Abstract
Dentinogenic ghost cell tumor (DGCT) is a rare, odontogenic neoplasm which is considered to be a solid variant of calcifying odontogenic cyst (COC) with locally aggressive behavior. It accounts for only 2–14% of all COCs. To the best of our knowledge, only 88 cases of DGCT have been reported in the literature from 1968 to 2014. Herewith, we report a case of DGCT in a 68-year-old male patient with clinical presentation as a soft tissue growth over alveolar ridge and histopathologically characterized by ameloblastomatous epithelium, abundance of eosinophilic material and ghost cells.
Keywords: Dentinogenic ghost cell tumor, dentinoid material, ghost cells, Van Gieson
INTRODUCTION
The dentinogenic ghost cell tumor (DGCT) is an uncommon odontogenic neoplasm, regarded as a solid variant of the calcifying odontogenic cyst (COC).[1] Only 2–14% of COC are solid tumors[2] and are considered to be DGCTs. It is characterized by ameloblastomatous odontogenic epithelium, presence of ghost cells and dentinoid material. Predominantly seen in older age group and can occur either as a central (intraosseous) lesion or peripheral (extraosseous in the soft tissues) lesion. The available literature to the best of our knowledge revealed that only 88 cases have been reported and published from 1968 till date.[3,4,5,6] Herewith, we report a case of central DGCT in a 68-year-old male patient with clinical presentation as a soft tissue growth over alveolar ridge.
CASE REPORT
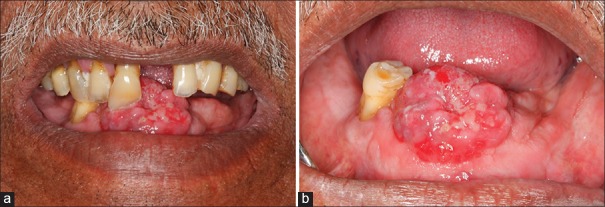
A 68-year-old male patient presented with chief complaint of a soft tissue growth in lower anterior region of the jaw since 3 years. The patient had history of loss of mandibular anterior teeth due to trauma 3 years back. Subsequently, he noticed an asymptomatic soft tissue growth in lower anterior edentulous region, which gradually increased to the present size. The patient also had habit of keeping tobacco quid with lime in lower anterior vestibule, for 5–6 times/day since last 50 years. Extraorally no obvious findings were noted. On intraoral examination a solitary, exophytic, sessile, globular soft tissue mass was present on mandibular anterior edentulous alveolar ridge, extending mesiodistally from 32 to 43 region (3.5 cm) and labio-lingually (2.5 cm) on the slopes of the edentulous alveolar ridge. The color of the overlying mucosa was pinkish red. The surface was irregular with an area of ulceration due to 11, covered with necrotic slough [Figure 1a and b]. The growth was firm in consistency and nontender on palpation with expansion of labial and lingual cortical plates and was provisionally diagnosed as reactive or neoplastic growth.
Figure 1.
(a) Solitary, exophytic, sessile, soft tissue mass, pinkish red in color present on mandibular anterior edentulous alveolar ridge. (b) Solitary, exophytic sessile soft tissue mass pinkish red in color with indentation of right maxillary central incisor
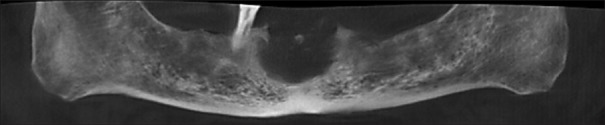
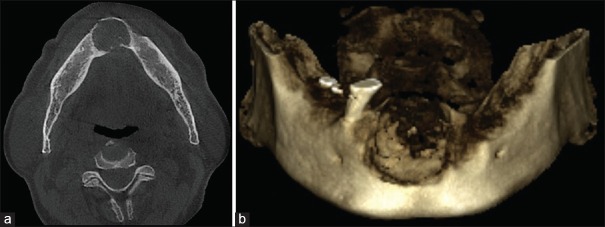
Orthopantomogram [Figure 2] and cone beam computed tomography [Figure 3a and b] showed a well-corticated unilocular radiolucent lesion with specks of radiopacities present in edentulous anterior region of mandible. The lesion extended from 33 to 43 mesio-laterally (23.2 mm), from the alveolar crest to 11 mm above the inferior border of the mandible superioinferiorly (20 mm). Periphery of the lesion was well defined and corticated with discontinuity at certain places. The lesion had caused expansion of superior, buccal and lingual cortices with the perforation of the same at certain places. Based on radiographic findings, diagnosis of benign tumor of anterior region of mandible was made. Central giant cell granuloma, benign odontogenic tumor and fibrosseous lesion (central ossifying fibroma) were considered in differential diagnosis.
Figure 2.
Reconstructed orthopantomography from cone beam computed tomography showing irregular radiolucent lesion in mandibular anterior region
Figure 3.
(a) Cone beam computed tomography showing expansion of both cortical plates in mandibular anterior region with lingual perforation. (b) Three-dimensional Cone beam computed tomography of mandible showing destructive lesion
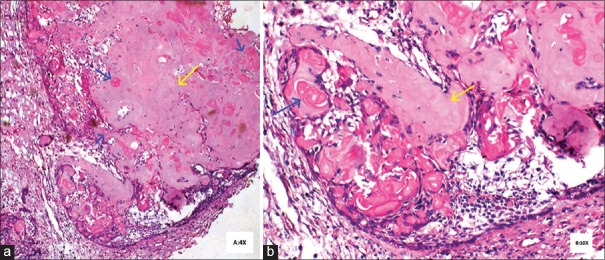
Routine blood investigations were done and incisional biopsy was performed. The hematoxylin and eosin (H and E) stained section showed proliferative epithelium without any dysplasia and one or two islands of odontogenic epithelium with eosinophilic dentinoid material in the connective tissue which led to the provisional diagnosis of benign odontogenic tumor. Excisional biopsy revealed parakeratinized stratified squamous surface epithelium with proliferative changes and an area of ulceration. The deeper connective tissue consisted of islands of odontogenic epithelium, abundant eosinophilic material resembling dentin and numerous ghost cells [Figures 4 and 5].
Figure 4.
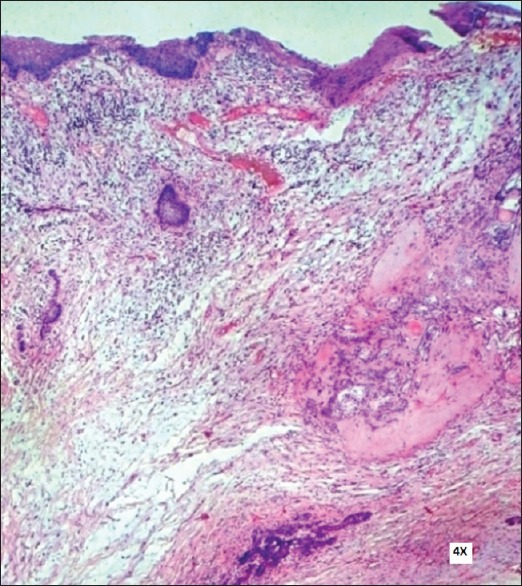
Photomicrograph showing surface epithelium and fibrocellular stroma with isolated odontogenic epithelial islands and eosinophilic dentinoid material (H&E stain, ×100)
Figure 5.
(a) Odontogenic epithelium with dentinoid material (yellow arrow) and ghost cells (blue arrow) (H&E stain, ×40) (b) High power view of odontogenic epithelium with dentinoid material (yellow arrow) and ghost cells (blue arrow) (H&E stain, ×100)
The odontogenic epithelium islands/follicles were lined by peripheral tall columnar cells and centrally placed stellate reticulum like cells with areas of keratinization and ghost cell formation. Mitoses was absent.
It is difficult to study dentinoid in H and E stained sections since it stains eosinophilic like most other structures surrounding it. Hence, special stains meant for connective tissue staining such as Van Gieson, Heidenhain, Goldner or Masson should be used to distinguish the ectodermal tissue from the mesodermal tissue. In the present case, sections were stained with Van Gieson [Figure 6] stain. The ghost cells stained yellow in color while the dentinoid material stained pink to reddish in color. Thus, the nature of both the eosinophilic materials, i.e. ghost cells and dentinoid could be identified and the lesion was further suggestive of DGCT.
Figure 6.
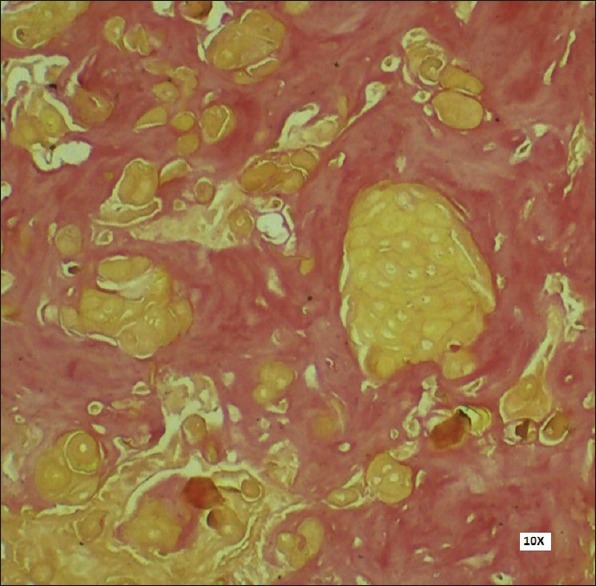
Positive Van Gieson stain showing ghost cells (yellow color) and dentinoid material (reddish pink) (Van Gieson stain, ×100)
DISCUSSION
According to the WHO,[7] the spectrum of odontogenic ghost cell tumors comprises DGCT, calcifying cystic odontogenic tumor (CCOT) and the ghost cell odontogenic carcinoma (GCOC). Based on a research of 215 COCs carried out by Buchner, it was found that COC represents 1–2% of all odontogenic tumors and of these only 2–14% were solid tumors, considered to be DGCTs.[2] DGCT is the solid, clinicopathologic variant of CCOT, which was first described by Gorlin et al.[8] as COC. However, Fejerskov and Krogh[9] in 1972 were of the opinion that the term COC is not entirely appropriate, for various reasons given below and suggested the term “calcifying ghost cell odontogenic tumor.”
The presence of the ghost cells which may subsequently show calcification
The possibility of cystic degeneration taking place in the center of proliferating epithelial islands rather than epithelial changes developing in a preexisting cyst wall
Growth proliferative potentiality of some lesions giving rise to lesions of considerable size.
In 1981, Praetorius et al.[1] suggested the term “DGCT” because of the presence of ghost cells and abundant dentinoid material. The WHO, in 2005, defined DGCT as, “A locally invasive neoplasm characterized by ameloblastoma-like islands of epithelial cells in a mature connective tissue stroma. Aberrant keratinization may be found in the form of ghost cells in association with varying amounts of dysplastic dentin.”[7]
DGCT may occur as an intraosseous lesion (Type 1, 83%) and less commonly as an extraosseous peripheral lesion arising in the gingiva or alveolar mucosa (Type 2, 17%).[3] The age may range from 12 to 75 years (mean 50 years) with slight male predilection.[10] The present case was seen in a male patient aged 68 years. The peripheral type occurs significantly later in life than the central type.[3] DGCT may occur in any tooth-bearing area or the edentulous region[3,7,10] of the jaws.
Intraosseous lesions predominantly occur in first molar to canine region.[2] The present case was noted in mandibular anterior edentulous region. The size of the intraosseous DGCT varies from 1 to more than 10 cm in diameter and is usually asymptomatic. The clinical signs of intraosseous DGCT variants may include expansion of the jaw, clinically visible swelling and obliteration of the maxillary sinus or infiltration of the soft tissues. Swelling can be painful or painless and occasionally accompanied by pus discharge, tooth displacement or mobility.[4] The present case which represented clinically as a soft tissue overgrowth is not a common finding. To our knowledge, only one case with similar findings has been reported.[10] The DGCT can be asymptomatic and can also present itself as coincidental radiographic finding during routine patient examination. It appears radiographically as a radiolucent, radiopaque or mixed lesion depending on the amount of calcification. Lesions can be unilocular or multilocular with either well-defined or ill-demarcated margins. Root resorption, displacement of adjacent teeth and presence of impacted teeth have been reported.[4,10]
The predominant location for peripheral type is anterior portion of mandible (73%) and appears as exophytic nodules confined to the gingival mucosa in dentate patients or the alveolar mucosa in edentulous patients.[3] This lesion may be confused with reactive or inflammatory lesions of gingiva, such as peripheral giant cell granuloma, pyogenic granuloma, irritation fibroma, epulis or parulis.[11] Very often it is of 0.5 cm to 1 cm in size with little variations.[8] Mild erosion or the saucerization of the underlying cortical bone may be seen in 20% of cases.[7]
Histogenesis
The histogenetic derivation of DGCT has been attributed to cell rests of Serre or the surface epithelium but currently remains unclear.[12] Missense mutation on codon 3 (ACT → TCT), i.e., threonine to serine of β-catenin gene suggests that β-catenin plays an important role in the tumorigenesis of DGCT by an improper differentiation process coordinated by Wnt signaling pathway.[13]
Histopathology
Histopathologically central and peripheral DGCT is characterized by sheets and rounded islands of odontogenic epithelial cells seen in a mature connective tissue. The epithelium of the tumor islands resembles that of an ameloblastoma. Mitoses is not seen. Minor cysts may form in the epithelial islands.[7]
A characteristic feature is the transformation of the epithelial cells into ghost cells. The ghost cells are swollen, ellipsoidal keratinized epithelial cells characterized by the loss of nuclei, preservation of basic cellular outlines, resistance to resorption, induction of foreign body granuloma and potential to calcify.[14] Ghost cells are thought to be derived either from transformation of epithelial cells, metaplastic transformation of odontogenic epithelium, squamous metaplasia with secondary calcification due to ischemia,[14] degeneration of epithelial cells[9] or as a result of apoptotic process.[10] For several researchers ghost cells represent an abnormal or incomplete keratinization process or as in an advanced stage of keratinization.[14] Coagulative necrosis has also been suggested as the origin of ghost cells.[14] David et al. suggested that ghost cells may represent abortive enamel matrix in odontogenic epithelium.[14] Although ghost cells are a basic prerequisite for the diagnosis of the DGCT, it must be stressed, that the presence of ghost cells alone is not pathognomonic, since they can also be identified in other neoplasms such as odontomas, ameloblastomas and ameloblastic fibro-odontomas. The latter tumor can be eliminated from the histopathological differential diagnosis by the presence of a cellular primitive ectomesenchyme resembling dental papilla.[10]
Numerous round homogeneously basophilic globules of calcification are also evident. The formation of dentinoid or osteoid material,[15] which is frequently described as being found in connection with masses of ghost cells, is another characteristic finding of the lesion. Gorlin et al. considered the appearance of dentinoid material in the COC to represent an inflammatory response of the body tissue toward masses of ghost cells. Abrams and Howell further stated that the masses of “ghost cells” induce granulation tissue to lay down juxtra-epithelial osteoid which may calcify. Contrary to these interpretations, Sauk postulated that it might be an inductive phenomenon. Ng et al. suggested that it represents a metaplastic change in the connective tissue without the participation of granulation tissue.[11]
Based on the study of H and E stained paraffin sections and ultrathin sections under light and electron microscope, Donath et al. in 1979 opined, that dentinoid or dysplastic dentin is not a product of mesodermal cells, but represents a hard type of keratin similar to that found in nails. Later on, Praetorius et al. suggested the material to be of a mesodermal origin based on the following findings:[1]
Dentinoid stains with connective tissue stainings such as Van Gieson, Heidenhain, Goldner and Masson like collagen
Dentinoid is usually not found in the luminal proliferations unless there is a disintegration of the basement membrane with outgrowth of connective tissue between the epithelial ghost cells.
Although the microscopic designation of DGCT is very clear, it can be confused with ameloblastoma, CCOT and odontogenic ghost cell carcinoma.[4] A diagnosis of a CCOT was excluded in the present case because of the very high amount of histologically confirmed dentinoid which accounted for the solid structure of a DGCT, in contrast to a cystic structure of a CCOT. The presence of dysplastic dentin and large amount of ghost cells seen in the present case differentiated it histologically from ameloblastoma and odontoameloblastoma. The absence of mitotic activity ruled out the diagnosis of GCOC.
Treatment, prognosis and predictive factors
Treatment is done by enucleation or surgical resection which can be segmental resection or enblock. Long-term patient follow-up is generally recommended as recurrences have been reported not only following local excision/enucleation but also 1–5 years after segmental mandibular resection and partial maxillectomy.[9,16] Central DGCTs are aggressive neoplasms that show locally invasive behavior and recurrence rates of up to 71%.[16] Compared to central, peripheral DGCTs are less aggressive in behavior and are not thought to recur.[9,16] Malignant transformation of a DGCT into an odontogenic ghost cell carcinoma has also been reported.[2] The present case was treated with local excision and was kept under regular follow-up with no evidence of recurrence, 7 months postoperatively.
CONCLUSION
DGCT is an uncommon odontogenic neoplasm, regarded as a solid variant of the COC. Only 2–14% of COC are solid tumors considered to be DGCTs. We report a case of central DGCT in a 68-year-old male patient with clinical presentation as a soft tissue growth over the alveolar ridge.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Praetorius F, Hjørting-Hansen E, Gorlin RJ, Vickers RA. Calcifying odontogenic cyst. Range, variations and neoplastic potential. Acta Odontol Scand. 1981;39:227–40. doi: 10.3109/00016358109162284. [DOI] [PubMed] [Google Scholar]
- 2.Buchner A. The central (intraosseous) calcifying odontogenic cyst: An analysis of 215 cases. J Oral Maxillofac Surg. 1991;49:330–9. doi: 10.1016/0278-2391(91)90365-s. [DOI] [PubMed] [Google Scholar]
- 3.Pommer B, Dvorak G, Rappersberger K, Jordan RC, Watzek G, Pogrel MA. Pyogenic granuloma mimicking peripheral dentinogenic ghost-cell tumour recurrence: Case report and meta-analytic literature update. Oral Surg. 2013;6:98–103. [Google Scholar]
- 4.Konstantakis D, Kosyfaki P, Ebhardt H, Schmelzeisen R, Voss PJ. Intraosseous dentinogenic ghost cell tumor: A clinical report and literature update. J Craniomaxillofac Surg. 2014;42:e305–11. doi: 10.1016/j.jcms.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 5.Etemad-Moghadam S, Baghaee F, Dadafarid Z, Alaeddini M. A 44-year analysis of ghost cell odontogenic tumour subtypes in an Iranian population. J Craniomaxillofac Surg. 2014;42:1154–9. doi: 10.1016/j.jcms.2014.01.048. [DOI] [PubMed] [Google Scholar]
- 6.Pulino BF, Siqueira CS, Duarte CM, Sousa SC. Expression of PCNA, p53 and Ki-67 in dentinogenic ghost cell tumors and calcifying cystic odontogenic tumors. Clin Lab Res Den. 2014;20:16–22. [Google Scholar]
- 7.Praetorius F, Ledesma-Montes C. Dentinogenic ghost cell tumour. In: Barnes L, Eveson JW, Reichart P, Sidransky D, editors. World Health Organization Classification of Tumours. Pathology and Genetics of Head and Neck Tumours. Lyon: IARC Press; 2005. p. 314. [Google Scholar]
- 8.Gorlin RJ, Pindborg JJ, Odont, Clausen FP, Vickers RA. The calcifying odontogenic cyst – A possible analogue of the cutaneous calcifying epithelioma of malherbe. An analysis of fifteen cases. Oral Surg Oral Med Oral Pathol. 1962;15:1235–43. doi: 10.1016/0030-4220(62)90159-7. [DOI] [PubMed] [Google Scholar]
- 9.Fejerskov O, Krogh J. The calcifying ghost cell odontogenic tumor – Or the calcifying odontogenic cyst. J Oral Pathol. 1972;1:273–87. doi: 10.1111/j.1600-0714.1972.tb01666.x. [DOI] [PubMed] [Google Scholar]
- 10.Juneja M, George J. Dentinogenic ghost cell tumor: A case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:e17–22. doi: 10.1016/j.tripleo.2009.01.033. [DOI] [PubMed] [Google Scholar]
- 11.Bello IO, Qannam A, Al-Zahrani A, AlDosari A. Peripheral dentinogenic ghost cell tumor: Report of a case and literature review. Int J Surg Pathol. 2012;20:494–9. doi: 10.1177/1066896911429299. [DOI] [PubMed] [Google Scholar]
- 12.Altini M, Farman AG. The calcifying odontogenic cyst. Eight new cases and a review of the literature. Oral Surg Oral Med Oral Pathol. 1975;40:751–9. doi: 10.1016/0030-4220(75)90444-2. [DOI] [PubMed] [Google Scholar]
- 13.Kim SA, Ahn SG, Kim SG, Park JC, Lee SH, Kim J, et al. Investigation of the β-catenin gene in a case of dentinogenic ghost cell tumor. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:97–101. doi: 10.1016/j.tripleo.2005.10.037. [DOI] [PubMed] [Google Scholar]
- 14.Hong SP, Ellis GL, Hartman KS. Calcifying odontogenic cyst. A review of ninety-two cases with reevaluation of their nature as cysts or neoplasms, the nature of ghost cells, and subclassification. Oral Surg Oral Med Oral Pathol. 1991;72:56–64. doi: 10.1016/0030-4220(91)90190-n. [DOI] [PubMed] [Google Scholar]
- 15.Tajima Y, Ohno J, Utsumi N. The dentinogenic ghost cell tumor. J Oral Pathol. 1986;15:359–62. doi: 10.1111/j.1600-0714.1986.tb00640.x. [DOI] [PubMed] [Google Scholar]
- 16.Sun G, Huang X, Hu Q, Yang X, Tang E. The diagnosis and treatment of dentinogenic ghost cell tumor. Int J Oral Maxillofac Surg. 2009;38:1179–83. doi: 10.1016/j.ijom.2009.06.016. [DOI] [PubMed] [Google Scholar]