of VAERS reports being reviewed within days of submission. Finally, CISA did not evaluate cases involving death or military personnel, which may have biased our results. Deaths related to VAERS, however, were reported in a previous publication [29].
It is important that the vaccine safety community continue to closely monitor all serious AEFI to detect any unrecognized potentially vaccine-related SAEs. A systematic review of individual AEFI using VAERS case reports may prove to be hypothesis generating for future epidemiological studies. For the 2009 H1N1 vaccine, several large scale epidemiological studies of AEFI were already in place at the time of this review. For AEFIs that have a known likely association with an etiologic agent, proper systematic causal assessments can be valuable in ruling out vaccines as a causal agent if a more likely cause for the adverse event can be identified [11]. Thus, causal assessment of serious AEFI reported through VAERS, although challenging, could be enhanced by development and introduction of structured protocols for the evaluation of specific AEFI to rule out other causes of the event. Providers would need to be educated on the importance of referring to these protocols if there is concern that an AEFI may be causally related to the administered vaccine. Improved communication with reporting providers at the time of the AEFI workup would greatly enhance the quality of information necessary for a more fully informed causal assessment utilizing thorough case histories, laboratory evaluation, imaging and electrophysiological testing. This approach could maximize the CCCR’s ability to address causality through VAERS reports more quickly and accurately in an effort to add to our overall knowledge of vaccine safety.
Acknowledgments
We would like to acknowledge the assistance of the following individuals for their support in this effort: Susan Swope, Dr. Rosanna Setse, Virginia Frontiero, Howard Choi, Dr. Nandini Bakshi, and Mari Griffioen. We would also like to acknowledge the contributions of the Vaccine Healthcare Centers Network, including Dr. Jay Montgomery, Dr. Limone Collins and Dr. Renata Engler. This work was supported by the Clinical Immunization and Safety Assessment (CISA) network through a subcontract with America's Health Insurance Plans (AHIP) under contract 200-2002-00732 from the Centers for Disease Control and Prevention (CDC).
Footnotes
Disclaimer: The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.Butler D. Portrait of a year-old pandemic. Nature. 2010 Apr 22;464(7292):1112–3. doi: 10.1038/4641112a. [DOI] [PubMed] [Google Scholar]
- 2.CDC. The 2009 H1N1 Pandemic: Summary Highlights, April 2009–April 2010, A Virus Emerges. 2010 [cited 11/24/2010]; Available from: http://www.cdc.gov/h1n1flu/cdcresponse.htm#virus_emerges.
- 3.Hidiroglu S, Ay P, Topuzoglu A, Kalafat C, Karavus M. Resistance to vaccination: The attitudes and practices of primary healthcare workers confronting the H1N1 pandemic. Vaccine. 2010 Oct 14; doi: 10.1016/j.vaccine.2010.09.104. [DOI] [PubMed] [Google Scholar]
- 4.Torun SD, Torun F, Catak B. Healthcare workers as parents: attitudes toward vaccinating their children against pandemic influenza A/H1N1. BMC Public Health. 2010;10:596. doi: 10.1186/1471-2458-10-596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.US Department of Health and Human Services. National Vaccine Advisory Committee Report on 2009 H1N1 Vaccine Safety Risk Assessment. [cited 1/13/2011]; Available from: http://www.hhs.gov/nvpo/nvac/reports/vsrawg_repot_may2010.html.
- 6.CDC VAERS - Vaccine Adverse Event Reporting System. [cited 12/23/2010]; Available from: http://vaers.hhs.gov/index.
- 7.Iskander JK, Miller ER, Chen RT. The role of the Vaccine Adverse Event Reporting system (VAERS) in monitoring vaccine safety. Pediatr Ann. 2004 Sep;33(9):599–606. doi: 10.3928/0090-4481-20040901-11. [DOI] [PubMed] [Google Scholar]
- 8.CDC. Vaccine Safety - Clinical Immunization Safety Assessment (CISA) Network. 2010 Feb 25; [cited 08/04/2010]; Available from: http://www.cdc.gov/vaccinesafety/Activities/cisa.html.
- 9.Schonberger LB, Bregman DJ, Sullivan-Bolyai JZ, Keenlyside RA, Ziegler DW, Retailliau HF, et al. Guillain-Barre syndrome following vaccination in the National Influenza Immunization Program, United States, 1976--1977. Am J Epidemiol. 1979 Aug;110(2):105–23. doi: 10.1093/oxfordjournals.aje.a112795. [DOI] [PubMed] [Google Scholar]
- 10.The Brighton Collaboration. [cited October 23, 2010]; Available from: https://brightoncollaboration.org/internet/en/index.html.
- 11.Halsey NA. The science of evaluation of adverse events associated with vaccination. Semin Pediatr Infect Dis. 2002 Jul;13(3):205–14. doi: 10.1053/spid.2002.125864. [DOI] [PubMed] [Google Scholar]
- 12.Collet JP, MacDonald N, Cashman N, Pless R. Monitoring signals for vaccine safety: the assessment of individual adverse event reports by an expert advisory committee. Advisory Committee on Causality Assessment. Bull World Health Organ. 2000;78(2):178–85. [PMC free article] [PubMed] [Google Scholar]
- 13.Kavadas FD, Bitnun A, MacGregor D, Heurter H, Ford Jones EL. Acute neurological events associated with influenza vaccination: are the WHO criteria for assessing causality adequate? Scand J Infect Dis. 2008;40(6–7):565–70. doi: 10.1080/00365540701793709. [DOI] [PubMed] [Google Scholar]
- 14.Rosenberg M, Sparks R, McMahon A, Iskander J, Campbell JD, Edwards KM. Serious adverse events rarely reported after trivalent inactivated influenza vaccine (TIV) in children 6–23 months of age. Vaccine. 2009 Jul 9;27(32):4278–83. doi: 10.1016/j.vaccine.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 15.FDA. Code of Federal Regulations; Postmarketing reporting of adverse drug experiences. 2010 Apr 01;2010 [cited 11/24/2010]; Available from: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=314.80. [Google Scholar]
- 16.van Doorn PA, Ruts L, Jacobs BC. Clinical features, pathogenesis, and treatment of Guillain-Barre syndrome. Lancet Neurol. 2008 Oct;7(10):939–50. doi: 10.1016/S1474-4422(08)70215-1. [DOI] [PubMed] [Google Scholar]
- 17.Hadden RD, Karch H, Hartung HP, Zielasek J, Weissbrich B, Schubert J, et al. Preceding infections, immune factors, and outcome in Guillain-Barre syndrome. Neurology. 2001 Mar 27;56(6):758–65. doi: 10.1212/wnl.56.6.758. [DOI] [PubMed] [Google Scholar]
- 18.Haber P, DeStefano F, Angulo FJ, Iskander J, Shadomy SV, Weintraub E, et al. Guillain-Barre syndrome following influenza vaccination. JAMA. 2004 Nov 24;292(20):2478–81. doi: 10.1001/jama.292.20.2478. [DOI] [PubMed] [Google Scholar]
- 19.Offit PA, editor. Vaccines. 5. Saunders Elsevier; 2008. [Google Scholar]
- 20.Wilson K, Potter B, Manuel D, Keelan J, Chakraborty P. Revisiting the possibility of serious adverse events from the whole cell pertussis vaccine: were metabolically vulnerable children at risk? Med Hypotheses. 2010 Jan;74(1):150–4. doi: 10.1016/j.mehy.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 21.Fleming TR. Identifying and addressing safety signals in clinical trials. N Engl J Med. 2008 Sep 25;359(13):1400–2. doi: 10.1056/NEJMe0807372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Black S, Eskola J, Siegrist CA, Halsey N, Macdonald N, Law B, et al. Importance of background rates of disease in assessment of vaccine safety during mass immunisation with pandemic H1N1 influenza vaccines. Lancet. 2009 Dec 19;374(9707):2115–22. doi: 10.1016/S0140-6736(09)61877-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deeks SL, Lim GH, Simpson MA, Rosella L, Mackie CO, Achonu C, et al. Estimating background rates of Guillain-Barre Syndrome in Ontario in order to respond to safety concerns during pandemic H1N1/09 immunization campaign. BMC Public Health. 2011;11:329. doi: 10.1186/1471-2458-11-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Noonan CW, Williamson DM, Henry JP, Indian R, Lynch SG, Neuberger JS, et al. The prevalence of multiple sclerosis in 3 US communities. Prev Chronic Dis. 2010 Jan;7(1):A12. [PMC free article] [PubMed] [Google Scholar]
- 25.Miller AE, Morgante LA, Buchwald LY, Nutile SM, Coyle PK, Krupp LB, et al. A multicenter, randomized, double-blind, placebo-controlled trial of influenza immunization in multiple sclerosis. Neurology. 1997 Feb;48(2):312–4. doi: 10.1212/wnl.48.2.312. [DOI] [PubMed] [Google Scholar]
- 26.Rutschmann OT, McCrory DC, Matchar DB. Immunization and MS: a summary of published evidence and recommendations. Neurology. 2002 Dec 24;59(12):1837–43. doi: 10.1212/wnl.59.12.1837. [DOI] [PubMed] [Google Scholar]
- 27.Schattner A. Consequence or coincidence? The occurrence, pathogenesis and significance of autoimmune manifestations after viral vaccines. Vaccine. 2005 Jun 10;23(30):3876–86. doi: 10.1016/j.vaccine.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 28.Liang XF, Li L, Liu DW, Li KL, Wu WD, Zhu BP, et al. Safety of influenza A (H1N1) vaccine in postmarketing surveillance in China. N Engl J Med. 2011 Feb 17;364(7):638–47. doi: 10.1056/NEJMoa1008553. [DOI] [PubMed] [Google Scholar]
- 29.Vellozzi C, Broder KR, Haber P, Guh A, Nguyen M, Cano M, et al. Adverse events following influenza A (H1N1) 2009 monovalent vaccines reported to the Vaccine Adverse Event Reporting System, United States, October 1, 2009-January 31, 2010. Vaccine. 2010 Oct 21;28(45):7248–55. doi: 10.1016/j.vaccine.2010.09.021. [DOI] [PubMed] [Google Scholar]
- 30.Dieleman J, Romio S, Johansen K, Weibel D, Bonhoeffer J, Sturkenboom M. Guillain-Barre syndrome and adjuvanted pandemic influenza A (H1N1) 2009 vaccine: multinational case-control study in Europe. BMJ. 2011;343:d3908. doi: 10.1136/bmj.d3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Institute of Medicine. Immunization Safety Review: Influenza Vaccines and Neurological Complications. 2003 [cited July 7, 2011]; Available from: http://www.iom.edu/Reports/2003/Immunization-Safety-Review-Influenza-Vaccines-and-Neurological-Complications.aspx. [PubMed]