Abstract
Modifications of cardiolipin (CL) levels or compositions are associated with changes in mitochondrial function in a wide range of pathologies. We have made the discovery that acetaminophen remodels CL fatty acids composition from tetralinoleoyl to linoleoyltrioleoyl-CL, a remodeling that is associated with decreased mitochondrial respiration. Our data show that CL remodeling causes a shift in electron entry from complex II to the β-oxidation electron transfer flavoprotein quinone oxidoreductase (ETF/QOR) pathway. These data demonstrate that electron entry in the respiratory chain is regulated by CL fatty acid composition and provide proof-of-concept that pharmacological intervention can be used to modify CL composition.
Keywords: Cardiolipin, mitochondria, electron transport chain, electron entry point, acetaminophen
1. Introduction
Cardiolipin (CL) is a diphosphatidylglycerol predominantly localized and exclusively synthesized in the mitochondria (Schlame and Haldar, 1993 and Hatch, 1998) and comprised of two diacylglycerol phosphate residues combined with four fatty acid chains (Sparagna et al., 2007). The presence of four acyl chains suggests a great number of combinations of fatty acids and therefore a wide variety of CLs. However, the fatty acid composition of CLs in a tissue may vary depending on the species (Schlame et al., 2005), or tissue type (Han et al., 2006), and only a few species of CLs are present in a specific tissue, organ or cell type. Indeed, in most mammalian tissues, CLs are principally composed of 18-carbon unsaturated acyl chains and 80% are typically linoleic acid. For example, the fatty acyl chain composition of CLs in the mammalian heart is highly specific, being predominantly comprised of tetralinoleoyl-CL (L4CL) (Schlame et al., 2005).
CL is localized in the inner membrane of mitochondria and has multiple roles in a wide range of mitochondrial functions, including apoptosis, mitochondrial dynamics and structure, and bioenergetics (Houtkooper and Vaz, 2008, Schug and Gottlieb, 2009 and Ren et al., 2014). Due to their localization to the inner mitochondrial membrane, CLs are well positioned to influence the electron transport chain and facilitate mitochondrial function. CLs play a role in the organization of the electron transport chain complexes by binding with high affinity complexes I, III, IV and V (Eble et al., 1990, Pfeiffer et al., 2003, Sharpley et al., 2006 and Arnarez et al., 2013) and by stabilizing complexes and ADP/ATP carrier in multiple supercomplexes (Beyer and Klingenberg, 1985, Zhang et al., 2002, Claypool et al., 2008 and Mileykovskaya and Dowhan, 2014). Moreover, the presence of cardiolipin, is critical for optimal activity of complex I (Fry and Green, 1981 and Paradies et al., 2002), complex II (Schwall et al., 2012), complex III (Fry and Green, 1981 and Gomez and Robinson, 1999), complex IV (Robinson, 1993) and complex V (ATP synthase) (Eble et al., 1990). This concerted evidence indicates that CLs are a key component of the respiratory chain.
Abnormalities in CL fatty acid composition have been reported in multiple disorders such as pulmonary hypertension (Saini-Chohan et al., 2011), heart failure (Sparagna et al., 2007 and Saini-Chohan et al., 2009), acute myocardial ischemia and reperfusion (Lesnefsky et al., 2001 and Petrosillo et al., 2003), and both insulin- and non insulin-dependent diabetes mellitus (Han et al., 2005 and Han et al., 2007). These modifications of CL levels or fatty acid composition are associated with changes in mitochondrial function (Petrosillo et al., 2003 and Heather et al., 2010). Furthermore, restoring normal CL content protects from myocardial ischemia and reperfusion injury (Petrosillo et al., 2003), suggesting that modification of CL content or composition may impact susceptibility to tissue injury associated with these pathologies. Therefore, developing molecules that modulate CL composition could represent a novel pharmacological approach to treat multiple diseases.
In a previous study, we showed that acetaminophen (ApAP), the most widely used pain relieving and fever reducing drug in the world, potently prevents cardiolipin oxidation induced by both cytochrome c/hydrogen peroxide in liposomes and tBid in mitochondria isolated from mouse liver (Yin et al., 2012). The most abundant cardiolipin species in liver and in other organs with high metabolic rate is tetralinoleoyl-cardiolipin (L4CL) (Han et al., 2006). Therefore, we selected Myeloid Progenitor Cells (MPCs) for cell culture experiments, because of their high metabolic rate and because tetralinoleoyl (L4CL) and linoleoyltrioleoyl-CL (LO3CL) are the most abundant CL species in these cells (Supplementary Figure S1). While performing these experiments with the MPCs, we have made the unexpected discovery that ApAP remodels CL fatty acids from L4CL to LO3CL. Using ApAP as a tool, we showed that CL rearrangement is associated with decreased mitochondrial respiration. Because CL is required for coenzyme Q electron transfer activity (Schwall et al., 2012 and Pöyry et al., 2013), we investigated the effect of ApAP-derived CL remodeling on electron entry point in the respiratory chain. Our data shows that this modification in mitochondrial oxygen consumption level is due to a shift in electron entry point from complex II to the β-oxidation electron transfer flavoprotein quinone oxidoreductase (ETF/QOR) pathway, which is accompanied by reduced production of mitochondrial superoxide.
2. Methods
2.1 Myeloid Progenitor Cells (MPCs)
Hox11-immortalized MPCs were cultured in IMDM medium (Invitrogen) supplemented with 20% FBS (Gemini), 100 U/ml penicillin-streptomycin (Gibco), 2 mM glutamine (Gibco), 0.1 mM β-mercaptoethanol (Sigma), and 10% conditioned medium from WEHI cells as a source of IL-3 (Zinkel et al., 2003).
To determine the effect of acetaminophen, MPCs were incubated in the presence or absence of 100 μM of ApAP for 2 hours. At this time, cells were harvested by centrifugation at 600 rcf for 10 minutes, and pelleted cells were used for CL extraction or for isolating mitochondria as described below.
The antioxidant effect of Vitamin C was tested by preincubating the cells for 2 hours with its precursor Dihydroascorbate (DHA) at 200 μM. Cells were harvested as described above for ApAP. Intracellular concentration of ascorbate was measured by HPLC according to the ion-pairing method of Pachla and Kissinger (1979) as modified for electrochemical detection (May et al., 1998).
2.2 Cardiolipin quantitation by liquid chromatography-electrospray ionization tandem mass spectrometry (LC/ESI/MS/MS)
Analysis of cardiolipin content was performed as previously described (Yin et al., 2012). Briefly, lipids were extracted from 6 × 106 cells by addition of 0.75% NaCl and chloroform:methanol (2:1, v:v) containing 0.1 mM butyrated hydroxytoluene, 0.1 mM triphenylphosphine, and 2.5 μg tetramyristeoyl-cardiolipin (M4CL) as an internal standard. The organic phase was evaporated and resuspended in methanol:acetonitrile:H2O (60:20:20, v:v:v) and stored at −80°C until analysis. The extracted lipids were separated online by UPLC using a Waters Acquity UPLC system (Waters) using a Phenomenex Luna C18 (150 × 2.00 mm, 5 μm) column. Analysis was performed using a Thermo Quantum Ultra triple quadrupole mass spectrometer (Thermo Scientific) operated in negative ion mode using selective reaction monitoring (SRM). Nitrogen was used as the sheath gas at 38 p.s.i. and the capillary temperature was 350°C. The spray voltage was 4.5 kV and the tube lens voltage was 100 V. Data analysis was performed using Xcalibur software, version 2.0. The following ions were monitored in SRM: M4CL, m/z 619.6-227.2; L4CL, m/z 723.6-279.2; and LO3CL, m/z 729.6-281.2. To quantitate the relative amounts of CL species, the area under the curve (AUC) was determined for M4CL, L4CL, and LO3CL and the AUC of L4CL or LO3CL were normalized to the AUC of M4CL. We present ratio (L4CL/M4CL and LO3CL/M4CL) because the internal standard used, M4CL, is different from the CL species measured (L4CL and LO3CL), and therefore, the difference in ionisation and fragmentation between the different CL species, precludes exact quantitation. However, the ratio between the internal standard and the CL species measured is preserved in each sample, allowing accurate determination of the L4CL/LO3CL ratio. The normalized values represent the relative abundance of L4CL and LO3CL within each sample, and normalized values were also used to calculate the L4CL/LO3CL ratio.
2.3 Mitochondrial Respiratory Evaluation
Mitochondrial oxygen consumption was studied using an Oroboros O2K oxygraph (High-resolution respirometer, Oroboros Instruments). Oxygen consumption rates (OCR) were performed in 2 × 106 MPCs placed under continuous stirring in an oxygraphic cell containing 2 ml of culture medium at 37°C. To determine basal respiration, the average OCR was measured over an interval of stable oxygen flux following addition of cells to the chamber.
The different electron entry points were assessed by measuring OCR in MPCs permeabilized by exposure to digitonin. Cells were permeabilized by the addition of digitonin (15 μg/106 cells) in MiRO5 buffer (110 mM sucrose, 60 mM K+-lactobionate, 0.5 mM EGTA, 3 mM MgCl2, 20 mM taurine, 10 mM KH2PO4, 20 mM HEPES adjusted to pH 7.1 with KOH at 37°C). The mixture was incubated for 2.5 minutes at room temperature and the digitonin was removed by two rinses in MiRO5 buffer containing 1 g/L BSA fatty acid free. OCR were measured in MiRO5 buffer containing BSA and supplemented with 10 mM glutamate, 4 mM malate, 2 mM malonate and 2 mM ADP (CoQ/complex I) or with 10 mM succinate, 1 μM rotenone and 2 mM ADP (CoQ/complex II) or with 2 mM malonate, 1 μM rotenone, 20 μM palmitoylcarnitine and 2 mM ADP (CoQ/ETF/QOR).
2.4 Mitochondrial Isolation
Mitochondria were isolated from cells as described previously (Pallotti and Lenaz, 2001). Briefly, 6 × 106 MPCs were washed with PBS, centrifuged at 600 rcf for 10 minutes and resuspended in isolation solution (250 mM sucrose, 20 mM HEPES-KOH, pH 7.5, 10 mM KCl, 1.5 mM MgCL2 1 mM EDTA, 1 mM EGTA, 1 mM dithiothreitol, 0.1 mM PMSF). Suspended cells were homogenized with a glass-glass dounce homogenizer with 20 passes of the pestle. The homogenized cells were centrifuged at 750 rcf for 10 minutes at 4°C and the supernatant was collected. The pellet was resuspended in isolation solution and centrifuged at 750 rcf for 10 minutes at 4°C. The two resulting supernatants were pooled and centrifuged at 10,000 rcf for 15 minutes at 4°C. The mitochondrial pellet was then resuspended in isolation solution and kept on ice.
2.5 ATP measurements
ATP was measured using the ATP Lite luminescence assay (Perkin Elmer) following the manufacturer’s directions. The total intracellular ATP amount was evaluated in 10 000 cells previously treated or not with ApAP.
The rate of ATP production by mitochondria isolated from MPCs was also monitored using the same luciferin–luciferase system. Isolated mitochondria were placed at 37°C in MiRO5 with 10 mM glutamate and 4 mM malate. Aliquots were taken every 10 seconds after addition of 2 mM ADP, for a total analysis time of 60 seconds.
2.6 Superoxide Evaluation
Production of mitochondrial superoxide was measured in cultured MPCs using the fluorescent probe MitoSOX (Ex/Em: 510/580 nm, Invitrogen). Cells were incubated with 2 μM MitoSOX for 20 minutes at 37° C in CO2 incubator (Nazarewicz et al., 2013). Following treatment with APAP or vehicle, MPCs were washed with Krebs-Hepes buffer and incubated with 2 μM/L MitoSOX for 20 minutes. Then media was aspirated and cells were collected with rubber policemen and transferred into methanol for extraction of superoxide specific product of MitoSOX, 2-OH-ethydium-mitoSOX (Zielonka and Kalyanaraman, 2010). Production of mitochondrial superoxide was measured by accumulation of OH-ethydium-mitoSOX using HPLC analysis as previously described (Dikolova et al., 2010).
2.7 Cardiolipin liposome preparation and treatments
To prepare cardiolipin liposomes, purified bovine heart cardiolipin (Aventi Polar Lipids) and 1,2-Dioleoyl-sn-glycero-3-phosphocholine (DOPC) (Aventi Polar Lipids) were combined in a 1:1 molar ratio and dried under argon. Lipids were then resuspended in complete culture medium (Fig. 5A and B) or in MiRO5 supplemented with specific substrates and inhibitors, 10 mM glutamate, 4 mM malate, 2 mM malonate and 2 mM ADP (CoQ/complex I) or 10 mM succinate, 1 μM rotenone and 2 mM ADP (CoQ/complex II) or 2 mM malonate, 1 μM rotenone, 20 μM palmitoylcarnitine and 2 mM ADP (CoQ/ETF/QOR) (Fig. 5C, D and E). The suspension was sonicated using a misonix ultrasonic liquid processor on a setting of 3 with two 10 second pulses.
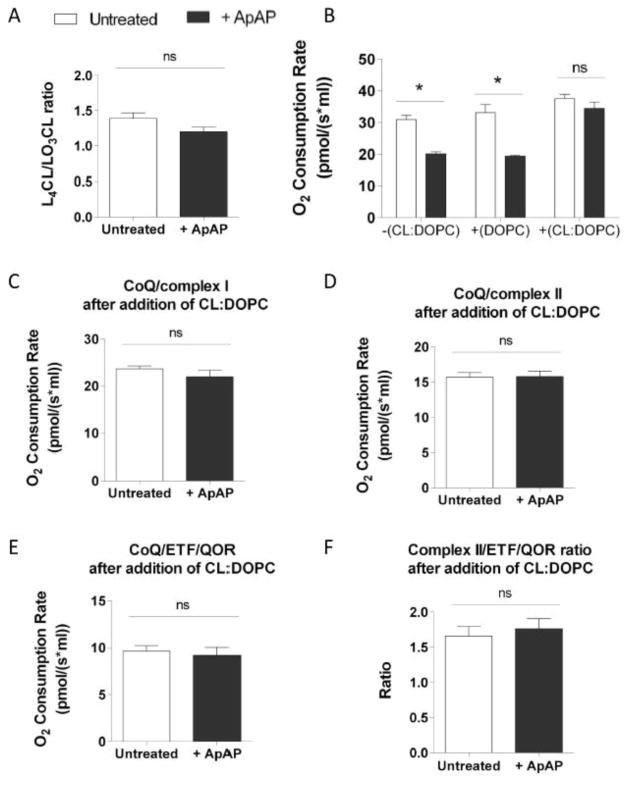
Figure 5. Addition of CL prevents modifications of oxygen consumption induced by ApAP.
Mitochondrial L4CL/LO3CL ratio measured in untreated MPCs and in MPCs treated with 100 μM of ApAP for 2 hours preincubated with CL:DOPC liposomes (A). Oxygen consumption rate in permeabilized MPCs treated or not with 100 μM of ApAP for 2 hours before and after 45 minutes treatment with CL:DOPC liposomes or DOPC liposomes (n=4) (B). O2 consumption rates evaluated in untreated MPCs and in MPCs treated with 100 μM of ApAP for 2 hours after 45 minutes of treatment with CL-liposomes in presence of 10 mM glutamate, 5 mM malate and 2 mM malonate (C) or in presence of 10 mM succinate and 1 μM rotenone (D) or in presence of 2 mM malonate, 1 μM rotenone and 20 μM palmitoylcarnitine (E) (n=5). Ratio between complex II and ETF/QOR-supported respiration measured in untreated MPCs and in MPCs treated with 100 μM of ApAP for 2 hours after 45 minutes treatment with CL:DOPC liposomes (F).Statistical significance was analyzed by Mann-Whitney test (error bars indicate SEM).
The effect of addition of CL-liposomes on CL composition was assessed. For this purpose, liposomes were added to cells to a final CL concentration of 125 μM (125 μM CL:125 μM DOPC). After 30 minutes of treatment with CL-liposomes, MPCs were washed twice with PBS to remove excess liposomes. Mitochondria were then isolated from cells (as described above) before lipids extraction. CL composition was measured in mitochondria isolated from MPCs by LC/ESI/MS/MS.
The effect of cardiolipin liposomes on mitochondrial function was studied by measuring oxygen consumption rate 45 minutes after addition of 125 μM CL:125 μM DOPC to permeabilized cells. The direct effect of DOPC on oxygen consumption rate was determined by treatment with 125 μM DOPC liposomes alone (Fig. 5B).
2.8 Statistical Analysis
Graphs and statistical analysis were completed using GraphPad Prism software. Data were analyzed by a Mann-Whitney test. For all figures: For statistical analysis of multiple means and multiple grouping parameters, a two-way ANOVA with bonferroni’s post test was used. * = P < 0.05, ** = P < 0.01, *** = P < 0.005, ns = non-significant and errors bars indicate SEM.
3. Results
3.1 Acetaminophen modifies cardiolipin fatty acid composition in mouse myeloid progenitor cells
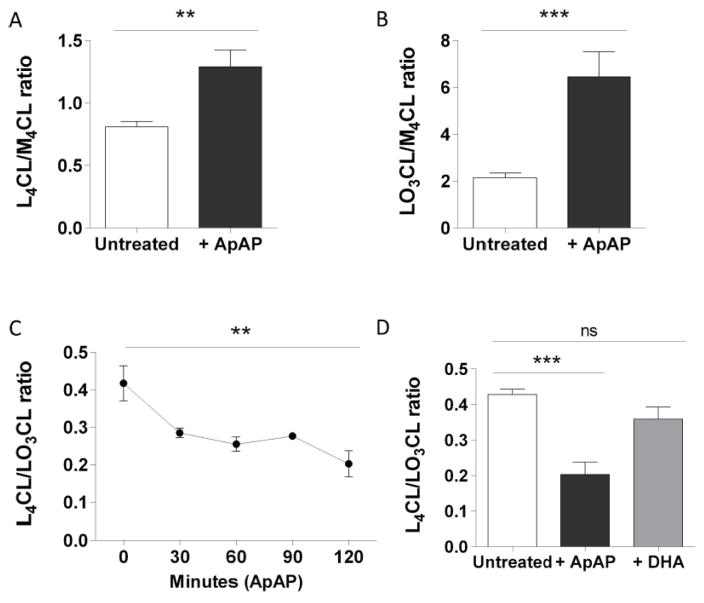
Using LC/ESI/MS/MS, we investigated whether ApAP could modify cardiolipin composition and levels in intact MPCs. We observed that ApAP treatment induces major modifications in the composition of two of them, tetralinoleoyl-CL (L4CL) and linoleoyltrileoyl-CL (LO3CL). Treatment of MPCs with 100 μM of ApAP for 2 hours caused increased levels of both L4CL and LO3CL (Fig. 1A and 1B). However, the L4CL/LO3CL ratio was decreased by 53 % and was almost complete after only 30 minutes (Fig. 1C), suggesting preclusion of gene transcription. Because ApAP has weak antioxidant properties, we assessed whether the ubiquitous antioxidant, vitamin c, caused similar CL remodeling. Our results indicate that treatment of MPCs with 200 μM of the vitamin c precursor DHA, increased intracellular vitamin c concentration by 1.69 folds but had no effect on CL composition, indicating that ApAP mediated-CL remodeling is not due to an antioxidant effect (Fig. 1D).
Figure 1. ApAP modifies cardiolipin composition in MPCs.
L4CL (A) and LO3CL (B) levels and L4CL/LO3CL ratio (C) measured in untreated MPCs and in MPCs treated with 100 μM of ApAP for 2 hours or for indicated time (n=6) or with 200 μM of DHA for 2 hours (D) (n=3). L4CL and LO3CL levels are expressed as the ratio to the internal standard, M4CL. Statistical significance was analyzed by Mann-Whitney test (error bars indicate SEM).
3.2 Rearrangement of CL induced by ApAP modifies mitochondrial respiration
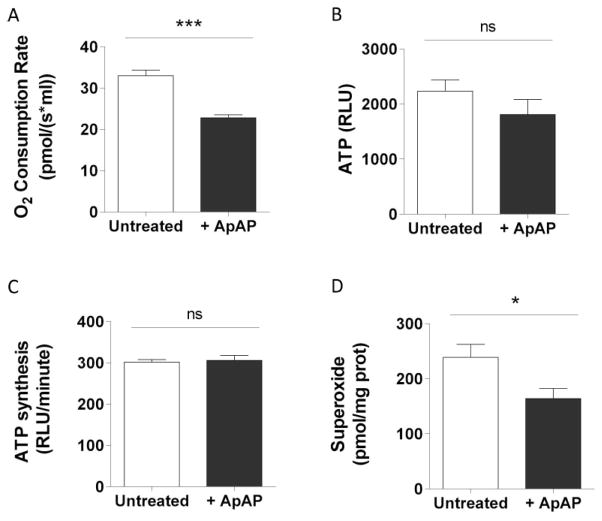
Cardiolipin is known to be required for normal mitochondrial function. Therefore, we investigated the consequence of the reduction in L4CL/LO3CL ratio induced by ApAP on mitochondrial respiration and ATP production. MPCs treated with ApAP displayed reduced basal oxygen consumption (Fig. 2A) without affecting ATP level measured in intact cells (Fig. 2B). We also evaluated ATP production rates in mitochondria isolated from untreated or ApAP treated MPCs, and showed that ATP production was not altered by ApAP (Fig. 2C). Because sustained ATP production following decreased respiration could be caused by increased efficiency of the respiratory chain, we measured superoxide levels and showed that they were decreased concomitantly with the reduction in oxygen consumption (Fig. 2D). Taken together, these results suggest that ApAP increases respiratory chain efficiency.
Figure 2. ApAP decreases mitochondrial respiration in MPCs without altering ATP production.
Oxygen consumption rate (A) and ATP level (B) in untreated MPCs and in MPCs treated with 100 μM of ApAP for 2 hours (n=6). ATP production by mitochondria isolated from untreated and treated MPCs (C) (n=3). Superoxide concentration (D) measured in untreated MPCs and in MPCs treated with 100 μM of ApAP for 2 hours using fluorescent dyes (MitoSox) (n=7). Statistical significance was analyzed by Mann-Whitney test (error bars indicate SEM).
3.3 ApAP induces a shift in the electron entry point from complex II to ETF/QOR
CLs are known to associate with respiratory complexes and facilitate their interaction with CoQ (Schwall et al., 2012 and Pöyry et al., 2013). This evidence together with our data showing that ApAP concomitantly modified CL fatty acid composition and respiration led us to hypothesize that the contribution of the different complexes to respiration could be regulated by ApAP. Accordingly, we used digitonin-permeabilized cells as well as specific electron transport chain substrates and inhibitors, in the presence of ADP as a phosphate acceptor (Hoffman and Brookes, 2009), to determine whether ApAP affected electron entry in the respiratory chain (Fig. 3).
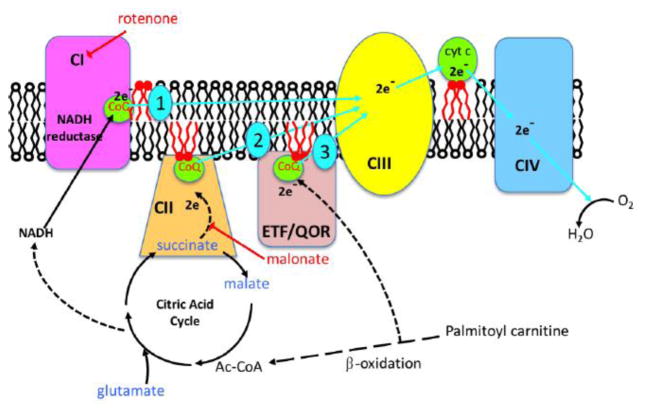
Figure 3.
Electron transport chain in the mitochondria showing the 3 electron entry points at complex I (1), complex II (2) and the ETF/QOR complex (3) (modified from Hoffman and Brookes, 2009).
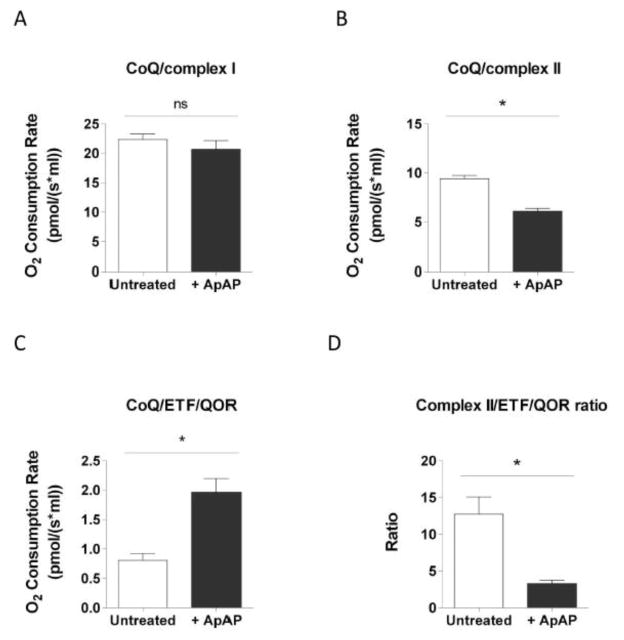
Respiratory rates measured in the presence of glutamate and malate as substrates were similar in ApAP-treated and untreated MPCs (Fig. 4A), indicating that electron entry through complex I is not affected by acetaminophen. Conversely, when feeding electrons into complex II using succinate as the respiratory substrate, ApAP treatment reduced oxygen consumption as compared with untreated cells (Fig. 4B). In contrast, MPCs treated with ApAP displayed a significant increase in oxygen consumption rate supported by palmitoylcarnitine (Fig. 4C). These data indicate that ApAP affects Complex II and ETF/QOR-supported respiration by shifting electron entry from complex II to the ETF/QOR pathway.
Figure 4. ApAP shifts electron entry point from complex II to ETF/QOR in MPCs.
The different electron entry points were assessed by measuring oxygen consumption rate in MiRO5 supplemented with 2 mM of ADP and specifics substrates and inhibitors. O2 consumption rate evaluated in untreated permeabilized MPCs and in permeabilized MPCs treated with 100 μM of ApAP for 2 hours incubated in presence of 10 mM glutamate, 5 mM malate and 2 mM malonate (A) or in presence of 10 mM succinate and 1 μM rotenone (B) or in presence of 2 mM malonate, 1 μM rotenone and 20 μM palmitoylcarnitine (C). Ratio between complex II and ETF/QOR-supported respiration measured in untreated MPCs and in MPCs treated with 100 μM of ApAP for 2 hours (D). Statistical significance was analyzed by Mann-Whitney test (n=4, error bars indicate SEM).
3.4 CL fatty acid remodeling composition mediates the changes in mitochondrial oxygen consumption
To assess whether the ApAP-induced decrease in oxygen consumption is due to the decreased L4CL/LO3CL ratio, we modified this ratio by the addition of exogenous L4CL. Liposomes composed of a 1:1 molar ratio of CL and the synthetic phospholipid dioleoylphosphatidylcholine (DOPC) were added to the culture medium for 30 minutes. Levels of L4CL after incubation with CL-liposomes were measured by LC/ESI/MS/MS in mitochondria isolated from untreated and ApAP-treated MPCs. Following addition of exogenous L4CL, mitochondrial levels of L4CL increased by 4 folds but those of LO3CL were unchanged (Supplementary Figure S2 and Fig. 1). Importantly, the L4CL/LO3CL ratio in MPCs preincubated with CL-liposomes increased to levels similar between those in untreated and ApAP-treated MPCs (Fig. 5A). The consequential modification in L4CL/LO3CL ratio was associated with a normalization of the mitochondrial oxygen consumption measured in digitonin-permealibilized cells (Fig. 5B, 5C, 5D and 5E). In contrast, DOPC-liposomes did not induce a significant change in mitochondrial oxygen consumption (Fig. 5B) demonstrating that restoration of normal respiration rate in CL-liposomes treated MPCs is CL-dependent.
The ratio of OCR between complex II and ETF/QOR-supported respiration decreased from 12.7 ± 2.3 to 3.3 ± 0.5 when ApAP was added (Fig. 4D). By comparison this ratio is lower in presence of exogenous L4CL (1.7 ± 0.1 in untreated cells and 1.8 ± 0.1 in ApAP-treated MPCs, Fig. 5F). The reduction of this ratio following L4CL addition indicates that CL modulates the electron entry point in the respiratory chain.
4. Discussion
Previous reports demonstrated that ApAP can decrease infarct size following cardiac ischemia/reperfusion (Merrill et al., 2001, Merrill and Goldberg, 2001, Merrill, 2002, Merrill et al., 2004 and Rork et al., 2004). The current model is that ApAP protects the heart against myocardial infarction via inhibition of the mitochondrial permeability transition pore and consequent cell death (Hadzimichalis et al., 2007). However, the mechanism of action of ApAP remains unknown. ApAP can affect mitochondrial function by modifying oxidative stress, protein modification, apoptosis or necrosis. In this manuscript, we describe a novel mechanism by which ApAP modifies mitochondrial function. Our data show that ApAP, independently of an antioxidant effect, causes profound remodeling of CL fatty acid composition, which is associated with a modification of the electron transport path in the mitochondria. Importantly, we have found that ApAP modulates CL composition at concentrations within the therapeutic range in humans, and we demonstrate for the first time that a pharmaceutical agent can affect CL fatty acid remodeling. Although the molecular pathway prior to CL remodeling still needs to be identified, our results could be explained by enhanced CL biosynthesis, impaired CL degradation, and/or modification of acyl chain remodeling. Moreover, given the specific mitochondrial localization of CL and its importance for mitochondrial morphology, this specific lipid is likely to influence the fluidity and the mechanical stability of the mitochondrial membrane. In particular, it has been demonstrated that CL level modulates packing of phospholipids found in the mitochondrial inner membrane (Unsay et al., 2013 and Zeczycki et al., 2014).
Our results showing that ApAP treatment reduces superoxide generation and increases respiration chain efficiency are in line with previous findings associating mitochondrial reactive oxygen species with increased proton (H+) leaks in the mitochondrial membrane, and a decrease in the respiratory control ratio. Increased proton leaks lead to enhanced state 4 oxygen consumption, indicating a compensatory increase in electron transfer which is however associated with the increased “electron leakage” from the electron transport chain to oxygen producing superoxide (Dikalova et al., 2010). ApAP improves cardiolipin composition and reduces cardiolipin oxidation (Yin et al., 2012), which reduces proton leaks and results in improved respiratory control ratio and diminished electron leakage to superoxide formation (Dikalova et al., 2015).
We have found that ApAP-induced reduction in basal mitochondrial respiration has no effects on ATP production or on total cellular ATP levels. This could be explained by the fact that MPCs, which are not undergoing significant stress or damage, do not experience changes in the ATP demand. However, in the context of oxidative stress or mitochondrial dysfunction, where mitochondria are not capable of supplying cells with adequate ATP, ApAP could have a beneficial effect on the cells by improving the respiratory chain efficiency, thus leading to decreased superoxide production and increased mitochondrial ATP production.
Loss of CL content, CL peroxidation, and changes in the CL acyl chain composition have been linked to a large variety of pathologies. Modifications of CL levels are associated with mitochondrial dysfunction and can have dramatic consequences on bioenergetics. CLs regulate mitochondrial functions through numerous mechanisms, including modification of respiratory complexes, membrane dynamics, supercomplex stabilization, and ATP synthase regulation. Most studies have focused on CL levels; however, recent evidence indicates that L4CL is important for complexes I and II activities by facilitating electron transfer through CoQ (Schwall et al., 2012 and Pöyry et al., 2013), suggesting that CL fatty acid composition may be as important as total CL levels. In this study, we show that, in MPCs, ApAP affects CL species differently. Indeed, ApAP leads to a 1.5 fold increase of L4CL levels but to a 3 fold increase in LO3CL levels, causing a 2 fold decrease of the L4CL/LO3CL ratio. Moreover, our results show that L4CL affects different respiratory complexes uniquely, and that L4CL may be used by the cells to regulate electron entry in the respiratory chain.
Mitochondrial respiration is controlled by a plethora of factors such as oxygen, ADP, and reducing equivalents (NADH, FADH2) availabilities. Our work demonstrates for the first time that CL fatty acid composition also affects electron entry in the respiratory chain through complex II and the β-oxidation electron transfer flavoprotein quinone oxidoreductase (ETF/QOR) pathway. Our results show that addition of exogenous CL increases L4CL levels by 4 folds. In contrast, exogenous CL does not affect levels of LO3CL or complex I-supported respiration. However, exogenous CL does increase complex II-supported respiration by 1.5 folds while increasing ETF/QOR-supported respiration by 10 folds. Taken together, these findings support our conclusion that CL fatty acid composition modulates electron entry in the respiratory chain through complex II and ETF/QOR. However, our results do not determine whether exogenous CL addition prevents ApAP modulation of the different respiratory complexes by maxing out their activities or by restoring a L4CL/LO3CL ratio similar to untreated cells.
In conclusion, our results provide proof-of-concept that pharmacological intervention can be used to modify CL fatty acids composition. Importantly, our work describes a novel role of cardiolipin in regulating the electron entry in the respiratory chain.
Supplementary Material
Highlights.
Acetaminophen causes remodeling of cardiolipin fatty acid side chains
Cardiolipin fatty acids composition modulates respiration chain efficiency
Cardiolipin fatty acids composition regulates electron entry in the respiratory chain
Cardiolipin fatty acids composition can be modified pharmacologically
Acknowledgments
The authors want to thank Dr. Joshua Fessel for giving us access to his Oroboros instrument and for his advice, and Qiong Shi to provide MPC cells for some experiments. The work described in this manuscript was in part funded by a grant from the NIH (GM15431).
Abbreviations
- CL
Cardiolipin
- ApAP
Acetaminophen
- MPCs
Myeloid Progenitor Cells
- CoQ
Coenzyme Q
- LC/ESI/MS/MS
Liquid Chromatography-Electrospray Ionization Tandem Mass Spectrometry
- L4CL
Tetralinoleoyl-cardiolipin
- LO3CL
Linoleoyltrioleoyl-cardiolipin
- M4CL
Tetramyristoyl-cardiolipin
- DOPC
Dioleoylphosphatidylcholine
- OCR
Oxygen Consumption Rate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnarez C, Marrink SJ, Periole X. Identification of cardiolipin binding sites on cytochrome c oxidase at the entrance of proton channels. Sci Rep. 2013;3:1263. doi: 10.1038/srep01263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer K, Klingenberg M. ADP/ATP carrier protein from beef heart mitochondria has high amounts of tightly bound cardiolipin, as revealed by 31P nuclear magnetic resonance. Biochemistry. 1985;24:3821–3826. doi: 10.1021/bi00336a001. [DOI] [PubMed] [Google Scholar]
- Claypool SM, Oktay Y, Boontheung P, Loo JA, Koehler CM. Cardiolipin defines the interactome of the major ADP/ATP carrier protein of the mitochondrial inner membrane. J Cell Biol. 2008;182:937–950. doi: 10.1083/jcb.200801152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikalova AE, Bikineyeva AT, Budzyn K, Nazarewicz RR, McCann L, Lewis W, Harrison DG, Dikalov SI. Therapeutic targeting of mitochondrial superoxide in hypertension. Circ Res. 2010;107:106–116. doi: 10.1161/CIRCRESAHA.109.214601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikalova AE, Kirilyuk IA, Dikalov SI. Antihypertensive effect of mitochondria-targeted proxyl nitroxides. Redox Biol. 2015;4:355–62. doi: 10.1016/j.redox.2014.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eble KS, Coleman WB, Hantgan RR, Cunningham CC. Tightly associated cardiolipin in the bovine heart mitochondrial ATP synthase as analyzed by 31P nuclear magnetic resonance spectroscopy. J Biol Chem. 1990;265:19434–19440. [PubMed] [Google Scholar]
- Fry M, Green DE. Cardiolipin requirement for electron transfer in complex I and III of the mitochondrial respiratory chain. J Biol Chem. 1981;256:1874–1880. [PubMed] [Google Scholar]
- Gomez B, Jr, Robinson NC. Phospholipase digestion of bound cardiolipin reversibly inactivates bovine cytochrome bc1. Biochemistry. 1999;38:9031–9038. doi: 10.1021/bi990603r. [DOI] [PubMed] [Google Scholar]
- Hadzimichalis NM, Baliga SS, Golfetti R, Jaques KM, Firestein BL, Merrill GF. Acetaminophen-mediated cardioprotection via inhibition of the mitochondrial permeability transition pore-induced apoptotic pathway. Am J Physiol Heart Circ Physiol. 2007;293:H3348–H3355. doi: 10.1152/ajpheart.00947.2007. [DOI] [PubMed] [Google Scholar]
- Han X, Yang J, Cheng H, Yang K, Abendschein DR, Gross RW. Shotgun lipidomics identifies cardiolipin depletion in diabetic myocardium linking altered substrate utilization with mitochondrial dysfunction. Biochemistry. 2005;44:16684–16694. doi: 10.1021/bi051908a. [DOI] [PubMed] [Google Scholar]
- Han X, Yang J, Yang K, Zhao Z, Abendschein DR, Gross RW. Alterations in myocardial cardiolipin content and composition occur at the very earliest stages of diabetes: a shotgun lipidomics study. Biochemistry. 2007;46:6417–28. doi: 10.1021/bi7004015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Yang K, Yang J, Cheng H, Gross RW. Shotgun lipidomics of cardiolipin molecular species in lipid extracts of biological samples. J Lipid Res. 2006;47:864–879. doi: 10.1194/jlr.D500044-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch GM. Cardiolipin: biosynthesis, remodeling and trafficking in the heart and mammalian cells (Review) Int J Mol Med. 1998;1:33–41. doi: 10.3892/ijmm.1.1.33. [DOI] [PubMed] [Google Scholar]
- Heather LC, Carr CA, Stuckey DJ, Pope S, Morten KJ, Carter EE, Edwards LM, Clarke K. Critical role of complex III in the early metabolic changes following myocardial infarction. Cardiovasc Res. 2010;85:127–136. doi: 10.1093/cvr/cvp276. [DOI] [PubMed] [Google Scholar]
- Hoffman DL, Brookes PS. Oxygen sensitivity of mitochondrial reactive oxygen species generation depends on metabolic conditions. J Biol Chem. 2009;284:16236–16245. doi: 10.1074/jbc.M809512200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houtkooper RH, Vaz FM. Cardiolipin, the heart of mitochondrial metabolism. Cell Mol Life Sci. 2008;65:2493–2506. doi: 10.1007/s00018-008-8030-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesnefsky EJ, Slabe TJ, Stoll MS, Minkler PE, Hoppel CL. Myocardial ischemia selectively depletes cardiolipin in rabbit heart subsarcolemmal mitochondria. Am J Physiol Heart Circ Physiol. 2001;280:H2770–H2778. doi: 10.1152/ajpheart.2001.280.6.H2770. [DOI] [PubMed] [Google Scholar]
- May JM, Qu ZC, Mendiratta S. Protection and recycling of alpha-tocopherol in human erythrocytes by intracellular ascorbic acid. Arch Biochem Biophys. 1998;349:281–289. doi: 10.1006/abbi.1997.0473. [DOI] [PubMed] [Google Scholar]
- Merrill G, McConnell P, Vandyke K, Powell S. Coronary and myocardial effects of acetaminophen: protection during ischemia-reperfusion. Am J Physiol Heart Circ Physiol. 2001;280:H2631–H2638. doi: 10.1152/ajpheart.2001.280.6.H2631. [DOI] [PubMed] [Google Scholar]
- Merrill GF. Acetaminophen and low-flow myocardial ischemia: efficacy and antioxidant mechanisms. Am J Physiol Heart Circ Physiol. 2002;282:H1341–H1349. doi: 10.1152/ajpheart.00716.2001. [DOI] [PubMed] [Google Scholar]
- Merrill GF, Goldberg E. Antioxidant properties of acetaminophen and cardioprotection. Basic Res Cardiol. 2001;96:423–430. doi: 10.1007/s003950170023. [DOI] [PubMed] [Google Scholar]
- Merrill GF, Rork TH, Spiler NM, Golfetti R. Acetaminophen and myocardial infarction in dogs. Am J Physiol Heart Circ Physiol. 2004;287:H1913–H1920. doi: 10.1152/ajpheart.00565.2004. [DOI] [PubMed] [Google Scholar]
- Mileykovskaya E, Dowhan W. Cardiolipin-dependent formation of mitochondrial respiratory supercomplexes. Chem Phys Lipids. 2014;179:42–48. doi: 10.1016/j.chemphyslip.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazarewicz RR, Bikineyeva A, Dikalov SI. Rapid and specific measurements of superoxide using fluorescence spectroscopy. J Biomol Screen. 2013;18:498–503. doi: 10.1177/1087057112468765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachla LA, Kissinger PT. Analysis of ascorbic acid by liquid chromatography with amperometric detection. Methods Enzymol. 1979;62:15–24. doi: 10.1016/0076-6879(79)62183-3. [DOI] [PubMed] [Google Scholar]
- Pallotti F, Lenaz G. Isolation and subfractionation of mitochondria from animal cells and tissue culture lines. Methods Cell Biol. 2001;65:1–35. doi: 10.1016/s0091-679x(01)65002-7. [DOI] [PubMed] [Google Scholar]
- Paradies G, Petrosillo G, Pistolese M, Ruggiero FM. Reactive oxygen species affect mitochondrial electron transport complex I activity through oxidative cardiolipin damage. Gene. 2002;286:135–141. doi: 10.1016/s0378-1119(01)00814-9. [DOI] [PubMed] [Google Scholar]
- Petrosillo G, Ruggiero FM, Di Venosa N, Paradies G. Decreased complex III activity in mitochondria isolated from rat heart subjected to ischemia and reperfusion: role of reactive oxygen species and cardiolipin. FASEB J. 2003;17:714–716. doi: 10.1096/fj.02-0729fje. [DOI] [PubMed] [Google Scholar]
- Pfeiffer K, Gohil V, Stuart RA, Hunte C, Brandt U, Greenberg ML, Schägger H. Cardiolipin stabilizes respiratory chain supercomplexes. J Biol Chem. 2003;278:52873–52880. doi: 10.1074/jbc.M308366200. [DOI] [PubMed] [Google Scholar]
- Pöyry S, Cramariuc O, Postila PA, Kaszuba K, Sarewicz M, Osyczka A, Vattulainen I, Róg T. Atomistic simulations indicate cardiolipin to have an integral role in the structure of the cytochrome bc1 complex. Biochim Biophys Acta. 2013;1827:769–778. doi: 10.1016/j.bbabio.2013.03.005. [DOI] [PubMed] [Google Scholar]
- Ren M, Phoon CKL, Schlame M. Metabolism and function of mitochondrial cardiolipin. Prog Lipid Res. 2014;55:1–16. doi: 10.1016/j.plipres.2014.04.001. [DOI] [PubMed] [Google Scholar]
- Robinson NC. Functional binding of cardiolipin to cytochrome c oxidase. J Bioenerg Biomembr. 1993;25:153–163. doi: 10.1007/BF00762857. [DOI] [PubMed] [Google Scholar]
- Rork TH, Van Dyke K, Spiler NM, Merrill GF. Acetaminophen in the hypoxic and reoxygenated guinea pig myocardium. Exp Biol Med (Maywood) 2004;229:1154–1161. doi: 10.1177/153537020422901110. [DOI] [PubMed] [Google Scholar]
- Saini-Chohan HK, Dakshinamurti S, Taylor WA, Shen GX, Murphy R, Sparagna GC, Hatch GM. Persistent pulmonary hypertension results in reduced tetralinoleoyl-cardiolipin and mitochondrial complex II + III during the development of right ventricular hypertrophy in the neonatal pig heart. Am J Physiol Heart Circ Physiol. 2011;301:H1415–H1424. doi: 10.1152/ajpheart.00247.2011. [DOI] [PubMed] [Google Scholar]
- Saini-Chohan HK, Holmes MG, Chicco AJ, Taylor WA, Moore RL, McCune SA, Hickson-Bick DL, Hatch GM, Sparagna GC. Cardiolipin biosynthesis and remodeling enzymes are altered during development of heart failure. J Lipid Res. 2009;50:1600–1608. doi: 10.1194/jlr.M800561-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlame M, Haldar D. Cardiolipin is synthesized on the matrix side of the inner membrane in rat liver mitochondria. J Biol Chem. 1993;268:74–79. [PubMed] [Google Scholar]
- Schlame M, Ren M, Xu Y, Greenberg ML, Haller I. Molecular symmetry in mitochondrial cardiolipins. Chem Phys Lipids. 2005;138:38–49. doi: 10.1016/j.chemphyslip.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Schug ZT, Gottlieb E. Cardiolipin acts as a mitochondrial signalling platform to launch apoptosis. Biochim Biophys Acta. 2009;1788:2022–2031. doi: 10.1016/j.bbamem.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Schwall CT, Greenwood VL, Alder NN. The stability and activity of respiratory Complex II is cardiolipin-dependent. Biochim Biophys Acta. 2012;1817:1588–1596. doi: 10.1016/j.bbabio.2012.04.015. [DOI] [PubMed] [Google Scholar]
- Sharpley MS, Shannon RJ, Draghi F, Hirst J. Interactions between phospholipids and NADH:ubiquinone oxidoreductase (complex I) from bovine mitochondria. Biochemistry. 2006;45:241–248. doi: 10.1021/bi051809x. [DOI] [PubMed] [Google Scholar]
- Sparagna GC, Chicco AJ, Murphy RC, Bristow MR, Johnson CA, Rees ML, Maxey ML, McCune SA, Moore RL. Loss of cardiac tetralinoleoyl cardiolipin in human and experimental heart failure. J Lipid Res. 2007;48:1559–1570. doi: 10.1194/jlr.M600551-JLR200. [DOI] [PubMed] [Google Scholar]
- Unsay JD, Cosentino K, Subburaj Y, García-Sáez AJ. Cardiolipin effects on membrane structure and dynamics. Langmuir. 2013;29:15878–15887. doi: 10.1021/la402669z. [DOI] [PubMed] [Google Scholar]
- Yin H, Vergeade A, Shi Q, Zackert WE, Gruenberg KC, Bokiej M, Amin T, Ying W, Masterson TS, Zinkel SS, Oates JA, Boutaud O, Roberts LJ., 2nd Acetaminophen inhibits cytochrome c redox cycling induced lipid peroxidation. Biochem Biophys Res Commun. 2012;423:224–228. doi: 10.1016/j.bbrc.2012.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeczycki TN, Whelan J, Hayden WT, Brown DA, Shaikh SR. Increasing levels of cardiolipin differentially influence packing of phospholipids found in the mitochondrial inner membrane. Biochem Biophys Res Commun. 2014;450:366–371. doi: 10.1016/j.bbrc.2014.05.133. [DOI] [PubMed] [Google Scholar]
- Zhang M, Mileykovskaya E, Dowhan W. Gluing the respiratory chain together. Cardiolipin is required for supercomplex formation in the inner mitochondrial membrane. J Biol Chem. 2002;277:43553–43556. doi: 10.1074/jbc.C200551200. [DOI] [PubMed] [Google Scholar]
- Zielonka J, Kalyanaraman B. Hydroethidine- and MitoSOX-derived red fluorescence is not a reliable indicator of intracellular superoxide formation: another inconvenient truth. Free Radic Biol Med. 2010;48:983–1001. doi: 10.1016/j.freeradbiomed.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkel SS, Ong CC, Ferguson DO, Iwasaki H, Akashi K, Bronson RT, Kutok JL, Alt FW, Korsmeyer SJ. Proapoptotic BID is required for myeloid homeostasis and tumor suppression. Genes Dev. 2003;17:229–239. doi: 10.1101/gad.1045603. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.