Abstract
Background
A previous study has reported that low-frequency (LF) electroacupuncture (EA) influences salivary secretory immunoglobulin A (sIgA) and the autonomic nervous system (ANS). The ANS is known to control the secretion volume of sIgA; however, the effect of high-frequency (HF) EA on salivary sIgA has not been determined. We investigated whether HF EA affects salivary sIgA levels and the ANS.
Method
Sixteen healthy subjects were randomly classified into two groups: a control group and an EA group. After a 5 min rest, subjects in the EA group received EA at 100 Hz bilaterally at LI4 and LI11 for 15 min before resting for a further 40 min post-stimulation. Subjects in the control group rested for a total of 60 min. Measurements of the ANS and sIgA levels in both groups were made before, immediately after, 20 min after, and 40 min after rest or 15 min EA treatment. HF and LF components of heart rate variability were analysed as markers of ANS function. LF/HF ratio and HF were taken as indices of sympathetic and parasympathetic nerve activity, respectively. Salivary protein concentrations and sIgA levels were determined by Bradford protein assay and ELISA, respectively.
Results
LF/HF ratio was significantly increased immediately after EA. HF was significantly increased at 20 min after EA and sIgA level was significantly increased at 40 min after EA. In addition, HF and salivary sIgA level were positively correlated with each another.
Conclusions
HF EA exerted sequential positive effects on sympathetic nerve activity, parasympathetic nerve activity, and salivary sIgA level (immediately and after 20 and 40 min, respectively). HF EA may increase salivary sIgA levels by influencing parasympathetic nerve activity.
Introduction
Acupuncture is used as a complementary or alternative therapy in many countries.1 Electroacupuncture (EA) is a form of acupuncture in which an electric current is passed between two acupuncture needles. EA and transcutaneous electrical nerve stimulation (TENS) can be classified as low-frequency (LF) or high-frequency (HF), which imply 2–5 and 100–250 Hz, respectively.2 3 Previous research has reported that HF and LF electrical stimulation have differential effects on the secretion of opioids.4 Furthermore, LF and HF stimulation excite Aδ and C nerve fibres, and Aδ and Aβ nerve fibres, respectively,3 with resultant effects on the autonomic nervous system (ANS), endocrine system, and immune system.5 These systems play an important role in the ability to cope with physical and mental stress. The sympathetic-adrenomedullary and hypothalamic-pituitary-adrenocortical systems are activated in response to stress, leading to increased adrenalin (epinephrine), noradrenaline (norepinephrine), and cortisol levels. These mediators act on all organs of the body and increase the ability to cope with stress by increasing pulse rate, blood pressure, and blood glucose levels.6 7 A previous study has shown that LF stimulation decreases heart rate, and that HF stimulation increases heart rate.8 Furthermore, it has been reported that electrical stimulation of Aβ nerve fibres in dogs increased parasympathetic nerve activity after increasing sympathetic nerve activity.9 10
Mucous membranes play an important protective role by preventing infection of pathogenic organisms located on the surface of the body. Various proteins contained in saliva, such as mucin or secretory immunoglobulin A (sIgA), provide protection against infection within the oral cavity.11 12 sIgA prevents adhesion and replication by binding to pathogenic microbes. IgA is a monomer secreted by plasma cells that is combined with a secretory component; therefore sIgA is secreted outside of the glands. Consequently, large amounts of sIgA are found in the oral cavity and intestinal tract. sIgA and mucin levels are controlled by the ANS. Stimulation of both the sympathetic and parasympathetic nerve systems has led to increased secretion of sIgA by submandibular and parotid glands, but the stimulatory effect on salivary sIgA was greater following stimulation of the sympathetic versus parasympathetic nervous systems.13–15 In addition, sIgA levels are known to fluctuate with physical or mental stress. Acute high-intensity exercise, long-term heavy training, and chronic mental stress lead to a decrease in sIgA.16 17 It has been reported that LF EA attenuates the decrease in salivary sIgA induced by high-strength exercise.16 18 However, the effect of HF EA on sIgA level and ANS function and their potential interactions have not yet been investigated. The latter is important as it may form the basis for deciding the appropriate EA frequency in clinical settings. The present study aimed to investigate whether markers of autonomic function and salivary sIgA parameters are impacted by HF EA in healthy volunteers.
Methods
Subjects
Sixteen healthy volunteers (aged 21.05±0.82 years) were randomly assigned to one of two groups: a control group and an EA group (n=8 each). This study was approved by the ethical committees of Teikyo Heisei University, Tokyo, Japan (reference no. 25-007). Informed consent was obtained from each healthy volunteer in accordance with the provisions of the Declaration of Helsinki. Subjects who smoked or were on medication in the month before study recruitment were excluded.
Experimental design
The subjects were asked to refrain from consuming alcoholic drinks from the day before the experiment, and caffeinated drinks such as coffee or tea on the day of the experiment. In addition, the subjects were requested to fast for 2 h before the experiment to limit carbohydrate intake, as acidic or high sugar foods can compromise salivary protein assay performance by lowering sample pH and influencing bacterial growth. Volunteers were instructed to arrive 15 min before the start of the experimental procedure to allow time for acclimatisation. To avoid the influence of circadian fluctuation in heart rate variability (HRV), salivary protein and sIgA, each experimental procedure was scheduled to take place in the morning between 09:00 and 12:00. In addition, the room temperature was maintained at 23–25°C. As calmness is important when measuring heart rate and assessing HRV, all external factors that might disturb the subject under investigation were kept to a minimum. All experiments were performed with the subject in a sitting position. After resting for 5 min, volunteers either continued to rest (control group) or underwent EA stimulation for 15 min (EA group), and then rested for a further 40 min. HRV was measured and saliva was collected at baseline (before) and at three time points following EA or rest: immediately, 20 min, and 40 min after treatment.
Electroacupuncture
Acupuncture points LI4 (Hegu) and LI11 (Quchi) were chosen based on previous research demonstrating a positive effect on sympathetic nerve activity and sIgA levels in saliva.18–20 A frequency of 100 Hz was selected as most HF EA studies have opted for this level of stimulation.21 22 In the EA group, stainless steel needles (50 mm long, 0.20 mm in diameter; Seirin, Shizuoka, Japan) were inserted bilaterally at LI4 and LI11 to a depth of approximately 10 and 15 mm, respectively. LI4 was located at the centre of the second metacarpal bone in the first dorsal interosseous muscle, and LI11 was located at the end of the lateral transverse elbow crease in the proximal part of the brachioradial muscle. EA was performed for 15 min at 100 Hz frequency using an electrostimulator (Ohm Pulser LFP-4000A; Zen Iryoki, Fukuoka, Japan). The control group subjects did not receive any stimulation.
Heart rate variability
To determine the effect of EA on cardiac sympathovagal tone, subjects were fitted with a disposable electrode for electrocardiogram (ECG) monitoring (Vitrode Bs 150; Nihon Kohden, Inc, Tokyo, Japan). HRV was recorded with a Marquette Holter recorder (LRR-03; GMS, Inc, Tokyo, Japan) at four time points: 5 min before, immediately after, 20 min after and 40 min after rest (control group) or 15 min EA. HRV data were processed using HRV analysis software (Crosswell, Inc, Tokyo, Japan). A power spectrum of this time series was then calculated using the maximum entropy method. LF and HF power were estimated within the 0.04−0.15 and 0.15−0.50 Hz frequency bands, respectively. The LF/HF ratio and HF were taken as indices of sympathetic and parasympathetic nerve activity, respectively.
Saliva collection
Saliva was collected before and after the EA period of 15 min (or rest) and every 20 min during the post-EA observation period of 40 min. Subjects were made to rinse their mouth thoroughly with 100 mL drinking water 10 min before sample collection, and then to chew a cotton sponge (Salivette; Sarstedt, Inc, Vumbrecht, Germany) for 2 min. Saliva was collected after centrifugation of the Salivette tube at g value=2000 for 10 min and stored at −20°C until analysis.
Protein measurement
Protein concentrations were measured by Bradford protein assay. A saliva sample or dose standard were added to each well of a Nunc-Immuno plate (NUNC, Roskilde, Denmark). Dye Reagent Concentrate (Bio Rad, Inc, Hercules, California, USA) was diluted 1:4 with distilled water and added to each well. The plate was incubated for 5 min and absorbance was measured using a micro-plate reader (iMark; Bio Rad, Inc) at 570 nm. Salivary protein levels were quantified using a standard curve method.
sIgA measurement
sIgA concentrations were measured by ELISA. Affinity-purified goat anti-human IgA (Bethyl Laboratories, Inc, Montgomery, Texas, USA) with coating buffer was added to each well of the plate, which was maintained at 4°C for >12 h. After the plate was washed with phosphate-buffered saline (PBS; Nissui Pharmaceutical, Inc, Tokyo, Japan) containing 0.05% Tween 20, PBS containing 1% bovine serum albumin (BSA; Sigma, Inc, St Louis, Missouri, USA) was added to the plate for blocking and maintained for 2 h at room temperature. Saliva samples were diluted 1:2000 with diluent (PBS containing BSA, 0.25% Tween). The dose standard consisted of human reference serum (Bethyl Laboratories, Inc). The diluted sample and dose standard were added to each well and incubated for 1 h at room temperature. After washing, horseradish peroxidase (HRP)-conjugated goat anti-human IgA (Bethyl Laboratories, Inc) was added to the plate and incubated for 1 h. After washing, 3,3′,5,5′-tetramethylbenzidine (TMB; KPL, Inc, Gaithersburg, Maryland, USA) solution was added to each well. After incubation at room temperature for 15 min, the reaction was terminated by adding 0.18 M sulfuric acid (H2SO4) and absorbance was measured using a micro-plate reader at 450 nm. Salivary sIgA levels were quantified using a standard curve method and corrected for salivary protein levels.
Analysis
All data are presented as mean±SD unless otherwise stated. Chronological changes in the LF/HF ratio, HF, protein, and salivary sIgA were examined using repeated measures two-way analysis of variance (ANOVA) followed by Tukey's post-hoc test. Differences between the control and EA groups were analysed by Student's t test at each time point and correlations between salivary sIgA, LF/HF ratio and HF indices were examined using Pearson's product-moment correlation coefficient in the EA group. The significance level of all statistical analyses was set at p<0.05.
Results
Heart rate variability
As illustrated in figure 1, relative to baseline, LF/HF ratio was significantly increased immediately after stimulation and 40 min later in the EA group (p=0.024 and p=0.003, respectively). There was no significant difference between baseline and the 20 min time point in the EA group and no significant change in LF/HF ratio at any point in the control group (p>0.05). Furthermore there were no significant differences between the EA and control groups at any stage.
Figure 1.
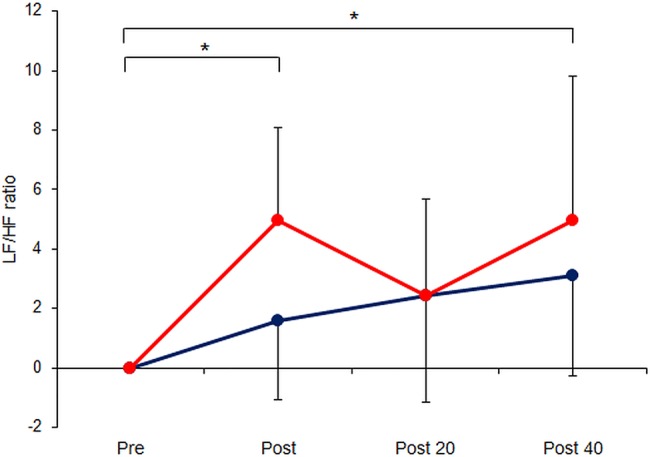
Serial measurements of low-frequency/high-frequency (LF/HF) ratio of heart rate variability, a marker of sympathetic nerve activity, in 16 healthy volunteers immediately before/after and 20/40 min following 15 min electroacupuncture (EA) at LI4+LI11 (EA group, red circles, n=8) or 15 min rest (control group, blue circles, n=8). Data are mean±SD. *p<0.05 comparing across time points (using repeated measures analysis of variance).
As shown in figure 2, HF was significantly greater at 20 min post-acupuncture than before the stimulation period in the EA group (p=0.049) but not immediately afterwards or at 40 min post-treatment. At this 20 min time point, HF was also significantly greater in the EA group than in the control group (p=0.001). No statistically significant changes in HF were seen in the control group over time (p>0.05).
Figure 2.
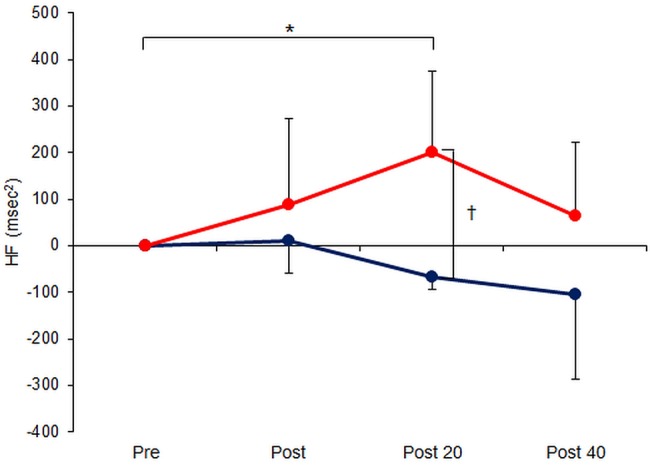
Serial measurements of high-frequency (HF) component of heart rate variability, a marker of parasympathetic nerve activity, in 16 healthy volunteers immediately before/after and 20/40 min following 15 min electroacupuncture (EA) at LI4+LI11 (EA group, red circles, n=8) or 15 min rest (control group, blue circles, n=8). Data are mean±SD. *p<0.05 comparing across time points (using repeated measures analysis of variance); †p<0.05 comparing across groups (using Student's t test).
Salivary protein and sIgA levels
Compared with baseline measurements, salivary protein levels did not differ significantly in either of the groups at any point (figure 3); neither were there any significant differences between the EA and control groups at any time. By contrast, sIgA levels were significantly greater at 40 min post-acupuncture than before treatment in the EA group (p=0.011) but not when assessed immediately post-stimulation or at 20 min (figure 4). At 40 min post-EA, the sIgA level was also significantly greater in the EA versus the control group (p=0.009). No significant changes were seen in the control group over time (p>0.05). Furthermore, the sIgA level at 40 min post-acupuncture correlated with HF at 20 min (r=0.752, n=8, p=0.031) but not with LF/HF ratio.
Figure 3.
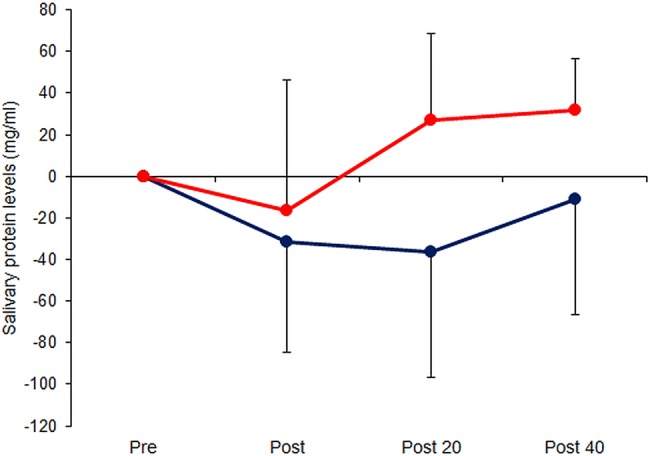
Serial measurements of salivary proteins, determined by the Bradford protein assay, in 16 healthy volunteers immediately before/after and 20/40 min following 15 min electroacupuncture (EA) at LI4+LI11 (EA group, red circles, n=8) or 15 min rest (control group, blue circles, n=8). Data are mean±SD.
Figure 4.
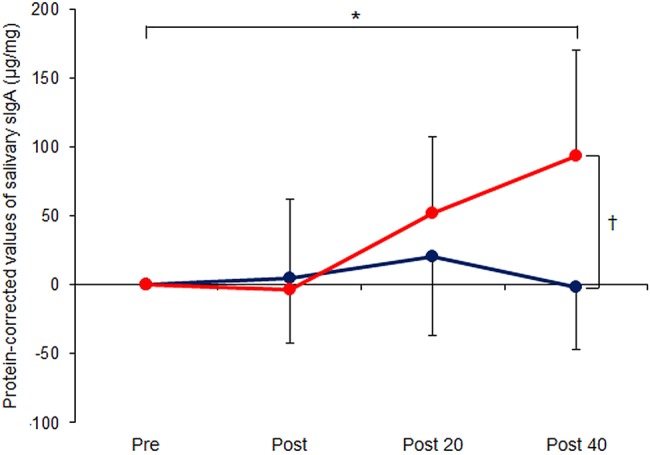
Serial measurements of salivary immunoglobulin A (sIgA), determined by ELISA and corrected for salivary protein levels, in 16 healthy volunteers immediately before/after and 20/40 min following 15 min electroacupuncture (EA) at LI4+LI11 (EA group, red circles, n=8) or 15 min rest (control group, blue circles, n=8). Data are mean±SD. *p<0.05 comparing across time points (using repeated measures analysis of variance); †p<0.05 comparing across groups (using Student's t test).
Discussion
In this study, we investigated whether EA influences the ANS and salivary sIgA level. There were significant increases in the LF/HF ratio immediately after EA, HF at 20 min after EA, and sIgA level at 40 min after EA, respectively. In addition, the increased HF at 20 min and sIgA levels at 40 min correlated with one another.
Previous research has shown that HF electrical stimulation excites Aβ nerve fibres3 and that this induces facilitation of vagal activity and long-term inhibition of sympathetic nerve activity by somato-vagal reflexes via the medulla oblongata.9 10 Parasympathetic nerves act on the salivary glands and control secretion of saliva and various proteins such as sIgA, and previous studies have shown that parasympathetic nerve stimulation increases saliva volume and salivary protein and sIgA levels through parasympathetic stimulation of the submandibular and parotid glands.13–15
In this study, levels of salivary protein and sIgA, both of which are important factors in defence mechanisms against infection, were elevated after a marker of parasympathetic nerve activity (HF) increased. Furthermore, the sIgA level at 40 min correlated with HF at 20 min. Therefore, we suggest that 100 Hz EA stimulation increased parasympathetic nerve activity, and the increased sIgA level was related to parasympathetic stimulation of the submandibular and parotid glands.
Previous studies have shown that EA at LI4 and LI11 increases sympathetic nerve activity,19–21 and that acupuncture stimulation at LI4 leads to activation of the hypothalamus.23 24 As the hypothalamus is the regulatory centre of the ANS, and its activation increases sympathetic nerve activity,24 25 we suspect that the increased sympathetic nerve activity observed following EA in the present study may have been associated with modulation of the hypothalamic function. In addition, sympathetic nerve stimulation has been associated with increased secretion of salivary sIgA by the submandibular and parotid glands,13–15 although the present study demonstrated no correlation between the LF/HF ratio and sIgA level. Although mental and physical stress increase sympathetic nerve activity in humans,17 26 27 and changes in sIgA levels can be induced by stress, no consensus has been reached about the association between sIgA levels and sympathetic nerve activity.16 17 Therefore, increased sympathetic nerve activity by EA may not be the most important factor affecting sIgA level. It is also possible that, despite the fact that the volunteers were resting, sympathetic nerve activity might have increased due to sitting for a long time, which itself can be stressful and may increase sympathetic tone. This is supported by the fact that mean values of LF/HF ratio increased slightly throughout the 40 min observation period in the control group, although not to a statistically significant degree.
Our results indicated that EA may increase sympathetic nerve activity immediately, parasympathetic nerve activity after 20 min, and salivary sIgA level after 40 min, respectively. However, this study has a few limitations. Our sample size was small and consisted of only healthy subjects. We believe that additional studies with a larger sample size, and including patients, are necessary to investigate further the physiological effects of acupuncture. In addition, the use of spectral indices of HRV may not completely reflect autonomic control28 and it would be useful to examine also the effects of acupuncture stimulation on components of the endocrine system, such as adrenalin, noradrenaline, and cortisol. However, in summary, our study indicates that HF EA may increase salivary sIgA levels by influencing parasympathetic nerve activity.
Footnotes
Contributors: TH, NY, HT, YM, YO, KU and HT conceived the study. SM contributed to data analysis. All authors critically edited drafts of this manuscript and approved the final manuscript.
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: Approved by the ethical committees of Teikyo Heisei University, Tokyo, Japan (reference no. 25-007).
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Eisenberg DM, Davis RB, Ettner SL. , et al. Trends in alternative medicine use in the United States, 1990-1997: results of a follow-up national survey. JAMA 1998;280:1569–75. 10.1001/jama.280.18.1569 [DOI] [PubMed] [Google Scholar]
- 2.Hamza MA, White PF, Ahmed HE, et al. Effect of the frequency of transcutaneous electrical nerve stimulation on the postoperative opioid analgesic requirement and recovery profile. Anesthesiology 1999;91:1232–8. 10.1097/00000542-199911000-00012 [DOI] [PubMed] [Google Scholar]
- 3.Koga K, Furue H, Rashid MH, et al. Selective activation of primary afferent fibers evaluated by sine-wave electrical stimulation. Mol Pain 2005;25:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han J-S. Acupuncture: neuropeptide release produced by electrical stimulation of different frequencies. Trends Neurosci 2003;26:17–22. 10.1016/S0166-2236(02)00006-1 [DOI] [PubMed] [Google Scholar]
- 5.Torres-Rosas R, Yehia G, Peña G. , et al. Dopamine mediates vagal modulation of the immune system by electroacupuncture. Nat Med 2014;20:291–5. 10.1038/nm.3479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamanaka Y, Honma K. Cardiovascular autonomic nervous response to postural change in 610 healthy Japanese subjects in relation to age. Auton Neurosci 2006;124:125–31. 10.1016/j.autneu.2005.12.008 [DOI] [PubMed] [Google Scholar]
- 7.Holsboer F. Stress, hypercortisolism and corticosteroid receptors in depression: implications for therapy. J Affect Disord 2001;62:77–91. 10.1016/S0165-0327(00)00352-9 [DOI] [PubMed] [Google Scholar]
- 8.Tallarida G, Baldoni F, Peruzzi G. , et al. Cardiorespiratory reflexes from muscles during dynamic and static exercise in the dog. J Appl Physiol 1985;58:844–52. [DOI] [PubMed] [Google Scholar]
- 9.Koizumi K, Terui N, Kollai M. Neural control of the heart: significance of double innervation re-examined. J Auton Nerv Syst 1983;7:279–94. 10.1016/0165-1838(83)90081-4 [DOI] [PubMed] [Google Scholar]
- 10.Terui N, Koizumi K. Responses of cardiac vagus and sympathetic nerves to excitation of somatic and visceral nerves. J Auton Nerv Syst 1984;10:73–91. 10.1016/0165-1838(84)90047-X [DOI] [PubMed] [Google Scholar]
- 11.Mazanec MB, Nedrud JG, Kaetzel CS. , et al. A three-tiered view of the role of IgA in mucosal defense. Immunol Today 1993;14:430–5. 10.1016/0167-5699(93)90245-G [DOI] [PubMed] [Google Scholar]
- 12.Lamm ME. Interaction of antigens and antibodies at mucosal surfaces. Annu Rev Microbiol 1997;51:311–40. 10.1146/annurev.micro.51.1.311 [DOI] [PubMed] [Google Scholar]
- 13.Carpenter GH, Garrett JR, Hartley RH. , et al. The influence of nerves on the secretion of immunoglobulin A into submandibular saliva in rats. J Physiol 1998;512(Pt 2):567–73. 10.1111/j.1469-7793.1998.567be.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Proctor GB, Carpenter GH. Neural control of salivary S-IgA secretion. Int Rev Neurobiol 2002;52:187–212. 10.1016/S0074-7742(02)52010-9 [DOI] [PubMed] [Google Scholar]
- 15.Proctor GB, Carpenter GH, Anderson LC. , et al. Nerve-evoked secretion of immunoglobulin A in relation to other proteins by parotid glands in anaesthetized rat. Exp Physiol 2000;85:511–18. 10.1111/j.1469-445X.2000.02060.x [DOI] [PubMed] [Google Scholar]
- 16.Akimoto T, Nakahori C, Aizawa K. , et al. Acupuncture and responses of immunologic and endocrine markers during competition. Med Sci Sports Exerc 2003; 35:1296–302. 10.1249/01.MSS.0000078934.07213.25 [DOI] [PubMed] [Google Scholar]
- 17.Willemsen G, Ring C, McKeever S. , et al. Secretory immunoglobulin A and cardiovascular activity during mental arithmetic: effects of task difficulty and task order. Biol Psychol 2000;52:127–41. 10.1016/S0301-0511(99)00028-9 [DOI] [PubMed] [Google Scholar]
- 18.Matsubara Y, Shimizu K, Tanimura Y. , et al. Effect of acupuncture on salivary immunoglobulin A after a bout of intense exercise. Acupunct Med 2010;28:28–32. 10.1136/aim.2009.001677 [DOI] [PubMed] [Google Scholar]
- 19.Knardahl S, Elam M, Olausson B. , et al. Sympathetic nerve activity after acupuncture in humans. Pain 1998;75:19–25. 10.1016/S0304-3959(97)00197-8 [DOI] [PubMed] [Google Scholar]
- 20.Yu DT, Jones AY. Physiological changes associated with de qi during electroacupuncture to LI4 and LI11: a randomised, placebo-controlled trial. Acupunct Med 2013;31:143–50. 10.1136/acupmed-2012-010280 [DOI] [PubMed] [Google Scholar]
- 21.Lee JH, Kim KH, Hong JW. , et al. Comparison of electroacupuncture frequency-related effects on heart rate variability in healthy volunteers: a randomized clinical trial. J Acupunct Meridian Stud 2011;4: 107–15. 10.1016/S2005-2901(11)60016-2 [DOI] [PubMed] [Google Scholar]
- 22.Zhao ZQ. Neural mechanism underlying acupuncture analgesia. Prog Neurobiol 2008;85:355–75. 10.1016/j.pneurobio.2008.05.004 [DOI] [PubMed] [Google Scholar]
- 23.Hsieh J-C, Tu C-H, Chen F-P. , et al. Activation of the hypothalamus characterizes the acupuncture stimulation at the analgesic point in human: a positron emission tomography study. Neurosci Lett 2001;307:105–8. 10.1016/S0304-3940(01)01952-8 [DOI] [PubMed] [Google Scholar]
- 24.Napadow V, Kettner N, Liu J. , et al. Hypothalamus and amygdala response to acupuncture stimuli in carpal tunnel syndrome. Pain 2007;130:254–66. 10.1016/j.pain.2006.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marsh AJ, Fontes MA, Killinger S. , et al. Cardiovascular responses evoked by leptin acting on neurons in the ventromedial and dorsomedial hypothalamus. Hypertension 2003;42:488–93. 10.1161/01.HYP.0000090097.22678.0A [DOI] [PubMed] [Google Scholar]
- 26.Borst C, Van Brederode JFM, Wieling W. , et al. Mechanisms of initial blood pressure response to postural change. Clin Sci 1984;67:321–7. 10.1042/cs0670321 [DOI] [PubMed] [Google Scholar]
- 27.Francis GS, Goldsmith SR, Ziesche S. , et al. Relative attenuation of sympathetic drive during exercise in patients with congestive heart failure. J Am Coll Cardiol 1985;5:832–9. 10.1016/S0735-1097(85)80420-4 [DOI] [PubMed] [Google Scholar]
- 28.Alraek T, Tan CO. Acupuncture and heart rate variability. Acupunct Med 2011;29:7–8. 10.1136/aim.2010.003665 [DOI] [PubMed] [Google Scholar]


