Abstract
Introduction
The NMDA receptor mediates a slow component of excitatory synaptic transmission, and NMDA receptor dysfunction has been implicated in numerous neurological disorders. Thus, interest in developing modulators that are able to regulate the channel continues to be strong. Recent research has led to the discovery of a number of compounds that hold therapeutic and clinical value. Deeper insight into the NMDA inter-subunit interactions and structural motifs gleaned from the recently solved crystal structures of the NMDA receptor should facilitate a deeper understanding of how these compounds modulate the receptor.
Areas covered
This article discusses the known pharmacology of NMDA receptors. A discussion of the patent literature since 2012 is also included, with an emphasis on those that claimed new chemical entities as regulators of the NMDA receptor.
Expert Opinion
The number of patents involving novel NMDA receptor modulators suggests a renewed interest in the NMDA receptor as a therapeutic target. Subunit-selective modulators continue to show promise, and the development of new subunit-selective NMDA receptor modulators appears poised for continued growth. Although a modest number of channel blocker patents were published, successful clinical outcomes involving ketamine have led to a resurgent interest in low-affinity channel blockers as therapeutics.
Keywords: channel blockers, deuterated analogs, glycine site antagonists, glycine transport inhibitors, NMDA receptor subunit-selective antagonists, NMDA receptor subunit-selective positive modulators
1. Introduction
Glutamate is the major excitatory neurotransmitter in the mammalian central nervous system (CNS) that acts as an agonist at two types of receptors: second messenger-linked metabotropic glutamate receptors (mGluRs) and ionotropic cation-selective ligand-gated channels (iGluRs). The NMDA (N-methyl D-aspartate) receptor, along with AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) and kainate receptors, comprise three classes of iGluRs [1]. These receptors have been linked to normal nervous system development and function and are implicated in a number of diseases, such as Parkinson’s disease [2], Alzheimer’s disease [3], schizophrenia [4–6], and depression [7,8]. The NMDA receptor is distinct from other ionotropic glutamate receptors in terms of its pharmacological properties. Selective agonists (such as NMDA) and antagonists (such as D-APV) are inactive at AMPA and kainate receptors, but active at the NMDA receptor. NMDA receptors also show substantial differences from AMPA and kainate receptors in amino acid sequence, and clear differences in terms of their structure [9–11]. The NMDA receptors are unique in that they show a high calcium permeability [12], voltage dependent-channel block by physiological concentrations of Mg2+ [13], requirement of the binding of two co-agonists for activation (glycine and glutamate), and multimeric subunit composition containing both GluN1 and GluN2 subunits [14]. These features, together with the ability of NMDA receptors to control synaptic plasticity and their involvement in neurological diseases, have created a therapeutic rationale driving the development of NMDA glutamate modulators. Indeed, enhancement or reduction of NMDA receptor function has been suggested as a potential treatment for a variety of neurological disorders. Due to their relative importance, a great deal is known about NMDA receptors, including the arrangement of different subunits within the tetrameric assembly, functional properties, and binding sites of various agonists, antagonists, and modulators [1,15].
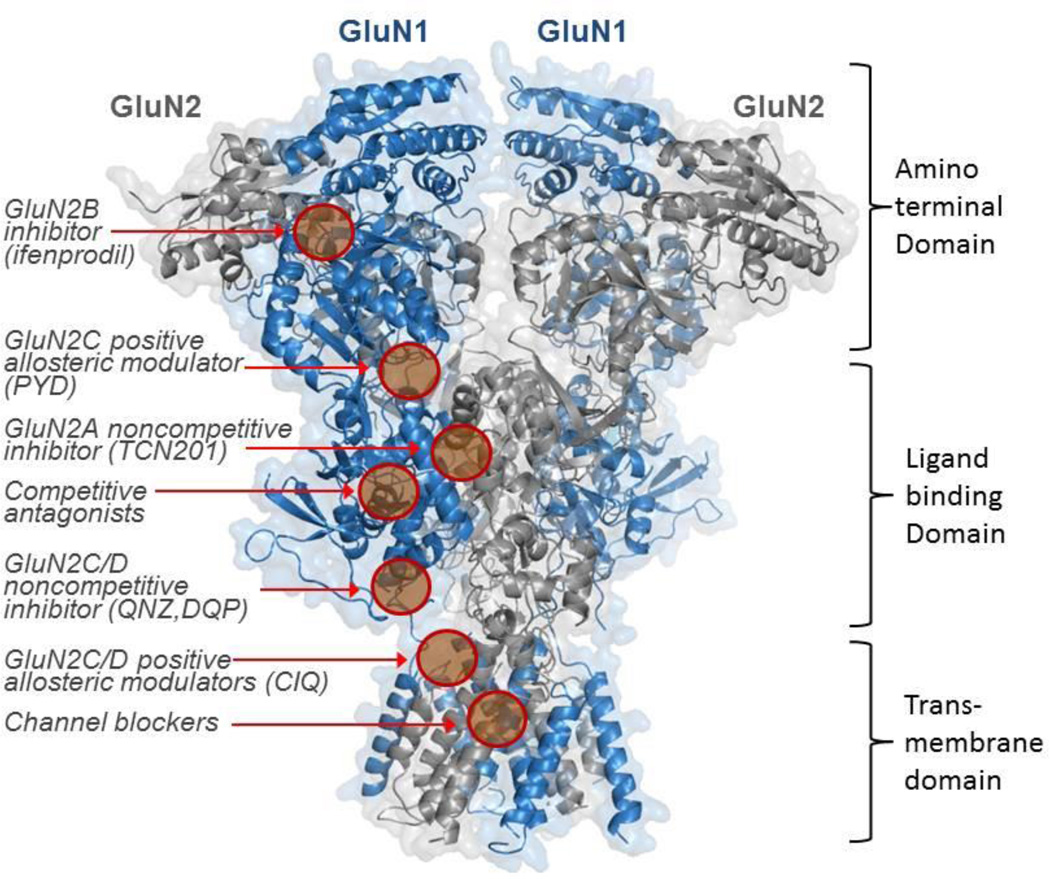
NMDA receptors mediate a slow Ca2+-permeable component of excitatory synaptic transmission [12] that requires the binding of the co-agonist glycine in addition to synaptically-released glutamate. Thus, systems that regulate concentrations of glycine, glutamate, and D-serine can influence NMDA receptor function. In addition to the simultaneous binding of glycine and glutamate [14], the opening of the channel must also be accompanied by a membrane depolarization to relieve channel block by external magnesium in order to allow current flow [13]. The NMDA receptor gating mechanism, which leads to the opening of the ion channel pore, takes on the order of 5–15 milliseconds, which is relatively slow compared to the submillisecond time scale for activation of AMPA receptors [16]. The NMDA receptor is typically composed of two GluN1 subunits and two GluN2 subunits, which are further divided into four types of subunits labeled GluN2A-GluN2D. Although not as well understood, two GluN3 subunits (GluN3A and GluN3B) can also be incorporated into the NMDA receptor complex, and may alter NMDA receptor properties. NMDA receptors comprised of GluN1, GluN2, and GluN3 subunits have been suggested to exist in the adult brain [17]. Receptors can contain two different GluN2 subunits, and these so-called triheteromeric NMDA receptors have unique properties [15,18]. The ionotropic glutamate receptors as a class have a somewhat similar sequence homology, suggesting that the three receptors share a similar architecture. The structure of all ionotropic glutamate receptor subunits includes four semiautonomous domains: an amino terminal domain (ATD), a ligand binding domain (LBD), a transmembrane domain (TMD), and a carboxyl terminal domain (CTD) (Figure 1). Each of the various NMDA subunits has been studied independently and in varying detail. Crystal structures of a GluN1-GluN2B ATD bound to both zinc [19] and ifenprodil [20] revealed a clamshell-like structure divided into two parts, R1 and R2. Although high sequence homology exists between NMDA receptors and non-NMDA receptors in terms of the LBD [21], the ATD of the NMDA receptor shares low sequence homology with non-NMDA receptors. In contrast to non-NMDA receptors, multiple binding sites for allosteric modulators that regulate receptor function have been described in the ATD of NMDA receptor [22]. Zn2+ binds to both the GluN2A and GluN2B subunits [23,24] within a pocket in the cleft of the GluN2B ATD clamshell [19], while the GluN2B-selective inhibitor ifenprodil binds at the interface between GluN1 and GluN2B ATD heterodimer, distinct from the zinc binding site [20] (Figure 1). The ATD of the NMDA receptor controls channel opening probability and the deactivation rate following glutamate removal, which is in contrast to the AMPA and kainate receptors, where the ATD does not appear to detectably regulate ion channel activity [25]. This may reflect the closer interaction of ATD with the LBD observed in NMDA receptors compared to non-NMDA receptors (discussed below).
Figure 1. Homology Model of an NMDA receptor highlighting known binding sites.
Ribbon diagram depicting the NMDA receptor where the GluN1 subunit is shown in blue and the GluN2 subunit is shown in grey. (The figure was generated using the GluN2B crystal structure, PDB 4PE5). Known or postulated binding sites are shown for several classes of ligands. The binding sites for zinc (the cleft of the GluN2A,B bilobed ATD), UPB compounds (the LBD), endogenous polyamines (the GluN2B ATD) are not shown. We also have not shown structural determinants of action for the endogenous neurosteroid pregnenolone sulfate, which has been suggested to involve helices J/K in the S2 portion of the LBD in addition to residues near the M4 transmembrane helix or the structural determinants for the negative allosteric modulation that appear to reside in the S2 region of the LBD.
Crystal structures of the isolated LBD region [14,26,27] of the NMDA receptor reveal a clamshell-like structure composed of two lobes, D1 and D2. The glutamate agonist binding pocket is located in the cleft between D1 and D2, and once glutamate binds, a conformational change takes place whereby the D2 lobe moves to produce a partial closure of the intralobe cleft [14]. Although sequence homology among NMDA and non-NMDA receptor LBD regions are high, evidence suggests that the conformational changes that lead to channel opening differ between NMDA and AMPA [26,27]. Modulators of glycine affinity bind at the GluN1-GluN2A heterodimer LBD interface, including the GluN2A-selective inhibitor TCN-201 [28] (Figure 1).
The transmembrane domain (TMD) forms the ion channel pore and is comprised of three transmembrane helices and a re-entrant pore loop, which resembles an inverted potassium channel [9]. The TMD is connected to the LBD by three short linkers associated with each of the transmembrane helices (M1, M3, and M4). M2 typically refers to a short reentrant loop that lines the inner pore of the channel and controls ion permeation and channel block [29]. Voltage dependent block of the NMDA receptor by extracellular Mg2+ ions occurs within the channel pore and is influenced by residues in the TMD [13], similar to uncompetitive antagonists known as channel blockers [29,30]. A large intracellular portion of the glutamate receptors comprises the carboxyl terminal domain (CTD), which influences membrane targeting and provides multiple sites for post-translational modifications that can alter receptor function and trafficking [1].
Recently two crystal structures of the heterotetrameric NMDA receptor ATD-LBD-TMD have been solved, revealing the structural details that differentiate the NMDA receptor from the closely related AMPA receptor (Figure 1) [10,11]. These crystal structures also allow a view of the inter-subunit and inter-domain interactions of the NMDA receptor, which should be valuable in understanding how molecules interact with the receptor to modulate activity. Indeed, three subunit-selective compounds appear to bind at domain interfaces (Figure 1). Both crystal structures were of an intact GluN1-GluN2B receptor, and both structures revealed that the ATD is much more tightly packed and in closer contact with the LBD in the NMDA receptor than in the AMPA receptor. The ATD and the LBD appear to be a single unit (Figure 1), while in AMPA receptors, a clear divide exists because of flexible linkers between the LBD and ATD. This may be the reason that NMDA receptors show pronounced regulation of ion channel function by the ATD [25]. Overall 2-fold symmetry of the receptor was observed for the extracellular portion of the glutamate receptor family, with subunits organized in a layered dimer-of-dimer arrangement, where 2-fold symmetry between the ATD and LBD exists, and pseudo-fourfold symmetry within the TMD (Figure 1). While the ATD and LBD portions of the receptor were elucidated in the two crystal structures, more studies in addition to higher resolution structures will be necessary to fully understand the ion-pore and TMD regions.
There are four GluN2 gene products: GluN2A, GluN2B, GluN2C, and GluN2D. Each GluN2 subunit imparts unique properties to the receptors, with differences in the deactivation time course, channel opening probability, channel open duration, and agonist sensitivity [14,15,31,32]. GluN2A-containing receptors have a much faster deactivation time course than the other GluN2 subtypes [33], and are also less sensitive to glycine and glutamate than the other subtypes. In terms of opening probability, recombinant GluN2A receptors have a high open probability, nearly 10-fold higher than GluN2C and GluN2D-containing receptors [34]. Channel properties including Mg2+blockade, Ca2+ permeability, and single-channel conductance vary between GluN2A/B and GluN2C/D receptors, and are determined by a single GluN2 residue in the M3 transmembrane region [35]. Not only are pharmacological properties dependent on GluN2 subunit composition, but also developmental expression levels and location in the brain. At the embryonic stage of a rat brain, low levels of GluN1, GluN2B, and GluN2D can be observed, whereas GluN2A and GluN2C are not prominently expressed. By birth, GluN2B is expressed in the cortex and hippocampus, while GluN2D is expressed in the thalamus, hypothalamus, and brain stem. GluN2A is expressed in the hippocampus and cortex and GluN2C is most highly expressed in the cerebellum [36–38]. In the adult rat brain, all NMDA receptor subunits are expressed, with GluN2A being most strongly expressed, while GluN2D is detected in the lowest amounts.
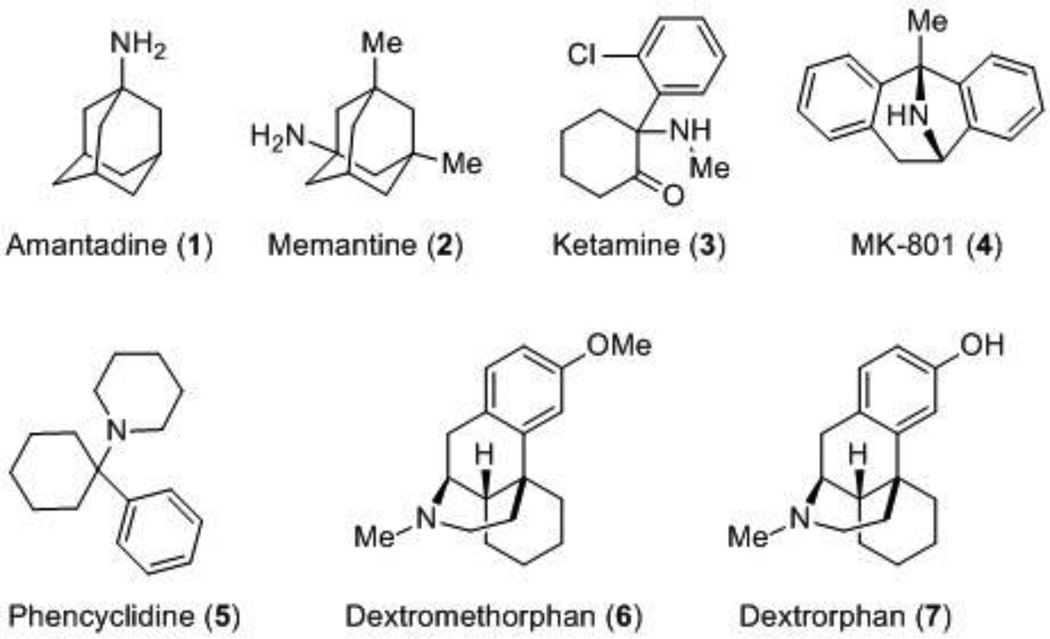
The diversity of functions mediated by many possible subunit combinations makes the NMDA receptor a strong candidate for a therapeutic target. Moreover, a variety of binding sites for antagonists and modulators with varying degrees of subunit-selectivity have been discovered and exploited. Uncompetitive antagonists, also known as channel blockers, bind deep in the ion pore (Figure 1) and require activation of the receptor prior to binding [30]. FDA-approved adamantine (1) and memantine (2) (Figure 2), in contrast to high-affinity channel blockers, have been well-tolerated in the clinic. This may reflect their fast unbinding-rates and relatively low affinity, which favors blockade of channels that are opened for prolonged periods of time over transiently activated receptors. Memantine and adamantine have been approved by the FDA for the symptomatic treatment of Alzheimer’s [39]. Ketamine (3) is an anesthetic that is typically used more often in children than adults [40], and it has also been effective in multiple trials for treatment-resistant depression [41]. Additionally, ketamine has been effective in preliminary clinical trials for obsessive compulsive disorder (OCD) [42] and post-traumatic stress disorder (PTSD) [43]. These initial successful outcomes will most likely lead to the testing of ketamine in additional clinical settings. Other well-studied channel blockers that share a similar binding site within the pore include MK-801 (4) and phencyclidine (PCP) (5). Dextromethorphan (6), the active ingredient in common over-the-counter (OTC) antitussives, and its primary metabolite, dextrorphan (7), are also noncompetitive NMDA receptor antagonists that act at a binding site within the pore [44–46]. While the demethylated metabolite 7 is more potent than dextromethorphan 6 at NMDA receptors [45,47], both compounds are also active at sigma 1 and sigma 2 receptors [48,49]. In 2010, the FDA approved a combination of dextromethorphan hydrobromide and quinidine sulfate, referred to as Nuedexta, for the treatment of pseudobulbar affect (PBA) [50]. Currently, available channel blockers are unable to differentiate between GluN2 subunits, with less than 10-fold selectivity described [51]. This lack of subunit-selectivity has been suggested to be partially responsible for the associated negative side effects, which include altered cardiovascular function, decreased motor function, hallucinations and delusions [52].
Figure 2.
NMDA receptor channel blockers.
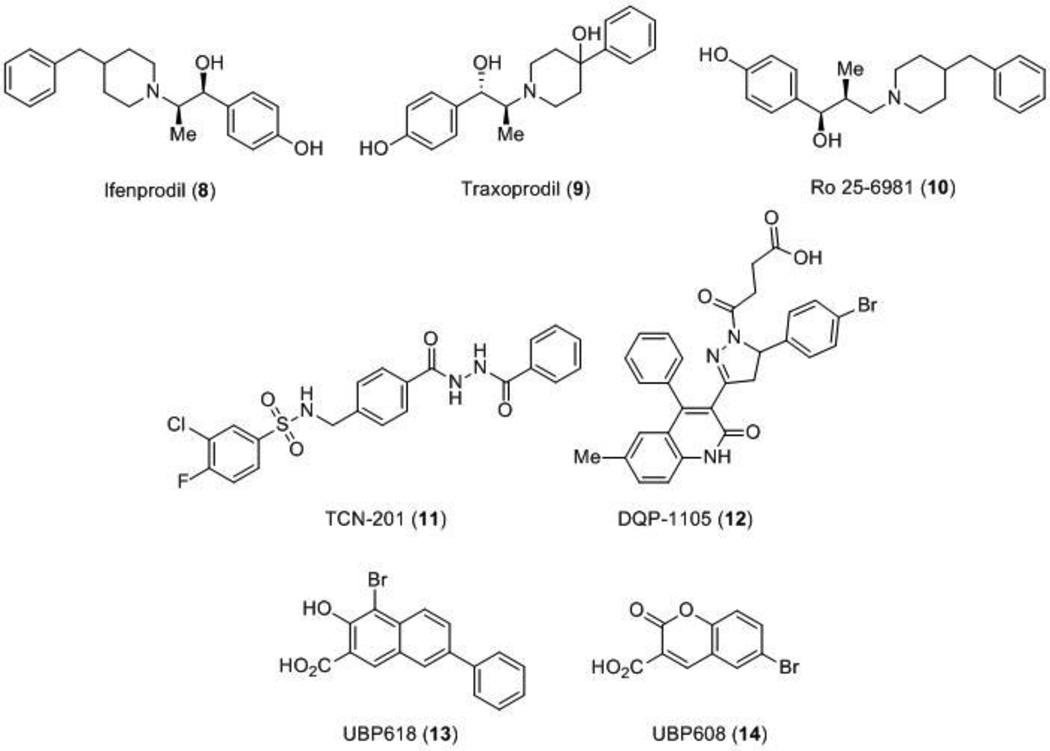
A breakthrough in the discovery of NMDA receptor modulators was the identification of the first subunit-selective NMDA receptor modulator, the noncompetitive antagonist ifenprodil (8), which is up to 400 times more selective for receptors that contain the GluN2B subunit than GluN2A, GluN2C, or GluN2D subunits [53,54]. Ifenprodil was originally reported to be a neuroprotectant and related compounds, such as traxoprodil (9) and Ro 25–6981 (10) (Figure 3), have been tested in advanced clinical trials for traumatic brain injury, neuropathic pain, and treatment-resistant depression [55–59]. GluN2B-selective inhibitors are typically better tolerated than channel blockers, although initial enthusiasm has been dampened due to observed side effects that are similar to behaviors observed with PCP use, raising concerns of abuse potential [61]. Further research is required, but the ifenprodil template and the binding mechanism continue to be a resource for finding new scaffolds that selectively modulate GluN2B-containing receptors.
Figure 3.
Subunit-selective antagonists.
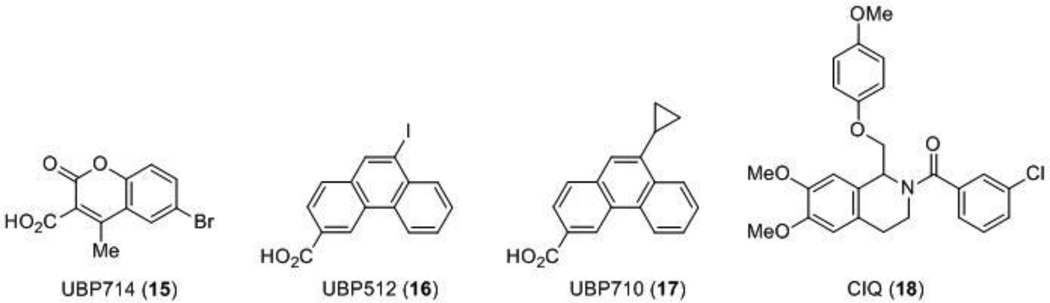
More recently, an allosteric modulator highly selective for GluN2A-containing receptors, TCN-201 (11), was identified [62]. TCN-201 acts as a noncompetitive modulator that depends on glycine; the binding of TCN-201 accelerates the dissociation of glycine, and thus is an allosteric regulator of glycine binding [63,64]. Although more work is necessary to elucidate the exact binding site of the compound, initial studies suggest that TCN-201 binds at the GluN1/GluN2 LBD interface, a novel site of NMDA receptor modulation [28] (Figure 1). An additional class of noncompetitive antagonists include the dihydroquinoline-pyrazolines such as DQP-1105 (12), compounds that show an IC50 increase following glutamate binding, but not glycine, and based on site-directed mutagenesis have been suggested to bind to the S2 region of the LBD [65] (Figure 1). A series of naphthalene and phenanthrene derivatives that act as positive and negative modulators of the NMDA receptor with varying degrees of potencies and selectivity have also been discovered (Figure 3 and 4). The naphthalene compound UBP618 (13) exhibits non-selective inhibition with approximate IC50 values of 2 µM at each of the subunits, while UBP608 (14) exclusively inhibits GluN2A-containing receptors [66]. The structurally related compound UBP714 (15) no longer exhibits any inhibition, but instead slight potentiation with a modest selectivity for GluN2A and GluN2B-containing receptors over GluN2D-containing receptors [67]. Additional selective potentiators include UBP512 (16) for GluN2A-containing receptors and UBP710 (17) for GluN2A and GluN2B-containing receptors. Compound 16 also shows inhibition at GluN2C and GluN2D-containing receptors, but no activity at GluN2B-containing receptors [66,68]. The structure activity relationship (SAR) studies [69] suggest that these compounds bind in the LBD region [66].
Three other classes, in addition to the naphthalene and phenanthrene (15–17) compounds, have been reported as small molecule subunit-selective allosteric potentiators. Tetrahydroisoquinoline compounds such as the prototype CIQ (18) are selective for GluN2C and GluN2D-containing receptors with EC50 values of 3–6 µM, and SAR studies have revealed that activity resides exclusively in the (+) enantiomer [70–72]. Site-directed mutagenesis suggests that the structural determinants of action for these compounds reside within the pre-M1-M1 region [73] (Figure 1). Recently, a pyrrolidinone (PYD) class of compounds has been reported that are ∼50-fold selective for the GluN2C subunit over GluN2A, GluN2B, or GluN2D, and this was recently described in the patent literature [74,75]. These compounds appear to target a new site at the interface between the LBD and ATD [76] (Figure 1). Additionally, The endogenous oxysterol and major cholesterol metabolite in the brain, 24-(S)-hydroxycholesterol (24(S)-HC), has also been identified as a potent positive allosteric modulator selective for the NMDA receptor over AMPA or GABA (gamma-Aminobutyric acid) receptors. Although the binding site of 24(S)-HC and its synthetic derivatives has not been fully elucidated, it does appear to be distinct from the neurosteroid pregnenolone sulfate [77]. Further studies regarding the mechanism of action behind these oxysterols revealed that a second endogenous oxysterol, 25-hydroxycholesterol (25-HC), non-competitively inhibited the potentiation of 24(S)-HC (but did not inhibit pregnenolone sulfate), suggesting two novel binding sites for this class of compounds [78].
The therapeutic significance of positive allosteric modulators has been argued from the NMDA receptor hypofunction hypothesis, which originated when studying the effects of the channel blocker PCP (5). The positive and negative symptoms associated with taking PCP, including hallucinations, delusions, lack of logical thinking, and lethargy are similar to the symptoms associated with schizophrenia. It stands to reason if reduction of NMDA receptor function can induce a syndrome almost indistinguishable from schizophrenia, perhaps NMDA receptor hypofunction may underlie some of the clinical features of this disease. The NMDA receptor hypofunction hypothesis proposes that these symptoms are a result of reduced NMDA receptor function and/or expression [6,79]. Subunit-selective potentiators have been suggested to potentially decrease schizophrenic symptoms by restoring normal NMDA receptor activity. In addition to the therapeutic value of positive allosteric modulators, others have argued that enhanced glycine concentrations could also be beneficial by enhancing NMDA receptor activation [80].
Endogenous compounds that selectively potentiate GluN2B-containing receptors include polyamines such as spermine, which has an EC50 value greater than 100 µM [81]. These compounds most likely bind in the GluN1-GluN2B ATD [82]. Sulfated neurosteroids have also been shown to selectively potentiate GluN2B subunits; pregnenolone sulfate in particular potentiates GluN2A and GluN2B-containing receptors over GluN2C and GluN2D-containing receptors by enhancing the open probability [83]. A steroid modulatory domain, SMD1, on the GluN2B subunit has been identified as the structural determinant of action for pregnenolone sulfate; this domain includes helices J/K in the S2 portion of the LBD along with residues in the M4 transmembrane helix [84]. Structural determinants of action for negative allosteric modulation by pregnenolone sulfate has been suggested to reside in the S2 region of the LBD [85]. Additionally, aminoglycoside antibiotics that are potent against Gram-negative bacteria selectivity potentiate GluN2B-containing receptors with EC50 values in the range of 38–134 µM. GluN2B potentiation has been suggested to improve cognition, which could be useful in mitigating some elements of age-dependent cognitive declines. It has been shown that as mice age, the expression of NMDA GluN2B receptors decreases, and it has been hypothesized that these decreased levels of synaptic plasticity and GluN2B expression are related to the cognitive decline that is associated with the aging process [86–89]. This speculation stems from data showing that GluN2B receptor overexpression in the forebrain of mice results in enhanced activation of NMDA receptors in response to stimulation [90].These mice show improved long-term memory andspatial performance, exhibit superior cued and contextual fear conditioning, and exhibit faster fear extinction [91–94]. Thus, GluN2B positive allosteric modulators not only have the potential to act as pharmacological probes, but also as potential means to target decreased memory performance [95].
This review aims to cover and summarize the patents that disclosed new NMDA antagonists and allosteric modulators that were filed and granted since the last review was published in 2012.
2. NMDA Receptor Channel Blockers
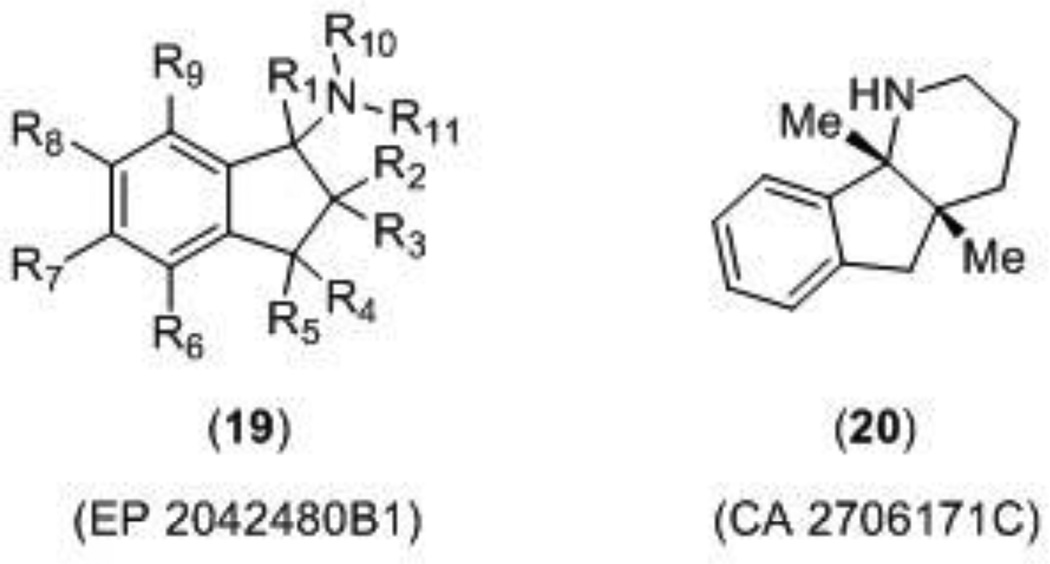
Two patents describing NMDA receptor channel blockers (Figure 5) were published in 2013 by Astellas Pharm Inc., one of which claimed a class of aminoindan derivatives (19) for the treatment of Alzheimer’s disease, dementia, Parkinson’s disease, and ischemic apoplexy [96]. Each compound was tested in a radiolabeled MK-801 binding assay and reported compounds gave Ki values in the range of 0.1 – 0.9 µM. Compounds were also tested in an intracellular Ca2+-flux assay (FLIPR), although this data was not reported. The second patent described a class of fused indane compounds, which were claimed for neurological disorders such as Parkinson’s disease, Alzheimer’s disease, dementia, pain, and depression [97]. Compounds were subjected to the radiolabeled MK-801 binding assay and the Ca2+- flux assay, but were also tested for maximal electroshock seizure (MES) inhibitory action. Compound 20 had a Ki value of 0.5 µM in the binding assay, an IC50 value of 1.8 µM in the Ca2+-flux assay, and displayed anti-seizure activity in mice following electroshock with an ED50 value of 4.2 mg/kg. The other enantiomer of compound 20 was active in all of the tests for antagonist activity as well, but was not as potent.
Figure 5.
Novel NMDA channel blockers.
3. Antagonists acting at the Glycine Site of the NMDA Receptor
Competitive antagonists specific for the GluN1 glycine binding site of the NMDA receptor have been studied for their therapeutic value with the rationale that a moderate dose of a glycine site antagonist would not completely shut down the activity of the receptor. Instead, these compounds would return the receptor to a level of normal synaptic transmission if the receptor were in a state of hyperactivation [98]. Early studies with glycine site antagonists suggested that these modulators would have less CNS side effects, such as those observed with PCP-like channel blockers including increases in locomotion, mylorelaxation, and psychosis [99]. Several studies have highlighted the potential for a greater therapeutic window with glycine site antagonists. For example, effects such as an increase in dopamine turnover [100] and vacuolization in cortical neurons [101] that were observed with MK-801 (4) (Figure 2) were absent with glycine site antagonists.
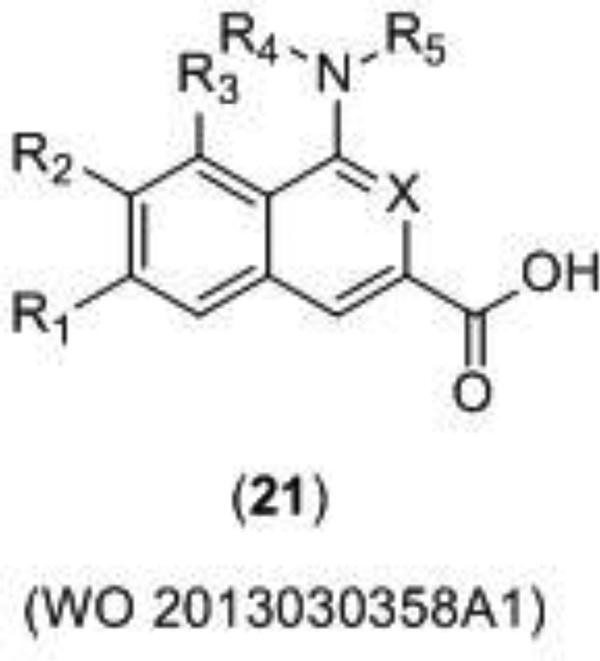
Merz Pharma recently disclosed a new series of glycine antagonists (21) (Figure 6) for a wide variety of neurological disorders including pain, peripheral neuropathy, neurodegenerative disorders, and psychiatric conditions [102]. The binding affinity of the compounds was characterized with a displacement study using radiolabeled MDL-105–519, a potent ligand for the glycine site. The potencies of the compounds were then calculated using electrophysiological whole cell patch clamp recording and/or a Functional Drug Screening System (FDSS 700) fluorometric Ca2+ imaging, although only the IC50 values for the Ca2+ imaging were given. Representative examples of potent compounds are given in Table 1.
Figure 6.
Merz Pharma developed glycine site antagonist.
Table 1.
Glycine-site specific antagonists disclosed by Merz Pharma.
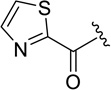
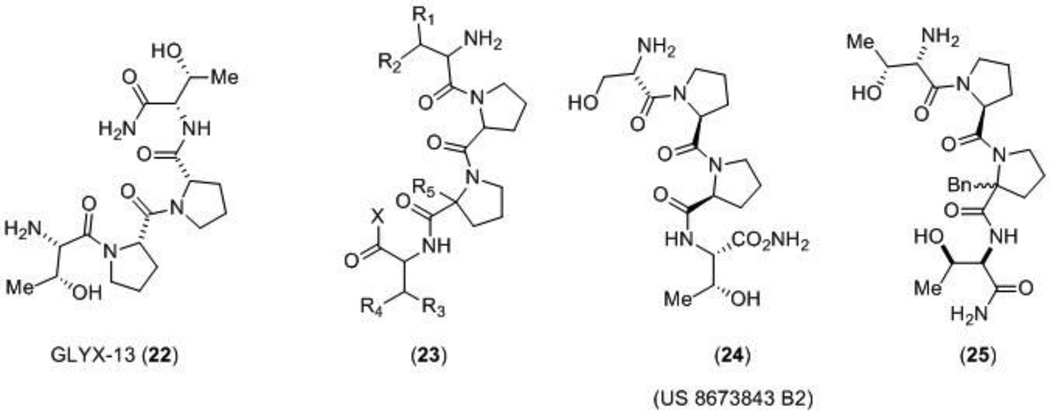
Northwestern University filed a patent disclosing analogs of peptide GLYX-13, which is suggested to be a glycine site functional partial agonist, (22) [103]. The tetrapeptide 22 (Figure 7) has unique effects on NMDA receptor function that have been suggested to involve actions of glycine, yet are distinct from conventional glycine site partial agonists. This modulator not only enhances long-term potentiation (LTP) in Schaffer collateral CA1 synapses in rat hippocampal slices, but also reduces long-term depression (LTD). This is in contrast to partial agonist D-cycloserine which only enhances LTP [104]. Although evaluation of GLYX-13 in hippocampal CA1 pyramidal neurons suggests that this compound acts similarly to a glycine site partial agonist [104–106], more research is required to determine the site and mechanism of action that results in these interesting effects on the NMDA receptor. Multiple lines of evidence suggest that GLYX-13 has therapeutic value including animal studies in cognitive enhancement [107,108], depression [105], and pain [109]. Additionally, GLYX-13 is currently in Phase II clinical trials for treatment-resistant depression [110]. Disclosed GLYX-13 analogs (23 – 25) were reported to be between 10 and 20-fold more potent than GLYX-13 as measured by burst-activated NMDA receptor-gated single neuron conductance (INMDA) in cultured hippocampal CA1 pyramidal neurons. Analogs were also characterized in a radiolabeled MK-801 binding assay, an assay to determine the effect of compounds on NMDA receptor currents, a LTP assay in Schaffer collateral-CA1 synapses in hippocampal slices, and a predictive test for antidepressant activity, a forced swimming model. Qualitative data was given for five analogs. Compound 24 caused a 79% increase at a concentration of 5 pM in the MK-801 binding affinity, had no effect on LTP, and caused a 90% reduction in floating time in the forced swimming model at a dose of 3 mg/kg administered by i.v.. Both diastereomers of derivative 25 (stereochemistry not defined) enhanced LTP and caused a reduction in floating time in the forced swimming model. While certain analogs enhanced LTP, an assay to determine whether any compounds were also able to diminish LTD was not reported. If these newer analogs do not diminish LTD in a similar fashion to GLYX-13, the analogs may not act at the same site or in the same fashion as GLYX-13. Additional studies are needed to further understand the site and mechanism of action.
Figure 7.
GLYX-13 analogs disclosed by Northwestern University.
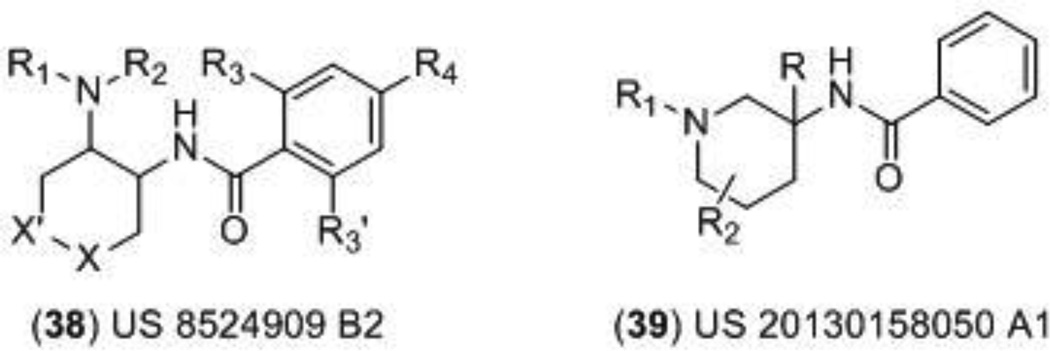
4. GluN2B-selective Antagonists
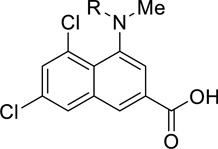
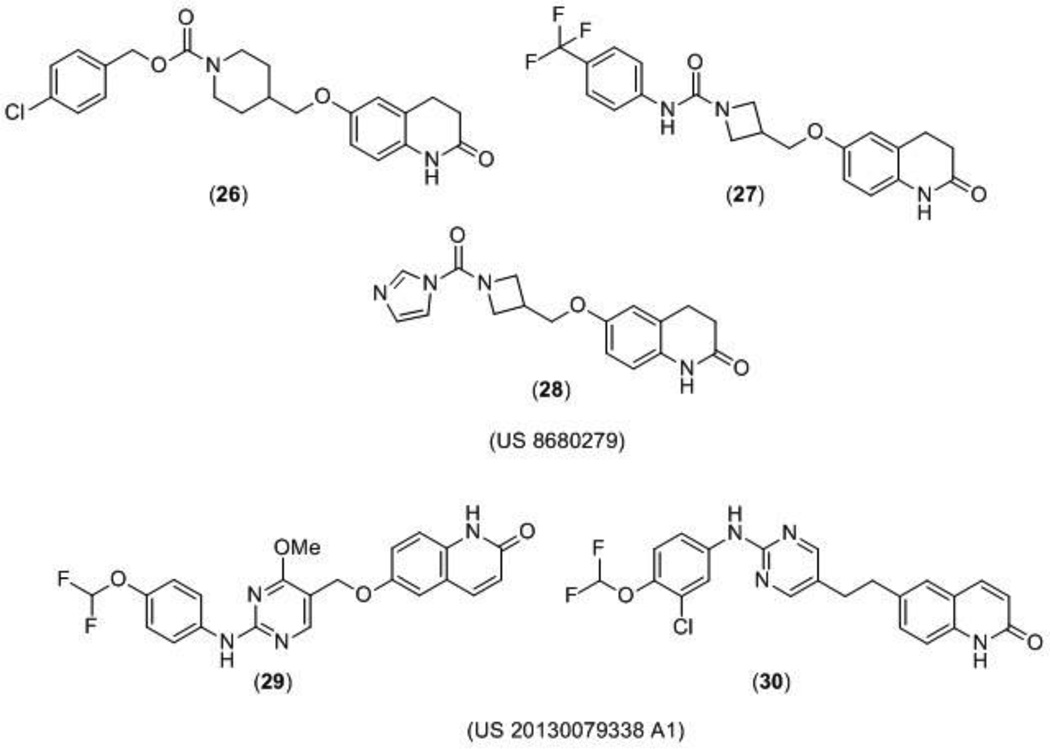
A US patent claiming 67 GluN2B-selective antagonists, including compounds 26, 27, and 28, (Figure 8) was published by NeurOp, Inc. The compounds showed enhanced activity in brain tissue having lower than normal pH, and were being proposed as potential new treatments for neuropsychiatric disorders, including depression and neurodegenerative disorders, such as Alzheimer’s disease, Huntington’s disease and Parkinson’s disease. Two electrode voltage-clamp recording from Xenopus laevis oocytes were conducted at pH 6.9 and 7.6, and compounds were more potent (had a lower IC50) at the acidic pH. This would have implications for the treatment of ischemia where the drug potency in healthy brain tissues with the pH closer to normal physiological range (pH 7.4) would be lower than in ischemic penumbra and the damaged tissue. Decreasing side effects should improve the therapeutic potential of this class of compounds and expand the therapeutic margins. Additionally, the compounds were consistently more potent at GluN2B-containing receptors at both pH levels than adrenergic α1 receptors and hERG channels. Plasma stability and brain penetration studies were conducted and many compounds were reported to have high blood-brain barrier penetration [111].
Figure 8.
GluN2B subunit-selective compounds disclosed by NeurOp Inc and Bristol-Myers Squibb.
An additional class of novel compounds acting as GluN2B-selective antagonists was patented for the treatment of major depressive disorder by Bristol-Myers Squibb (BMS) Company. In this patent 156 compounds were synthesized, but only 24 of them were claimed with biological data. In particular, compounds 29 and 30 (Figure 8) were reported as the most efficacious compounds with IC50 values of 5 nM and 2.5 nM, respectively, in the electrophysiological assay using Xenopus oocytes [112].
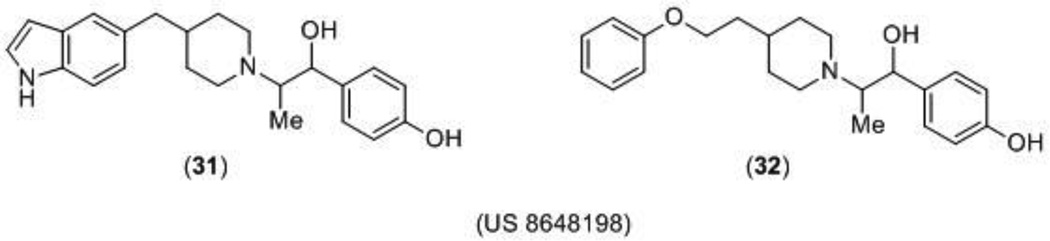
A series of phenylethanolamine-based compounds was patented by Cold Spring Harbor Laboratory as enhanced NMDA receptor antagonists using an in silico method. The atomic coordinates of the heterodimers GluN1 and GluN2B subunits bound to ifenprodil (8) or Ro 25–6981 (10) (Figure 3) were used to computationally screen for novel phenylethanolamine-based compounds that also bind to GluN1/GluN2B with a higher affinity than ifenprodil. Researchers used the previously obtained three-dimensional X-ray coordinates of the GluN1 and GluN2B subunits when bound to ifenprodil [20] to construct new phenethanolamine compounds with enhanced activity by attaching a hydrophobic moiety group to the ifenprodil template. These new compounds, including compounds 31 and 32 (Figure 9), were proposed to inhibit NMDA receptor function by physically interacting with hydrophobic residues on the GluN2B subunit of the NMDA receptor. No biological data was reported for the 16 novel compounds and in vitro or in vivo testing of the proposed compounds would still be necessary to confirm the in silico models [113].
Figure 9.
Ifenprodil based compounds disclosed by Cold Springs Harbor Laboratory.
5. NMDA receptor positive modulators
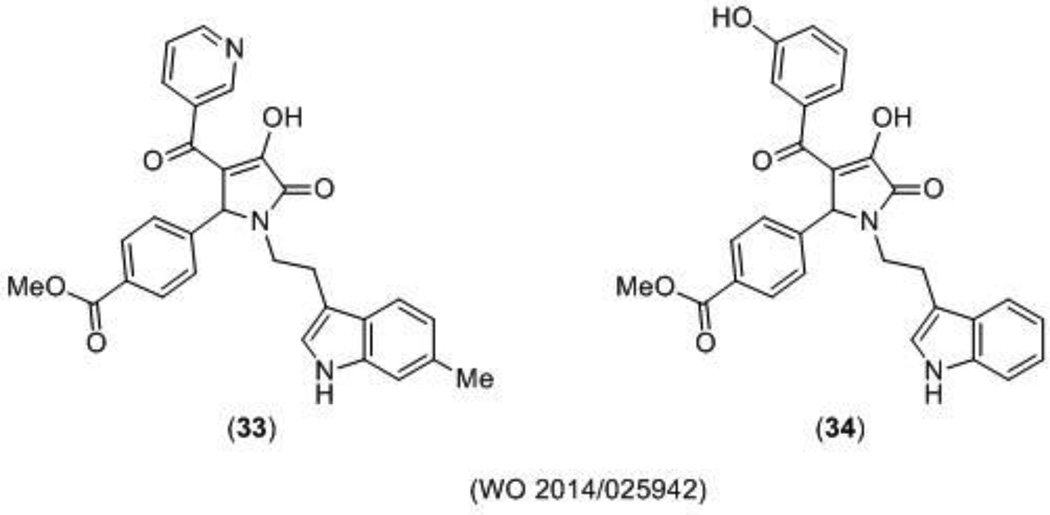
NMDA receptor potentiators have therapeutic potential in neurological disorders such as schizophrenia [114], and research with the partial agonist D-cycloserine (DCS) has suggested NMDA receptor dysfunction is implicated in PTSD [115,116]. It has also been suggested that potentiation of the NMDA receptor could help to restore age-dependent cognitive loss [90]. Recently, a novel series of pyrrolidinones (PYD), which selectively potentiate GluN2C-containing subunit NMDA potentiators was filed by Emory University. Approximately 83 novel compounds were disclosed and 17 of them were characterized using a two-electrode voltage clamp assay. Compound 33 (Figure 10) was reported as the most potent compound with an EC50 of 4 µM at GluN2C-containing receptors exclusively. Notably, compound 34 also exhibited potency at GluN2C-containing receptors with an EC50 of 5 µM, but caused inhibition of GluN2B- and GluN2D-containing receptors with an IC50 of 56 µM and 52 µM, respectively [75]. After additional medicinal chemistry efforts, the SAR was further advanced and evidence suggests that the class is stereoselective. This class of molecules represents the first diheteromeric GluN2C selective allosteric potentiators [74], which appears to act at a new modulatory site on the NMDA receptor between the ATD and the LBD [76]. These compounds also sense the subunit stoichiometry, potentiating receptors with two copies of GluN2C, but not those with one copy of GluN2A and one copy of GluN2C [76].
Figure 10.
Novel GluN2C subunit-selective potentiators disclosed by Emory University.
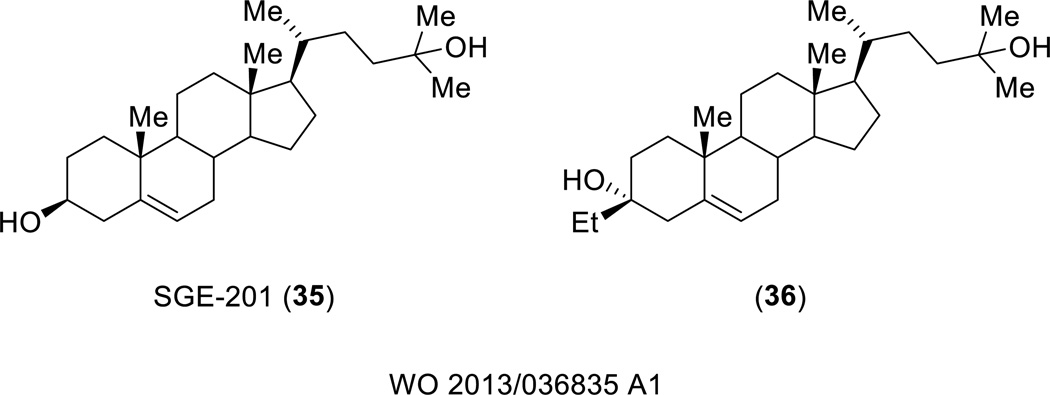
Sage Therapeutics filed a patent disclosing derivatives of SGE-201 (35), an oxysterol that is a synthetic derivative of 24(S)-HC, the major cholesterol metabolite in the brain [117]. 24(S)-HC and its synthetic derivatives, including SGE-201, are positive allosteric modulators that have a distinct binding site from the structurally related pregnenolone sulfate [77]. A number of SGE-201 analogs were tested in a whole-cell patch clamp assay in HEK- cells expressing the GluN1/GluN2A NMDA receptor at concentrations of 0.1 µM and 1.0 µM. An example of a potent compound includes compound 36, (Figure 11) which caused a 181% increase in potentiation at 0.1 µM and 286% increase at 1.0 µM. Compounds were developed for the treatment of a number of neurological disorders including schizophrenia, stroke, Parkinson’s disease, and Alzheimer’s disease.
Figure 11.
Positive allosteric oxysterols disclosed by Sage Therapeutics.
6. Unknown Site modulators
Although most of the compounds discussed as NMDA receptor modulators target a specific site of the NMDA receptor, some therapeutic effects do not require such selectivity. One example of such therapeutic intervention, described in a patent filed by Nihon University, is in the prevention of nerve cell necrosis by inhibiting calcium influx through the NMDA receptor [118]. The patent described a broad class of biguanide derivatives, as shown in Table 2, with antagonistic activity against both the NMDA receptor and the acid-sensing ion channel 1a (ASIC1a). ASIC1a, expressed in neurons throughout mammalian central and nervous peripheral systems, is a Ca2+ permeable ion channel that is involved in synaptic plasticity and acid induced cell death [119]. Activation of the NMDA receptor leads to an influx of Ca2+, which activates Ca2+/calmodulin-dependent protein kinase II (CAMKII) that then phosphorylates and activates the ASIC1a channel. Once ASIC1a is activated, this leads to an influx of Na+, which causes further depolarization of the cell. Evidence suggests that the activation of NMDA receptors and ASIC1a plays a role in ischemic neuronal cell death [120,121], and interest has been expressed in inhibiting this activation pathway. The patent described 27 compounds with dual IC50 values under 30 µM, and the most potent (compound 3 on Table 2) exhibited an inhibitory concentration of 210 nM at ASIC1a and 300 nM at GluN2A-containing receptors as measured by a two-electrode voltage clamp. No data was given for the GluN2B-, GluN2C-, or GluN2D-containing receptors, although the authors attributed this choice to the higher prevalence of the GluN2A subunit. Although the patent describes an inhibitor of both NMDA receptors and ASIC1a ion channels, the specific binding site or mechanism of action was not described.
Table 2.
Biguanide derivatives as unknown site NMDA antagonist/ASIC1a channel blockers.
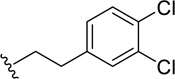
Another therapeutic effect that does not require specificity is in broadly modulating dopaminergic and glutamatergic neurotransmission. Integrative Research Laboratories recently patented a series of phenoxy-ethyl-amine compounds that modulate the cortical basal ganglia dopaminergic and NMDA receptor glutamatergic neurotransmission [122]. The phenoxy-ethyl-amine core (37) (Figure 12) has demonstrated in vivo activity against drug induced hyperactivity (amphetamine and MK-801) suggesting therapeutic relevance for the treatment of neurologically related movement disorders like Huntington’s disease. The site and mechanism of action was not described.
Figure 12.
Phenoxy-ethyl-amine derivatives disclosed by Integrative Research Laboratories.
7. Indirect site Modulators
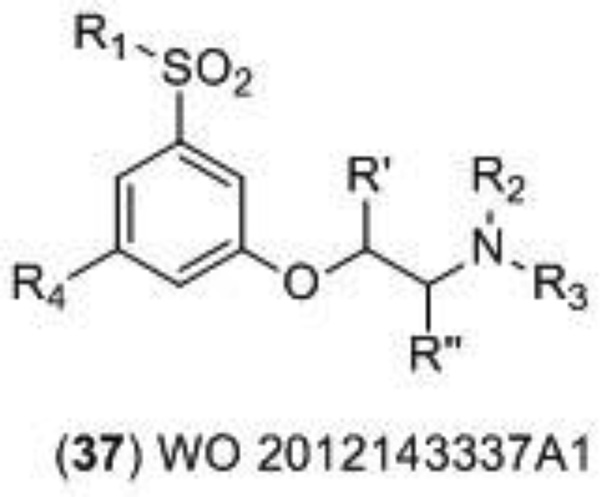
In addition to channel blockers, competitive antagonists, and allosteric modulators, the activity of the NMDA receptor can be indirectly regulated. Recent patent literature and clinical trials have focused on developing and testing inhibitors of the indirect modulator glycine transporter I (GlyT1) as a means to increase activity of the NMDA receptor [123]. GlyT1 plays an essential role in controlling the level of glycine at the excitatory synapses of the NMDA receptor and prevents high levels of glycine that would lead to saturation of the glycine site. GlyT1 inhibition should increase the concentration of glycine at the NMDA receptor within synapses, but activation of the receptor would still rely on glutamate release. In contrast to positive allosteric modulators that enhance the maximally effective response of NMDA receptors, GlyT1 inhibitors instead increase the open probability of the NMDA receptor only under certain physiological conditions that led to non-saturating concentrations of glycine or related glycine site agonists [124,125]. This unique mechanism of action of GlyT1 inhibitors may have clinical benefits in disease states that have been proposed to stem from a deficiency of NMDA receptor activity, such as schizophrenia. Hoffmann La-Roche developed the GlyT1 inhibitor Bitopertin (also referred to as RO4917838 and RG1678), which had advanced to Phase III clinical trials after the successful completion of two Phase II trials [126]. This compound would have been the first in its class of third generation schizophrenia drugs, but unfortunately the first two Phase III clinical trials meant to test the effect of Bitopertin on the negative symptoms of schizophrenia failed to meet their primary endpoints [127]. Despite this information, the interest in GlyT1 inhibitors is still high and this interest has been reflected in the recent patent literature. For example, Hoffman La-Roche published two patents claiming over 200 structurally similar GlyT1 inhibitors in 2013. Tetrahydropyran derivatives [128] (38) and piperidine derivatives [129] (39) (Figure 13) were both tested in a glycine uptake inhibition assay (mGlyT-1b) and the typical IC50 value of the covered compounds was below 0.2 µM. Notable examples from both patents are given Table 3.
Figure 13.
GlyT1 inhibitors disclosed by Hoffmann La-Roche
Table 3.
GlyT1 inhibitors disclosed by Hoffman La-Roche.
| US 8524909B2 | mGlyT-1b IC50 (µM) | |
| 47 | 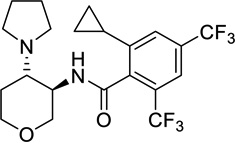 |
0.016 |
| 56 | 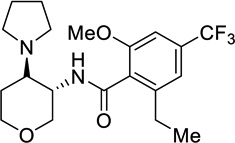 |
0.0155 |
| US2013/0158050A1 | ||
| 38 | 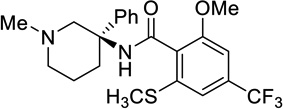 |
0.0101 |
| 121 | 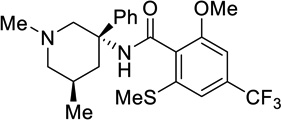 |
0.0165 |
8. Deuterated Analogs
The replacement of hydrogen with deuterium only inflicts a small steric effect, but has the potential to drastically slow down or even prevent particular metabolic pathways. This can have the effect of either pushing metabolism towards a potent species or limiting the build-up of a toxic metabolite [130,131]. This approach has been utilized in an attempt to improve pharmacokinetic properties in drugs and drug-leads. A compound referred to as Neudexta, a combination of the NMDA antagonist dextromethorphan (6) and quinidine sulfate (Figure 2), was approved by the FDA in 2010 for the treatment of pseudobulbar affect (PBA), and the clinical trials surrounding this approval were discussed in an earlier review [132]. Notably, in February 2013, AVP-786, a deuterated version of dextromethorphan in combination with quinidine sulfate, was studied in a Phase 1 clinical trial to determine the single and multiple-dose pharmacokinetics, safety, and tolerability with and without quinidine sulfate [133]. Results were not provided, but the combination of d6-dextromethorphan quinidine sulfate has advanced to Phase II clinical trials for the use in patients with treatment-resistant depression. The clinical trial was verified in May 2014, but at this time has not begun recruiting participants [134]. The deuterated form of the drug has been reported to exhibit more resistance towards CYP2D6 metabolism [135].
9. New Therapeutic Uses of Known NMDA receptor modulators
In terms of therapeutic indications for NMDA receptor modulators, many additional patents since 2012 were filed or granted involving disease states that have been extensively studied in relationship to the NMDA receptor, including neuropathic pain, depression, and Alzheimer’s disease. However, the recent literature also included patents which described new therapeutic applications for previously studied NMDA modulators. Two examples include patents describing sickle-cell anemia and visual system disorders.
A patent issued in early 2014 from the University of Zurich described the use of NMDA blockers as a method for the treatment of sickle-cell anemia [136]. A commonly occurring complication of sickle cell disease (SCD) is vaso-occlusive crisis, a painful condition caused by the occlusion of blood flow by sickled red blood cells [137]. Pain associated with this condition is often treated with opioids, however, frequent opioid use can potentially lead to tolerance and opioid induced hyperalgesia (OIH) [138]. The NMDA receptor has been indicated to play a role in the mechanism of tolerance through a pathway that involves the upregulation of protein kinase C, which leads to phosphorylation and subsequent enhanced activation of the NMDA receptor [139–142]. Additionally, inhibition of the NMDA receptor leads to a reduction in OIH [143]. These study results suggest that blockage of the NMDA receptor could reduce tolerance and OIH, and therefore that NMDA antagonists could be useful for pain management associated with SCD [144,145]. Ketamine, used in conjunction with opiates, has been studied in small case studies for the treatment of pain associated with SCD in children, and has shown an acceptable short-term safety profile [146] in addition to the reduction of opioid use and decreased reported pain [147]. Recently a Phase II clinical trial was completed where a low-dose of ketamine in addition to treatment involving opioids was studied for pain stemming for SCD, although no results were provided [148]. While the patent issued from the University of Zurich does not involve the use of opioids, it does claim that MK-801 can be used in treating sickle-cell anemia. The studies provided in the patent behind the claim show that red blood cells from patients with SCD contain more NMDA receptors than healthy patients and that the patients with SCD had a higher flux rate of potassium and calcium as measured by radioactive tags, which may play a role in the deformation of the red blood cell observed in sickle cell anemia. Treatment of the diseased cells with MK-801 (4) or memantine (2) (Figure 2) was able to decrease the high flux rates, and red blood cell morphological changes in patients with SCD were measured when the agonist NMDA was delivered with and without a pretreatment of MK-801. NMDA delivery resulted in an increase in the sickling of cells for the SCD patients, while pretreatment with MK-801 or memantine appeared to alleviate the sickling compared to NMDA alone and no treatment [136]. Although MK-801 is unlikely to progress toward development, more clinical and pre-clinical studies might reveal whether NMDA channel blockers are a viable option in treating complications associated with SCD.
Another extensively studied NMDA receptor modulator, D-serine, was the subject of a second new use patent filed by Allergan Inc. involving visual system deficits [149]. NMDA receptors located in the retinal ganglion cells play a role in visual processing and more specifically, contrast coding [150]. The NMDA receptor modulates synaptic plasticity in the visual cortex, a process that contributes to visual function [151]. D-serine is present in the retina and has been shown to enhance NMDA receptor responses in retinal glial cells [152]. In the patent filed in December of 2012 [149], D-serine was shown to facilitate a cellular model of synaptic plasticity (LTP) in the visual cortex of rats. Additionally, the patent claimed that either at a dose of 600 mg/kg for sixteen weeks or 100 mg/kg for fifty weeks, D-serine improved contrast sensitivity in mice that had been impaired by blue-light treatment, a model for visual system degradation. D-serine is intended to be used as a therapy to combat visual impairment that is related to age or neurological disorders that could lead to degenerative eyesight. However, more studies are required in this area, including tests of whether enhancement of the NMDA receptor could lead to neurodegeneration. A second patent published by Allergan Inc. claimed using D-serine transport inhibitors as a means to combat visual system disorders [153]. The patent suggests that D-serine transport inhibitors would increase the extracellular levels of D-serine, and this would result in an increase of NMDA receptor activity that could compensate for the impairment of vision associated with retinal diseases such as glaucoma and macular degeneration. A number of amino acids were shown to inhibit D-serine transporters, and two inhibitors enhanced visual function in rats and rabbits. In addition contrast sensitivity was improved in mice that had been impaired by blue light [153].
10. Conclusion
The NMDA receptor is a complex ligand-gated ion channel that has garnered much attention over the past 30 years for its value as a therapeutic target, and the recent patent literature has continued to reflect this interest. Two patents were filed that disclosed new compounds as channel blockers for a variety of neurological disorders, such as Parkinson’s disease, Alzheimer’s disease, and depression. The positive results from clinical trials involving ketamine for treatment-resistant depression and OCD have played a significant role in driving recent interest in the field. Glycine-site antagonists, such as the compounds developed by Merz Pharma still hold promise as well, although the tetrapeptide GLYX-13 (22) will require more research to determine the site and mechanism of action that is allowing these compounds to exert effects on the NMDA receptor responses.
The past two years also continued to see advances in subunit-selective modulators, compounds that should decrease side effects and have increased safety profiles. Although early GluN2B subunit-selective compounds were unsuccessful in the clinic when they were originally introduced, the ifenprodil template and binding site continues to catalyze the development of novel compounds, such as those disclosed by NeurOp Inc., BMS, and Cold Springs Harbor Laboratories. Cold Spring Harbor filed a patent on compounds that had been developed using in silico methods with the previously obtained crystal structure of ifenprodil bound to the GluN1-GluN2B ATD region. With the recent description of the ATD-LBD-TMD NMDA receptor crystal structure, in silico methods are likely to become increasingly useful to identify how newly discovered modulators interact with the NMDA receptor. The first di-heteromeric GluN2C selective compound, filed by Emory University, was also discovered.
In addition to allosteric potentiators, advances were made in the area of indirect modulators, specifically GlyT1 inhibitors. The mechanism of action behind these compounds should make GlyT1 inhibitors therapeutically valuable in schizophrenia. Despite initial optimism, compounds developed by Hoffmann La-Roche failed to meet primary endpoints in Phase III clinical trials. Nevertheless, some research in this area of indirect NMDA receptor modulators will most likely continue. A second compound that saw advances in the clinic was AVP-786, a combination of d6-dextromethorphan and quinidine sulfate. This result is not surprising since the original, nondueterated compound Neudexta was approved by the FDA in 2010 for PBA.
11. Expert Opinion
There were a significant number of patents filed and granted over the past two years claiming new chemical entities or the new use of an existing compound as an antagonist, agonist, or modulator of the NMDA receptor. This, together with continued discovery in the academic arena of novel modulators and antagonists, suggests a sustained resurgent interest in NMDA receptor pharmacology. A key advance during this period was the elucidation of the structure of the NMDA receptor, which should catalyze discovery of new ligands as well as advance our understanding of existing ligands. Thus, the NMDA receptor remains an active area for pharmacological research and drug development, and continues to garner efforts that have yielded development of new intellectual property. The ultimate goal of this research remains to exploit new pharmacological modulators for therapeutic gain to improve quality of life of patients. The substantial advances in terms of subunit selective modulators as well as refinement of existing classes of channel blockers and GluN2B-selective noncompetitive inhibitors holds promise that this target may eventually yield therapeutically useful compounds.
Several trends appear evident from the published patent literature. First, among the patents, there remains strong interest in allosteric modulators, as evidenced by subunit-selective modulators for GluN2B and GluN2C-containing receptors, small molecule modulators with mixed subunit selectivity, as well as the interesting peptide modulators that have been discussed. Subunit-selectivity may allow for the isolation of desired effects from non-specific effects, and lead to development of compounds that are better tolerated and more effective. GluN2B selective noncompetitive inhibitors appear to produce fewer problems than non-selective competitive antagonists or channel blockers. New GluN2B-selective antagonists developed have the interesting feature that they can detect biochemical changes that should enhance on-target effects, while minimizing receptor block in healthy tissue, thereby reducing side effects. GluN2C-selective modulators appear to act at a new binding site not previously identified. The identification of new binding sites for compounds could lead to a wealth of medicinal chemistry efforts that yield new classes of compounds. Furthermore, the existence of the cavity in which this new class appears to bind on other NMDA receptor subunits raises the possibility of the development of new classes of subunit selective modulators that target other receptor subunits. A second trend emerging from this body of work is the sustained interest in systems that indirectly alter NMDA receptor function, such as the glycine transport system. A host of enzymes handling production and disposition of D-serine are viable candidates for therapeutic modulation, as are endogenous systems that yield competitive glutamate receptor antagonists via the kynurenate pathway [154]. One might predict future work will explore these avenues, despite disappointing results with clinical trials of GlyT1 inhibitors. Lastly, there is renewed interest in development of channel blockers despite a disappointing track record in terms of clinical development, followed by subsequent loss of support over the past decade. We anticipate this interest is driven by the remarkable results obtained with ketamine use for treatment-resistant depression. It is now generally accepted that the ketamine results are reliable and reproducible, and the interest in channel blockers seems connected to the idea that fast dissociating lower affinity channel blockers may provide an opportunity to gain therapeutic effects with more tolerable side-effect profiles. Due to the success of ketamine towards treatment-resistant depression, ketamine and other NMDA receptor channel blockers will most likely be considered for additional clinical settings.
Figure 4.
Subunit-selective allosteric modulators.
Article highlights.
Two crystal structures of the ATD-LBD-TMD NMDA receptor were recently solved, which should enhance the understanding of how modulators regulate the receptor.
A review of the relevant patent literature from 2012 is discussed, highlighting new chemical entities as channel blockers, glycine site antagonists, subunit-selective modulators, and indirect modulators of NMDA receptor function.
Novel compounds developed around the ifenprodil template show the promising ability to sense extracellular pH and thereby selectivity target unhealthy tissue during ischemia.
The first di-heteromeric GluN2C selective positive allosteric modulator has been disclosed and appears to interact with a novel binding site.
GlyT-1 inhibitors developed for schizophrenia failed to meet primary endpoints in Phase III clinical trials
Acknowledgments
The authors would like to thank D Menaldino and F Menniti for their comments and suggestions, and also P Burger for his assistance in generating Figure 1. This work was supported by the NIH (NS036654, NS065371 SFT).
Footnotes
Declaration of Interest
Some of the authors (KL Strong, Y Jing, AR Prosser, SF Traynelis, DC Liotta) are inventors on Emory-owned IP involving NMDA receptor modulators. Authors have an equity position (SF Traynelis, DC Liotta), are Board members (DC Liotta), and are paid consultants (SF Traynelis) for NeurOp, Inc., a company that is developing NMDA receptor modulators.
References
- 1.Traynelis SF, Wollmuth LP, McBain CJ, et al. Glutamate Receptor Ion Channels: Structure, Regulation, and Function. Pharmacol Rev. 2010;62(3):405–496. doi: 10.1124/pr.109.002451.**An extensive review covering ionotropic glutamate receptor structure, function, assembly, and detailed pharmacology
- 2.Hallett PJ, Standaert DG. Rationale for and use of NMDA receptor antagonists in Parkinson’s disease. Pharmacol Ther. 2004;102(2):155–174. doi: 10.1016/j.pharmthera.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Reisberg B, Doody R, Stöffler A, et al. Memantine in Moderate-to-Severe Alzheimer’s Disease. N Engl J Med. 2003;348(14):1333–1341. doi: 10.1056/NEJMoa013128. [DOI] [PubMed] [Google Scholar]
- 4.Coyle JT. NMDA Receptor and Schizophrenia: A Brief History. Schizophr Bull. 2012;38(5):920–926. doi: 10.1093/schbul/sbs076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balu DT, Li Y, Puhl MD, Bennyworth MA, et al. Multiple risk pathways for schizophrenia converge in serine racemase knockout mice, a mouse model of NMDA receptor hypofunction. Proc Natl Acad Sci. 2013;110(26):E2400–E2409. doi: 10.1073/pnas.1304308110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olney JW, Newcomer JW, Farber NB. NMDA receptor hypofunction model of schizophrenia. J Psychiatr Res. 1999;33(6):523–533. doi: 10.1016/s0022-3956(99)00029-1. [DOI] [PubMed] [Google Scholar]
- 7.Murrough JW. Ketamine as a Novel Antidepressant: From Synapse to Behavior. Clin Pharmacol Ther. 2012;91(2):303–309. doi: 10.1038/clpt.2011.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Skolnick P, Popik P, Trullas R. Glutamate-based antidepressants: 20 years on. Trends Pharmacol Sci. 2009;30(11):563–569. doi: 10.1016/j.tips.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Sobolevsky AI, Rosconi MP, Gouaux E. X-ray structure, symmetry and mechanism of an AMPA-subtype glutamate receptor. Nature. 2009;462(7274):745–756. doi: 10.1038/nature08624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karakas E, Furukawa H. Crystal structure of a heterotetrameric NMDA receptor ion channel. Science. 2014;344(6187):992–997. doi: 10.1126/science.1251915.**The first published paper describing the ATD-LBD-TMD crystal structure of the NMDA receptor
- 11.Lee C-H, Lü W, Michel JC, Goehring A, et al. NMDA receptor structures reveal subunit arrangement and pore architecture. Nature. 2014;511(7508):191–197. doi: 10.1038/nature13548.**A detailed description of the ATD-LBD-TMD crystal structure of the NMDA receptor
- 12.MacDermott AB, Mayer ML, Westbrook GL, Smith SJ, et al. NMDA-receptor activation increases cytoplasmic calcium concentration in cultured spinal cord neurones. Nature. 1986;321(6069):519–522. doi: 10.1038/321519a0. [DOI] [PubMed] [Google Scholar]
- 13.Mayer ML, Westbrook GL, Guthrie PB. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984;309(5965):261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- 14.Furukawa H, Singh SK, Mancusso R, Gouaux E. Subunit arrangement and function in NMDA receptors. Nature. 2005;438(7065):185–192. doi: 10.1038/nature04089. [DOI] [PubMed] [Google Scholar]
- 15.Paoletti P, Bellone C, Zhou Q. NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nat Rev Neurosci. 2013;14(6):383–400. doi: 10.1038/nrn3504.***Detailed review of subunit diversity in terms of structure, function, and impact of subunit composition on disease states
- 16.Forsythe ID, Westbrook GL. Slow excitatory postsynaptic currents mediated by N-methyl-D-aspartate receptors on cultured mouse central neurones. J Physiol. 1988;396:515–533. doi: 10.1113/jphysiol.1988.sp016975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rauner C, Köhr G. Triheteromeric NR1/NR2A/NR2B receptors constitute the major N-methyl-D-aspartate receptor population in adult hippocampal synapses. J Biol Chem. 2011;286(9):7558–7566. doi: 10.1074/jbc.M110.182600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hansen KB, Ogden KK, Yuan H, Traynelis SF. Distinct Functional and Pharmacological Properties of Triheteromeric GluN1/GluN2A/GluN2B NMDA Receptors. Neuron. 2014;81(5):1084–1096. doi: 10.1016/j.neuron.2014.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karakas E, Simorowski N, Furukawa H. Structure of the zinc-bound amino-terminal domain of the NMDA receptor NR2B subunit. EMBO J. 2009;28(24):3910–3920. doi: 10.1038/emboj.2009.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karakas E, Simorowski N, Furukawa H. Subunit arrangement and phenylethanolamine binding in GluN1/GluN2B NMDA receptors. Nature. 2011;475(7355):249–253. doi: 10.1038/nature10180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Furukawa H, Gouaux E. Mechanisms of activation, inhibition and specificity: crystal structures of the NMDA receptor NR1 ligand-binding core. EMBO J. 2003;22(12):2873–2885. doi: 10.1093/emboj/cdg303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paoletti P, Neyton J. NMDA receptor subunits: function and pharmacology. Curr Opin Pharmacol. 2007;7(1):39–47. doi: 10.1016/j.coph.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 23.Paoletti P, Ascher P, Neyton J. High-Affinity Zinc Inhibition of NMDA NR1-NR2A Receptors. J Neurosci. 1997;17(15):5711–5725. doi: 10.1523/JNEUROSCI.17-15-05711.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rachline J, Perin-Dureau F, Goff AL, Neyton J, et al. The Micromolar Zinc-Binding Domain on the NMDA Receptor Subunit NR2B. J Neurosci. 2005;25(2):308–317. doi: 10.1523/JNEUROSCI.3967-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hansen KB, Furukawa H, Traynelis SF. Control of assembly and function of glutamate receptors by the amino-terminal domain. Mol Pharmacol. 2010;78(4):535–549. doi: 10.1124/mol.110.067157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yao Y, Harrison CB, Freddolino PL, Schulten K, et al. Molecular mechanism of ligand recognition by NR3 subtype glutamate receptors. EMBO J. 2008;27(15):2158–2170. doi: 10.1038/emboj.2008.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vance KM, Simorowski N, Traynelis SF, Furukawa H. Ligand-specific deactivation time course of GluN1/GluN2D NMDA receptors. Nat Commun. 2011;2:294. doi: 10.1038/ncomms1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hansen KB, Ogden KK, Traynelis SF. Subunit-selective allosteric inhibition of glycine binding to NMDA receptors. J Neurosci. 2012;32(18):6197–6208. doi: 10.1523/JNEUROSCI.5757-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Talukder I, Borker P, Wollmuth LP. Specific sites within the ligand-binding domain and ion channel linkers modulate NMDA receptor gating. J Neurosci. 2010;30(35):11792–11804. doi: 10.1523/JNEUROSCI.5382-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huettner JE, Bean BP. Block of N-methyl-D-aspartate-activated current by the anticonvulsant MK-801: selective binding to open channels. Proc Natl Acad Sci. 1988;85(4):1307–1311. doi: 10.1073/pnas.85.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cull-Candy SG, Leszkiewicz DN. Role of Distinct NMDA Receptor Subtypes at Central Synapses. Sci Signal. 2004;2004(255):re16. doi: 10.1126/stke.2552004re16. [DOI] [PubMed] [Google Scholar]
- 32.Paoletti P. Molecular basis of NMDA receptor functional diversity. Eur J Neurosci. 2011;33(8):1351–1365. doi: 10.1111/j.1460-9568.2011.07628.x. [DOI] [PubMed] [Google Scholar]
- 33.Vicini S, Wang JF, Li JH, Zhu WJ, et al. Functional and Pharmacological Differences Between RecombinantN-Methyl-d-Aspartate Receptors. J Neurophysiol. 1998;79(2):555–566. doi: 10.1152/jn.1998.79.2.555. [DOI] [PubMed] [Google Scholar]
- 34.Erreger K, Dravid SM, Banke TG, Wyllie DJA, et al. Subunit-specific gating controls rat NR1/NR2A and NR1/NR2B NMDA channel kinetics and synaptic signalling profiles. J Physiol. 2005;563(2):345–358. doi: 10.1113/jphysiol.2004.080028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siegler Retchless B, Gao W, Johnson JW. A single GluN2 subunit residue controls NMDA receptor channel properties via intersubunit interaction. Nat Neurosci. 2012;15(3):406–413. doi: 10.1038/nn.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Monyer H, Burnashev N, Laurie DJ, Sakmann B, et al. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12(3):529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- 37.Akazawa C, Shigemoto R, Bessho Y, Nakanishi S, et al. Differential expression of five N-methyl-D-aspartate receptor subunit mRNAs in the cerebellum of developing and adult rats. J Comp Neurol. 1994;347(1):150–160. doi: 10.1002/cne.903470112. [DOI] [PubMed] [Google Scholar]
- 38.Watanabe M, Inoue Y, Sakimura K, Mishina M. Developmental changes in distribution of NMDA receptor channel subunit mRNAs. NeuroReport. 1992;3(12):1138–1140. doi: 10.1097/00001756-199212000-00027. [DOI] [PubMed] [Google Scholar]
- 39.Chen H-SV, Lipton SA. The chemical biology of clinically tolerated NMDA receptor antagonists. J Neurochem. 2006;97(6):1611–1626. doi: 10.1111/j.1471-4159.2006.03991.x. [DOI] [PubMed] [Google Scholar]
- 40.Mellon RD, Simone AF, Rappaport BA. Use of Anesthetic Agents in Neonates and Young Children. Anesth Analg. 2007;104(3):509–520. doi: 10.1213/01.ane.0000255729.96438.b0. [DOI] [PubMed] [Google Scholar]
- 41.Hasselmann H. Ketamine as Antidepressant? Current State and Future Perspectives. Curr Neuropharmacol. 2014;12(1):57–70. doi: 10.2174/1570159X113119990043.**Review of the studies behind ketamine as a treatment for depression, along with a pertinent literature review
- 42.Rodriguez CI, Kegeles LS, Levinson A, Feng T, et al. Randomized Controlled Crossover Trial of Ketamine in Obsessive-Compulsive Disorder: Proof-of-Concept. Neuropsychopharmacology. 2013;38(12):2475–2483. doi: 10.1038/npp.2013.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feder A, Parides MK, Murrough JW, Perez AM, et al. Efficacy of intravenous ketamine for treatment of chronic posttraumatic stress disorder: A randomized clinical trial. JAMA Psychiatry. 2014;71(6):681–688. doi: 10.1001/jamapsychiatry.2014.62. [DOI] [PubMed] [Google Scholar]
- 44.Church J, Lodge D, Berry SC. Differential effects of dextrorphan and levorphanol on the excitation of rat spinal neurons by amino acids. Eur J Pharmacol. 1985;111(2):185–190. doi: 10.1016/0014-2999(85)90755-1. [DOI] [PubMed] [Google Scholar]
- 45.Church J, Jones MG, Davies SN, Lodge D. Antitussive agents as N-methylaspartate antagonists: further studies. Can J Physiol Pharmacol. 1989;67(6):561–567. doi: 10.1139/y89-090. [DOI] [PubMed] [Google Scholar]
- 46.Franklin PH, Murray TF. High affinity [3H]dextrorphan binding in rat brain is localized to a noncompetitive antagonist site of the activated N-methyl-D-aspartate receptor-cation channel. Mol Pharmacol. 1992;41(1):134–146. [PubMed] [Google Scholar]
- 47.Pechnick RN, Poland RE. Comparison of the Effects of Dextromethorphan, Dextrorphan, and Levorphanol on the Hypothalamo-Pituitary-Adrenal Axis. J Pharmacol Exp Ther. 2004;309(2):515–522. doi: 10.1124/jpet.103.060038. [DOI] [PubMed] [Google Scholar]
- 48.Shin E-J, Nah S-Y, Chae JS, Bing G, et al. Dextromethorphan attenuates trimethyltin-induced neurotoxicity via σ1 receptor activation in rats. Neurochem Int. 2007;50(6):791–799. doi: 10.1016/j.neuint.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 49.Musacchio J, Klein M, Canoll PD. Dextromethorphan and sigma ligands: Common sites but diverse effects. Life Sci. 1989;45(19):1721–1732. doi: 10.1016/0024-3205(89)90510-9. [DOI] [PubMed] [Google Scholar]
- 50.Pseudobulbar Affect | NUEDEXTA. [[Last accessed 2014 Aug 12]]; Available from: https://www.nuedexta.com/
- 51.Dravid SM, Erreger K, Yuan H, Nicholson K, et al. Subunit-specific mechanisms and proton sensitivity of NMDA receptor channel block. J Physiol. 2007;581(1):107–128. doi: 10.1113/jphysiol.2006.124958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lipton SA. Failures and Successes of NMDA Receptor Antagonists: Molecular Basis for the Use of Open-Channel Blockers like Memantine in the Treatment of Acute and Chronic Neurologic Insults. NeuroRx. 2004;1(1):101–110. doi: 10.1602/neurorx.1.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Williams K. Ifenprodil discriminates subtypes of the N-methyl-D-aspartate receptor: selectivity and mechanisms at recombinant heteromeric receptors. Mol Pharmacol. 1993;44(4):851–859. [PubMed] [Google Scholar]
- 54.Chenard BL, Bordner J, Butler TW, Chambers LK, et al. (1S,2S)-1-(4-Hydroxyphenyl)-2-(4-hydroxy-4-phenylpiperidino)-1-propanol: A Potent New Neuroprotectant Which Blocks N-Methyl-D-Aspartate Responses. J Med Chem. 1995;38(16):3138–3145. doi: 10.1021/jm00016a017. [DOI] [PubMed] [Google Scholar]
- 55.Gotti B, Duverger D, Bertin J, Carter C, et al. Ifenprodil and SL 82.0715 as cerebral anti-ischemic agents. I. Evidence for efficacy in models of focal cerebral ischemia. J Pharmacol Exp Ther. 1988;247(3):1211–1221. [PubMed] [Google Scholar]
- 56.Taniguchi K, Shinjo K, Mizutani M, Shimada K, et al. Antinociceptive activity of CP-101,606, an NMDA receptor NR2B subunit antagonist. Br J Pharmacol. 1997;122(5):809–812. doi: 10.1038/sj.bjp.0701445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chizh BA, Headley PM, Tzschentke TM. NMDA receptor antagonists as analgesics: focus on the NR2B subtype. Trends Pharmacol Sci. 2001;22(12):636–642. doi: 10.1016/s0165-6147(00)01863-0. [DOI] [PubMed] [Google Scholar]
- 58.Yurkewicz L, Weaver J, Bullock MR, Marshall LF. The effect of the selective NMDA receptor antagonist traxoprodil in the treatment of traumatic brain injury. J Neurotrauma. 2005;22(12):1428–1443. doi: 10.1089/neu.2005.22.1428. [DOI] [PubMed] [Google Scholar]
- 59.Preskorn SH, Baker B, Kolluri S, Menniti FS, et al. An innovative design to establish proof of concept of the antidepressant effects of the NR2B subunit selective N-methyl-D-aspartate antagonist, CP-101,606, in patients with treatment-refractory major depressive disorder. J Clin Psychopharmacol. 2008;28(6):631–637. doi: 10.1097/JCP.0b013e31818a6cea. [DOI] [PubMed] [Google Scholar]
- 60.Boyce S, Wyatt A, Webb JK, O’Donnell R, et al. Selective NMDA NR2B antagonists induce antinociception without motor dysfunction: correlation with restricted localisation of NR2B subunit in dorsal horn. Neuropharmacology. 1999;38(5):611–623. doi: 10.1016/s0028-3908(98)00218-4. [DOI] [PubMed] [Google Scholar]
- 61.Nicholson KL, Mansbach RS, Menniti FS, Balster RL. The phencyclidine-like discriminative stimulus effects and reinforcing properties of the NR2B–selective N-methyl-D-aspartate antagonist CP-101 606 in rats and rhesus monkeys. Behav Pharmacol. 2007;18(8):731–743. doi: 10.1097/FBP.0b013e3282f14ed6. [DOI] [PubMed] [Google Scholar]
- 62.Bettini E, Sava A, Griffante C, Carignani C, et al. Identification and characterization of novel NMDA receptor antagonists selective for NR2A- over NR2B–containing receptors. J Pharmacol Exp Ther. 2010;335(3):636–644. doi: 10.1124/jpet.110.172544. [DOI] [PubMed] [Google Scholar]
- 63.Edman S, McKay S, Macdonald LJ, Samadi M, et al. TCN 201 selectively blocks GluN2A-containing NMDARs in a GluN1 co-agonist dependent but non-competitive manner. Neuropharmacology. 2012;63(3):441–449. doi: 10.1016/j.neuropharm.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McKay S, Griffiths N, Butters P, Thubron E, et al. Direct pharmacological monitoring of the developmental switch in NMDA receptor subunit composition using TCN 213, a GluN2A-selective, glycine-dependent antagonist. Br J Pharmacol. 2012;166(3):924–937. doi: 10.1111/j.1476-5381.2011.01748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hansen KB, Traynelis SF. Structural and Mechanistic Determinants of a Novel Site for Noncompetitive Inhibition of GluN2D-Containing NMDA Receptors. J Neurosci. 2011;31(10):3650–3661. doi: 10.1523/JNEUROSCI.5565-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Costa BM, Irvine MW, Fang G, Eaves RJ, et al. A Novel Family of Negative and Positive Allosteric Modulators of NMDA Receptors. J Pharmacol Exp Ther. 2010;335(3):614–621. doi: 10.1124/jpet.110.174144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Irvine MW, Costa BM, Volianskis A, Fang G, et al. Coumarin-3-carboxylic acid derivatives as potentiators and inhibitors of recombinant and native N-methyl-d-aspartate receptors. Neurochem Int. 2012;61(4):593–600. doi: 10.1016/j.neuint.2011.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Monaghan DT, Irvine MW, Costa BM, Fang G, et al. Pharmacological modulation of NMDA receptor activity and the advent of negative and positive allosteric modulators. Neurochem Int. 2012;61(4):581–592. doi: 10.1016/j.neuint.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Costa BM, Irvine MW, Fang G, Eaves RJ, et al. Structure-activity relationships for allosteric NMDA receptor inhibitors based on 2-naphthoic acid. Neuropharmacology. 2012;62(4):1730–1736. doi: 10.1016/j.neuropharm.2011.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mullasseril P, Hansen KB, Vance KM, Ogden KK, et al. A subunit-selective potentiator of NR2C- and NR2D–containing NMDA receptors. Nat Commun. 2010;1:90. doi: 10.1038/ncomms1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Santangelo Freel RM, Ogden KK, Strong KL, Khatri A, et al. Synthesis and Structure Activity Relationship of Tetrahydroisoquinoline-Based Potentiators of GluN2C and GluN2D Containing N-Methyl-D-aspartate Receptors. J Med Chem. 2013;56(13):5351–5381. doi: 10.1021/jm400177t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Santangelo Freel RM, Ogden KK, Strong KL, Khatri A, et al. Correction to Synthesis and Structure Activity Relationship of Tetrahydroisoquinoline-Based Potentiators of GluN2C and GluN2D Containing N-Methyl-D-aspartate Receptors. J Med Chem. 2014;57(11):4975–4975. doi: 10.1021/jm500710w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ogden KK, Traynelis SF. Contribution of the M1 Transmembrane Helix and Pre-M1 Region to Positive Allosteric Modulation and Gating of N-Methyl-D-Aspartate Receptors. Mol Pharmacol. 2013;83(5):1045–1056. doi: 10.1124/mol.113.085209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zimmerman SS, Khatri A, Garnier-Amblard EC, Mullasseril P, et al. Design, Synthesis, and Structure-Activity Relationship of a Novel Series of GluN2C-Selective Potentiators. J Med Chem. 2014;57(6):2334–2356. doi: 10.1021/jm401695d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Traynelis SF, Mullasseril P, Garnier EC, Liotta DC, et al. Nmda receptor modulators and uses related thereto. WO2014025942 A1. 2014 [Google Scholar]
- 76.Khatri A, Burger PB, Swanger SA, Hansen KB, et al. Structural Determinants and Mechanism of Action of a GluN2C-selective NMDA Receptor Positive Allosteric Modulator. Mol Pharmacol: published online 9 September 2014. doi: 10.1124/mol.114.094516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Paul SM, Doherty JJ, Robichaud AJ, Belfort GM, et al. The major brain cholesterol metabolite 24(S)-hydroxycholesterol is a potent allosteric modulator of N-methyl-D-aspartate receptors. J Neurosci. 2013;33(44):17290–17300. doi: 10.1523/JNEUROSCI.2619-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Linsenbardt AJ, Taylor A, Emnett CM, Doherty JJ, et al. Different oxysterols have opposing actions at N-methyl-d-aspartate receptors. Neuropharmacology. 2014;85:232–242. doi: 10.1016/j.neuropharm.2014.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tsai G, Coyle JT. Glutamatergic mechanisms in schizophrenia. Annu Rev Pharmacol Toxicol. 2002;42:165–179. doi: 10.1146/annurev.pharmtox.42.082701.160735. [DOI] [PubMed] [Google Scholar]
- 80.Coyle JT, Tsai G. The NMDA receptor glycine modulatory site: a therapeutic target for improving cognition and reducing negative symptoms in schizophrenia. Psychopharmacology. 2004;174(1):32–38. doi: 10.1007/s00213-003-1709-2. [DOI] [PubMed] [Google Scholar]
- 81.Traynelis SF, Hartley M, Heinemann SF. Control of proton sensitivity of the NMDA receptor by RNA splicing and polyamines. Science. 1995;268(5212):873–876. doi: 10.1126/science.7754371. [DOI] [PubMed] [Google Scholar]
- 82.Mony L, Zhu S, Carvalho S, Paoletti P. Molecular basis of positive allosteric modulation of GluN2B NMDA receptors by polyamines. EMBO J. 2011;30(15):3134–3146. doi: 10.1038/emboj.2011.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Malayev A, Gibbs TT, Farb DH. Inhibition of the NMDA response by pregnenolone sulphate reveals subtype selective modulation of NMDA receptors by sulphated steroids. Br J Pharmacol. 2002;135(4):901–909. doi: 10.1038/sj.bjp.0704543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jang M-K, Mierke DF, Russek SJ, Farb DH. A steroid modulatory domain on NR2B controls N-methyl-D-aspartate receptor proton sensitivity. Proc Natl Acad Sci. 2004;101(21):8198–8203. doi: 10.1073/pnas.0401838101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Horak M, Vlcek K, Petrovic M, Chodounska H, et al. Molecular Mechanism of Pregnenolone Sulfate Action at NR1/NR2B Receptors. J Neurosci. 2004;24(46):10318–10325. doi: 10.1523/JNEUROSCI.2099-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wenk GL, Barnes CA. Regional changes in the hippocampal density of AMPA and NMDA receptors across the lifespan of the rat. Brain Res. 2000;885(1):1–5. doi: 10.1016/s0006-8993(00)02792-x. [DOI] [PubMed] [Google Scholar]
- 87.Clayton DA, Mesches MH, Alvarez E, Bickford PC, et al. A Hippocampal NR2B Deficit Can Mimic Age-Related Changes in Long-Term Potentiation and Spatial Learning in the Fischer 344 Rat. J Neurosci. 2002;22(9):3628–3637. doi: 10.1523/JNEUROSCI.22-09-03628.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bai L, Hof PR, Standaert DG, Xing Y, et al. Changes in the expression of the NR2B subunit during aging in macaque monkeys. Neurobiol Aging. 2004;25(2):201–208. doi: 10.1016/s0197-4580(03)00091-5. [DOI] [PubMed] [Google Scholar]
- 89.Magnusson KR, Brim BL, Das SR, Magnusson KR, et al. Selective vulnerabilities of N-methyl-D-aspartate (NMDA) receptors during brain aging. Front Aging Neurosci. 2010;2:11. doi: 10.3389/fnagi.2010.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tang Y-P, Shimizu E, Dube GR, Rampon C, et al. Genetic enhancement of learning and memory in mice. Nature. 1999;401(6748):63–69. doi: 10.1038/43432. [DOI] [PubMed] [Google Scholar]
- 91.Cao X, Cui Z, Feng R, Tang Y-P, et al. Maintenance of superior learning and memory function in NR2B transgenic mice during ageing. Eur J Neurosci. 2007;25(6):1815–1822. doi: 10.1111/j.1460-9568.2007.05431.x. [DOI] [PubMed] [Google Scholar]
- 92.Jacobs SA, Tsien JZ. Genetic Overexpression of NR2B Subunit Enhances Social Recognition Memory for Different Strains and Species. PLoS ONE. 2012;7(4):e36387. doi: 10.1371/journal.pone.0036387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yihui Cui, Jing Jin, Xuliang Zhang, Hao Xu, et al. Forebrain NR2B Overexpression Facilitating the Prefrontal Cortex Long-Term Potentiation and Enhancing Working Memory Function in Mice. PLoS ONE. 2011;6(5):1–10. doi: 10.1371/journal.pone.0020312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.White TL, Youngentob SL. The effect of NMDA-NR2B receptor subunit over-expression on olfactory memory task performance in the mouse. Brain Res. 2004;1021(1):1–7. doi: 10.1016/j.brainres.2004.05.114. [DOI] [PubMed] [Google Scholar]
- 95.Brim BL, Haskell R, Awedikian R, Ellinwood NM, et al. Memory in aged mice is rescued by enhanced expression of the GluN2B subunit of the NMDA receptor. Behav Brain Res. 2013;238:211–226. doi: 10.1016/j.bbr.2012.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hayashibe S, Yamasaki S, Watanabe K, Shiraishi N, et al. Aminoindan derivative or salt thereof. EP2042480 B1. 2013 [Google Scholar]
- 97.Hayashibe S, Yamasaki S, Shiraishi N, Hoshii H, et al. Fused indane compound. CA2706171 C. 2013 [Google Scholar]
- 98.Danysz W, Parsons CG. Glycine and N-Methyl-D-Aspartate Receptors: Physiological Significance and Possible Therapeutic Applications. Pharmacol Rev. 1998;50(4):597–664. [PubMed] [Google Scholar]
- 99.Ginski MJ, Witkin JM. Sensitive and rapid behavioral differentiation of N-methyl-d-aspartate receptor antagonists. Psychopharmacology. 1994;114(4):573–582. doi: 10.1007/BF02244987. [DOI] [PubMed] [Google Scholar]
- 100.Tricklebank MD, Bristow LJ, Hutson PH, Leeson PD, et al. The anticonvulsant and behavioural profile of L-687,414, a partial agonist acting at the glycine modulatory site on the N-methyl-D-aspartate (NMDA) receptor complex. Br J Pharmacol. 1994;113(3):729–736. doi: 10.1111/j.1476-5381.1994.tb17054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hargreaves RJ, Rigby M, Smith D, Hill RG. Lack of effect of L-687,414 ((+)-cis-4-methyl-HA-966), an NMDA receptor antagonist acting at the glycine site, on cerebral glucose metabolism and cortical neuronal morphology. Br J Pharmacol. 1993;110(1):36–42. doi: 10.1111/j.1476-5381.1993.tb13768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Abel U, Hansen A, Wolter FE, Krueger B, et al. Glycine b antagonists. WO2013030358 A1. 2013 [Google Scholar]
- 103.Moskal J, Khan MA. NMDA receptors modulators and uses thereof. US8673843 B2. 2014 [Google Scholar]
- 104.Zhang X, Sullivan JA, Moskal JR, Stanton PK. A NMDA receptor glycine site partial agonist, GLYX-13, simultaneously enhances LTP and reduces LTD at Schaffer collateral-CA1 synapses in hippocampus. Neuropharmacology. 2008;55(7):1238–1250. doi: 10.1016/j.neuropharm.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Burgdorf J, Zhang X, Nicholson KL, Balster RL, et al. GLYX-13, a NMDA Receptor Glycine-Site Functional Partial Agonist, Induces Antidepressant-Like Effects Without Ketamine-Like Side Effects. Neuropsychopharmacology. 2013;38(5):729–742. doi: 10.1038/npp.2012.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Moskal JR, Kuo AG, Weiss C, Wood PL, et al. GLYX-13: A monoclonal antibody-derived peptide that acts as an N-methyl-d-aspartate receptor modulator. Neuropharmacology. 2005;49(7):1077–1087. doi: 10.1016/j.neuropharm.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 107.Burgdorf J, Zhang X, Weiss C, Matthews E, et al. The N-methyl-D-aspartate receptor modulator GLYX-13 enhances learning and memory, in young adult and learning impaired aging rats. Neurobiol Aging. 2011;32(4):698–706. doi: 10.1016/j.neurobiolaging.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Burgdorf J, Kroes RA, Weiss C, Oh MM, et al. Positive emotional learning is regulated in the medial prefrontal cortex by GluN2B-containing NMDA receptors. Neuroscience. 2011;192:515–523. doi: 10.1016/j.neuroscience.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wood PL, Mahmood SA, Moskal JR. Antinociceptive action of GLYX-13: an N-methyl-D-aspartate receptor glycine site partial agonist. NeuroReport. 2008;19(10):1059–1061. doi: 10.1097/WNR.0b013e32830435c9. [DOI] [PubMed] [Google Scholar]
- 110.Phase 2, Double-Blind, Placebo Controlled, Randomized Withdrawal, Parallel Efficacy and Safety Study of GLYX-13 in Subjects With Inadequate/Partial Response to Antidepressants During the Current Episode of Major Depressive Disorder. [[Last accessed on 6 August 2014]]; Available from: http://www.clinicaltrials.gov/ct2/show/NCT01684163.
- 111.Liotta DC, Snyder JP, Traynelis SF, Wilson L, et al. NMDA receptor antagonists for neuroprotection. US8420680 B2. 2013 [Google Scholar]
- 112.King D, Macor JE, Olson RE, Iwuagwu CI, et al. Selective NR2B Antagonists. US20130079338 A1. 2013 [Google Scholar]
- 113.Furukawa H, Karakas E. Phenylethanolamine-based NMDA receptor antagonists. US8648198 B2. 2014 [Google Scholar]
- 114.Shim SS, Hammonds MD, Kee BS. Potentiation of the NMDA receptor in the treatment of schizophrenia: focused on the glycine site. Eur Arch Psychiatry Clin Neurosci. 2008;258(1):16–27. doi: 10.1007/s00406-007-0757-8. [DOI] [PubMed] [Google Scholar]
- 115.Ressler KJ, Rothbaum BO, Tannenbaum L, Anderson P, et al. Cognitive enhancers as adjuncts to psychotherapy: use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry. 2004;61(11):1136–1144. doi: 10.1001/archpsyc.61.11.1136. [DOI] [PubMed] [Google Scholar]
- 116.Richardson R, Ledgerwood L, Cranney J. Facilitation of fear extinction by D-cycloserine: theoretical and clinical implications. Learn Mem Cold Spring Harb N. 2004;11(5):510–516. doi: 10.1101/lm.78204. [DOI] [PubMed] [Google Scholar]
- 117.Upasani RB, Harrison BL, Askew BC, Dodart J-C, et al. Neuroactive steroids, compositions, and uses thereof WO2013036835 A1. 2013 [Google Scholar]
- 118.Miyake M, Kusama T, Masuko T. Biguanide derivative compound. US20130281464 A1. 2013 [Google Scholar]
- 119.Waldmann R, Champigny G, Bassilana F, Heurteaux C, et al. A proton-gated cation channel involved in acid-sensing. Nature. 1997;386(6621):173–177. doi: 10.1038/386173a0. [DOI] [PubMed] [Google Scholar]
- 120.Gao J, Duan B, Wang D-G, Deng X-H, et al. Coupling between NMDA receptor and acid-sensing ion channel contributes to ischemic neuronal death. Neuron. 2005;48(4):635–646. doi: 10.1016/j.neuron.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 121.Xiong Z-G, Zhu X-M, Chu X-P, Minami M, et al. Neuroprotection in Ischemia: Blocking Calcium-Permeable Acid-Sensing Ion Channels. Cell. 2004;118(6):687–698. doi: 10.1016/j.cell.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 122.Sonesson C, Karlsson J, Svensson P. Novel modulators of cortical dopaminergic- and nmda-receptor-mediated glutamatergic neurotransmission. US20140128360 A1. 2014 [Google Scholar]
- 123.Harvey RJ, Yee BK. Glycine transporters as novel therapeutic targets in schizophrenia, alcohol dependence and pain. Nat Rev Drug Discov. 2013;12(11):866–885. doi: 10.1038/nrd3893. [DOI] [PubMed] [Google Scholar]
- 124.Eulenburg V, Armsen W, Betz H, Gomeza J. Glycine transporters: essential regulators of neurotransmission. Trends Biochem Sci. 2005;30(6):325–333. doi: 10.1016/j.tibs.2005.04.004.**Detailed review of the rationale for therapeutic endeavors behind GLYT1 inhibitors and a discussion of clinical development of recent compounds
- 125.Cubelos B, Giménez C, Zafra F. Localization of the GLYT1 Glycine Transporter at Glutamatergic Synapses in the Rat Brain. Cereb Cortex. 2005;15(4):448–459. doi: 10.1093/cercor/bhh147. [DOI] [PubMed] [Google Scholar]
- 126.Umbricht D, Alberati D, Martin-Facklam M, Borroni E, et al. Effect of Bitopertin, a Glycine Reuptake Inhibitor, on Negative Symptoms of Schizophrenia: A Randomized, Double-Blind, Proof-of-Concept Study. JAMA Psychiatry. 2014;71(6):637–646. doi: 10.1001/jamapsychiatry.2014.163. [DOI] [PubMed] [Google Scholar]
- 127.Roche provides update on the first two of six phase III studies of bitopertin in schizophrenia. [[Last accessed on 19 July 2014]];Media Release. 2014 Available from: http://www.roche.com/media/media_releases/med-cor-2014-01-21.htm.
- 128.Kolczewski S, Pinard E. Tetrahydro-pyran derivatives. US8524909 B2. 2013 [Google Scholar]
- 129.Kolczewski S, Pinard E, Stalder H. Piperidine derivatives. US20130158050 A1. 2013 [Google Scholar]
- 130.Foster AB. Deuterium isotope effects in studies of drug metabolism. Trends Pharmacol Sci. 1984;5:524–527. [Google Scholar]
- 131.Harbeson SL, Tung RD. Chapter 24 - Deuterium in Drug Discovery and Development. Ann Rep Med Chem. 2011;46:403–417. [Google Scholar]
- 132.Santangelo RM, Acker TM, Zimmerman SS, Katzman BM, et al. Novel NMDA Receptor Modulators: An Update. Expert Opin Ther Pat. 2012;22(11):1337–1352. doi: 10.1517/13543776.2012.728587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pharmacokinetics (PK) and Tolerability of AVP-786 in Healthy Volunteers. [[Last accessed 9 July 2014]]; Available from: https://clinicaltrials.gov/ct2/show/NCT01787747?term=AVP%3D786&rank=2.
- 134.Efficacy, Safety, and Tolerability Study of AVP-786 as an Adjunctive Therapy in Patients With Major Depressive Disorder With an Inadequate Response to Antidepressant Treatment. [[Last accessed 9 July 2014]]; Available from: https://clinicaltrials.gov/ct2/show/NCT02153502?term=AVP%3D786&rank=1.
- 135.Tung R. Morphinan compounds. US8541436 B2. 2013 [Google Scholar]
- 136.Bogdanova AY, Gassman M, Goede J. Methods of treating sickle cell anemia-related conditions with MK-801 or memantine. US8680042 B2. 2014 [Google Scholar]
- 137.Embury SH. Sickle Cell Disease: Basic Principles and Clinical Practice. Lippincott-Raven; 1994. [Google Scholar]
- 138.Lee M, Silverman Sanford M, Hansen H, Patel V, Manchikanti L. A Comprehensive Review of Opioid-Induced Hyperalgesia Focused Review. Pain Physician. 14(2):145–161. [PubMed] [Google Scholar]
- 139.Larcher A, Laulin JP, Celerier E, Le Moal M, et al. Acute tolerance associated with a single opiate administration: involvement of N-methyl-d-aspartate-dependent pain facilitatory systems. Neuroscience. 1998;84(2):583–589. doi: 10.1016/s0306-4522(97)00556-3. [DOI] [PubMed] [Google Scholar]
- 140.Chen L, Huang Marine L-YM. Sustained potentiation of NMDA receptor-mediated glutamate responses through activation of protein kinase C by a µ opioid. Neuron. 1991;7(2):319–326. doi: 10.1016/0896-6273(91)90270-a. [DOI] [PubMed] [Google Scholar]
- 141.Chen L, Mae Huang L-Y. Protein kinase C reduces Mg2+ block of NMDA-receptor channels as a mechanism of modulation. Nature. 1992;356(6369):521–523. doi: 10.1038/356521a0. [DOI] [PubMed] [Google Scholar]
- 142.Mao J, Price DD, Mayer DJ. Thermal hyperalgesia in association with the development of morphine tolerance in rats: Roles of excitatory amino acid receptors and protein kinase C. J Neurosci. 1994;14(4):2301–2312. doi: 10.1523/JNEUROSCI.14-04-02301.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mao J. NMDA and opioid receptors: their interactions in antinociception, tolerance and neuroplasticity. Brain Res Rev. 1999;30(3):289–304. doi: 10.1016/s0165-0173(99)00020-x. [DOI] [PubMed] [Google Scholar]
- 144.Neri CM, Pestieau SR, Darbari DS. Low-dose ketamine as a potential adjuvant therapy for painful vaso-occlusive crises in sickle cell disease. Pediatr Anesth. 2013;23(8):684–689. doi: 10.1111/pan.12172. [DOI] [PubMed] [Google Scholar]
- 145.Wolfgang K, Reinhard S, Scheuber K, Alsheimer M, et al. Differential Modulation of Remifentanil-induced Analgesia and Postinfusion Hyperalgesia by S-Ketamine and Clonidine in Humans. Anesthesiology. 2013;99(1):152–159. doi: 10.1097/00000542-200307000-00025. [DOI] [PubMed] [Google Scholar]
- 146.Neri CM. Low-dose Ketamine for Children and Adolescents with Acute Sickle Cell Disease Related Pain: A Single Center Experience. J Anesth Clin Res. 2014;5:394. [Google Scholar]
- 147.Zempsky WTM, Loiselle KAB, Corsi JMB, Hagstrom JNM. Use of Low-dose Ketamine Infusion for Pediatric Patients With Sickle Cell Disease-related Pain: A Case Series. J Pain. 2010;26(2):163–167. doi: 10.1097/AJP.0b013e3181b511ab. [DOI] [PubMed] [Google Scholar]
- 148.Low-Dose Ketamine Infusion for Children With Sickle Cell Disease-Related Pain. [[Last accessed 8 July 2014]]; Available from: https://clinicaltrials.gov/ct2/show/NCT00595530?term=NMDA++sickle+cell&rank=1.
- 149.Li Y, Foster AC, Staubli U, Donello JE, et al. serine for the treatment of visual system disorders. WO2012174243 A8. 2013 [Google Scholar]
- 150.Manookin MB, Weick M, Stafford BK, Demb JB. NMDA receptor contributions to visual contrast coding. Neuron. 2010;67(2):280–293. doi: 10.1016/j.neuron.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cooke SF, Bear MF. Visual Experience Induces Long-Term Potentiation in the Primary Visual Cortex. J Neurosci. 2010;30(48):16304–16313. doi: 10.1523/JNEUROSCI.4333-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Stevens ER, Esguerra M, Kim PM, Newman EA, et al. d-serine and serine racemase are present in the vertebrate retina and contribute to the physiological activation of NMDA receptors. Proc Natl Acad Sci. 2003;100(11):6789–6794. doi: 10.1073/pnas.1237052100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Foster AC, Li Y-X, Staubli U, Viswanath V, et al. D-serine transporter inhibitors as pharmaceutical compositions for the treatment of central nervous system disorders. US20120329851 A1. 2012 [Google Scholar]
- 154.Schwarcz R, Bruno JP, Muchowski PJ, Wu H-Q. Kynurenines in the mammalian brain: when physiology meets pathology. Nat Rev Neurosci. 2012;13(7):465–477. doi: 10.1038/nrn3257. [DOI] [PMC free article] [PubMed] [Google Scholar]