Abstract
Background and Objective:
Tissue microarray (TMA) is a method of harvesting small disks of tissue from a range of standard paraffin tissue blocks and placing them in an array on a recipient paraffin block such that hundreds of cases can be analyzed simultaneously by using only a few microliters of antibody in immunohistochemistry as a single experiment. The TMA construction done with the help of automated tissue arrayer or commercially available rubber molds are expensive. This study involved the fabrication of TMA using rubber-based additional silicone mold constructed in the department and comparison of this method with two other methods of fabricating TMA.
Materials and Methods:
The TMA mold was fabricated using silicone material in the department. The recipient blocks were prepared. The tissue core prepared from donor blocks were inserted into the recipient blocks. The sections taken from this were compared with the TMA using double-sided adhesive tape technique and TMA by punching out holes in prefabricated dummy paraffin recipient block for insertion of tissue core.
Results:
The TMA using a mold made of silicone showed more advantages than other two methods.
Conclusion:
Fabricating TMA mold using silicone in the department is inexpensive and yet efficient.
Keywords: Multiple small tubular structures, silicone molds, tissue microarray
INTRODUCTION
Tissue microarrays (TMAs) are the paraffin blocks composed of multiple specimens. With TMAs, multiple specimens can be simultaneously investigated with different in situ techniques under identical laboratory conditions, resulting in dramatic time and cost reduction compared with conventional pathologic studies. Furthermore, this technology is less exhausting for the finite original donor material, allowing for a significantly increased number of assays per each case. The numerous advantages of this technology are obvious and have thus stimulated many constructors to evolve and improve different technical approaches.[1]
TMA technology is a new method used to analyze several tissues, especially tumor samples on a single slide.[2] It involves core needle biopsies of multiple preexisting paraffin-embedded tissue blocks and re-embedding them in the form of an arrayed master block. Thus, it means biopsy of a biopsy.[2,3]
The origin of TMAs can be attributed to Dr. Hector Battifora's “multitumor sausage blocks” in which a number of tissues, typically from different organs, were thrown together in the same block and tissue distribution of a particular antigen/protein was assessed.[4] Battifora and Mehta developed a method with the alignment of the tissue specimens in a Cartesian coordinate system (checkerboard pattern) popularly known as “checker board tissue block” method.[5] Kononen used a cast of a small amount of melted paraffin to record the position of each punch specimen. This led to the development of a TMA precision microarray instrument with an X-Y Guide. This enabled real high throughput analysis with arraying of up to 1000 cores in the same block.[6]
Most of the applications of the TMA technology have come from the field of cancer research. Examples include analysis of the frequency of molecular alterations in large tumor materials, exploration of tumor progression, identification of predictive or prognostic factors and validation of newly discovered genes as diagnostic and therapeutic targets.[7] It can be used to correlate lymph node positive and negative tumors, for molecular classification of tumors, for rapid linking of molecular changes to clinical endpoints and for predicting the response of chemotherapeutics or hormonotherapy.[8,9] Since it provides studying a parameter for 100–1000 samples on a single slide, community-based retrospective cohort studies could be made possible.[10] The recent development of TMA technology has potentiated large-scale retrospective cohort studies using archival formalin fixed, paraffin-embedded tissues.[11]
Commercially available rubber molds and automated tissue microarrayer are expensive ranging from few thousands to lakhs of rupees. Hence, manual TMA construction has been introduced to avoid the high cost of automated and semi-automated techniques. Here, we present an alternative method for the construction of TMA blocks that can be performed by any pathology laboratory; at low cost with a minimum requirement of skill and time using rubber-based additional silicone material.
MATERIALS AND METHODS
Materials required
Emptied ball point pen refills with an approximate diameter of 3 mm, stainless steel base mold, modeling wax, embedding ring, paraffin wax embedding station, dermal biopsy punch with stylets and microtome for sectioning.
Method
We constructed ten TMA blocks of the same measurement with different tissues being embedded in each of the ten blocks. The following steps were followed for the construction of TMA silicone mold and TMA.
Step 1: Construction of wax base
A wax base with the dimensions exactly to that of stainless steel mold which is used for normal paraffin wax embedding was prepared by pouring molten molding wax into the stainless steel mold. After setting, the wax was removed from the stainless steel mold. This wax acts as a base for the wax mold. The number of the tissue core required in the TMA was decided, for example, 5 × 3 or 5 × 5. The distance between each core of 2 mm was marked on the base wax. A ball point pen refill cut in 4 mm length was just pressed against the markings made by melting the surface of the wax base. The 3 mm height wall was built around the wax base [Figure 1].
Figure 1.
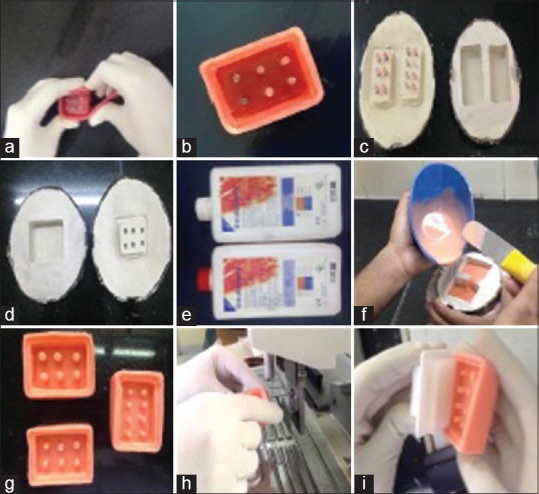
(a) Custom made wax mold for tissue microarray, (b) dewaxing of custom made wax mold, (c) negative replica of custom made wax mold, (d) A single negative replica of custom made wax mold, (e) Monomer and polymer of rubber-base addition silicone used to prepare tissue microarray molds, (f) pouring of rubber-base addition silicone material, (g) replica of recipient block mold made using rubber-base addition silicone material, (h) liquid wax being poured into rubber mold and (I) removal of recipient block after setting of rubber mold
Step 2: Construction of silicon mold
The wax mold was then subjected for dewaxing in a normal denture flask. A negative replica of wax mold was obtained. Rubber-based additional silicone material was mixed and poured into the negative replica of wax mold, packed and allowed to set for 1 h in a denture flask. Additional silicone material can withstand temperatures from −55 to +300°C. After deflasking, a positive replica of the wax mold of rubber base material was obtained [Figure 1].
Step 3: Construction of recipient block
This rubber-based mold could now be used as a tissue array mold which is used for making recipient blocks. Liquid wax was poured into the rubber molds and after setting the recipient block was removed from the rubber mold [Figure 1].
Step 4: Construction of tissue microarray
The paraffin tissue blocks of interest were collected and numbered accordingly. The donor tissue cores were prepared from different paraffin wax tissue blocks by punching the area of interest with the help of dermal punch biopsy needle. The diameter of punch biopsy used was of similar dimension to that of the recipient holes. The donor tissue cores were inserted into the recipient blocks carefully. During this procedure, care was taken to number the recipient blocks with respect to donor tissue cores and the position of each core was recorded properly in a separate sheet. After inserting the tissue cores, the surface of the recipient block was pressed against the heated glass slide so that the tissue cores merges with the recipient block uniformly and was kept at 45°C for 10 min [Figure 2].
Figure 2.
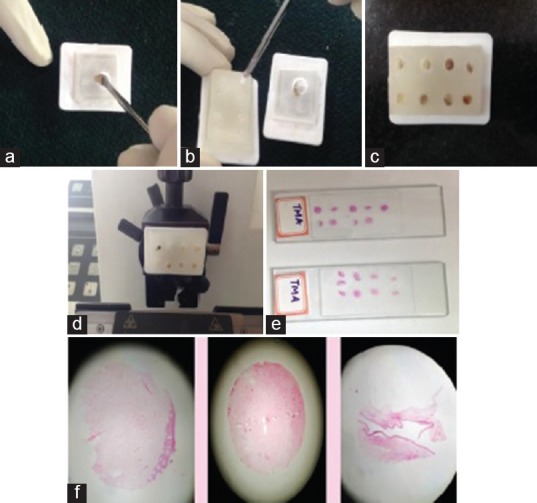
(a) Punching out tissue core with the help of bone biopsy needle, (b) inserting the tissue cores from donor block to the recipient block, (c) recipient block ready for sectioning and (d, e & f) Obtaining H&E stained sections of the tissue microarray
Step 5: Sectioning of tissue microarray
Now, the TMA block was sectioned in the routine method, stained and numbered accordingly [Figure 2].
In contrast, two other techniques such as double-sided adhesive tape technique [Figure 3] and dummy paraffin recipient block technique were also tried in the department.
Figure 3.
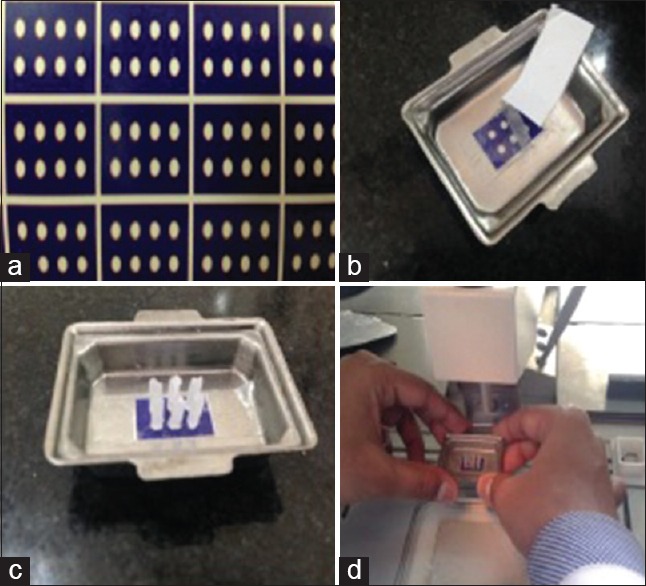
(a) Double-sided adhesive tape made using Corel draw software. (b) double-sided tape being applied to the stainless steel mold, (c) tissue cores obtained from various donor blocks placed on double-sided adhesive tape and (d) embedding of the tissue cores in double sided adhesive tape method
A grid using the drawing software Corel Draw was designed along these lines: 1 mm white circles were drawn and aligned leaving 1 mm space between them, on a colorful background; the grid was printed in the plain blue paper [Figure 3]. Paper grids were attached to stainless steel molds by means of a double face adhesive tape, Scotch 3M, 12 mm wide. The tape should be wider than the grid so that the free border of the adhesive tape bottom surface could attach the paper grid into the mold and the whole upper surface should be available for attaching the tissue cores. Following this, to extract the core the bevel from the conventional hypodermic needles were removed and the core were punched from the donor block, the extracted cores were attached to the tape, overlying the selected white circles on the paper grid, in an orderly fashion, melted paraffin was gently poured into the mold. It is important to make sure that all cores are perfectly vertical at this step. From this point, blocks were handled according to routine histopathological procedures. This procedure was adopted from method followed by Pires et al.[12]
The other method was making a dummy recipient block by pouring the molten paraffin wax into the stainless steel molds. After removing the block, the number of cores was decided and marking was made on the block with a uniform distance between each core area and the cores were punched on the markings using the punch biopsy needle. The donor cores were punched from the donor block and were inserted into these holes. Once the TMA were ready, sectioning and staining were done in the routine manner.
RESULTS
The TMA using a mold made of rubber-based addition silicone material worked out to be very cheap. We could perform many recipient blocks from the single mold, which was fabricated. It was found that the technique is more standardized than other two techniques. In the double-sided adhesive technique, we experienced the difficulty when pouring the wax over the adhered tissue core, the core tilt and floatation were common. In the dummy recipient block method, the difficulty was in punching the uniform core. During punching the core would break or the depth of the hole would vary. Hence, the TMA block showed many tilted and sunken tissue core and the sectioning was difficult.
DISCUSSION
In 1965, Lilie first described the “special blocking and trimming procedure for cross-sections of multiple small tubular structures” in his histopathologic technique and practical histochemistry. Since then, an evolutionary process has begun to refine and optimize the technical application of TMAs.[13]
It was Battifora and Mehta, who introduced the “multitumor sausage block” and a few years later, “checkerboard tissue block.”[5,6]
Using a cannula to directly punch out tissue cores from archival paraffin blocks was described first by Wan et al.[14] in 1987. This technical improvement abolished the laborious rod cutting and re-embedding of the tissues designated for a TMA and introduced a new generation of TMA.
The original Beecher manual tissue microarrayer was improved in a little while to become a computer numerical control arrayer with still manual or fully automatic transfer of the punched tissue core biopsies [PTCBs] (automated tissue arrayer ATA-27, Beecher Instruments, Inc.).[15,16]
Furthermore, an arrayer was designed with a reflecting microscope to improve the selection of the tissue from the donor block (e.g. VTA-100 Tissue Arrayer [about 55,000 US$], Veridiam, Oceanside, CA, USA).[17]
There are two types of TMA technique, automated and manual. In automated method, we can mark, edit and save punch coordinates using an on-screen display and software tools, while visual selection can be performed during punching, using magnifying glass or a stereomicroscope ASA guide. It is faster in the automated method with respect to punch and speed. The block capacity is 7 times more than manual method. Video merge unit displays premarked slide images side-by-side to the donor block image in the automated method, while pathologist marks regions of interest to slides by hand before arraying in manual method. Punch sets of 0.6, 1.0, 1.5 and 2.0 mm are available. Like automated Tissue Microarrayers, Manual Tissue Arrayers are also commercially available. Automated evaluation is also possible with a DNA microarray scanner.[3]
Microarray is a technique for organizing minute amounts of biological samples on a solid support.[18] TMAs are composite paraffin blocks constructed by extracting cylindrical tissue core “biopsies” from different paraffin donor blocks and re-embedding these into a single recipient (microarray) block at defined array coordinates.[19]
A meaningful database must record the relevant patient data, allowing for a reliable connection to clinical follow-up databases and also all relevant pathologic data such as tissue type, tumor or disease entity, grading and staging to each case and most importantly all data for each tissue core in every TMA. There are existing web-based database structures to handle clinical and pathological data for each patient in a TMA, easily facilitating intra-institutional and inter-institutional collaborations. Data management is crucial and should be well established before any substantial application of TMAs is begun.[1]
The costs of constructing a TMA differ from a few to thousands of Euros depending on the technique/equipment to be used. Remarkably, high-quality TMAs can also be achieved by low-cost techniques.[20,21]
Such one technique was employed in our laboratory by utilizing the rubber-based silicone material easily available in the Department of Prosthodontics. The TMA using a mold made of rubber-based addition silicone material worked out to be very cheap. Once fabricated, we could perform many recipient blocks from the single mold. It was found that the technique is more standardized than other two techniques such as the double-sided adhesive technique and the dummy recipient block method.
CONCLUSION
The disadvantages were:
Choosing the representative area on the donor block requires patience and experience
The disadvantage of the destruction of the donor block while making the punch. Hence, leading to loss of archival tissue (note: As TMA is being used more often for research purpose than for diagnostic purposes, it is always advised to use tissue from the excisional biopsy specimens as the donor block).
The advantages were as follows:
Once the mold is fabricated, the molds can be used many times and is less cumbersome than the other two methods
The depth of the tissue cores will get standardized; hence, the level of all the tissue core will be in the same plane
No question of core tilt
No fracture of inter-core bridge as was noticed with the dummy recipient method
Thus, even with low-cost techniques high density and precise TMAs can be constructed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Mengel M, Kreipe H, von Wasielewski R. Rapid and large-scale transition of new tumor biomarkers to clinical biopsy material by innovative tissue microarray systems. Appl Immunohistochem Mol Morphol. 2003;11:261–8. doi: 10.1097/00129039-200309000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Schoenberg Fejzo M, Slamon DJ. Frozen tumor tissue microarray technology for analysis of tumor RNA, DNA, and proteins. Am J Pathol. 2001;159:1645–50. doi: 10.1016/S0002-9440(10)63011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aktas S. Tissue microarray: Current perspectives in pathology. Aegean Pathology Journal. 2004;1:27–32. [Google Scholar]
- 4.Battifora H. The multitumor (sausage) tissue block: Novel method for immunohistochemical antibody testing. Lab Invest. 1986;55:244–8. [PubMed] [Google Scholar]
- 5.Battifora H, Mehta P. The checkerboard tissue block. An improved multi tissue control block. Lab Invest. 1990;63:722–4. [PubMed] [Google Scholar]
- 6.Kononen J, Bubendorf L, Kallioniemi A, Bärlund M, Schraml P, Leighton S, et al. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med. 1998;4:844–7. doi: 10.1038/nm0798-844. [DOI] [PubMed] [Google Scholar]
- 7.Kallioniemi OP, Wagner U, Kononen J, Sauter G. Tissue microarray technology for high-throughput molecular profiling of cancer. Hum Mol Genet. 2001;10:657–62. doi: 10.1093/hmg/10.7.657. [DOI] [PubMed] [Google Scholar]
- 8.Hu YC, Komorowski RA, Graewin S, Hostetter G, Kallioniemi OP, Pitt HA, et al. Thymidylate synthase expression predicts the response to 5-fluorouracil-based adjuvant therapy in pancreatic cancer. Clin Cancer Res. 2003;9:4165–71. [PubMed] [Google Scholar]
- 9.Bubendorf L, Kononen J, Koivisto P, Schraml P, Moch H, Gasser TC, et al. Survey of gene amplifications during prostate cancer progression by high-throughout fluorescence in situ hybridization on tissue microarrays. Cancer Res. 1999;59:803–6. [PubMed] [Google Scholar]
- 10.Kluger HM, Dolled-Filhart M, Rodov S, Kacinski BM, Camp RL, Rimm DL. Macrophage colony-stimulating factor-1 receptor expression is associated with poor outcome in breast cancer by large cohort tissue microarray analysis. Clin Cancer Res. 2004;10(1 Pt 1):173–7. doi: 10.1158/1078-0432.ccr-0699-3. [DOI] [PubMed] [Google Scholar]
- 11.Camp RL, Charette LA, Rimm DL. Validation of tissue microarray technology in breast carcinoma. Lab Invest. 2000;80:1943–9. doi: 10.1038/labinvest.3780204. [DOI] [PubMed] [Google Scholar]
- 12.Pires AR, Andreiuolo Fda M, de Souza SR. TMA for all: A new method for the construction of tissue microarrays without recipient paraffin block using custom-built needles. Diagn Pathol. 2006;1:14. doi: 10.1186/1746-1596-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lilie RD. 3rd ed. New York, USA: McGraw-Hill Book Co; 1965. Histopathologic Technic and Practical Histochemistry. [Google Scholar]
- 14.Wan WH, Fortuna MB, Furmanski P. A rapid and efficient method for testing immunohistochemical reactivity of monoclonal antibodies against multiple tissue samples simultaneously. J Immunol Methods. 1987;103:121–9. doi: 10.1016/0022-1759(87)90249-3. [DOI] [PubMed] [Google Scholar]
- 15.Sun Prairie, WI: Beecher Instruments, Inc; [Last accessed on 2014 Jan 09]. Manual Tissue Arrayer MTA-1: Instruction Manual. Available from: http://www.beecherinstruments.com/ [Google Scholar]
- 16.Estonia: Estigen Tissue Science; [Last accessed on 2014 Jan 09]. Manual Tissue Arrayer MTA-1. Available from: http://www.estigen.com/ [Google Scholar]
- 17.San Diego (USA): Veridiam; [Last accessed on 2014 Jan 09]. Veridiam Tissue Arrayer. Available from: http://www.veridiamtissuearrayer.com/ [Google Scholar]
- 18.Giltnane JM, Rimm DL. Technology insight: Identification of biomarkers with tissue microarray technology. Nat Clin Pract Oncol. 2004;1:104–1119. doi: 10.1038/ncponc0046. [DOI] [PubMed] [Google Scholar]
- 19.Wang H, Wang H, Zhang W, Fuller GN. Tissue microarrays: Applications in neuropathology research, diagnosis, and education. Brain Pathol. 2002;12:95–107. doi: 10.1111/j.1750-3639.2002.tb00426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilkens L. Method and Apparatus for preparation of tissue samples. German Patent. 2003 DE 102 03 524 A1. [Google Scholar]
- 21.Vogel UF, Bueltmann BD. Low-cost tissue microarrays (TMA) constructed with a common microcompound table. Mod Pathol. 2004;17:363A. doi: 10.1309/F2Q38DXN1V1V4GQM. [DOI] [PubMed] [Google Scholar]


