Abstract
Background
Fracture risk in men and women with type 1 diabetes (type 1 DM) has not been studied in a large prospective well designed cohort.
Objective
A systematic review and meta-analysis of observational studies were conducted to assess the association between type 1 DM and fractures.
Data source
Data were selected from Medline and Embase and abstract from annual scientific meeting of various diabetes and bone and mineral societies.
Study selection
Published studies reporting fracture risk in subjects with type 1 DM in comparison with subjects without diabetes between 1990 and July, 2014 and abstracts from various annual meeting (2005 onwards) were included for this meta-analysis.
Data extraction
Data were extracted from text of included publication or abstract of conferences.
Data synthesis
Fourteen studies that met inclusion criteria reported 2,066 fracture events among 27,300 subjects with type 1 DM (7.6%) and 136,579 fracture events among 4,364,125 subjects without diabetes (3.1%). The pooled relative risk (RR) of any fracture in subjects with type 1 DM was 3.16 (95% CI 1.51–6.63, p=0.002). Women and men with type 1 DM had four and two times higher risk for any fractures, respectively, compared to subjects without diabetes. The pooled RR of hip fractures and spinal fractures were 3.78 (95%CI; 2.05–6.98, p<0.001) and 2.88 (1.71–4.82, p<0.001), respectively, among subjects with type 1 DM.
Conclusion
Our meta-analysis suggests that both men and women with type 1 DM might have an increased risk for any fractures. A large prospective epidemiological study is needed to confirm our findings.
Introduction
Osteoporotic fractures result in increased morbidity and mortality that is preventable with effective screening and early treatment (1, 2). Low bone mineral density (BMD) has been consistently associated with high risk for fractures especially in postmenopausal women (3). Impairment in glucose homeostasis has been shown to alter BMD and bone structure (4–6). Most, but not, all studies have shown that BMD is decreased in people with type 1 diabetes (type 1 DM) (5–8). Nevertheless, studies have reported type 1 DM is associated with two- to six-fold higher risks for fractures, especially hip fracture (9–22). Type 1 DM has been associated with higher risk for fractures even compared to type 2 diabetes (17, 23). Furthermore, the risk for fractures in people with type 1 DM is higher even after adjustment with multiple variables such as age, gender, duration of diabetes and complications of diabetes (9, 17, 23).
As a result of better glycemic control and dramatic declines in acute and long-term complications, survival and life expectancy has improved for people with type 1 DM in the last 2 decades (24, 25). Therefore, more people with type 1 DM are older and at risk for osteoporosis fractures. To minimize the morbidity and mortality associated with fractures, the National Osteoporosis Foundation (NOF) guidelines recommend screening for osteoporosis in the general population for women aged 65 years or older and men aged 70 years and older (26). The U.S. Preventive Services Task Force (USPSTF) has a recommendation for screening of osteoporosis in women similar to that of NOF, but the USPSTF did not recommend screening for osteoporosis in men (27).
Currently, there are no guidelines or recommendations for screening of osteoporosis in people with type 1 DM despite the number of studies reporting higher risk for fractures (28). To our knowledge, no large prospective epidemiological study has been carried out to answer three important questions: 1) is the risk for fracture in people with type 1 DM higher than people without diabetes?, 2) is there a difference in risk for fractures at hip and spine?, and 3) is there any difference in the risk for fracture among men and women with type 1 DM ? Therefore, we conducted a meta-analysis aimed to answer these three essential questions.
Research Design and Methods
Data Sources and Searches
We followed a standardized protocol to do this meta-analysis as per the guidelines and similar to our previous study (29, 30). We conducted a systematic search for the articles from MEDLINE and Embase on the association between type 1 DM and fractures published between 1990 and July, 2014, using the following search terms: type 1 DM, insulin dependent diabetes mellitus (IDDM), insulin requiring diabetes, AND fracture, bone mineral density, osteopenia, or osteoporosis. “Abstracts” from annual scientific meetings (2005 onward) of the American Society for Bone and Mineral Research (ASBMR), American Diabetes Association (ADA), European Association for the Study of Diabetes (EASD), and the European Calcified Tissue Society (ECTS) were also searched. We also searched studies from cross references of the included studies.
Study selection
Two authors (VNS, CSS) independently screened abstracts of the studies reporting fractures in type 1 DM using search criteria mentioned above. The abstracts of the studies were reviewed further if the following criteria were fulfilled: 1) recruitment of subjects with type 1 DM as case and non-diabetic subjects as control groups, and 2) reported risk of fracture or incidence of fracture. The studies were excluded if they: a) enrolled only subjects with type 2 diabetes, b) did not classify diabetes type, c) did not reported incidence or risk of fracture, d) had incomplete or missing data, e) included no control group, f) included cases with post-transplant diabetes or diabetes subjects who underwent kidney or kidney-pancreas transplant, g) included intervention with any osteoporotic medication or surgical procedure. The studies were included after agreement between two authors (VNS, CSS) and disagreement was resolved with discussion and opinion of third author (JSK). The only studies published in English literature were included.
We have analyzed the risk for various fractures sites defined as major osteoporotic fractures by NOF (26). We excluded the fractures of ribs, face, skull, toes, fingers, stress fracture, neuropathic (Charcot’s) foot related fracture, and fracture as a result of hypoglycemic seizure.
Data extraction and quality assessment
From each study we extracted the name of first author, year of publication, country, criteria for defining type 1 DM, number of subjects with type 1 DM and number of controls, incidence of fractures among type 1 DM patients, and controls, and fracture sites (Table-1). The third author (JKS) verified the extracted data. The methodological quality of the included studies was assessed using the Newcastle-Ottawa Scale (31). The instrument used a star system to assess the study quality based on three criteria: (i) participants' selection (4 stars), (ii) comparability of study groups (2 stars) and (iii) assessment of exposure (3 stars). The highest total score for a study was nine. Higher quality study was defined if the study scored 7 or more stars.
Table 1.
Characteristics and quality assessment of the studies included for fracture risk in type 1 diabetes
| First author, year, (Ref) |
Study design | Risk measure | Gender | Age ● | Country | Study period |
Number of T1DM/ Control |
Fracture events T1DM /Control |
Fracture type |
Fracture events by fracture types, age and gender (T1DM/Control) |
Fracture assessment |
Definition of T1DM |
Variables adjusted |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hothersall et al, 2014, (9) |
Registry based population retrospective cohort study |
Incidence risk ratio (IRR) |
M & F |
20–84 | Scotland | 2005– 2007 |
21,708 / 3660000 € |
105/11733 | Hip | Men 20–39 (7/143) 40–49 (7/188) 50–59 (4/369) 60–69 (11/697) 70–79 (11/1314) 80–84 (3/872) |
Women 20–39 (2/39) 40–49 (5/121) 50–59 (13/459) 60–69 (13/1141) 70–79 (19/3341) 80–84 (10/3049) |
ICD-10 codes Δ |
Scottish Care information- Diabetes Collaboration data base |
Age, calendar year |
| Zhukouskaya et al, 2013 (10) |
Cross- sectional |
Prevalence (%) | M & F |
20–55 | Belarus | 2007– 2011 |
82/82 | 20/ 5 | Vertebral | Men 6/1 |
Women 14/4 |
Vertebral fracture assessment software by DXA |
American Diabetes Association criteria |
Age, sex, BMI, lumber spine BMD, score of diabetes complications, physical activity |
| Nicodemus et al, 2001 (11) |
Prospective Cohort |
RR | F | 55–69 | USA | 1986– 1997 |
47/30,377 | 5/ 452 | Hip | NR | Self-report | DM diagnosis≤30 year and Insulin use |
Age, BMI, smoking, use of estrogen, physical activity, intake of vitamin A and D, calcium, protein, alcohol, caffeine |
|
| Forsen et al, 1999 (12) |
Prospective Cohort |
RR | M & F |
> 50 | Norway | 1986– 1995 |
54 /33,594 | 4/1533 | Hip | Men 50–74 (1/217) >74 (0/204) |
Women 50–74 (3/565) >74 (0/547) |
ICD-9 code | DM diagnosis≤40 year and Insulin use |
Age, BMI, smoking |
| Vestergaard et al, 2005 (13) |
Registry based Case-control |
OR | M & F |
43± 27 |
Denmark | 2000 | 4369/484615 | 1703/119694 | Any fracture. However the hip, spine and forearm were analyzed separately. |
NR | ICD-10 codes | WHO criteria | Disease and medication affecting fracture, number of contact with health services, social variables such as working, income, living alone or with another person |
|
| Janghorbani et al, 2006 (14) |
Prospective Cohort |
RR | F | 34 – 59 |
USA | 1980– 2002 |
292/101,343 | 18/1255 | Hip | NR | Self-report | DM diagnosis≤30 year and insulin use or ketosis prone |
BMI, postmenopausal status, use of HRT, smoking, physical activity, history of osteoporosis, intake on thiazide, calcium, vitamin D, A and K, calcium, alcohol, caffeine |
|
| Murray et al, 2007 (15) |
Cross- sectional (matched) |
Prevalence (%) | F | 12 – 15 |
USA | NR | 11/11 | 6/0 | Any | NR | Self-report | NR | NR | |
| Melchior et al, 1994 (16) |
Retrospective cohort study |
RR | F | > 40 | Denmark | 1989– 1990 |
274¥/26,564 | 112/ 622*** | Hip Colles’ |
Colles 40–49 (13/106) 50–59 (28/101) 60–69 (23/149) 70–79 (13/103) >80 (5/50) |
Hip*** 40– 49(2/8) 50– 59(6/16) 60–69 (12/15) 70–79 (5/41) >80 (5/62) |
Registry, Self-report & Medical report, fracture after the age of 40 years |
Age <30 years with Insulin use |
NR |
| Ahmed et al, 2006 (17) |
Population based Cohort |
RR | M & F |
25 – 98 |
Norway | 1994– 2000 |
81/26704 | 8/1209 | All non- vertebral |
Hip Men Women 3/ 65 1/163 |
All Men Women 5/432 3/ 777 |
Radiological archives from hospital registry |
WHO criteria confirmed with ICD code |
Age, BMI, smoking for hip And age, BMI, smoking, BP, HDL and TG for non- vertebral |
| Danielson et al, 2009 (18) ≠ |
Matched, nested cross- sectional study (from population based cohort) |
Prevalence (%), OR |
F | 18 – 50 |
USA | 2005 | 75/75 | 28/18 | Any fracture except skull, face, metacarpals, fingers and toes |
NR | self-reported and confirmed by physician or X-ray |
Polyuria and Polydipsia with insulin use |
NR | |
| Neumann et al, 2011 (19) |
Matched cross- sectional study |
Prevalence(%), OR |
M & F |
20 – 70 $ |
Germany | 2006 | 128/77 | 29/9 | Upper extremities except rib, fingers and toes |
Men 14/5 |
Women 15/4 |
Self-report | NR | Age, BMI, family history of osteoporosis, smoking, contraceptive use, physical activity, vitamin D deficiency, A1c |
| Tuominen et al, 1999 (20) ≠ |
Cross- sectional |
Prevalence (%) | M & F |
57 – 72 |
Finland | 1984– 1990 |
56/498 | 5/34 | Wrist or forearm |
Men 0/6 |
Women 5/28 |
NR | C- peptide <0.20 nmol/l |
NR |
| Joshi et al, 2013 (21) ≠ |
Matched cross-sectional |
Prevalence (%) | M & F |
12 – 45 |
India | NR | 75/140 | 9/1 | Any fracture except skull, face, metacarpals, fingers and toes |
NR | Self-reported fracture at any sites in past 3 years |
NR | NR | |
| Roggen et al, 2013 (22) ≠ |
Cross- sectional |
Prevalence (%) | M & F |
16 – 24 |
Belgium | 2005– 2010 |
48/45## | 14/14 | Any site | Men 8/5 |
Women 6/9 |
Self-reported | NR | NR |
Abbreviations: T1DM; Type 1 Diabetes mellitus; NR; not reported; ICD; International classification of diseases codes, BMI; body mass index, BMD; bone mineral density, DXA; dual-X-ray absorptiometry, M; male, F; Female, RR; relative risk, OR; odds ratio, IRR; incident risk ratio, SD; standard deviation,
The data were provided by the investigators via personal communication.
only hospitalized cases of hip fractures have been reported
; 274 subjects out of 838 insulin treated subjects had insulin dependent diabetes.
age in range or mean ± SD;
, These studies did not have fracture incidence as primary end point though they reported fracture events,
a total of 622 females from the background population were admitted with Hip (n=142) and Colles’ (n=509) fracture over a period of 22 months $ the inclusion criteria for the study was age between 20–70 years however, mean age for men was 45.2±9.7 and for women 41.5±7.5 years.
Total number of subjects with T1DM was 56 but they reported fracture events from only 48 T1DM subjects.
Statistical methods
Data were analyzed using Comprehensive Meta-Analysis version 2.2 (Englewood, NJ, USA). As fracture is a rare event, the odds ratio mathematically approximates the relative risk (RR) and therefore, we presented all data as RR. We calculated RR using MedCalc® version 12.2.1 (MedCalc Software bvba, Mariakerke, Belgium) if the effect estimate was not reported and the raw data allowed RR calculations. Heterogeneity was assessed by I2 statistic to estimate variation across studies. I2 values of 25% or less, 50–74%, and 75% or higher represent low, moderate and high inconsistency, respectively. Random-effects model was chosen over a fixed-effect model when significant heterogeneity was observed (32). Tests for interaction using summary estimates were performed, using the method described by Altman and Bland (33). Publication bias was assessed using funnel plot and adjusted rank correlation tests (34, 35). All statistical tests were two-sided and P < 0.05 was considered statistically significant, except otherwise specified. Pre-specified Subgroup analyses were performed according to (i) cohort studies, (ii) higher quality studies, (iii) gender (male and female), (iv) fracture site (hip and spine), and (v) gender specific fracture types.
Results
Study selection
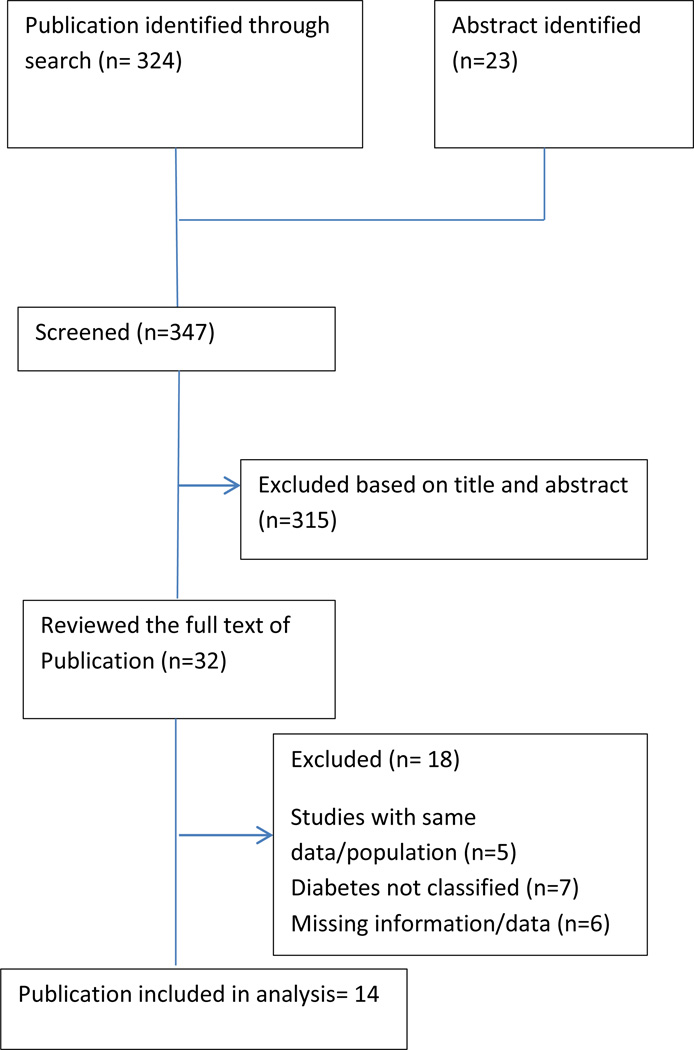
Of 324 publications and 23 abstracts presented at various scientific meetings identified initially, 315 were excluded based on titles and abstracts of the studies [Figure-1]. After full text review of 32 selected articles, 18 articles were excluded that did not qualify inclusion criteria. The excluded studies are shown in supplemental table-1. Finally, 14 studies were included for the analysis. Of these 14 studies, 13 were published articles and one was paper presented at scientific meeting.
Figure 1.
Flow of study selection and search
Study characteristics and description of study quality
Table-1 contains the characteristics of the studies including name of the first author, year of publication, country, and number of subjects with type 1 DM and without diabetes, fracture incidence, definition of type 1 DM, fracture assessment methods and adjusted variables. Of these 14 studies, six were cohort studies (9, 11, 12, 14, 16, 17), seven were cross-sectional studies (10, 15, 18–22) and one registry based case-control study (13). There were only three prospective cohort studies (11, 12, 14).Of the 14 studies, 10 studies had fracture event as primary end point (9–17, 19). There were 2,066 fracture events among 27,300 subjects with type 1 DM (7.6%) compared to 136,579 fracture events among 4,364,125 subjects without diabetes (3.1%). The studies by Hothersall et al and Melchoir et al reported the fracture events from hospital records (9, 16). Three studies identified fracture events based on International Classification of Disease (ICD) codes for fractures (9,12,13); one study reported spinal fractures based on vertebral fracture assessment software from DXA study (10); two studies have confirmed self-reported fracture either by physician or medical records (16,18) and six studies reported fractures events as self-reported without further confirmation (11,14,15,19,21,22). Four studies were conducted in US populations (11,14,15,18) and nine were in European populations (9,10,12,13,16,17,19,20,22). Seven studies reported hip fractures, two studies reported spinal (vertebral) fractures, and two studies reported wrist or forearm fractures [Table-1]. Four studies reported fracture events only in women (11, 14–16, 18). None of these studies stratified fractures by ethnicity. All but four have reported how they defined type 1 DM. The definition of type 1 DM and fracture assessment is detailed in Table-1. The quality of the studies was heterogeneous. Six studies were of higher quality (Newcastle-Ottawa Scale >7).
Type 1 diabetes and fracture risk
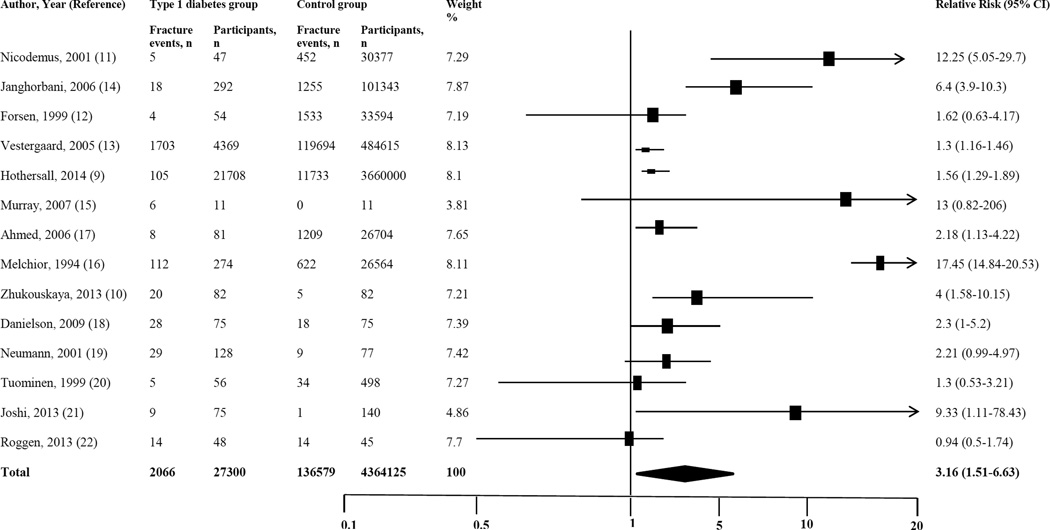
Fracture risk was calculated from all 14 studies that included 27,300 subjects with type 1 DM and 4,364,125 subjects without diabetes. The proportion of fracture events reported in subjects with type 1 DM was 7.6% compared to 3.1% in subjects without diabetes. The pooled relative risk (RR) of any fracture in subjects with type 1 DM was 3.16 (95% CI 1.51–6.63, p=0.002) compared to subjects without diabetes [Figure-2]. There was high heterogeneity across the studies (I2=98.25, p<0.001).
Figure 2.
Forest plot for fracture risk in subjects with type 1 diabetes
The sensitivity and subgroups analysis was carried out as specified in methods and presented in Table-2. After excluding outliers, the risk for any fracture was 1.5 times higher in subjects with type 1 DM (RR 1.54; 1.27–1.88, p<0.001). In a sensitivity analysis, including six cohort studies showed 4.45 fold higher risk for any fractures (RR 4.45; 1.33–14.89, p<0.01) in type 1 DM. Similarly, the risk was two times higher among subjects with type 1 DM in a sensitivity analysis including seven cross-sectional studies (RR 2.13; 1.24–3.67, p=0.006). In a random effect model with six higher-quality studies, the pooled RR of type 1 DM and any fracture was 2.96 (CI; 1.8–4.86, p<0.001).
Table 2.
Stratified and sensitivity analyses of the association of type 1 diabetes and risk of fractures
| Variable | Studies included N, (References) |
RR (95%CI), p | Heterogeneity (I2) |
|---|---|---|---|
| Sensitivity analysis | |||
| After excluding outliers | 9 (9,10,12,13,17–20,22) | 1.54 (1.27–1.88), P<0.001 | 41.89 |
| Cohort studies | 6 (9,11,12,14,16,17) | 4.45(1.33–14.89), P = 0.01 | 98.67 |
| Cross sectional studies | 7 (10,15,18–22) | 2.13 (1.24–3.67), P=0.006 | 51.39 |
| Higher quality studies | 6 (9–11, 13, 14, 17,) | 2.96 (1.80–4.86), P<0.001 | 92.58 |
| Stratified by fractures | |||
| Hip | 7 (9, 11, 12, 13, 14, 16, 17) | 3.78 (2.05–6.98), P<0.001 | 93.42 |
| Vertebral (spine) | 2 (10, 13) | 2.88(1.71–4.82)#, P<0.001 | 0.0 |
| Stratified by gender and fracture type | |||
| Men | |||
| Any fracture | 7 (9, 10, 12, 17, 19, 20, 22) | 1.79 (1.38–2.33)#, P<0.001 | 0 % |
| Hip Fracture | 3 (9, 12, 17) | 4.05 (0.99–16.47), P=0.051 | 81.43 |
| Fracture other than Hip | 3 (10, 17, 20) | 1.73 (0.59–5.09)#, P=0.317 | 0.00 |
| Women | |||
| Any fracture | 12 (9–12, 14–20, 22,) | 4.10 (1.79–9.38), P<0.001 | 96.22 |
| Hip Fracture | 6 (9, 11, 12, 14, 16, 17) | 5.19(2.22–12.11), P<0.001 | 92.80 |
| Fracture other than Hip | 4 (10, 15, 17, 20) | 2.65 (1.38–5.13)#, P= 0.004 | 4.84 |
| Country wise | |||
| USA | 4 (11,14, 15, 18) | 5.89 (4.05–8.58), P<0.001 | 62.73 |
| Europe | 9 (9–10, 12–13, 16–17, 19–20, 22) | 2.25 (0.89–5.62), P=0.08 | 98.87 |
Random effect model used for all analysis except marked as # where fixed model used because of low heterogeneity.
RR; Relative risk, N; number.
The pooled RR of hip fracture was 3.78 (95%CI; 2.05–6.98, p<0.001) while pooled RR for spinal fracture was 2.88 (1.71–4.82, p<0.001) among subjects with type 1 DM compared to subjects without diabetes. Since the study by Hothersall et al included the largest number of subjects with type 1 DM and captured only hip fracture events from patients who were hospitalized, it is quite possible that this study might have underestimated fracture risk and might affect the risk for hip fracture in our meta-analysis. Therefore, a sensitivity analysis was carried out after excluding the study by Hothersall et al, and the pooled relative risk for hip fracture was 4.51 (2.11–9.66, P<0.001).
The pooled RR for any fracture was higher in women with type 1 DM (RR 4.10; 1.79–9.38, p<0.001) compared to women without diabetes. Nevertheless, the risk for any fracture was also higher in men with type 1 DM compared to men without diabetes (1.79; 1.38–2.33, p<0.001). The risk for hip fracture and fractures other than hip was also higher in men and women with type 1 DM compared to people without diabetes [Table-2]. The pooled RR for any fracture in subjects with type 1 DM was higher compared to subjects without diabetes irrespective of studies with US populations (5.89; 4.05–8.58, p<0.001) or studies with European populations (2.25; 0.89–5.62, p=0.08).
No publication bias was observed among studies using adjusted rank correlation (p = 0.25) and the funnel plot showed minimal asymmetry (32, 33).
Discussion
Findings of this meta-analysis reveal that type 1 DM is associated with three-fold higher risk for any fracture. The relative risk for hip fracture and spinal fracture is 3.78 times and 2.88 times higher in type 1 DM, respectively. The risk for hip fracture is five times higher in women with type 1 DM and four times higher in men with type 1 DM. The risk for any fracture is higher in both US and European populations.
Our study showed that type 1 DM was associated with a three times higher risk for any fracture and the higher risk persisted even after sensitivity analysis including cohort studies and higher quality studies. Osteoporosis and fracture risk in subjects with type 1 DM is poorly understood as many factors influence bone health in people with type 1 DM (28). Studies have shown that BMD is lower in children and adolescents with type 1 DM (5, 7, 24). The inadequate accrual of bone mass may result into osteoporosis in later life (36) and may be one of the reasons for higher risk for fracture in subjects with type 1 DM. Poor glycemic control and longer duration of diabetes have been associated with low BMD and higher risk for fracture in subjects with type 1 DM in some studies (19, 23) but, not in other studies (10, 17, 21). None of the studies have stratified the fracture events as per the glycemic status or duration of type 1 DM hence; it was not possible for us to do sensitivity analysis based on these factors. Studies have also demonstrated higher risk for fractures in presence of microvascular complications especially nephropathy (23). Studies have found that risk for fracture is higher in patients with type 1 DM compared to type 2 diabetes (17, 37). A plausible reason may be that people with type 2 diabetes have higher insulin levels as a result of insulin resistance and therefore have higher BMD while type 1 DM are insulin deficient (28). Recently it has been shown that insulin increases bone density and strength via direct or indirect effect on bone formation and thus acting as anabolic agent to bone. (38). However, it is not known whether intensive insulin therapy helps in preserving BMD and reducing the risk for fractures. A study in type 1 DM showed stable BMD with intensive insulin therapy over 7 years (39) while a large randomized trial in type 2 diabetes showed no difference in fracture risk with intensive insulin therapy (40).
People with type 1 DM have increased risk for other autoimmune diseases such as thyroid disease and celiac disease. Presence of thyroid or celiac disease is an independent risk factor for fracture (41, 42). Most of the studies included in our analysis did not adjust the fracture risk for the presence of either thyroid or celiac disease; hence, contributions of these autoimmune diseases to fracture risk among subjects with type 1 DM may not be ruled out.
Studies have shown that the life-time risk for osteoporotic fractures is higher in elderly women compared to men (43, 44). Therefore, the guidelines by various associations and organizations uniformly recommend screening of osteoporosis in postmenopausal women at the age of 65 or greater, but there is no consensus among these guidelines on screening for osteoporosis in men (26, 27, 45–47). However, our meta-analysis revealed that risk for any fracture, hip fracture, and spinal fracture is higher in both men and women with type 1 DM compared to people without diabetes. Therefore, both men and women with type 1 DM should be considered as a risk factor for fracture and might be considered for screening of osteoporosis.
The risk for hip and spine fracture is higher in subjects with type 1 DM in our meta-analysis. However, it is difficult to comment on the risk for forearm fractures because only a few studies have reported the fracture events at these locations in subjects with type 1 DM. Similarly, it is difficult to comment on age and risk for fractures in subjects with type 1 DM as only three studies stratified fracture events as per age (9,12,16). Furthermore, the small number of fracture events in these studies made it difficult to explore association between type 1 DM and fracture risk with age stratification.
Our study has several limitations which should be noted. First, the studies included in this meta-analysis are observational studies and therefore, the findings of this meta-analysis should be interpreted with caution (48). Furthermore, the potential bias, confounding factors, and heterogeneity among the included studies might affect the findings of this study (48). However, we have taken care to address these limitations by reporting sensitivity analysis for fracture events after excluding outliers and analyzing the cohort studies and high quality studies separately. Second, the definition of type 1 DM in the studies varies. Therefore, it is quite possible that there may be misclassification of diabetes in many cases. However, we have taken adequate measures to reduce this error by excluding studies where either diabetes was not classified or authors have acknowledged misclassification of type 1 DM (supplemental Table-1). Similarly, fracture assessment was heterogeneous among the selected studies. The risk for hip fracture in our study might have been influenced by the study of Hothersall et al as they had the largest number of type 1 DM subjects and reported only hospitalized hip fractures events; therefore it might underestimate the fracture risk. Since the non-hospitalization of hip fracture is rare and the risk for hip fracture was almost similar even after excluding Hothersall study, we feel that the bias or influence of Hothersall study is minimal and unlikely to affect the result of our study. Third, all the studies except one were carried out in Caucasian populations. It is known that African Americans populations have higher BMD and less fracture risk (49). Since the studies have not reported the facture events stratified by ethnicity, it was not possible for us to comment on that. Therefore, the interpretation and findings of this meta-analysis should be limited to Caucasian populations. Fourth, most studies included in this meta-analysis have a relatively small number of subjects with type 1 DM compared to type 2 diabetes and non-diabetic subjects. Furthermore, the fracture events were also small resulting in wide confidence interval.
Despite these limitations, our study has important clinical and research implications. The guidelines by various organizations such as NOF, USPSTF, World Health Organization, American College of Physicians, and American College of Obstetricians and Gynecologists recommends BMD testing in women aged 65 or older or postmenopausal women younger than 65 in presence of other risk factors (26,27,43–45). Furthermore, many guidelines do not recommend testing BMD for osteoporosis screening in men (27). The findings of our study indicate that both men and women with type 1 DM are at higher risk for fractures compared to individuals without diabetes. This might have major public health implication in screening subjects with type 1 DM for osteoporosis. A prospective epidemiological study is necessary to recommend screening for osteoporosis among people with type 1 DM at younger ages than current recommendations.
In summary, our meta-analysis suggests that type 1 DM might be associated with increased risk for any fractures. The risk for fracture is higher in both men and women with type 1 DM. Further studies are needed to confirm these findings.
Supplementary Material
Acknowledgments
We acknowledge the help of Neeharik Mareedu, research intern at MD Anderson Cancer Center, Houston, TX, for data extraction, Lisa Meyers, Dawn White and Elizabeth Staton for editorial assistance, and Satish Garg, MD, and Aaron Michels, MD, at Barbara Davis Center for Diabetes for their review and inputs.
Funding: None
Footnotes
Conflict of interest: None
References
- 1.Leslie WD, Morin SN. Osteoporosis epidemiology 2013: implications for diagnosis, risk assessment, and treatment. Curr Opin Rheumatol. 2014;26:440–446. doi: 10.1097/BOR.0000000000000064. [DOI] [PubMed] [Google Scholar]
- 2.Hiligsmann M, Kanis JA, Compston J, et al. Health technology assessment in osteoporosis. Calcif Tissue Int. 2013;93:1–14. doi: 10.1007/s00223-013-9724-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siris ES, Miller PD, Barrett-Connor E, et al. Identification and fracture outcomes of undiagnosed low bone mineral density in postmenopausal women: results from the National Osteoporosis Risk Assessment. JAMA. 2001;286:2815–2822. doi: 10.1001/jama.286.22.2815. [DOI] [PubMed] [Google Scholar]
- 4.Krakauer JC, McKenna MJ, Buderer NF, Rao DS, Whitehouse FW, Parfitt AM. Bone loss and bone turnover in diabetes. Diabetes. 1995;44:775–782. doi: 10.2337/diab.44.7.775. [DOI] [PubMed] [Google Scholar]
- 5.Heilman K, Zilmer M, Zilmer K, Tillmann V. Lower bone mineral density in children with type 1 diabetes is associated with poor glycemic control and higher serum ICAM-1 and urinary isoprostane levels. J Bone Miner Metab. 2009;27:598–604. doi: 10.1007/s00774-009-0076-4. [DOI] [PubMed] [Google Scholar]
- 6.Loureiro MB, Ururahy MA, Freire-Neto FP, et al. Low bone mineral density is associated to poor glycemic control and increased OPG expression in children and adolescents with type 1 diabetes. Diabetes Res Clin Pract. 2014;103:452–457. doi: 10.1016/j.diabres.2013.12.018. [DOI] [PubMed] [Google Scholar]
- 7.Mastrandrea LD, Wactawski-Wende J, Donahue RP, Hovey KM, Clark A, Quattrin T. Young women with type 1 diabetes have lower bone mineral density that persists over time. Diabetes Care. 2008;31:1729–1735. doi: 10.2337/dc07-2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gallacher SJ, Fenner JA, Fisher BM, et al. An evaluation of bone density and turnover in premenopausal women with type 1 diabetes mellitus. Diabet Med. 1993;10:129–133. doi: 10.1111/j.1464-5491.1993.tb00029.x. [DOI] [PubMed] [Google Scholar]
- 9.Hothersall EJ, Livingstone SJ, Looker HC, et al. Contemporary risk of hip fracture in type 1 and type 2 diabetes: a national registry study from Scotland. J Bone Miner Res. 2014;29:1054–1060. doi: 10.1002/jbmr.2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhukouskaya VV, Eller-Vainicher C, Vadzianava VV, et al. Prevalence of morphometric vertebral fractures in patients with type 1 diabetes. Diabetes Care. 2013;36:1635–1640. doi: 10.2337/dc12-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nicodemus KK, Folsom AR Iowa Women's Health Study. Type 1 and type 2 diabetes and incident hip fractures in postmenopausal women. Diabetes Care. 2001;24:1192–1197. doi: 10.2337/diacare.24.7.1192. [DOI] [PubMed] [Google Scholar]
- 12.Forsén L, Meyer HE, Midthjell K, Edna TH. Diabetes mellitus and the incidence of hip fracture: results from the Nord-Trøndelag Health Survey. Diabetologia. 1999;42:920–925. doi: 10.1007/s001250051248. [DOI] [PubMed] [Google Scholar]
- 13.Vestergaard P, Rejnmark L, Mosekilde L. Relative fracture risk in patients with diabetes mellitus, and the impact of insulin and oral antidiabetic medication on relative fracture risk. Diabetologia. 2005;48:1292–1299. doi: 10.1007/s00125-005-1786-3. [DOI] [PubMed] [Google Scholar]
- 14.Janghorbani M, Feskanich D, Willett WC, Hu F. Prospective study of diabetes and risk of hip fracture: the Nurses' Health Study. Diabetes Care. 2006;29:1573–1578. doi: 10.2337/dc06-0440. [DOI] [PubMed] [Google Scholar]
- 15.Murray MA. Fracture incidence in adolescent girls with type 1 diabetes and relation to age at onset, metabolic control, bone characteristics and one turnover. Bone. 2007;40:S22–S89. [Google Scholar]
- 16.Melchoir TM, Sorensen H, Torp-Pedersen C. Hip and distal arm fracture rates in peri- and postmenopausal insulin-treated diabetic females. J Intern Med. 1994;236:203–208. doi: 10.1111/j.1365-2796.1994.tb01284.x. [DOI] [PubMed] [Google Scholar]
- 17.Ahmed LA, Joakimsen RM, Berntsen GK, Fønnebø V, Schirmer H. Diabetes mellitus and the risk of non-vertebral fractures: the Tromsø study. Osteoporos Int. 2006;17:495–500. doi: 10.1007/s00198-005-0013-x. [DOI] [PubMed] [Google Scholar]
- 18.Danielson KK, Elliott ME, LeCaire T, Binkley N, Palta M. Poor glycemic control is associated with low BMD detected in premenopausal women with type 1 diabetes. Osteoporos Int. 2009;20:923–933. doi: 10.1007/s00198-008-0763-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neumann T, Sämann A, Lodes S, et al. Glycaemic control is positively associated with prevalent fractures but not with bone mineral density in patients with Type 1 diabetes. Diabet Med. 2011;28:872–875. doi: 10.1111/j.1464-5491.2011.03286.x. [DOI] [PubMed] [Google Scholar]
- 20.Tuominen JT, Impivaara O, Puukka P, Ronnemaa T. Bone mineral density in patients with type 1 and type 2 diabetes. Diabetes Care. 1999;22:1196–1200. doi: 10.2337/diacare.22.7.1196. [DOI] [PubMed] [Google Scholar]
- 21.Joshi A, Varthakavi P, Chadha M, Bhagwat N. A study of bone mineral density and its determinants in type 1 diabetes mellitus. J Osteoporos. 2013;2013:397–814. doi: 10.1155/2013/397814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roggen I, Gies I, Vanbesien J, Louis O, De Schepper J. Trabecular bone mineral density and bone geometry of the distal radius at completion of pubertal growth in childhood type 1 diabetes. Horm Res Paediatr. 2013;79:68–74. doi: 10.1159/000346686. [DOI] [PubMed] [Google Scholar]
- 23.Vestergaard P, Rejnmark L, Mosekilde L. diabetes and its complications and their relationship with risk of fracture in type 1 and type 2 diabetes. Calcif Tissue Int. 2009;84:45–55. doi: 10.1007/s00223-008-9195-5. [DOI] [PubMed] [Google Scholar]
- 24.Pambianco G, Costacou T, Ellis D, Becker DJ, Klein R, Orchard TJ. The 30-year natural history of type 1 diabetes complications: the Pittsburgh Epidemiology of Diabetes Complications Study experience. Diabetes. 2006;55:1463–1469. doi: 10.2337/db05-1423. [DOI] [PubMed] [Google Scholar]
- 25.Miller RG, Secrest AM, Sharma RK, Songer TJ, Orchard TJ. Improvements in the life expectancy of type 1 diabetes: the Pittsburgh Epidemiology of Diabetes Complications study cohort. Diabetes. 2012;61:2987–2992. doi: 10.2337/db11-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Osteoporosis Foundation. Clinician’s guide to prevention and treatment of osteoporosis. Washington, DC: National Osteoporosis Foundation; 2013. [Accessed on September 9, 2014]. Available at http://nof.org/files/nof/public/content/file/2237/upload/878.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.U.S. Preventive Services Task Force. Screening for Osteoporosis: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2011;154:356–364. doi: 10.7326/0003-4819-154-5-201103010-00307. [DOI] [PubMed] [Google Scholar]
- 28.Dheon P, Shah VN. Type 1 Diabetes and Osteoporosis: A Review of Literature. Indian J Endocrinol Metab. 2014;18:159–165. doi: 10.4103/2230-8210.129105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shah VN, Shah CS, Bhadada SK, Rao DS. Effect of 25 (OH) D replacements in patients with primary hyperparathyroidism (PHPT) and coexistent vitamin D deficiency on serum 25(OH) D, calcium and PTH levels: a meta-analysis and review of literature. Clin Endocrinol (Oxf) 2014;80:797–803. doi: 10.1111/cen.12398. [DOI] [PubMed] [Google Scholar]
- 30.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 31.Wells GA, Shea B, O'Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of non-randomised studies in meta-analyses. Ottawa: Ottawa Hospital Research Institute; 2011. [accessed 16 February 2014]. Available: www.ohri.ca/programs/clinical_epidemiology/oxford.htm. [Google Scholar]
- 32.Song F, Sheldon TA, Sutton AJ, Abrams KR, Jones DR. Methods for exploring heterogeneity in meta-analysis. Eval Health Prof. 2001;24:126–151. doi: 10.1177/016327870102400203. [DOI] [PubMed] [Google Scholar]
- 33.Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ. 2003;326:219. doi: 10.1136/bmj.326.7382.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 35.Sterne JA, Egger M, Smith GD. Systematic reviews in health care: Investigating and dealing with publication and other biases in meta-analysis. BMJ. 2001;323:101–105. doi: 10.1136/bmj.323.7304.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bonjour JP, Chevalley T. Pubertal timing, bone acquisition, and risk of fracture throughout life. Endocr Rev. 2014;35:820–847. doi: 10.1210/er.2014-1007. [DOI] [PubMed] [Google Scholar]
- 37.Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes--a meta-analysis. Osteoporos Int. 2007;18:427–444. doi: 10.1007/s00198-006-0253-4. [DOI] [PubMed] [Google Scholar]
- 38.Thrailkill KM, Lumpkin CK, Jr, Bunn RC, Kemp SF, Fowlkes JL. Is insulin an anabolic agent in bone? Dissecting the diabetic bone for clues. Am J Physiol Endocrinol Metab. 2005;289:E735–E745. doi: 10.1152/ajpendo.00159.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Campos Pastor MM, López-Ibarra PJ, Escobar-Jiménez F, Serrano Pardo MD, García-Cervigón AG. Intensive insulin therapy and bone mineral density in type 1 diabetes mellitus: a prospective study. Osteoporos Int. 2000;11:455–459. doi: 10.1007/s001980070114. [DOI] [PubMed] [Google Scholar]
- 40.Schwartz AV, Margolis KL, Sellmeyer DE, et al. Intensive glycemic control is not associated with fractures or falls in the ACCORD randomized trial. Diabetes Care. 2012;35:1525–1531. doi: 10.2337/dc11-2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wirth CD, Blum MR, da Costa BR, et al. Subclinical thyroid dysfunction and the risk for fractures: a systematic review and meta-analysis. Ann Intern Med. 2014;161:189–199. doi: 10.7326/M14-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heikkilä K, Pearce J, Mäki M, Kaukinen K. Coeliac disease and bone fractures: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2014 Oct 3; doi: 10.1210/jc.2014-1858. jc20141858. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 43.Hannan MT, Felson DT, Dawson-Hughes B, Tucker KL, Cupples LA, Wilson PWF, Kiel DP. Risk Factors for Longitudinal Bone Loss in Elderly Men and Women: The Framingham Osteoporosis Study. J Bone Miner Res. 2000;15:710–720. doi: 10.1359/jbmr.2000.15.4.710. [DOI] [PubMed] [Google Scholar]
- 44.Nguyen ND, Ahlborg HG, Center JR, Eisman JA, Nguyen TV. Residual Lifetime Risk of Fractures in Women and Men. J Bone Miner Res. 2007;22:781–788. doi: 10.1359/jbmr.070315. [DOI] [PubMed] [Google Scholar]
- 45.Qaseem A, Snow V, Shekelle P, Hopkins R, Jr, Forciea MA, Owens DK. Clinical Efficacy Assessment Subcommittee of the American College of Physicians. Screening for osteoporosis in men: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2008;148:680–684. doi: 10.7326/0003-4819-148-9-200805060-00008. [DOI] [PubMed] [Google Scholar]
- 46.American College of Obstetricians and Gynecologists. Osteoporosis. Washington DC: American College of Obstetricians and Gynecologists; 2012. [Accessed on September 9, 2014]. Available at http://www.acog.org/About-ACOG/News-Room/News-Releases/2012/Osteoporosis-Guidelines-Issued. [Google Scholar]
- 47.WHO Study Group on Assessment of Fracture Risk and its Application to Screening for Postmenopausal Osteoporosis. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: report of a WHO study group. Geneva: World Health Organization; 1994. [Accessed on September 9, 2014]. (Technical Report Series, No. 843). Available at http://whqlibdoc.who.int/trs/WHO_TRS_843.pdf. [PubMed] [Google Scholar]
- 48.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 49.Cauley JA, Lui LY, Ensrud KE, Zmuda JM, Stone KL, Hochbeg MD, Cummings SR. Bone mineral density and the risk of incident Non-spinal fractures in black and white women. JAMA. 2005;293:2102–2108. doi: 10.1001/jama.293.17.2102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.