Abstract
Context:
Microbial contamination, which occurs during dental procedures, has been a potential threat to dental professionals and individuals. There has been a growing concern over the role of bioaerosols in spread of various airborne infections and also to reduce the risk of bioaerosol contamination.
Aims:
This study was to analyze the number of colony forming units (CFUs) in bioaerosols generated during ultrasonic scaling procedure as well as to evaluate the efficacy of chlorhexidine 0.12% (CHX) preprocedural mouth rinse and high volume evacuator (HVE) in minimizing the bioaerosol contamination.
Methods:
About 45 individuals were divided into three Groups A, B and C. These groups underwent ultrasonic scaling before and after the use of CHX (0.12%), HVE and combination of CHX (0.12%) and HVE. Bioaerosols were collected on blood agar plates which were incubated at 37°C for 48 h, and the CFUs were counted with manual colony counting device. A comparison was also done between A versus B, B versus C and A versus C groups.
Statistical Analysis Used:
Student's t-test.
Results:
We found a significant reduction in the CFUs when CHX (0.12%) preprocedural rinse (P < 0), or HVE (P < 0.001) or combination of both CHX (0.12%) and HVE were employed (P < 0.001). Maximum reduction in CFUs was observed when CHX (0.12%) and HVE were used in combination as compared to their individual use. A moderate significance was seen between A versus C groups but not with B versus C groups and A versus B groups.
Conclusion:
From our study, we conclude that individual methods such as CHX (0.12%) and HVE were useful to reduce the dental bioaerosols; however, combination of both CHX (0.12%) and HVE is more efficient to reduce dental bioaerosols than individual method.
Keywords: Bioaerosols, chlorhexidine, colony forming units, high volume evacuator
INTRODUCTION
Spread of infection in the dental set-up is of major concern to the dental community mainly because it carries a possible risk of transmission of infectious agents and their potential effects on the health of the dental personnel and the individuals.[1] Many dental procedures that use mechanical instrumentations such as ultrasonic scalers, handpieces, air polishing device and air abrasion units are known to produce bioaerosols from the operating site.[2] Bioaerosols are defined as particles <50 μm in diameter containing microorganisms in saliva, nasopharyngeal secretions and blood; plaque that are small enough to stay airborne for an extended period of time before they settle down on the environmental surface or enter the respiratory tract.[1] To effectively minimize bioaerosol contamination, American Dental Association (ADA) and the center of disease control and prevention (CDC) have recommended the use of universal barrier techniques for all dental procedures. These include gloves, mouth mask, rubber dam and high volume evacuator (HVE).[3] Preprocedural rinse mainly chlorhexidine (CHX) in varying concentrations, (0.12% normally) are also being used to reduce the bacterial contamination before ultrasonic scaling and periodontal surgery.[4] Hence, it seemed prudent to evaluate the efficacy of simple and inexpensive methods such as CHX (0.12%) preprocedural rinse and HVE in reducing bacterial contamination in bioaerosols generated during ultrasonic scaling procedure in dental setup by assessing the colony forming units (CFUs) before and after their use.
SUBJECTS AND METHODS
For this study, a good ventilated room measuring about 20 × 15 feet was selected; it was equipped with an autoclave and a single dental chair attached with an ultrasonic scaler and HVE. Before ultrasonic scaling procedure, the room was cleaned and fumigated with formaldehyde and potassium permanganate crystals for 2 days (48 h). Following fumigation, blood agar plates were kept for 10 min in the four corners and in the middle of the closed room to collect bioaerosol samples. After 10 min, agar plates were incubated at 37°C for 48 h to allow for the growth of bacteria. Following this, the culture plates were assessed for CFUs using manual colony counting device. Fumigation was repeated until there was a substantial reduction in contaminated bioaerosols in the room. The dental chair was disinfected with surface disinfectant (bacilol-25) and instruments such as mouth mirror, probe, kidney tray and scaler tips were sterilized in the autoclave.
Individuals were randomly selected for the study and medically compromised individuals were excluded from the study, they were evaluated for systemic diseases. EMS ultrasonic scaler was used and 5 min time was taken for oral prophylaxis of each quadrant.
A total of ninety bioaerosol samples were collected on the blood agar plates from 45 individuals in the department of periodontics during supragingival ultrasonic scaling procedure as shown in Figure 1. These individuals were divided into three Groups A, B and C each group comprising of15 individuals. Split mouth design technique was used to assess and compare the CFUs, without and with the use of 10 ml CHX 0.12% preprocedural rinse for 30 s and the use of HVE during supragingival scaling procedures. While using HVE, pressure was maintained according to the norms of confident dental chair, i.e. 30–40 psi kg/cm2 [Figure 2].
Figure 1.

Position of the blood agar plate in the fiberglass box during the procedure
Figure 2.
Position of the high volume evacuator during procedure
Group A consists of 15 individuals with 1st and 4th quadrants undergoing supragingival scaling without preprocedural rinse (0.12% CHX), 2nd and 3rd quadrants undergoing supragingival scaling after preprocedural rinse with 0.12% CHX. The cultures obtained with samples from 1 and 4 quadrants are presented in Figure 3 and those obtained with pre-procedural rinse with CHX are presented in Figure 4.
Figure 3.
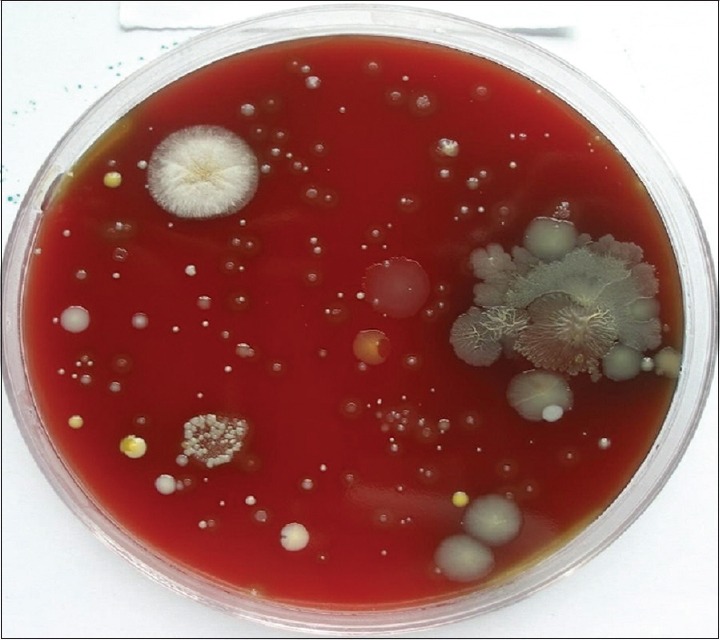
Colony forming units without the use of chlorhexidine (0.12%) preprocedural rinse
Figure 4.
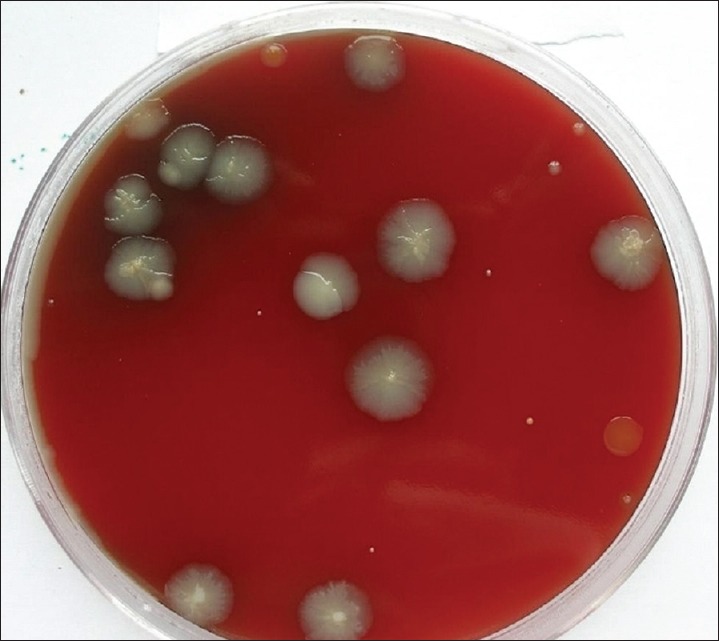
Reduction of colony forming units after the use of chlorhexidine (0.12%) preprocedural rinse
Group B consists of 15 individuals with 1st and 4th quadrants undergoing supragingival scaling without HVE, 2nd and 3rd quadrants undergoing supragingival scaling with HVE. The cultures obtained with samples from 1 and 4 quadrants are presented in Figure 5 and those obtained with the use of HVE are presented in Figure 6. Group C consists of 15 individuals with 1st and 4th quadrants undergoing supragingival scaling without preprocedural rinse (0.12% CHX) and HVE, 2nd and 3rd quadrants undergoing supragingival scaling with preprocedural rinse using 0.12% CHX and HVE. The cultures obtained with samples from 1 and 4 quadrants are presented in Figure 7 and those obtained with pre-procedural rinse with CHX and HVE are presented in Figure 8. All the collected bioaerosols samples were cultured in the Department of Oral Pathology.
Figure 5.
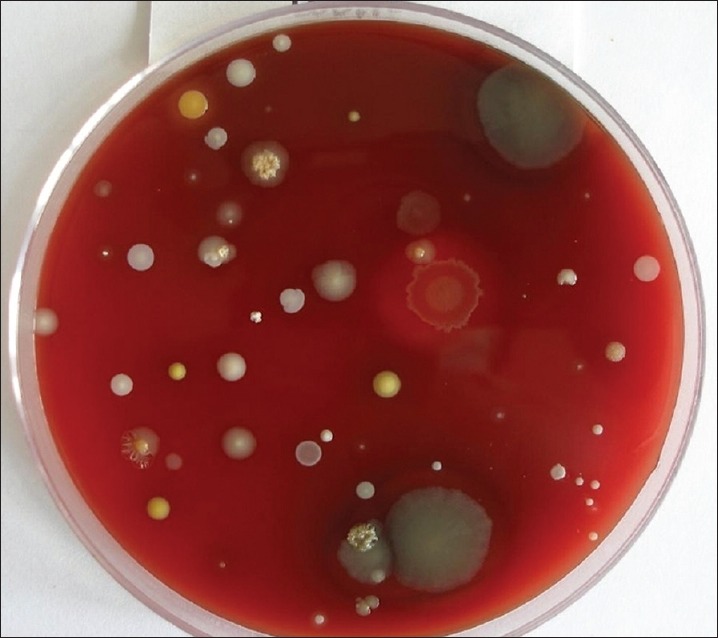
Colony forming units without the use of high volume evacuator
Figure 6.
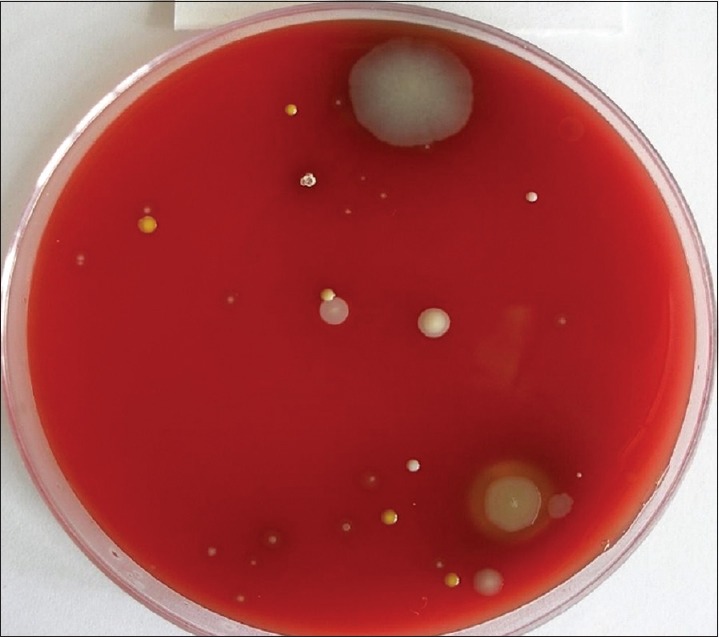
Reduction of colony forming units after the use of high volume evacuator
Figure 7.

Colony forming units without the use of both chlorhexidine (0.12%) preprocedural rinse and high volume evacuator
Figure 8.
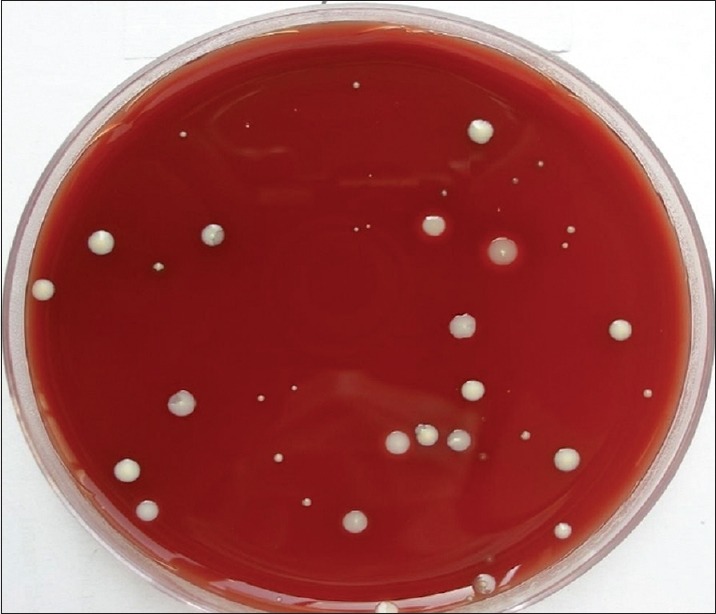
Significant reduction of colony forming units with the use of chlorhexidine (0.12%) preprocedural rinse and high volume evacuator
RESULTS
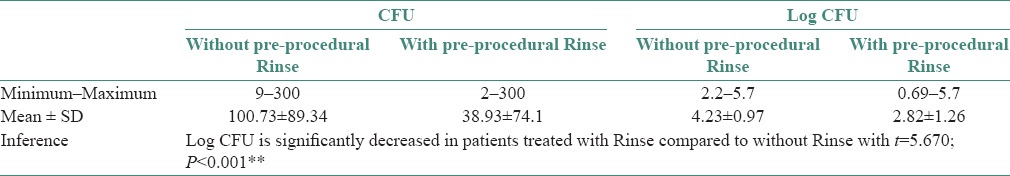
In the Group A individuals, the efficacy of CHX (0.12%) preprocedural rinse alone in reducing bioaerosol contamination during ultrasonic scaling procedure was assessed. Results showed that a regimen of 30s preprocedural rinse with 10 ml CHX (0.12%) before the ultrasonic scaling procedure significantly reduced the CFUs than when preprocedural rinse was not used [P < 0.001, Table 1 and Graph 1]. In Group B individuals who underwent ultrasonic scaling without and with the use of HVE, CFUs were significantly reduced when HVE was used compared to when HVE was not used [P < 0.0001, Table 2 and Graph 2]. In Group C individuals, CFUs were compared between without the use of CHX (0.12%) and HVE versus when a combination of both CHX (0.12%) and HVE was used during ultrasonic scaling procedure. CFUs were significantly reduced in individuals when treated with a combination of CHX (0.12%) preprocedural rinse and HVE [P < 0.001, Table 3 and Graph 3].
Table 1.
Comparison of Colony forming units from bioaerosols between without pre-procedural rinse and with pre-procedural rinse
Graph 1.
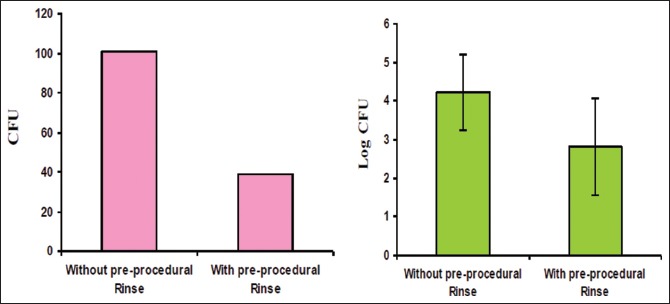
Log Colony Forming Units with and without use of CHX
Table 2.
Comparison of Colony forming units from bioaerosols between with and without the use of high volume evacuator
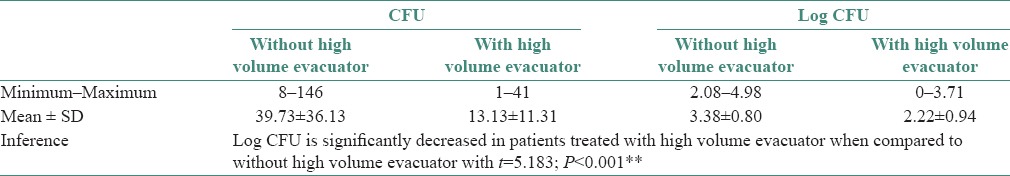
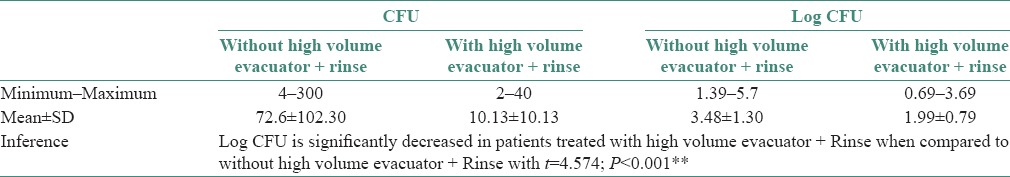
Table 3.
Comparison of colony forming units from bioaerosols between with and without the use of high volume evacuator and rinse
Graph 2.
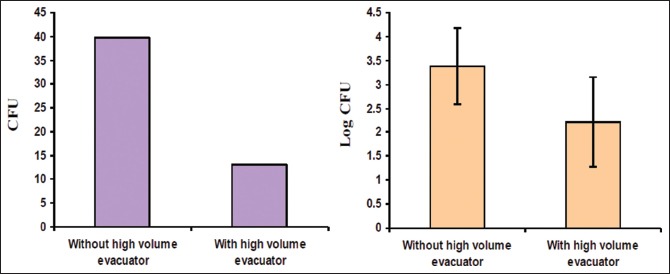
Log Colony Forming Units with and without use of HVE
Comparison of CFUs was also done between A versus B, B versus C and A versus C groups. Statistical significance was not found between A versus B groups (P = 0.135) and B versus C groups (P = 0.411); however, a moderate statistical significance was found between A versus C groups (P = 0.043) during ultrasonic scaling procedure. ANOVA is preferred to compare log CFUs between A, B and C groups.
DISCUSSION
Air contamination caused by different procedures carried out in the dental office has raised great concern and has prompted a continuous search for alternatives and methods to maintain the health of both dentists and individuals undergoing dental treatment.[5,6]
A probable cause of infection dissemination to the dentists, auxiliary personnel and individuals is intimately related to the generation of particles, aerosols, gases and splatter during the dental procedures.[1] Therefore, the dental practitioners have been strongly recommended to put into practice the control of bioaerosols contamination in their routine infection control protocol.[2]
ADA and the CDC have recommended several guidelines to minimize bioaerosol contamination. Sterilization of instruments, treatment of dental unit water lines, universal barrier protection such as gloves, face masks, rubber dam, preprocedural rinse and HVE should be used to reduce airborne contamination during dental procedures.[2,6] Use of expensive methods such as high-efficiency particulate air and ultraviolet chambers in the ventilation system have also been recommended.[7]
The aim of this study was to evaluate the role of simple and inexpensive methods such as 0.12% CHX perprocedural rinse and HVE in reducing the quantity of bacterial contamination in bioaerosols generated during oral prophylaxis using ultrasonic scalers.
In the Group A individuals, the CFUs were reduced to certain extent with the use of CHX (0.12%) preprocedural rinse alone [Table 1 and Graph 1]. This can be attributed to CHX (0.12%) being an effective antiseptic against free floating bacteria such as those found in the saliva and those loosely adhering to mucous membranes.[8]
Several studies have evaluated the efficacy of CHX (0.12%) preprocedural rinse in reducing the bacterial contamination.[9] A study using a similar protocol during scaling procedures showed that a 2 min prerinse with CHX significantly reduced the number of CFUs. Fine et al. reported that there was a 94.1% reduction in CFUs with 20 ml CHX (0.12%) mouth rinse for 30s when compared with non-rinse control and the difference was statistically significant. Similarly, another study done using saliva samples showed that two consecutive 30s preprocedural rinsing with CHX (0.12%) had a profound effect on the bacterial flora of the oral cavity.[10,11,12]
CFUs were significantly reduced with the use of HVE in Group B individuals when compared to Group A individuals [Table 2 and Graph 2]. The above results of our study are comparable with the findings of Harrel et al. who also found that HVE was effective in minimizing the bioaerosol contamination during ultrasonic scaling. The HVE used in dentistry has a large opening about 8 mm or greater and is attached to an evacuation system that helps to remove a large volume of air up to 100 cubic feet of air per minute.[13,14] These characteristics of HVE could also be the possible reason why HVE has shown to reduce contamination caused from operating site by more than 90%.[15,16,17] However, there are reports where only a narrow difference was observed when either HVE or conventional suction device (CDS) was used to reduce bioaerosol contamination.[18,19]
In Group C individuals, CFUs were significantly reduced when treated with a combination of CHX (0.12%) preprocedural rinse and HVE [Table 3 and Graph 3]. To the best of our knowledge, this is the first ever study done where CFUs were assessed when a combination of CHX (0.12%) and HVE have been used and a significant reduction in CFUs was found.
Graph 3.
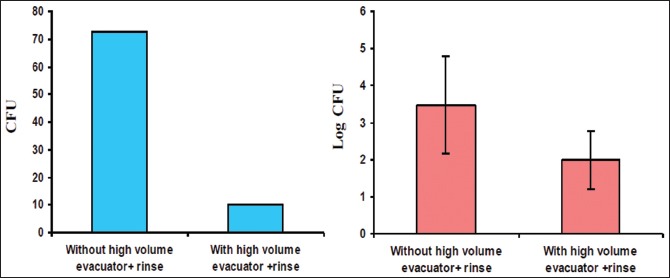
Log CFU with and without use of both CHX and HVE
Comparison of CFUs was also done between A versus B, B versus C and A versus C groups. Statistical significance was not found in the first two groups, whereas a moderate statistical significance was found between Groups A versus C during ultrasonic scaling procedure.
In certain circumstances due to lack of assistance, it may not be possible to use HVE in the dental settings.[1] In such situations use of preprocedural rinse with 0.12% CHX alone would be helpful in minimizing the bioaerosol contamination as has been seen in Group A individuals.
In our study, we have only assessed the role of CHX (0.12%) preprocedural rinse and HVE in reducing contamination by aerobic bacteria in bioaerosols. Any bacteria that require special media or growth conditions such as mycobacteria or strict anaerobes that are common in periodontal pockets will not grow on the media used in this study. Furthermore, viruses such as rhinoviruses, influenza and Severe Acute Respiratory Syndrome (SARS) virus do not grow on the type of media used for bacteria and hence, these viral particles cannot be cultured.[1,18] Further studies need to be done to investigate the role of CHX (0.12%) preprocedural rinse and HVE in effectively reducing the contamination by these microorganisms.
While it is impossible to completely eliminate the risk posed by dental bioaerosols, it is possible to minimize the risk by using simple and inexpensive methods such as personal protection barriers such as mouth mask, gloves, eye gear; by using preprocedural CHX (0.12%) mouth rinse;[15,18] and by using HVE. Unfortunately, many dental practitioners appear to use only the personal protection barrier as a single approach to reduce the risk of infection.[20] As we have observed in our study, a significant reduction in the CFUs when a combination of both CHX (0.12%) and HVE were used, we emphasize the need to use both CHX (0.12%) and HVE along with the personal protective barriers in routine dental infection control thereby minimizing any legal or regulatory risks that may exist in dental practice.[1,20]
CONCLUSION
The bioaerosols generated during dental procedures pose a potential risk for the spread of infections to dental personnel and individuals. The control of these contaminated bioaerosols has not been emphasized enough in dental infection control protocol.
Combination of both, CHX (0.12%) preprocedural rinse and HVE reduced bacterial contamination of bioaerosol more effectively than individual methods during ultrasonic scaling procedure. Moderate statistical significance was found in A group versus C group during ultrasonic scaling procedure. In some situations due to lack of assistance or infrastructure, use of HVE is difficult. In such situations use of CHX (0.12%) preprocedural rinse alone can be used to minimize the contaminated bioaerosols to a certain accepted levels.
We hereby emphasize the need to use a combination of both CHX (0.12%) preprocedural rinse and HVE along with the universal protective barriers in routine dental infection control in general dental practice. This in turn will help to reduce the risk of transmission of airborne infections to dental personnel and individuals in the dental setup.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Harrel SK, Molinari J. Aerosols and splatter in dentistry: A brief review of the literature and infection control implications. J Am Dent Assoc. 004;;35:429–37. doi: 10.14219/jada.archive.2004.0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harrel SK. Airborne spread of disease – The implications for dentistry. J Calif Dent Assoc. 2004;32:901–6. [PubMed] [Google Scholar]
- 3.Barnes JB, Harrel SK, Rivera-Hidalgo F. Blood contamination of the aerosols produced by in vivo use of ultrasonic scalers. J Periodontol. 1998;9:434–8. doi: 10.1902/jop.1998.69.4.434. [DOI] [PubMed] [Google Scholar]
- 4.Walmsley AD, Walsh TF, Laird WRE, Williams AR. Effect of cavitational activity on the root surfaces of teeth during ultrasonic scaling. J Clin Periodontol. 1990;17:306–12. doi: 10.1111/j.1600-051x.1990.tb01094.x. [DOI] [PubMed] [Google Scholar]
- 5.Trenter SC, Walmsley AD. Ultrasonic dental scaler: Associated hazards. J Clin Periodontol. 2003;30:95–101. doi: 10.1034/j.1600-051x.2003.00276.x. [DOI] [PubMed] [Google Scholar]
- 6.Leggat PA, Kedjarune U. Bacterial aerosols in the dental clinic: A review. Int Dent J. 2001;51:39–44. doi: 10.1002/j.1875-595x.2001.tb00816.x. [DOI] [PubMed] [Google Scholar]
- 7.Al Maghlouth A, Al Yousef Y, Al Bagieh N. Qualitative and quantitative analysis of bacterial aerosols. J Contemp Dent Pract. 2004;5:91–100. [PubMed] [Google Scholar]
- 8.Molinari JA. Infection control: Its evolution to the current standard precautions. J Am Dent Assoc. 2003;134:569–74. doi: 10.14219/jada.archive.2003.0222. [DOI] [PubMed] [Google Scholar]
- 9.Muzzin KB, King TB, Berry CW. Assessing the clinical effectiveness of an aerosol reduction device for the air polisher. J Am Dent Assoc. 999;;30:1354–9. doi: 10.14219/jada.archive.1999.0407. [DOI] [PubMed] [Google Scholar]
- 10.Szymanska J. Dental bioaerosol as an occupational hazard in a dentist's workplace. Ann Agric Environ Med. 2007;14:203–7. [PubMed] [Google Scholar]
- 11.Grenier D. Quantitative analysis of bacterial aerosols in two different dental clinic environments. Appl Environ Microbiol. 1995;61:3165–8. doi: 10.1128/aem.61.8.3165-3168.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bharathi P, Harsh P, Shashidhar A, Bhatt M, Ballal M. Efficacy of pre-procedural rinsing in reducing aerosol contamination during dental rocedures. J Infect Prev. 2009;10:190. [Google Scholar]
- 13.Azari MR, Ghadjari A, Nejad MR, Nairee NF. Airborne microbial contamination of dental units. Tanaffos. 2008;7:54–7. [Google Scholar]
- 14.Dorothy A, Perry R. Aerosol management policy for powered scalers. CDHA J. 2004;18:17–20. [Google Scholar]
- 15.Berlutti F, Testarelli L, Vaia F, De Luca M, Dolci G. Efficacy of anti-retraction devices in preventing bacterial contamination of dental unit water ines. J Dent. 2003;31:105–10. doi: 10.1016/s0300-5712(03)00004-6. [DOI] [PubMed] [Google Scholar]
- 16.Akande OO, Alada AR, Aderinokun GA, Ige AO. Efficacy of different brands of mouth rinses on oral bacterial load count in healthy adults. Afr Biomed Res. 2004;7:125–8. [Google Scholar]
- 17.Fine DH, Korik I, Furgang D, Myers R, Olshan A, Barnett ML, et al. Assessing pre-procedural subgingival irrigation and rinsing with an antiseptic mouthrinse to reduce bacteremia. J Am Dent Assoc. 1996;127:641–6. doi: 10.14219/jada.archive.1996.0276. [DOI] [PubMed] [Google Scholar]
- 18.Milejczak CB. Optimum travel distance of dental aerosols in the dental hygiene practice. ADHA. 2005;79:1. [Google Scholar]
- 19.Timmerman MF, Menso L, Steinfort J, van Winkelhoff AJ, van der Weijden GA. Atmospheric contamination during ultrasonic scaling. J Clin Periodontol. 2004;31:458–62. doi: 10.1111/j.1600-051X.2004.00511.x. [DOI] [PubMed] [Google Scholar]
- 20.Fine DH, Mendieta C, Barnett ML, Furgang D, Meyers R, Olshan A, et al. Efficacy of preprocedural rinsing with an antiseptic in reducing viable bacteria in dental aerosols. J Periodontol. 1992;63:821–4. doi: 10.1902/jop.1992.63.10.821. [DOI] [PubMed] [Google Scholar]


