Abstract
Introduction and Objectives:
Oral squamous cell carcinoma (OSCC) is an epithelial neoplasm generally beginning as focal overgrowth of altered stem cells near the basement membrane, moving upward and laterally, replacing the normal epithelium. Histopathological grading has been used for many decades in an attempt to predict the clinical behavior of oral squamous cell carcinoma. In the present study, Forty biopsies were studied for histological grading and p53 expression. The p53 expression was studied in relation to clinical parameters such as age, sex of patient and site of tumors. Relation between histological grade of malignancy and p53 protein expression was analysed. All cases were classified according to Anneroth's histological malignancy grading system (1987).
Materials and Methods:
40 cases of OSCC were assessed for clinical parameters, Anneroth's histological grading and immunohistochemically stained with p53 protien.
Statistical Analysis:
The results obtained were analyzed using Spearman's Co-relation.
Observations and Results:
The positive expression of p53 was found in 62% of carcinomas studied. Positivity of p53 showed correlation with histological grade of malignancy and with individual parameters like degree of keratinization, nuclear polymorphism, number of mitoses and lymphoplasmacytic infiltration while showed a negative correlation with pattern of invasion.
Conclusion:
Our study showed a significant correlation between parameters of tumor cell population, lymphoplasmacytic infiltration and p53 expression. A significant association between high grade of malignancy and p53 overexpression and insignificant correlation of p53 with age, sex of the patient and site of the tumor was found.
Keywords: Malignancy grading system, oral cancer, p53
INTRODUCTION
Cancer is older than humanity. S. U. Williston, in early 1920's, found a bone tumor on skeleton of dinosaur in Wyoming. Since that period, larger number of books about cancer and its treatment are available.[1]
Oral squamous cell carcinoma (SCC) is one of the tenth most common cancers in the world and shows marked geographic differences in occurrence. Oral cancer is common where betel quid chewing, bidi smoking and alcohol consumption are high. It is common cancer in Southeast Asia, where more than 100,000 new cases are reported every year.[2] Oral SCC is life-threatening disease of oral cavity and most common histopathological type of cancer accounting for 91% of oral malignancies.[3] Oral cancer is an epithelial neoplasm generally beginning as focal overgrowth of altered stem cells near the basement membrane, moving upward and laterally, replacing the normal epithelium.[4]
Histopathological grading has been used for many decades in an attempt to predict the clinical behavior of SCC of head and neck region.[5]
Broders’ initiated quantitative grading of oral SCC with a system originally based on proportions of highly differentiated cells within the entire tumor cell population.[6]
The new grading system of head and neck carcinoma was originally introduced by Jakobsson et al.[7] and recently modified by Anneroth et al., who proposed a grading system based on the degree of keratinization, nuclear polymorphism, pattern of invasion, host response and mitotic activity.[8,9]
In the 20th century, cancer research has taken a large step forward with discovery of human oncogenes, doors have been opened to the understanding of molecular pathogenesis of cancer.
Development of oral SCC is a multistep process requiring the accumulation of multiple genetic alterations, influenced by patient's genetic predisposition as well as by environmental factors including tobacco, alcohol, chronic inflammation and viral infection.[10]
Understanding the pathogenesis of cancer may well lead to improved diagnostic tests, preventive treatment and management. Every year the approach to the laboratory diagnosis of cancer is becoming more complex, sophisticated and specialized. The routine diagnostic method includes histologic and cytologic methods. The advanced diagnostic methods include immunohistochemistry, molecular diagnosis, flow cytometry and tumor markers.[11] Histopathology is the gold standard in diagnosis though it is time consuming.
Among all these new techniques, immunohistochemistry has become a powerful tool in the armamentarium of pathologist, as it provides insight into tumor histopathogenesis and has contributed to more accurate determination of patient's prognosis.
The p53 tumor suppressor gene has come to forefront of cancer research as it is commonly mutated in human cancer and spectrum of p53 mutation in these cancers is providing clues to the biology and molecular pathogenesis of neoplasia.[12] p53, a 393 amino acid nuclear phosphoprotein, was first detected because it formed a tight complex with SV40 large T antigen. It is located on short arm of chromosome 17. It gets activated by exogenous and endogenous stresses such as DNA damage, hypoxia and activation of oncogenes. It's exact biochemical function is yet unknown, but it is postulated that it has a role in both cell regulation and transcription of gene that suppress cell proliferation, perhaps those affecting passage from late G1 to S phase of cell cycle and has biological function as G1 checkpoint control allowing the repair of DNA damage.
This study is aimed to assess the p53 expressions in patients of oral SCC and to correlate it with individual parameter of malignancy grading system.
MATERIALS AND METHODS
Forty-four biopsy samples of clinically diagnosed oral SCC in the year 2009–2010 were selected. Detailed case history of each patient was recorded. All the tissues were fixed in 40% formalin and processed using manual tissue processing technique and paraffin-embedded block were prepared. Out of 44 samples, 40 blocks of biopsy specimen of acceptable quality were selected for the study. Tumour, node and metastasis status of patient was unknown. All the cases were reviewed and classified according to modified histological malignancy grading system [Table 1]. Each morphological parameter in classification system was graded on the basis of 1–4 points. The final score for each case was defined as the total sum of points attributed to the parameters evaluated. As incisional biopsies were used in this study, the “stage of invasion” was omitted.
Table 1.
Modified Anneroth's histological malignancy grading system
Three sections of 4–6 μ in thickness were cut, of which two sections were stained with hematoxylin and eosin stain for determining Anneroth grading and immunohistochemical staining for p53 was carried out on one section with autoimmunostainer (benchmark) to assess its expression. The analysis of p53 positive cells was performed on IHC stained sections. Only staining of nucleus of epithelial cells was observed; nuclei with clear brown color regardless of staining intensity were regarded as p53 positive. The slide was scanned from one end to other and those areas which had no labeling at all were excluded. The slides were scanned until total 1000 nuclei were reached and in this study only the percentage of cells with p53 positive expression was quantified.[13] Counting of the cells was done with graticule eyepiece.
RESULTS
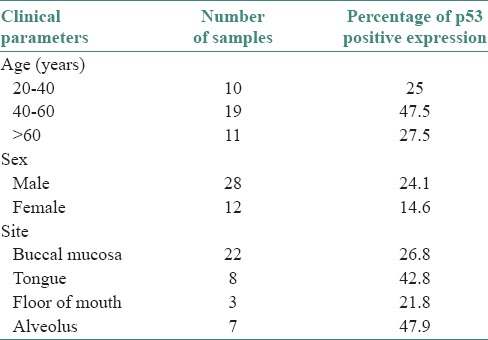
p53 positivity was detected in 65% of tumor samples. In general, in well-differentiated cases, p53 staining was seen in peripheral cells, whereas in poorly differentiated carcinoma staining was observed consistently [Figures 1–6]. p53 expression was studied in relation to clinical parameters such as age, sex of patient and tumor site. The relationship between p53 expression and various clinical parameters are summarized in Table 2. No correlation was found between age, sex and tumor site with p53 accumulation. Similarly, p53 immunopositivity was studied in relation to each parameter of modified Anneroth's histological malignancy grading system and with total malignancy grade [Graph 1]. Nuclear accumulation of p53 was related to the total histological grade of malignancy (r = 0.059, P < 0.05). This correlation was detected for degree of keratinization (r = 0.536, P < 0.05), nuclear polymorphism (r = 0.589, P < 0.05), number of mitose (r = 0.439, P < 0.05) and lymphoplasmacytic infiltration (r = 0.412, P < 0.05) with p53 immunoexpression [Table 3].
Figure 1.
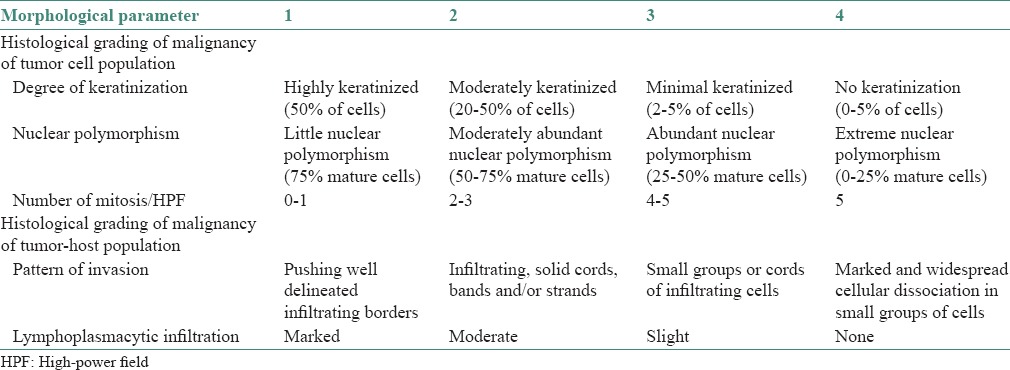
Photomicrograph showing cellular dissociation in Grade III oral squamous cell carcinoma (H&E stain, ×100)
Figure 6.
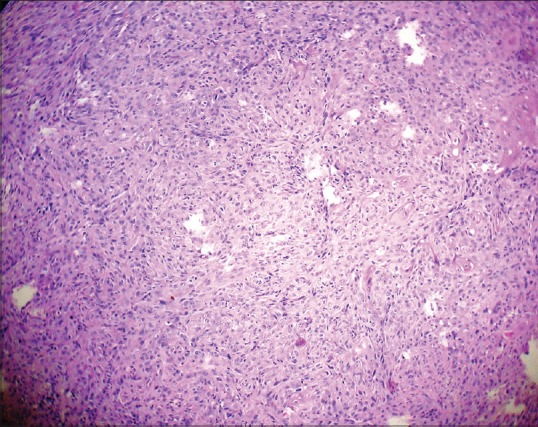
Photomicrograph showing tumor invasion in small solid cords and strands (H&E stain, ×100)
Table 2.
The relationship between p53 expression and various clinical parameters are summarized
Graph 1.
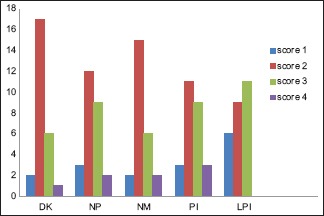
Correlation of individual parameter of modified Anneroth's histological malignancy grading system with p53 positive expression. DK: Degree of Keratinization, NP: Nuclear polymorphism, NM: Number of mitoses, PI: Pattern of invasion, LPI: Lymphoplasmacytic infiltration
Table 3.
The different parameters and their significance
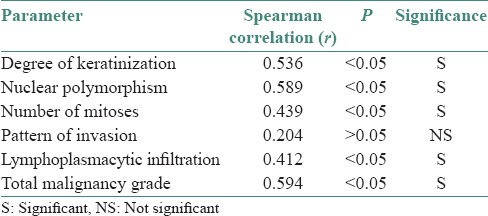
Figure 2.
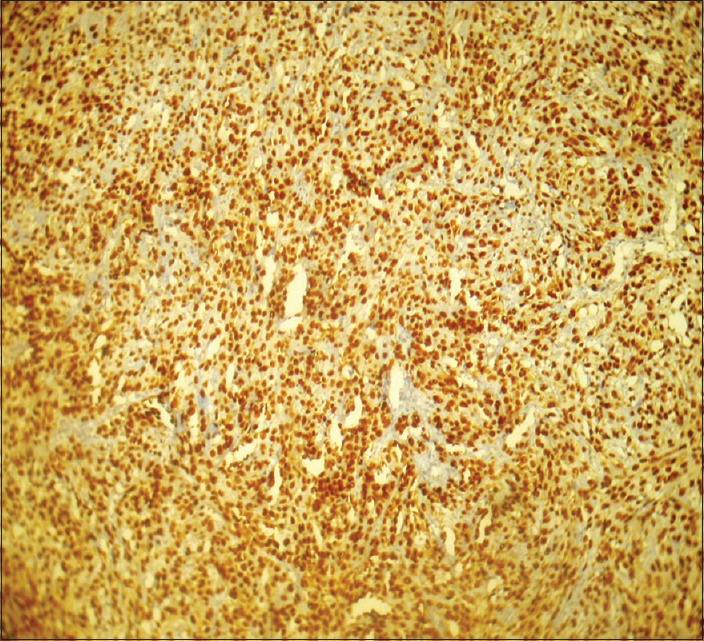
Photomicrograph showing intense and extensive p53 staining in Grade III tumors (IHC stain, ×100)
Figure 3.
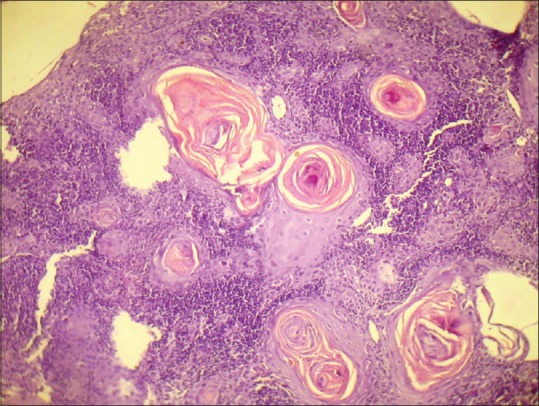
Photomicrograph showing keratin pearl formation in Grade I oral squamous cell carcinoma (H&E stain, ×100)
Figure 4.
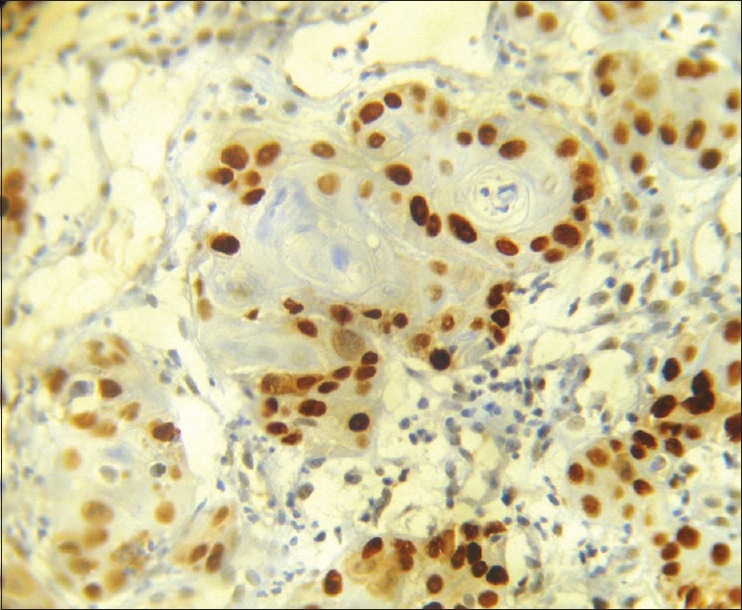
Photomicrograph showing P53 positivity to peripheral undifferentiated cells, whereas central keratinized cells are spared (IHC stain, ×400)
Figure 5.
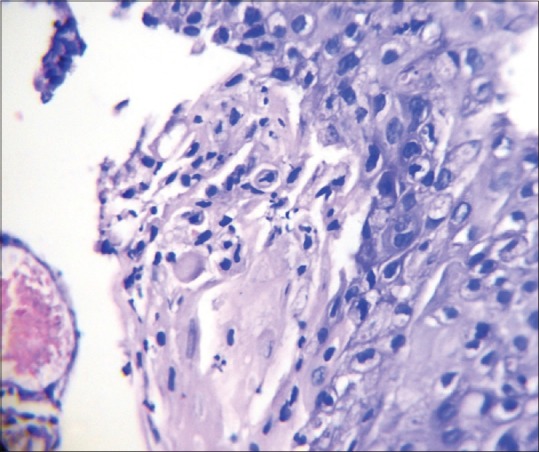
Photomicrograph showing abnormal mitotic figure (H&E stain, ×100)
No correlation was found between pattern of invasion and p53 expression (r = 0.204, P > 0.05).
DISCUSSION
SCC accounts for 90% of all oral malignancies. Globally, it represents 5% and 2% of all cancer in males and females, respectively.[14] This disease is now accepted to be caused by genetic damage to mucosal cells in the form of accumulated mutations, causing the cells to proliferate rapidly in an uncontrolled manner.[15]
p53 is a tumor suppressor gene that plays an important role in cell cycle control and apoptotic response to radiation and other causes of cell damage.[16] This gene gets commonly mutated in oral cancer.
The wild type of p53 is believed to be present in low concentration in cells because of its short half-life of about 20 min and it is therefore not demonstrated under physiologic conditions.[17] Mutations affecting p53 gene will result in structural changes, which increases the stability and hence accumulation of mutant p53 protein, which can be detected immunohistochemically.[5]
The initial alteration seems to occur at the basal cell layer under the influence of smoke, alcohol and other carcinogens and may involve deactivation of TP53 and other tumor suppressors. The transition from normal epithelium to invasive cancer is more often accompanied by multiple hits which promote proliferation, angiogenesis, local invasion and eventually distant metastasis.[10]
In this study, out of 40 patients of SCC, 28 (70%) were males and 12 (30%) were females. This study showed a clear male predominance, with the sex ratio being 7:3. Such findings were in consonance with those obtained by Kazi[18] (4:1), Yazdi and Khalili[19] (3:2) and Pimenta Amaral et al.[20] (3:1).
The patients selected for this study were in the age range of 23–87 years, with highest patient in the age range of 40–60 years (47.5%). These findings were similar to those of Kazi,[18] in whose study the most frequently occurring age group was 40–50 years [Table 2].
Histologic grading has been used for many decades in an attempt to predict the clinical behavior of SCC.
Broders initiated quantitative grading of carcinoma with a system originally based on the degree of cell differentiation. He graded the OSCC cases from 1 to 4 depending upon the proportion of differentiated and undifferentiated cells, independent of clinical history.[6] On the basis of Broder's classification, Holm et al.[21] classified cancers as well differentiated, moderately differentiated and poorly differentiated.
However, Broder's degree of differentiation of SCC and their correlation with prognosis proved to be of little significance. The lack of correlation found between Broder's grade and prognosis of head and neck SCCs has been explained by the fact that SCCs usually exhibits heterogeneous populations with probable difference in invasive and metastatic behavior.
Jakobsson's[7] multifactorial grading system including both tumor cell population and tumor-host relationship has been used in recent years and this system reveals a close relationship between total malignancy score and the final outcome of the disease.
Anneroth et al. modified Jakobsson's grading system and evaluated three tumor cell features in the less differentiated parts of the tumor. He included six variables, which were degree of keratinization, nuclear polymorphism, number of mitoses, pattern of invasion, stage of invasion and leucocyte infiltration. In this study, stage of invasion was not included as punch biopsies were used.[8,9]
In this study, p53 expression was studied and compared with five different parameters of Anneroth histological malignancy grading system. The stage of invasion was excluded, as punch biopsies were used and accordingly, the grading was modified [Table 3].
In our study, out of 40 samples, 26 (65%) samples showed positive p53 expression and remaining 14 (35%) samples showed negative p53 expression. In oral SCC, p53 expression was reported with a range from 11% to 94% in various previous studies. Our results were similar to those of De Araújo et al.[22] and Watling et al.[23] (62.5% positivity) and Ogden et al.[24] (54% positivity).
Mean index of p53 expression in males was 24.1% while that of females was 14.6%. Mean index of p53 staining was found to be less in females than in males. These results were in contrast to Vora et al.,[25] who demonstrated that none of the female patients in their study showed p53 immunoexpression. Statistically, no significant correlation was found between sex of the patient and p53 expression (r = −0.176, P > 0.05).
In our study, the highest mean percentage of p53expression was found in the age range of 40–60 years, which was 47.5% [Table 2]. Statistically, no significant correlation was found between age of the patient and p53expression (r = −0.084, P > 0.05). Previous studies done by Chen et al.,[26] Daniel et al.[27] and Vora et al.[25] showed no correlation between age of the patient and p53 expression.
With respect to various anatomical sites, highest p53 immunoexpression was seen in cancer of alveolus (47.9%). Our results were in contrast to the study done by Sa et al.,[28] who found the highest p53 expression in tongue. Statistically, no significant correlation was found between p53 expression with tumor site (P > 0.05). Similar findings were reported by Chiang et al.,[29] Sa et al.[28] (2006).
Histological grades of oral SCC and p53 overexpression was studied for their significance as determinants of prognosis.
p53 expression was studied in relation to the histologic grade of malignancy. p53 positivity increased as the grade of malignancy increased. These findings were similar to those shown by Watling et al.[23] who demonstrated a positive correlation between higher grade of malignancy and p53 overexpression.
Boyle et al.[30] and Nylander et al.[31] have described the accumulation of p53 protein levels as associated with increasing histological severity. Chiang et al.[29] also studied the positive relation with increasing grade of malignancy and p53 staining. In contrast to this study, Abbas et al.[32] did not find any relation between grade of malignancy and p53 expression.
While correlating each parameter of malignancy grading system with p53 expression, we got positive correlation for degree of keratinization (r = 0.536, P < 0.05) and similarly significant correlation for nuclear polymorphism (r = 0.589, P < 0.05), number of mitoses (r = 0.05, P < 0.05) and lymphoplasmacytic infiltration (r = 0.412, P < 0.05). Whereas negative correlation was found between pattern of invasion (r = 0.204, P > 0.05) and p53 overexpression.[22,33]
Jayade et al.[34] also demonstrated same significant correlation between degree of keratinization, number of mitoses and nuclear polymorphism with p53 overexpression. However, he did not find a positive correlation between p53 expression and lymphoplasmacytic infiltration. Odell et al.[35] also showed positive correlation between degree of keratinization and prognosis.
Our studies were in contrast to other studies done previously, where Jayade et al.[34] showed positive correlation for pattern of invasion with p53 expression and Gonzalez-Moles et al.,[36] who found a significant correlation between pattern of invasion and p53 overexpression.
Altogether our study showed a significant correlation between parameters of tumor cell population and p53 expression and also for lymphoplasmacytic infiltration. This correlation was not found for tumor architecture. These reports were in conflict with previous other studies. However, a significant association between high grade of malignancy and p53 overexpression and insignificant correlation of p53 with age, sex of the patient and site of the tumor was found.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Ladik J, Forner W. 1st ed. Berlin, Heidelberg, NewYork: Springer-Verlag; 1994. The beginning of cancer in the cell: An interdisciplinary Approach. [Google Scholar]
- 2.Control of oral cancer in developing countries. A WHO meeting. Bull World Health Organ. 1984;62:817–30. [PMC free article] [PubMed] [Google Scholar]
- 3.Sankaranarayanan R. Oral cancer in India: An epidemiologic and clinical review. Oral Surg Oral Med Oral Pathol. 1990;69:325–30. doi: 10.1016/0030-4220(90)90294-3. [DOI] [PubMed] [Google Scholar]
- 4.Shah JP, Jhonson NW, Batsakis JG. 1st ed. London: Taylor and Francis Group; 2003. Oral Cancer. [Google Scholar]
- 5.Kumar V, Abbas AK, Fausto N, Mitchell RM. 8th ed. Philadelphia: Elsevier Slander Publication; 2007. Robins and Cotran's. Pathologic Basis of Diseases. [Google Scholar]
- 6.Broders AC. Squamous cell epithelioma of the lip. J Am Med Assoc. 1920;74:656–6. [Google Scholar]
- 7.Jakobsson PA, Eneroth CM, Killander D, Moberger G, Mårtensson B. Histologic classification and grading of malignancy in carcinoma of the larynx. Acta Radiol Ther Phys Biol. 1973;12:1–8. doi: 10.3109/02841867309131085. [DOI] [PubMed] [Google Scholar]
- 8.Anneroth G, Batsakis J, Luna M. Review of the literature and a recommended system of malignancy grading in oral squamous cell carcinomas. Scand J Dent Res. 1987;95:229–49. doi: 10.1111/j.1600-0722.1987.tb01836.x. [DOI] [PubMed] [Google Scholar]
- 9.Anneroth G, Batsakis JG, Luna M. Malignancy grading of squamous cell carcinoma in the floor of the mouth related to clinical evaluation. Scand J Dent Res. 1986;94:347–56. doi: 10.1111/j.1600-0722.1986.tb01773.x. [DOI] [PubMed] [Google Scholar]
- 10.Tsantoulis PK, Kastrinakis NG, Tourvas AD, Laskaris G, Gorgoulis VG. Advances in the biology of oral cancer. Oral Oncol. 2007;43:523–34. doi: 10.1016/j.oraloncology.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 11.Jordan RC, Daniels TE, Greenspan JS, Regezi JA. Advanced diagnostic methods in oral and maxillofacial pathology. Part II: immunohistochemical and immunofluorescent methods. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;93:56–74. doi: 10.1067/moe.2002.119567. [DOI] [PubMed] [Google Scholar]
- 12.Gasco M, Crook T. The p53 network in head and neck cancer. Oral Oncol. 2003;39:222–31. doi: 10.1016/s1368-8375(02)00163-x. [DOI] [PubMed] [Google Scholar]
- 13.Hall PA, Lane DP. p53 in tumour pathology: Can we trust immunohistochemistry? Revisited! J Pathol. 1994;172:1–4. doi: 10.1002/path.1711720103. [DOI] [PubMed] [Google Scholar]
- 14.Mehta FS, Pindborg JJ, Gupta PC, Daftary DK. Epidemiologic and histologic study of oral cancer and leukoplakia among 50,915 villagers in India. Cancer. 1969;24:832–49. doi: 10.1002/1097-0142(196910)24:4<832::aid-cncr2820240427>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 15.Scully C. Oncogenes, onco-suppressors, carcinogenesis and oral cancer. Br Dent J. 1992;173:53–9. doi: 10.1038/sj.bdj.4807936. [DOI] [PubMed] [Google Scholar]
- 16.Lane DP. Cancer. p53, guardian of the genome. Nature. 1992;358:15–6. doi: 10.1038/358015a0. [DOI] [PubMed] [Google Scholar]
- 17.Nylander K, Dabelsteen E, Hall PA. The p53 molecule and its prognostic role in squamous cell carcinomas of the head and neck. J Oral Pathol Med. 2000;29:413–25. doi: 10.1034/j.1600-0714.2000.290901.x. [DOI] [PubMed] [Google Scholar]
- 18.Kazi RA. Carcinoma of tongue: A retrospective study of 110 cases. Internet J Otorhinolaryngol. 2003;2:33–38. [Google Scholar]
- 19.Yazdi IN, Khalili M. Grading of oral cancer and comparison of different system with respect to lymph-node metastasis in tongue squamous cell carcinoma. Arch Iran Med. 1999;2(2) Avaliable from: http://www.ams.ac.ir/AIM/9922/yazdi9922.html . [Google Scholar]
- 20.Pimenta Amaral TM, Da Silva Freire AR, Carvalho AL, Pinto CA, Kowalski LP. Predictive factors of occult metastasis and prognosis of clinical stages I and II squamous cell carcinoma of the tongue and floor of the mouth. Oral Oncol. 2004;40:780–6. doi: 10.1016/j.oraloncology.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 21.Holm LE, Lundquist PG, Silfverswärd C, Sobin A. Histological grading of malignancy in squamous cell carcinoma of the oral tongue. Acta Otolaryngol. 1982;94:185–92. doi: 10.3109/00016488209128904. [DOI] [PubMed] [Google Scholar]
- 22.De Araújo VC, Loyola AM, Pinto Júnior DD, Borra RC, De Araújo NS. p53 in biopsies of oral squamous cell carcinoma. A comparative study with a malignancy grading system. Oral Oncol. 1997;33:5–9. doi: 10.1016/s0964-1955(96)00055-3. [DOI] [PubMed] [Google Scholar]
- 23.Watling DL, Gown AM, Coltrera MD. Overexpression of p53 in head and neck cancer. Head Neck. 1992;14:437–44. doi: 10.1002/hed.2880140603. [DOI] [PubMed] [Google Scholar]
- 24.Ogden GR, Kiddie RA, Lunny DP, Lane DP. Assessment of p53 protein expression in normal, benign, and malignant oral mucosa. J Pathol. 1992;166:389–94. doi: 10.1002/path.1711660411. [DOI] [PubMed] [Google Scholar]
- 25.Vora HH, Trivedi TI, Shukla SN, Shah NG, Goswami JV, Shah PM. p53 expression in leukoplakia and carcinoma of the tongue. Int J Biol Markers. 2006;21:74–80. doi: 10.1177/172460080602100202. [DOI] [PubMed] [Google Scholar]
- 26.Chen YK, Huse SS, Lin LM. Differential expression of p53, p63 and p73 proteins in human buccal squamous-cell carcinomas. Clin Otolaryngol Allied Sci. 2003;28:451–5. doi: 10.1046/j.1365-2273.2003.00743.x. [DOI] [PubMed] [Google Scholar]
- 27.Siegelmann-Danieli N, Ben-Izhack O, Hanlon A, Ridge JA, Stein ME, Khandelwal V, et al. P53 alteration in oral tongue cancer is not significantly associated with age at diagnosis or tobacco exposure. Tumori. 2005;91:346–50. doi: 10.1177/030089160509100412. [DOI] [PubMed] [Google Scholar]
- 28.Sa CT, Fonseca LM, Cardoso SV, Aguiar MC, Carmo MA. p53 immunoexpression in oral squamous cell carcinomas from different anatomical sites: A comparative study. Int J Morphol. 2006;24:231–8. [Google Scholar]
- 29.Chiang CP, Huang JS, Wang JT, Liu BY, Kuo YS, Hahn LJ, et al. Expression of p53 protein correlates with decreased survival in patients with areca quid chewing and smoking-associated oral squamous cell carcinomas in Taiwan. J Oral Pathol Med. 1999;28:72–6. doi: 10.1111/j.1600-0714.1999.tb01999.x. [DOI] [PubMed] [Google Scholar]
- 30.Boyle JO, Hakim J, Koch W, van der Riet P, Hruban RH, Roa RA, et al. The incidence of p53 mutations increases with progression of head and neck cancer. Cancer Res. 1993;53:4477–80. [PubMed] [Google Scholar]
- 31.Nylander K, Stenling R, Gustafsson H, Zackrisson B, Roos G. p53 expression and cell proliferation in squamous cell carcinomas of the head and neck. Cancer. 1995;75:87–93. doi: 10.1002/1097-0142(19950101)75:1<87::aid-cncr2820750115>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 32.Abbas NF, Labib El-Sharkawy S, Abbas EA, Abdel Monem El-Shaer M. Immunohistochemical study of p53 and angiogenesis in benign and preneoplastic oral lesions and oral squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:385–90. doi: 10.1016/j.tripleo.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 33.Yan JJ, Tzeng CC, Jin YT. Overexpression of p53 protein in squamous cell carcinoma of buccal mucosa and tongue in Taiwan: An immunohistochemical and clinicopathological study. J Oral Pathol Med. 1996;25:55–9. doi: 10.1111/j.1600-0714.1996.tb00192.x. [DOI] [PubMed] [Google Scholar]
- 34.Jayade BV, Bhat K, Patil BR, Nayak R, Sant A. Histological significance of p53 gene expression in squamous cell carcinoma of the buccal mucosa. J Maxillofac Oral Surg. 2009;8:205–10. doi: 10.1007/s12663-009-0051-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Odell EW, Jani P, Sherriff M, Ahluwalia SM, Hibbert J, Levison DA, et al. The prognostic value of individual histologic grading parameters in small lingual squamous cell carcinomas. The importance of the pattern of invasion. Cancer. 1994;74:789–94. doi: 10.1002/1097-0142(19940801)74:3<789::aid-cncr2820740302>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 36.Gonzalez-Moles MA, Galindo P, Gutierrez-Fernandez J, Sanchez-Fernandez E, Rodriguez-Archilla A, Ruiz-Avila I, et al. P53 protein expression in oral squamous cell carcinoma. Survival analysis. Anticancer Res. 2001;21:2889–94. [PubMed] [Google Scholar]


