Abstract
Aims and Objectives:
The aim of this study was to evaluate and correlate the relationship between mast cells counts and different stages of human periodontal diseases.
Materials and Methods:
The study sample comprised 50 patients, which were divided into three groups, consisting of 10 cases of clinically healthy gingival tissues (control group) 20 cases of dental plaque-induced gingivitis with no attachment loss and 20 cases of localized chronic periodontitis (LCP) characterized by the loss of periodontal support. The samples for control group were obtained during tooth extractions for orthodontic reasons. The specimens were immediately fixed in 10% neutral buffered formalin.
Conclusion:
In this study, LCP cases had higher mast cell counts compared to gingivitis sites or healthy tissues. Increased mast cell counts in the progressing sites of periodontal diseases may indicate the importance of these cells in the progression of chronic periodontitis.
Keywords: Inflammation, mast cells, periodontal disease
INTRODUCTION
The bacterial plaque has been implicated as the primary etiologic factor in inflammatory periodontal disease, but recently, several studies have focused on the role of the immune system in the evolution of the periodontal disease, indicating that bacterial antigens trigger an immunopathologic reaction and that the susceptibility of the patients determines the ultimate outcome of the disease process.[1]
Mast cells are also known as “unicellular endocrine glands” due to their ability to release a wide variety of chemical mediators that have a potent biological action on target tissues. In inflammatory lesions of the oral cavity, neo-vascularization and presence of inflammatory cells is an expected finding.[2] Substantial evidence has implicated certain immune and inflammatory responses as destructive mechanisms in the periodontal disease process. One possible host reaction against periodontal breakdown may be mediated by mast cell release. Some investigators have proposed a role for mast cell constituents in periodontal destruction.[3]
The aim of this study was to evaluate and correlate the relationship between mast cell count in different stages of human periodontal diseases.
MATERIALS AND METHODS
The American Academy of Periodontology (AAP)[4] guidelines were followed for classification of the periodontal disease and conditions.
Based on AAP guidelines samples were divided into three groups:
Group 1 - Control group comprising of normal oral mucosa probing depth (PD) <3 mm with no bleeding on probing
Group 2 - Dental plaque induced gingivitis [DPIG] PD <3 mm and clinical attachment loss (CAL) <1 mm with bleeding on probing
Group 3 - Localized chronic periodontitis [LCP] PD and CAL >4 mm with bleeding on probing.
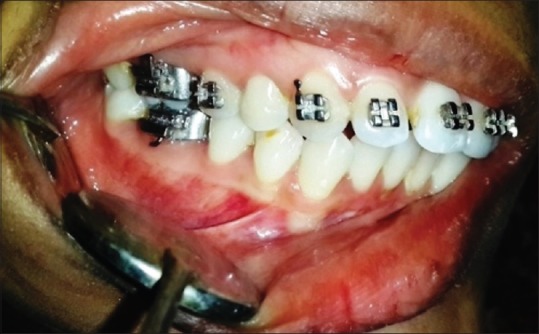
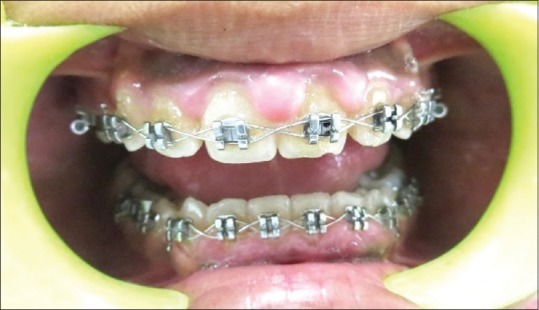
The study sample comprised 50 patients, which were divided into three groups, consisting of 10 cases of clinically healthy gingival tissues (control group) [Figure 1], 20 cases of DPIG with no attachment loss [Figure 2] and 20 cases of LCP characterized by the loss of periodontal support [Figure 3].
Figure 1.
Clinical image of a case in periodontally healthy group
Figure 2.
Clinical image of a case in dental plaque-induced gingivitis group
Figure 3.
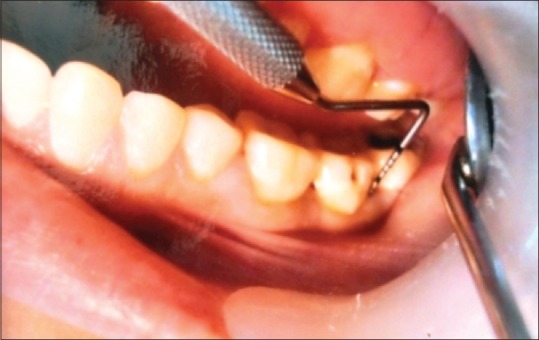
Clinical image of a case in localised chronic periodontitis group
The study was undertaken after approval from the Institutional Ethical Committee. Patients were selected from the outpatient department of Department of Periodontology and Implantology. Informed consent was taken from the patient after explaining the biopsy procedure. The healthy gingival tissue was obtained during tooth extractions for orthodontic reasons. The samples of groups DPIG and LCP were obtained from patients undergoing periodontal surgery (modified Widman flap surgery and gingivectomy). Clinical parameters such as plaque index,[5] gingival index,[6] PD and CAL were recorded for each of the cases.
The biopsied specimens were immediately fixed in 10% neutral buffered formalin for further processing and then dehydrated, cleared and embedded in paraffin wax. Tissue sections of 5 μm were sectioned using semi-automatic soft tissue microtome. Two tissue sections of 5 μm thickness were cut from each tissue. One section was stained with hematoxylin and eosin and categorized to the particular groups and the second section was stained with special stain, i.e., 1% toluidine blue used specifically to demonstrate mast cells.
Hematoxylin and eosin stained tissue sections from the surgical specimens fixed in 10% formalin and embedded in paraffin wax were reviewed to confirm the histological diagnosis and representative sites on tissue sections blocks were selected. Examination was carried out on Olympus BX 51 trinocular light microscope with provision for photomicrograph with Olympus E331 SLR digital camera.
Mast cells were analyzed quantitatively by counting the total number of mast cells as well as qualitatively by counting the number of intact and degranulated mast cells in toluidine blue stained sections. Mast cells were counted using an oculometer grid under a magnification of ×40 [Figures 4–6]. The area of the microscopic field was calibrated-with an ocular grid fitted inside the eyepiece with an area of 0.04 mm2. The mast cells were counted throughout each of the tissue sections in 10 representative and consecutive grid fields (×40). The mean of ten values was calculated and expressed as mean ± standard deviation (SD) per mm2. The field was analyzed in a step-ladder fashion and care was taken to prevent the overlapping of fields. The mast cell counts of each field were re-assessed after 4 weeks by same observer and other observer in random order to observe intra- and inter-observer variability.
Figure 4.
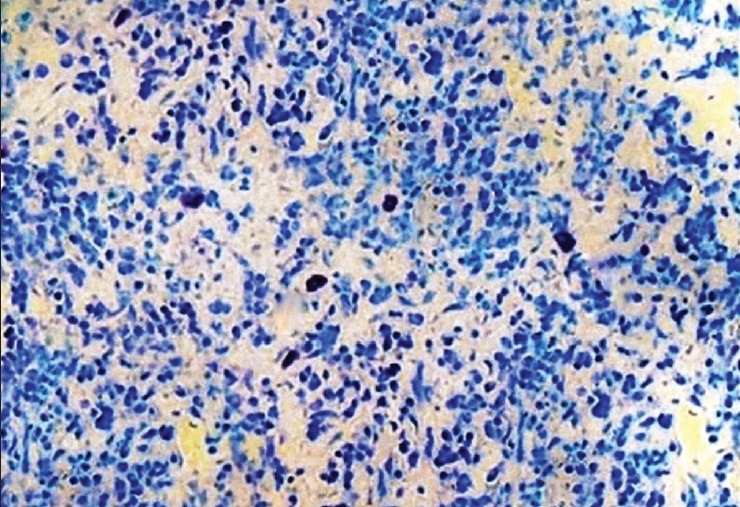
Histological section showing mast cells in a case of periodontally healthy group (Toluidine blue stain, ×400)
Figure 6.

Histological section showing mast cells in localized chronic periodontitis with occulometer grid (Toluidine blue stain, ×400)
Figure 5.
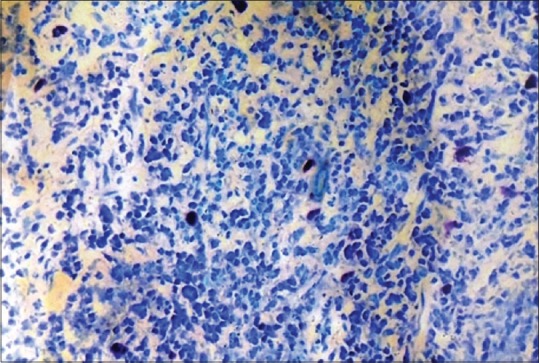
Histological section showing mast cells in dental plaque-induced gingivitis (Toluidine blue stain, ×400)
Statistical analysis
The continuous data were summarized as mean ± SD while discrete (categorical) in number and percentage continuous groups were compared using analysis of variance and the significance of the mean difference between the groups was done by Tukey's post hoc test. Categorical groups were compared using Chi-square test. The intra- and inter-observer variability were analyzed using intraclass correlation coefficient and Pearson correlation coefficient analyses, respectively. A two-tailed (α = 2) P < 0.05 was considered statistically significant.
Number of positively stained mast cells in the periodontal tissues was determined in 10 consecutive microscopic high power (×800; objective ×64; eyepiece ×12.5; tube factor ×1) fields (each field has an area of 0.017 gg 25 mm2, obtained from the mathematical expression A = [πd2]/4, where P = 3.14, d = 0.15) in a representative section of each specimen.
RESULTS
Table 1 shows that the mean mast cell counts of LCP group was the highest followed by DPIG group and control group the least (control < DPIG < LCP). On statistical analysis using SPSS software (Windows version 18.0), we found significantly (P < 0.001) high intra- and inter-observer reliability/reproducibility of mast cell counts.
Table 1.
The mast cell counts (mean±standard deviation) of three groups

The observed mean PD and CAL of three groups are summarized in Table 2.
Table 2.
The mean probing depth and clinical attachment loss of three groups
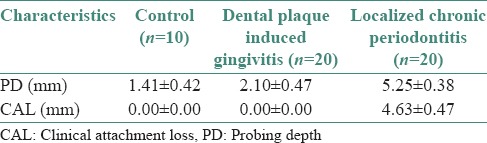
The correlation between PD and mast cell counts of all three groups are summarized graphically in [Figure 7]. Pearson correlation analysis revealed a significant and positive (direct) correlation between PD and mast cells counts (r = 0.90, P < 0.001).
Figure 7.
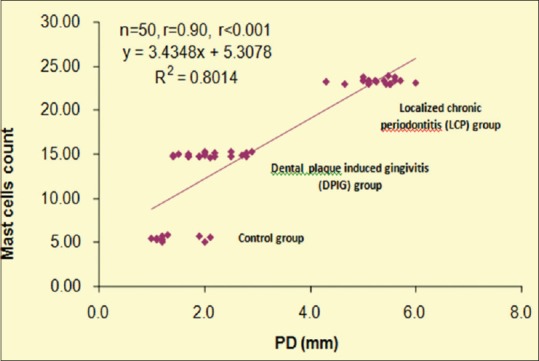
Correlation between probing depth and mast cell counts
The correlation between CAL and mast cell counts of LCP group are summarized in [Figure 8]. Pearson correlation analysis revealed a significant and positive (direct) correlation between CAL and mast cells counts (r = 0.90, P < 0.001).
Figure 8.
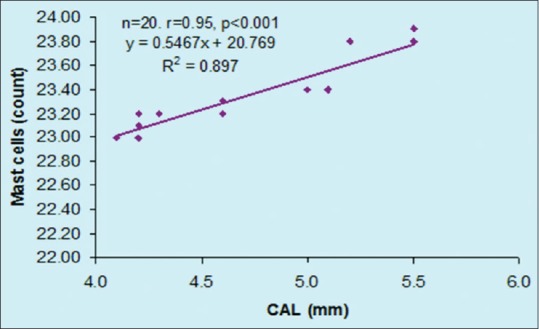
Correlation between clinical attachment loss and mast cell counts
DISCUSSION
Mast cells originate from pluripotential hematopoietic cells in the bone marrow, undergo part of differentiation in this site, then enter the circulation and complete their differentiation in peripheral mucosal or connective tissue microenvironments.[7]
Mast cells are scattered throughout gingival connective tissue, often in close association with endothelial cells, but are also found sub- and intra-epithelially. In inflamed and in healing gingiva, number of mast cells are found to be increased. Mast cells are characterized by oval to round nuclei with the cytoplasm densely packed with bright red granules. They can be stained with Giemsa stain or toludine blue stain. Mast cells may be round, oval, or spindle with abundant cytoplasm or thin and elongated resembling fibroblast. Each mast cell typically contains between 80 and 300 granules. When activated, mast cells may either undergo explosive degranulation and then resynthesize their granules or they may release solitary granules into their environment on an on-going basis, a process termed “piecemeal degranulation” that has been observed in both the oral mucosa and skin.[8]
Following degranulation, mast cell mediators are deposited in large quantities in the extracellular environment, where they exert effects on endothelial cells and other cell types. Mast cells may subsequently synthesize and secrete additional mediators that are not preformed in their granules. Key mediators that are preformed in mast cells are serine proteases tryptase, chymase cathepsin G, histamine, heparin, serotonin, acid hydrolases and the cytokines tumor necrosis factor- -α and interleukin- 16 (IL-16).[9] Following activation, mast cells can synthesize a range of mediators, including the ILs IL-1, IL-3, IL-4, IL-5, IL-6, IL-8, IL-10, IL-13, and IL-16, together with granulocyte macrophage colony-stimulating factor, platelet activating factor, RANTES, macrophage inflammatory protein-1a and the arachidonic acid metabolites prostaglandin 2 and leukotriene C4.[10]
The results of our study indicates that mast cells have higher counts in chronic periodontitis compared to healthy/gingivitis cases, which is consistent with the results of studies carried out by Batista et al., Vahabi et al., Lagdive et al.
Gemmell et al., using the same immunohistochemistry marker, observed that the numbers of tryptase-positive mast cells were decreased in chronic periodontitis tissue sections compared with healthy/gingivitis samples. One possible explanation for this discrepancy could be that the clinically healthy and marginal gingivitis samples were grouped together (healthy/gingivitis) in Gemmell's study, while we used separate samples of DPIG and clinically healthy gingival tissues (health).[11]
The results of this study suggest that mast cell counts may be associated with periodontitis. This finding suggests a significant role of mast cells in chronic periodontal tissue breakdown. One of the biological and biochemical factors is histamine, which breaks down the tissue barrier, causes edema and helps cellular infiltration. In addition, mast cells are believed to contain most of the body's histamine.[12] Another reason is that the expression of matrix metalloproteinases (MMPs) 1, 2 and 8 are strongest in mast cells. MMPs are crucial in the degradation of the main components in extracellular matrices.[13] Furthermore, tryptase can cleave the third component of collagen and activate latent collagenase that can participate in tissue destruction in periodontitis. Furthermore, it has been indicated that tryptase activity is confined to mast cell granules.[3]
A change from gingivitis to periodontitis involves a shift from predominantly T -cell lesion to a B - cell/plasma cell lesion. Mast cells seem to be able to present antigens to T-cells. The resultant T-cell activation would activate mast cells, leading to both degranulation and cytokine release. In our study, we found an increase in number of mast cells in inflamed site as compared to periodontally healthy site, suggesting important dynamic alterations in the migration and localization of mast cells in the evolution of periodontal disease, which need to be more precisely speculated.[7]
Based on our results, the increase of mast cells speculates the possible participation of mast cells in the defense mechanism and destructive events both as effector and responsive cells in chronic inflammation, as well as the possible functional relationship between mast cells and immunocompetent cell populations in periodontal lesions.[8]
Based on the concept that mast cells play a critical role in the induction of inflammation, it is logical to use therapeutic agents to alter mast cell function and secretion, to prevent inflammation in its earliest phase.[9] We put forward a concept where in mast cell stabilizers as a group of drugs, can be used in the prevention of periodontal disease. Mast cell stabilizers can be used to advantage for host modulation therapy in periodontitis: Mast cell stabilizers are currently being used successfully in prophylactic treatment of bronchial asthma. Some of the commonly used mast cell stabilizers are sodium cromoglycate and nedocromil sodium (NS). These drugs stabilize the mast cell membrane thus preventing mast cell degranulation.[14] NS not only reduces allergic inflammation by inhibiting mediator release from mast cells but also exhibits a wide range of additional anti-inflammatory activities, including inhibition of increased vascular permeability induced by individual mediators such as histamine.[15]
For longer bioavailability of mast cell stabilizers in the periodontal pocket, they can be incorporated into a biodegradable matrix of cross-linked hydrolyzed gelatine and made into a chip. After insertion into the pocket, the chip will come into contact with the gingival crevicular fluid and swell, which prevents the chip from falling out of the pocket. In the first 24 h, there will be a burst effect and a portion of drug will be released into the pocket. Thereafter, the release profile of the drug will be in zero order (constant release rate), up to 10 days. Within 7–10 days, it provides optimal levels of the drug in the periodontal pocket. At the same time, the chip is biodegraded within 7–10 days due to enzymatic degradation. Placement of the mast cell stabilizer incorporated in a bioadhesive local drug delivery mode, will result in higher bioavailability of the drug for a longer period of time. Hence, the best option of delivery of the drug into the narrow periodontal pocket environment is through local drug delivery systems. Placement of the drug in the periodontal pocket environment followed by sealing the periodontal pocket environment with cyanoacrylate glue or a periodontal dressing would be advised.[16]
CONCLUSION
The possible relationships between mast cells and pathogenesis of periodontal diseases is putforth in this study. Further research is needed to elucidate the cellular interactions and immunological and dynamic aspects of the disease so that the pathogenesis of periodontitis might be elucidated more clearly and effective treatment approaches can be suggested. The therapeutic implications of the findings and suggestions here in presented include strategies directed toward the possible use of drugs to influence mast cell secretion and there by prevent inflammation and maintenance of chronicity or even with the aim of improving periodontal regeneration. Further studies on mast cells can prove to be beneficial in determining the efficacy and effectiveness of mast cell stabilizers in limiting the progression of periodontal diseases.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Batista AC, Rodini CO, Lara VS. Quantification of mast cells in different stages of human periodontal disease. Oral Dis. 2005;11:249–54. doi: 10.1111/j.1601-0825.2005.01113.x. [DOI] [PubMed] [Google Scholar]
- 2.Sudhakar R, Ramesh V, Balamurali PD, Nirima O, Premalatha B, Karthikshree V. Incidence of mast cells in oral inflammatory lesions. J Oral Pathol Microbiol. 2005;9:12–5. [Google Scholar]
- 3.Vahabi S, Rezazadeh F, Movaghar E, Sepideh NB. Relationship between mast cell counts and different types of periodontitis. J Periodontol Implant Dent. 2010;2:56–60. [Google Scholar]
- 4.Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 5.Silness J, Loe H. Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condtion. Acta Odontol Scand. 1964;22:121–35. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 6.Loe H, Silness J. Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontol Scand. 1963;21:533–51. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- 7.Steinsvoll S, Helgeland K, Schenck K. Mast cells – A role in periodontal diseases? J Clin Periodontol. 2004;31:413–9. doi: 10.1111/j.1600-051X.2004.00516.x. [DOI] [PubMed] [Google Scholar]
- 8.Lagdive SS, Lagdive SB, Mani A, Anarthe R, Pendyala G, Pawar B, et al. Correlation of mast cells in periodontal diseases. J Indian Soc Periodontol. 2013;17:63–7. doi: 10.4103/0972-124X.107500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walsh LJ. Mast cells and oral inflammation. Crit Rev Oral Biol Med. 2003;14:188–98. doi: 10.1177/154411130301400304. [DOI] [PubMed] [Google Scholar]
- 10.Walsh LJ, Davis MF, Xu LJ, Savage NW. Relationship between mast cell degranulationand infiammation in the oral cavity. J Oral Pathol Med. 1995;24:266–272. doi: 10.1111/j.1600-0714.1995.tb01180.x. [DOI] [PubMed] [Google Scholar]
- 11.Gemmell E, Carter CL, Seymour GJ. Mast cells in human periodontal disease. J Dent Res. 2004;83(5):384–387. doi: 10.1177/154405910408300506. [DOI] [PubMed] [Google Scholar]
- 12.Aeschlimann CR, Kaminski EJ, Robinson PJ. The effects of periodontal therapy on mast cell population in gingival tissues. J Periodontol. 1980;51:193–8. doi: 10.1902/jop.1980.51.4.193. [DOI] [PubMed] [Google Scholar]
- 13.Naesse EP, Schreurs O, Helgeland K, Schenck K, Steinsvoll S. matrix metalloproteinases and their inhibitors in gingival mast cells in persons with and without human immunodeficiency virus infection. J Periodont Res. 2003;38:575–582. doi: 10.1034/j.1600-0765.2003.00687.x. [DOI] [PubMed] [Google Scholar]
- 14.Steinbacher DM, Glick M. The dental patient with asthma. An update and oral health considerations. J Am Dent Assoc. 2001;132:1229–39. doi: 10.14219/jada.archive.2001.0365. [DOI] [PubMed] [Google Scholar]
- 15.Raud J, Konrad D, Dahlen SE. Delayed anti-inflammatory action of nedocromil sodium in the rat paw is dependent on de novo protein synthesis. Eur J Pharmacol. 1995;282:207–11. doi: 10.1016/0014-2999(95)00327-h. [DOI] [PubMed] [Google Scholar]
- 16.Mombelli A. Antibiotics in periodontal therapy. In: Niklaus P, Lang, Jan Lindhe, editors. Clinical periodontology and Implant dentistry. 5th edition. Oxford: Munksgaard; 2008. pp. 882–93. [Google Scholar]


