Abstract
Purpose:
To report visual outcomes and corneal biomechanical changes after femtosecond-assisted Intacs SK implantation in keratoconic eyes.
Methods:
This prospective interventional case series is comprised of 32 keratoconic eyes of 25 patients with mean age of 23.8 ± 5.4 years. Uncorrected (UDVA) and corrected (CDVA) distance visual acuity, refraction, manifest refraction spherical equivalent (MRSE), keratometry, central corneal thickness (CCT), corneal hysteresis (CH) and corneal resistance factor (CRF) were measured preoperatively, and 1, 3 and 6 months postoperatively.
Results:
Mean UDVA improved from 0.81 ± 0.3 LogMAR preoperatively to 0.53 ± 0.2 LogMAR six months postoperatively (P < 0.001). At 6 months, MRSE was significantly reduced only in eyes with moderate KCN (mean change, +2.61 ± 0.54 diopter [D]; P< 0.001). A significant improvement in sphere (mean change, +1.92 ± 0.37 D; P< 0.001) and mean keratometry (mean change, -3.34 ± 0.47D; P< 0.001) were observed. CCT increased from 446.1 ± 38 μm preoperatively to 462.2 ± 50 μm at six months (P < .001). CRF decreased from 6.5 ± 1.6 mmHg to 5.9 ± 1.1 mmHg six months after surgery (P = 0.02). CDVA, refractive cylinder and CH did not change significantly (P = 0.48, 0.203 and 0.55, respectively). Linear regression analysis disclosed that a decrease in CCT and moderate KCN are associated with higher CRF (standardized B,-0.513 and 0.314;P= 0.004 and 0.024, respectively; Adjusted R square = 0.353).
Conclusion:
Visual, refractive and keratometric indices remarkably improved in a parallel fashion. CRF was inversely associated with CCT. Changes in CRF represent the trend of changes in corneal biomechanics and thickness during the early postoperative months.
Keywords: Corneal Biomechanic, Corneal Hysteresis, Corneal Resistance Factor, Femtosecond Laser, Intacs SK, Intracorneal Ring Segment, Keratoconus
INTRODUCTION
Keratoconus (KCN) is a progressive non-inflammatory condition resulting in conical protrusion of the cornea with ophthalmologic complications ranging from mild myopia and irregular astigmatism to severe opacity and scarring of the corneal apex.[1] The treatment of choice highly differs according to the stage of the disease. In the early stages, optical correction is well attained using spectacles and contact lenses. However, later on when a proper optical correction is no longer accomplished or contact lenses are no longer tolerated, surgical treatments are the only viable options.[1] Intracorneal ring segments (ICRSs) appear as a promising surgical alternative to at least postpone, if not entirely obviate, the need for lamellar or penetrating keratoplasty in moderate to advanced KCN.[2]
Numerous studies have revealed the efficacy of different ICRSs to correct low myopia thanks to an “arc-shortening” effect of these segments on corneal lamellae.[3,4] According to the Barraquer law of thickening, these segments gently operate by thickening the peripheral cornea that subsequently flattens the central cornea as a result of coupling forces exerted on different parts of the same cornea. The flattening effect of the ICRS is directly proportional to its thickness but inversely associated with its diameter.[4,5] Intacs SK (Addition Technology Inc., California, USA) has an elliptical shape and an inner diameter of 6 mm that is positioned closer to the visual axis and central cornea, consequently resulting in greater spherocylindrical changes.
While flattening the center of the cornea and changing its structural pattern, ICRSs may also serve as a mechanical support by creating a second limbus of smaller diameter in the middle of the cornea. ICRSs are made of polymethylmethacrylate (PMMA), and reinforce and alter corneal shape, thus they may affect biomechanical parameters. Even assuming that implantation of ICRSs does not directly alter corneal biomechanical properties, it has been shown that this procedure significantly alters curvature and redistributes stress leading to improvement in corneal biomechanics over time.[6]
Indeed, keratoconic corneal tissue has a thinner structure compared to the normal corneas and hence can be flattened with more ease. On the negative side, the disrupted arrangement of collagen fibrils within the apical scar of a keratoconic cornea leads to highly unpredictable visual and refractive outcomes. Moreover, the cornea has viscoelastic properties and its response to any exerted force depends on the magnitude and velocity of that force, as well as intrinsic corneal biomechanical properties.[7]
Until recently few studies have focused on assessing corneal biomechanical variations in keratoconic eyes after ICRS insertion. Dauwe et al[8] reported no change in viscoelastic corneal biomechanics after Intacs insertion, but recently a few studies have showed minor variations in these parameters during long-term follow up of patients who had received different types of ICRSs.[7,9]
The current study was designed to assess the visual, refractive and keratometric outcomes along with corneal biomechanical variations during a postoperative period of 6 months after Intacs SK implantation in patients with moderate to advanced KCN.
METHODS
Patients
This prospective case series was comprised of 32 keratoconic eyes of 25 consecutive patients who had received Intacs SK between July 2012 and December 2012 at Farabi Eye Hospital, Tehran, Iran. The Research Council of Tehran University of Medical Sciences reviewed and approved the study protocol. In accordance with the tenets of Declaration of Helsinki, informed consent was obtained from all participants.
Diagnosis of KCN was based on slit lamp clinical findings and corneal topography. Inclusion criteria were the presence of moderate to severe keratoconus (stages II, III or IV based on Amsler-Krumeich classification)[10] with a clear cornea, 18 years of age or older, contact lens intolerance or decreased tolerance, and minimum corneal thickness of 450 μm at the site of segment implantation. Exclusion criteria consisted of mean keratometry exceeding 60 diopter (D), history of corneal/intraocular surgery, previous ocular diseases (i.e., uveitis, glaucoma and active vernal disease), and any type of connective tissue diseases.
Examination and Follow-up
Preoperatively, all eyes underwent a complete ocular examination including measurement of visual acuity (uncorrected distance visual acuity [UDVA] and corrected distance visual acuity [CDVA]) using standard Snellen chart, slit lamp examination, subjective manifest refraction (sphere and cylinder) and keratometry (AutoKR 8900, Topcon, Japan). Corneal topography (Orbscan II Topography System, Bausch and Lomb, NY, USA) was used to measure pre-and postoperative keratometry readings, corneal astigmatism, and anterior and posterior elevations of the cornea. Ultrasonic pachymetry (Micropach 200 P+, Sonomed, Cleveland, Ohio, USA) with a hand-held solid probe was performed to measure central corneal thickness (CCT). The cornea was anesthetized with topical benoxinate hydrochloride 0.4% and 3 consecutive measurements were made. Prior to the study, the pachymeter was calibrated according to the manufacturer's instruction manual and was tested with an appropriate test block. All ultrasonic measurements were performed by the same investigator, who applied the probe tip as perpendicularly as possible on the central cornea. The mean value of the 3 measurements was calculated as the final CCT reading. In all cases, peripheral pachymetry was assessed using either Orbscan II or the Pentacam (Oculus Optikgeräte GmbH, Wetzlar, Germany) pachymetry map to ensure sufficient corneal thickness at the incision site and appropriate depth for placement of the Intacs. All eyes underwent evaluation with the Ocular Response Analyzer (ORA; Reichert Ophthalmic Instruments, Buffalo, NY, USA) to determine the biomechanics of the cornea in terms of corneal hysteresis (CH) and corneal resistance factor (CRF). The same procedure was applied at postoperative visits 1, 3 and 6 months after surgery.
Surgical Technique
The procedures were performed by either of two experienced cornea subspecialty surgeons (M.A.Z and H.B). The eye to be operated was prepared with a topical anesthetic agent and then a standard prepping and draping was performed.
The pupil center was marked with a Sinskey hook as the reference point. We used the Technolas femtosecond workstation 520F (Technolas Perfect Vision GmbH, Munich, Germany) to create a 360-degree tunnel for inserting the segments. To minimize decentralization, the docking ring of the laser platform was carefully centered on cornea and then suction was applied. The infrared Nd: Glass laser beam was centered to create a channel at a predetermined mean corneal depth of 75%. The tunnel was created in the stroma during a 15 seconds timeline, with an entry cut thickness of 1 µm. At the maximum pulse energy of 4.0 µJ, the inner and outer diameters of the tunnel were preset at 5.9 and 7.7 mm, respectively. A temporal radial incision 1.3 mm in length was created in the cornea. Shortly afterwards, suction was brought to a halt, and the cone, docking ring and lid speculum were withdrawn from the eye. Channel dissection was completed by interconnection of the cavitation and micro-bubbles within the cornea.
After opening the incision with a Sinskey hook, segments were implanted using the Intacs SK inserter forceps followed by closing the entry with a 10-0 nylon suture. We employed the Colin's approach for creating the radial incisions[2](i.e. a temporal incision either at the steep axis in pellucid-like cones or perpendicular to the steep axis in asymmetric bow-tie patterns to embrace the ectatic area). To reduce irregular astigmatism and associated myopia induced by keratoconus, both segments (Intacs SK 450 µm, arch length 150°) were inserted asymmetrically.
Finally, a bandage contact lens was fitted on the operated eye for one week. Postoperative medications consisted of ciprofloxacin hydrochloride 0.3% eye drops, betamethasone sodium phosphate 0.1% eye drops, and non-preserved tear substitute eye drops, 4 times daily each for 7 days. The suture was removed 4 weeks after the operation.
Statistical Analysis
Analysis was performed employing SPSS software version 17 (SPSS, Inc., Chicago, Illinois, USA). Snellen visual acuity data (UDVA and CDVA) were transformed into logarithm of minimum angle of resolution (LogMAR) notations. However, for a clinically meaningful comparison, LogMAR values were reverted to Snellen acuity. The Kolmogorov-Smirnov test was used to ascertain normal distribution of data. Paired Samples T-test was employed to evaluate the significance of alteration of each variable during successive examinations. Wilcoxon-Signed Ranks were applied to compare categorical data. Association of categorical variables with the outcomes was assessed by either the Independent Samples T-test or ANOVA test. Association of continuous variables with categorical factors was assessed using parametric analyses (ANOVA test). K-independent-sample analysis (Kruskal-Wallis H test) was used to assess associations of categorical variables with UDVA and CDVA. Correlation coefficients (Pearson or Spearman Rho, depending on whether normal distribution of data could be assumed) were used to evaluate correlation between different variables. Multiple linear regression analysis was employed to assess the effect of independent variables (change in central corneal thickness, severity of keratoconus, age and gender) on CRF changes. Backward procedure was applied for variable selection. All statistical tests were two sided, and P values less than 0.05 were considered to indicate significance.
RESULTS
The study included 32 eyes of 25 patients including 14 male and 11 female subjects with mean age of 23.8 ± 5.4 (range, 18-41) years. Moderate and advanced keratoconus were present in 22 and 10 eyes, respectively. No patient was lost to follow-up.
Visual Acuity
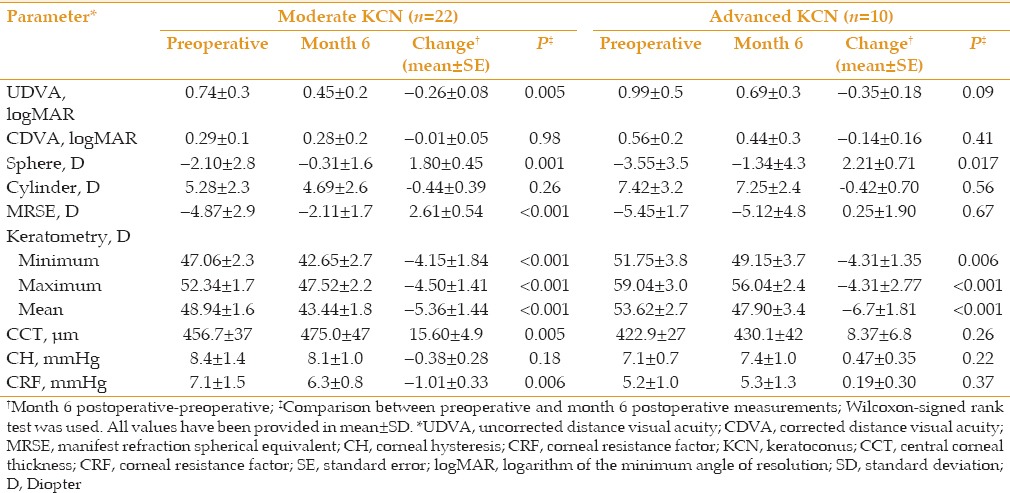
Preoperative UDVA ranged from 0.22 to 1.6 LogMAR (≈20/32 to <20/400 Snellen acuity). Mean UDVA was significantly improved from 0.81 ± 0.3 LogMAR preoperatively to 0.57 ± 0.3 LogMAR at 3 months and 0.53 ± 0.2 LogMAR at 6 months (P = 0.005 and <0.001, respectively, Table 1). As demonstrated in Table 2, eyes with moderate and advanced KCN experienced a clinically significant improvement in UDVA 6 months after the operation (mean change of-0.26 ± 0.08 LogMAR [P = 0.005] and -0.35 ± 0.18 LogMAR [P = 0.09], respectively). Mean preoperative CDVA ranged from 0.04 to 1.15 LogMAR (≈20/20 to ≈20/320 Snellen acuity). Mean CDVA remained stable from a preoperative value of 0.37 ± 0.2 LogMAR to 0.32 ± 0.2 LogMAR at month 6 (P = 0.48; Table 1).
Table 1.
Pre- and post-operative (months 1, 3 and 6) data for 32 keratoconic eyes with Intacs SK implantation
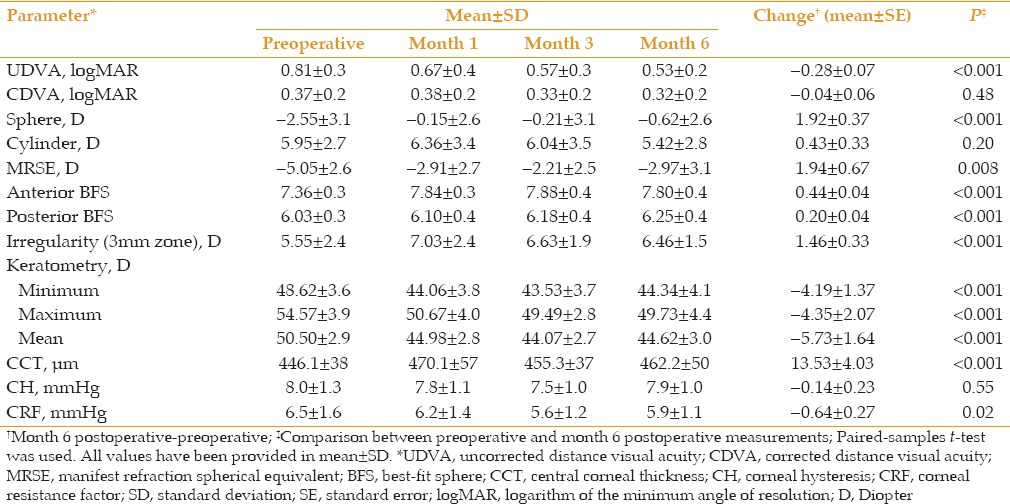
Table 2.
Pre- and 6 month post-operative data for 32 keratoconic eyes regarding the severity of keratoconus
UDVA showed progressive improvement during follow up (P<.001); however, this trend was not observed for CDVA (P = 0.48, Figure 1). After 6 months of follow-up, 5 eyes (16%) lost 2 or more lines of UDVA and 19 eyes (60%) gained 2 or more lines of UDVA [Figure 2]. Regarding CDVA, 5 eyes (16%) lost at least 2 lines while 8 eyes (25%) gained 2 or more lines at 6 months. Even when comparing preoperative CDVA with postoperative UDVA, 16% of the eyes achieved 2 lines or more.
Figure 1.
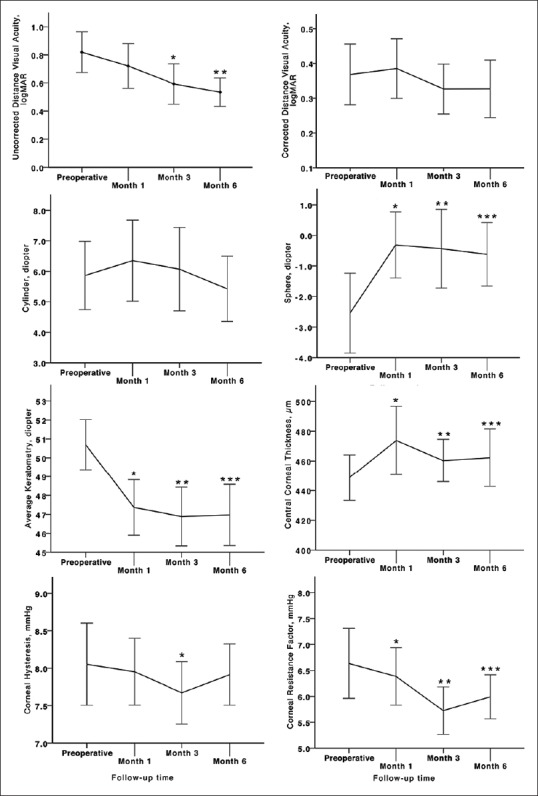
Trend of visual, refractive, keratometric, corneal thickness and corneal biomechanical changes during 6 months of follow up in keratoconic patients who underwent Intacs SK implantation (asterisks represent statistically significant differences between pre-and postoperative measurements).
Figure 2.
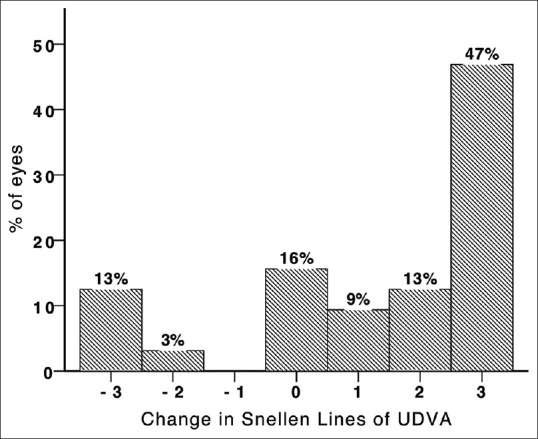
Change in Snellen lines of uncorrected distance visual acuity (UDVA) 6 months after Intacs SK implantation in keratoconic eyes.
Refractive Changes
The sphere was significantly decreased from a preoperative value of -2.55 ± 3.1 D preoperatively to -0.15 ± 2.6 D at month one (2.41 ± 2.6 D reduction, P < 0.001) and remained stable until month six at a mean value of-0.62 ± 2.6 D (1.92 ± 0.37 D reduction, Table 1, P < 0.001).
Mean preoperative corneal cylinder was 5.95 ± 2.7 D. Corneal cylinder power showed a trend towards lower values one month postoperatively [Figure 1]. Six months after surgery, this value was reduced to 5.42 ± 2.8 D; however changes were not statistically significant (all P values > 0.05, Table 1).
Mean manifest refraction spherical equivalent (MRSE) was significantly decreased from -5.05 ± 2.6 D preoperatively to -2.97 ± 3.1 D, six months following the operation (P = 0.008). Mean decrease in MRSE was 2.8 ± 2.9 D and 1.94 ± 0.67 D at postoperative months 3 and 6, respectively [Table 1]. However, no significant change (0.25 ± 1.90 D, P = 0.89) was observed in MRSE in eyes with advanced keratoconus [Table 2].
Keratometry
Mean keratometry readings revealed a sustained and significant reduction during all postoperative examinations (all P values < 0.05; Figure 1). Mean differences between preoperative readings and month 3 and 6 readings were -6.23 ± 1.7 D and -5.73 ± 1.6 D, respectively [Table 1]. A similar trend was observed for minimum and maximum keratometry readings (all P values < 0.05; Table 1). Irregularity at the 3-mm zone increased significantly at month 6 postoperatively (1.46 ± 0.33 D, P < 0.001; Table 1).
As shown in Table 1, the flattening effect of Intacs SK (evident in the radius of best fit spheres) was more prominent in the anterior surface of the cornea (mean difference; 0.44 ± 0.04 mm) than in the posterior surface (mean difference; 0.20 ± 0.04 mm).
Corneal Thickness
CCT was increased by 5.3% (24.0 ± 39.3 µm) at postoperative month 1. Afterwards, a slight decrease in corneal thickness was observed [Figure 1], but measurements at months 3 and 6 were still significantly higher than preoperative values (P values < 0.05; Table 1). The increase in corneal thickness was higher in eyes with moderate KCN than those with advanced disease (15.60 ± 4.9 versus 8.37 ± 6.8 µm, respectively; Table 2).
Corneal Hysteresis and Corneal Resistance Factor
There was a comparable trend in the operated eyes regarding corneal hysteresis (CH) and corneal resistance factor (CRF) [Figure 1]. Preoperatively, mean CH was 8.0 ± 1.3 mmHg and reached its minimum value (7.5 ± 1.0 mmHg, P = 0.015) three months after the operation [Table 1]. Likewise, CRF was decreased postoperatively and reached its lowest value at three months (5.6 ± 1.2 mmHg, P < 0.001). At month 6, CRF showed a slight increase, but was still significantly less than its preoperative value (P = 0.02). As shown in Table 2, this reduction was only significant in eyes with moderate KCN (P = 0.006). Linear regression analysis disclosed an inverse association between CCT and severity of KCN with CRF changes (standardized B, -0.513 and 0.314; P = 0.004 and 0.024, respectively; adjusted R square, 0.353; Figure 3).
Figure 3.
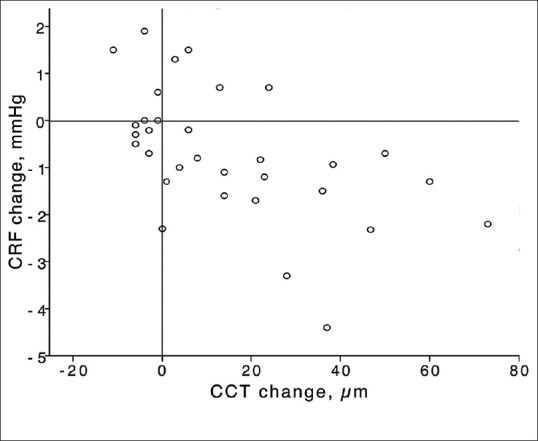
Changes in central corneal thickness (CCT) and corneal resistance factor (CRF); note the inverse linear correlation between CCT and CRF in keratoconic eyes with Intacs SK implantation.
Complications
Aqueous leakage occurred in 2 eyes while attempting to open the radial incision with the Sinskey hook. The operation was halted and the incision site was closed with one 10-0 nylon suture ensued by prescription of ciprofloxacin hydrochloride 0.3% eye drops for the next 5 postoperative days. One eye was scheduled for rechanneling after 2 months, and the other eye was scheduled for lamellar keratoplasty which was deemed necessary due to severe corneal thinning. Two eyes were complicated by segment migration leading to corneal melting and segment extrusion. The problem was managed by segment removal. Corneal deposits adjacent to the channels were noted in 5 cases 6 months postoperatively. These deposits caused no adverse effects [Figure 4].
Figure 4.
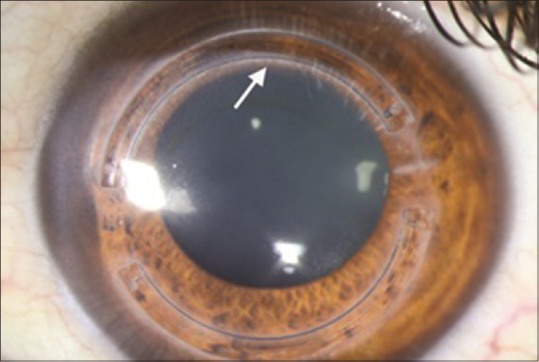
Deposits around the ring channel (arrow) at month 6 of follow-up; note that deposits are more concentrated around the inner rim of the superior segment without affecting patient's quality of vision.
DISCUSSION
Intrastromal segments and ring implantation has an established position in the continuum of care for keratoconus.[11] In the current study, UDVA demonstrated steady and significant improvement after 6 months of follow up. In contrast to the studies by Ertanet al,[12] Sansanayudh et al[13] and Fahdet al,[14] we noticed a greater increase in UDVA than in CDVA. An improvement in CDVA is attributed to reduction in corneal aberrations. However, this association cannot be investigated in the present study because we did not measure higher-order aberrations. An improvement in UDVA is attributable mostly to a reduction in lower order aberrations (sphere and MRSE values in our study). Some authors have observed greater improvements in CDVA than UDVA, while others reported the opposite. These variations could be ascribed to variations in the structure of the implant, its shape and distance from the center, as well as orientation and symmetric/asymmetric insertion of the rings.[7,12,15] However, baseline disease severity and the pattern of ectasia are so heterogeneous in keratoconic eyes, that generalizations are not possible.
A large subgroup of our patients gained lines of UDVA; at final follow-up, 60% and 34% of eyes gained 2 or more lines of UDVA and CDVA, respectively. The small number of cases with loss of 2 lines of UDVA and CDVA (15.6 and 6.2%, respectively) observed in our study are in accordance with previous reports.[11,12]
Following a remarkable initial keratometric flattening, a regression was observed between months 3 and 6 after surgery. This change did not reach statistical significance, but was consistent with the trend of changes in MRSE after surgery. Intacs SK was shown to exert significant flattening on both the anterior and posterior corneal surfaces, although the flattening effect was more anteriorly focused. This flattening effect could be related to the depth of ICRS implantation.
We observed an unexpected and significant increase in CCT after Intacs SK implantation. This observation has already been reported but not discussed in the literature.[16] Although one might simply attribute this change to epithelial thickening following corneal flattening, Steinert et al detected central epithelial thinning with the use of very high-frequency digital ultrasound (Artemis; ArcScan Inc., Morrison, Colorado, USA).[16] Moreover, such a change can be attributed to corneal edema likely caused by a change in endothelial cell function (especially in the area behind the implants). But the authors prefer to propose the hypothesis of “collagen crowding and stromal infolding”: It is known that the implant walls off and flattens a certain volume of the central cornea (i.e. the cone); this will compact tissue in a shorter span, translating into an increase in corneal thickness.
Several studies have found that when corneal tissue is weakened as a result of a corneal wound such as refractive surgery or surgical incisions, or because of disease processes such as KCN, corneal biomechanics are lower than expected.[17,18,19] The baseline values of CH (8.0 ± 1.3 mmHg) and CRF (6.5 ± 1.6 mmHg) in our study were comparable to those reported in previous studies on keratoconic eyes.[19] We found that CH and CRF were significantly decreased in first 3 months after the operation and then gradually approached preoperative values by the end of 6-months. Both CH and CRF demonstrated significant reductions at month 3, although only the decrease in CRF remained significant at the end of the follow-up period. Similarly, Kucumen et al stated that corneal biomechanical parameters decreased temporarily after phacoemulsification, then gradually increased and reached their preoperative values within 3 months.[18] These findings are consistent with prior studies in which CRF has been reported to decrease more than CH after a surgical procedure supporting the notion that viscous properties of the cornea are less affected by the procedure than its elastic properties.[20] Previous studies have demonstrated that CRF, as compared to CH, is more correlated with variations in CCT.[21,22] This may explain why we observed a significant reduction in CRF (as opposed to CH) with increasing CCT. Nevertheless, the fact that no significant difference was observed in CH at the end of the follow-up period may suggest that the ORA is not accurate enough to detect subtle changes in viscous properties of the cornea.
In a more rational scenario, we believe that the introduction of ICRS into the cornea does not instantly modify the structural and mechanical properties of the tissue, an explanation first suggested by Pinero and his colleagues.[7] Dauwe et al investigated morphological and biomechanical corneal responses at 6 months in corneas with mechanically implanted Intacs ICRSs. The authors found that Intacs implantation did not alter the viscoelastic biomechanical parameters of cornea (CH and CRF).[8] However, they did not refer to the likely alterations between different follow-up intervals (i.e., month 1, month 3 and month 6). This discrepancy can be explained by the design of the implanted material (Intacs SK is elliptical, Intacs is hexagonal in cross-section); more importantly, Intacs SK is implanted more centrally (6 mm vs. 7 mm optical zone). It is known that the ORA is focused on the central cornea; it is possible that when segments are implanted closer to the center of the cornea, corneal weakening due to dissection of the central lamellae increases, and therefore more significant reductions in CH and CRF are observed in early postoperative period. Gorgun et al used the even more centrally oriented Keraring Segments (5.0-mm optical zone) and reported greater reduction in CRF and CH than our study.[9]
Theoretically, such biomechanical variations can be attributed to: (1) KCN progression (i.e. keratocytes activation and apoptosis, and stromal remodeling); (2) corneal histopathological changes such as stromal edema; and (3) the tension exerted by ICRSs on the cornea. It has been shown that biomechanical properties of keratoconic corneas decrease with more advanced disease. But, the two latter causes seem more plausible for accounting for changes observed in our study because during follow up, we observed regression of viscoelastic biomechanical parameters toward baseline values, although they did not reach the preoperative values by the end of the 6th month.
The issue of lower corneal biomechanics in unusually edematous corneas has been addressed in previous reports.[23] In fact, in normal individuals, corneal biomechanics should increase with increasing CCT.[19] However, in ocular pathologies or non-intact eyes, controversial data have been generated on the relationship between corneal biomechanical properties measured by the ORA and CCT.[20,22] In a study by Laiquzzamanet al, post-PK eyes had higher mean CCT than normal eyes, while CH and CRF were lower than normal.[24] We observed an increase in CCT during the early phase of our study (up to the first month), alongside a marked decrease in both CRF and CH, though more marked with CRF which is, as mentioned earlier, more correlated with CCT.[25] Previous investigations have shown that lower levels of edema (e.g. due to contact lens wear) may increase corneal rigidity,[26,27] whereas higher levels of edema (e.g. due to surgical intervention) may be associated with reduced rigidity.[28,29] We observed a combination of both patterns in our patients; during the first 3 months after the operation, the trend of changes in CCT, CRF and CH followed the latter pattern, but during the final 3 months of follow up edema decreased while CH and CRF increased.
An important factor which may affect corneal biomechanics is the tensile force exerted on the cornea by ICRS. It can be assumed that insertion of ICRS resets corneal biomechanical characteristics in a way that better tensile force (rigidity) is achieved for flattening the cornea.[6] This effect, however, is primarily hampered by the extensive inflammatory and edematous response of the corneal stroma following the operation. Thus, the observed biphasic (downward and then upward) variation in CH and CRF can be attributed to uncovering the effects of ICRS with inflammation and edema resolution. On the other hand, it has been discussed that after collagen corneal cross-linking[30] and photorefractive keratectomy,[31] stromal keratocyte repopulation is completed by 6 months and is accompanied by disappearance of stromal edema. In this regard, longer follow up should be recommended to provide more information for assessing stability of visual and refractive outcomes as well as better investigation of corneal biomechanical variations after ICRS insertion in KCN.
We employed the femtosecond technology to create the tunnels for ICRS implantation. We believe the long term visual and refractive outcomes of our study are comparable, if not superior, to those with manual tunneling, as confirmed by previous reports.[32] The patients in the present study, unlike most previous studies were homogenous, i.e. were operated by the same surgeon using the same surgical protocol and all interventions took place at the same eye hospital.
While further studies are required to determine the optimal alignment for segments, it has been implied that maximal flattening of the central cornea is approached at 90 degrees from the incision site. To the present date, three known approaches for segment incision have been recommended: Colin's approach (as was used in this study) favors a temporal incision.[2,33,34,35] Advocates of the second approach claim that the best location for incision is in the steepest meridian of the cornea.[36,37] However, recent studies reported good results with implantation of ICRS guided by the comatic axis.[38]
Consistent with previous reports, we found that Intacs SK implantation a safe and effective surgical modality for reducing corneal steepening and improving visual acuity in KCN. Five eyes (15%) were detected to develop deposits around the ring channel later during follow up. A higher concentration of deposits was a prominent characteristic around the inner edge of the tunnel rim, albeit not interfering with patients' quality of vision. Five (15%) eyes lost 2 or more lines of UDVA. Preoperative indices in these eyes were comparable to eyes that gained of UDVA. Thus, regardless of the severity of keratoconus, the possibility of such conditions should be discussed with patients prior to obtaining informed consent.
In conclusion, Intacs SK implantation is an effective surgical modality for management of both moderate and advanced keratoconus. Visual, refractive and keratometric indices remarkably improved in a parallel fashion and remained stable during follow-up. Biomechanical parameters of the cornea were inversely correlated with CCT. Fluctuations in corneal hydration might explain the trend of changes in biomechanical parameters and CCT during the early postoperative months. The role of keratoconus progression and corneal biomechanical response to the tension exerted by the rings could be better revealed through longer periods of follow-up.
Financial Support and Sponsorship
Nil.
Conflicts of Interest
There are no conflicts of interest.
REFERENCES
- 1.Rabinowitz YS. Keratoconus. Surv Ophthalmol. 1998;42:297–319. doi: 10.1016/s0039-6257(97)00119-7. [DOI] [PubMed] [Google Scholar]
- 2.Colin J, Cochener B, Savary G, Malet F. Correcting keratoconus with intracorneal rings. J Cataract Refract Surg. 2000;26:1117–1122. doi: 10.1016/s0886-3350(00)00451-x. [DOI] [PubMed] [Google Scholar]
- 3.Cochener B, Savary-LeFloch G, Colin J. Effect of intrastromal corneal ring segment shift on clinical outcome: One year results for low myopia. J Cataract Refract Surg. 2000;26:978–986. doi: 10.1016/s0886-3350(00)00537-x. [DOI] [PubMed] [Google Scholar]
- 4.Patel S, Marshall J, Fitzke FW., 3rd Model for deriving the optical performance of the myopic eye corrected with an intracorneal ring. J Refract Surg. 1995;11:248–252. doi: 10.3928/1081-597X-19950701-08. [DOI] [PubMed] [Google Scholar]
- 5.Burris TE, Ayer CT, Evensen DA, Davenport JM. Effects of intrastromal corneal ring size and thickness on corneal flattening in human eyes. Refract Corneal Surg. 1991;7:46–50. [PubMed] [Google Scholar]
- 6.Roberts CJ. Biomechanics in keratoconus. In: Barbara A, editor. Textbook on Keratoconus: New Insights. India: JP Medical Ltd.; 2012. pp. 29–32. [Google Scholar]
- 7.Piñero DP, Alio JL, Barraquer RI, Michael R. Corneal biomechanical changes after intracorneal ring segment implantation in keratoconus. Cornea. 2012;31:491–499. doi: 10.1097/ICO.0b013e31821ee9f4. [DOI] [PubMed] [Google Scholar]
- 8.Dauwe C, Touboul D, Roberts CJ, Mahmoud AM, Kérautret J, Fournier P, et al. Biomechanical and morphological corneal response to placement of intrastromal corneal ring segments for keratoconus. J Cataract Refract Surg. 2009;35:1761–1767. doi: 10.1016/j.jcrs.2009.05.033. [DOI] [PubMed] [Google Scholar]
- 9.Gorgun E, Kucumen RB, Yenerel NM. Influence of intrastromal corneal ring segment implantation on corneal biomechanical parameters in keratoconic eyes. Jpn J Ophthalmol. 2011;55:467–471. doi: 10.1007/s10384-011-0057-8. [DOI] [PubMed] [Google Scholar]
- 10.Krumeich JH, Daniel J. Live epikeratophakia and deep lamellar keratoplasty for I-III stage-specific surgical treatment of keratoconus. Klin Monbl Augenheilkd. 1997;211:94–100. doi: 10.1055/s-2008-1035103. [DOI] [PubMed] [Google Scholar]
- 11.Ertan A, Colin J. Intracorneal rings for keratoconus and keratectasia. J Cataract Refract Surg. 2007;33:1303–1314. doi: 10.1016/j.jcrs.2007.02.048. [DOI] [PubMed] [Google Scholar]
- 12.Ertan A, Kamburoglu G. Intacs implantation using a femtosecond laser for management of keratoconus: Comparison of 306 cases in different stages. J Cataract Refract Surg. 2008;34:1521–1526. doi: 10.1016/j.jcrs.2008.05.028. [DOI] [PubMed] [Google Scholar]
- 13.Sansanayudh W, Bahar I, Kumar NL, Shehadeh-Mashour R, Ritenour R, Singal N, et al. Intrastromal corneal ring segment SK implantation for moderate to severe keratoconus. J Cataract Refract Surg. 2010;36:110–113. doi: 10.1016/j.jcrs.2009.07.040. [DOI] [PubMed] [Google Scholar]
- 14.Fahd DC, Jabbur NS, Awwad ST. Intrastromal corneal ring segment SK for moderate to severe keratoconus: A case series. J Refract Surg. 2012;28:701–705. doi: 10.3928/1081597X-20120921-05. [DOI] [PubMed] [Google Scholar]
- 15.Tu KL, Sebastian RT, Owen M, Batterbury M, Kaye SB. Quantification of the surgically induced refractive effect of intrastromal corneal ring segments in keratoconus with standardized incision site and segment size. J Cataract Refract Surg. 2011;37:1865–1870. doi: 10.1016/j.jcrs.2011.04.025. [DOI] [PubMed] [Google Scholar]
- 16.Reinstein DZ, Srivannaboon S, Holland SP. Epithelial and stromal changes induced by intacs examined by three-dimensional very high-frequency digital ultrasound. J Refract Surg. 2001;17:310–318. doi: 10.3928/1081-597X-20010501-04. [DOI] [PubMed] [Google Scholar]
- 17.Ortiz D, Piñero D, Shabayek MH, Arnalich-Montiel F, Alió JL. Corneal biomechanical properties in normal, post-laser in situ keratomileusis, and keratoconic eyes. J Cataract Refract Surg. 2007;33:1371–1375. doi: 10.1016/j.jcrs.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 18.Kucumen RB, Yenerel NM, Gorgun E, Kulacoglu DN, Oncel B, Kohen MC, et al. Corneal biomechanical properties and intraocular pressure changes after phacoemulsification and intraocular lens implantation. J Cataract Refract Surg. 2008;34:2096–2098. doi: 10.1016/j.jcrs.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 19.Shah S, Laiquzzaman M, Bhojwani R, Mantry S, Cunliffe I. Assessment of the biomechanical properties of the cornea with the ocular response analyzer in normal and keratoconic eyes. Invest Ophthalmol Vis Sci. 2007;48:3026–3031. doi: 10.1167/iovs.04-0694. [DOI] [PubMed] [Google Scholar]
- 20.Kotecha A. What biomechanical properties of the cornea are relevant for the clinician? Surv Ophthalmol. 2007;52(Suppl 2):S109–S114. doi: 10.1016/j.survophthal.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 21.Franco S, Lira M. Biomechanical properties of the cornea measured by the ocular response analyzer and their association with intraocular pressure and the central corneal curvature. Clin Exp Optom. 2009;92:469–475. doi: 10.1111/j.1444-0938.2009.00414.x. [DOI] [PubMed] [Google Scholar]
- 22.Touboul D, Roberts C, Kérautret J, Garra C, Maurice-Tison S, Saubusse E, et al. Correlations between corneal hysteresis, intraocular pressure, and corneal central pachymetry. J Cataract Refract Surg. 2008;34:616–622. doi: 10.1016/j.jcrs.2007.11.051. [DOI] [PubMed] [Google Scholar]
- 23.Luce DA. Determining in vivo biomechanical properties of the cornea with an ocular response analyzer. J Cataract Refract Surg. 2005;31:156–162. doi: 10.1016/j.jcrs.2004.10.044. [DOI] [PubMed] [Google Scholar]
- 24.Laiquzzaman M, Tambe K, Shah S. Comparison of biomechanical parameters in penetrating keratoplasty and normal eyes using the ocular response analyser. Clin Experiment Ophthalmol. 2010;38:758–763. doi: 10.1111/j.1442-9071.2010.02353.x. [DOI] [PubMed] [Google Scholar]
- 25.Edelhauser HF. The resiliency of the corneal endothelium to refractive and intraocular surgery. Cornea. 2000;19:263–273. doi: 10.1097/00003226-200005000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Lu F, Xu S, Qu J, Shen M, Wang X, Fang H, et al. Central corneal thickness and corneal hysteresis during corneal swelling induced by contact lens wear with eye closure. Am J Ophthalmol. 2007;143:616–622. doi: 10.1016/j.ajo.2006.12.031. [DOI] [PubMed] [Google Scholar]
- 27.Hamilton KE, Pye DC, Hali A, Lin C, Kam P, Ngyuen T. The effect of contact lens induced corneal edema on Goldmann applanation tonometry measurements. J Glaucoma. 2007;16:153–158. doi: 10.1097/01.ijg.0000212277.95971.be. [DOI] [PubMed] [Google Scholar]
- 28.Kamiya K, Shimizu K, Ohmoto F, Amano R. Time course of corneal biomechanical parameters after phacoemulsification with intraocular lens implantation. Cornea. 2010;29:1256–1260. doi: 10.1097/ICO.0b013e3181d9284b. [DOI] [PubMed] [Google Scholar]
- 29.Huang Y, Zhang M, Huang C, Chen B, Lam DS, Zhang S, et al. Determinants of postoperative corneal edema and impact on goldmann intraocular pressure. Cornea. 2011;30:962–967. doi: 10.1097/ICO.0b013e3182035884. [DOI] [PubMed] [Google Scholar]
- 30.Mazzotta C, Balestrazzi A, Traversi C, Baiocchi S, Caporossi T, Tommasi C, et al. Treatment of progressive keratoconus by riboflavin-UVA-induced cross-linking of corneal collagen: Ultrastructural analysis by Heidelberg retinal tomograph II in vivo confocal microscopy in humans. Cornea. 2007;26:390–397. doi: 10.1097/ICO.0b013e318030df5a. [DOI] [PubMed] [Google Scholar]
- 31.Møller-Pedersen T, Li HF, Petroll WM, Cavanagh HD, Jester JV. Confocal microscopic characterization of wound repair after photorefractive keratectomy. Invest Ophthalmol Vis Sci. 1998;39:487–501. [PubMed] [Google Scholar]
- 32.Piñero DP, Alio JL, El Kady B, Coskunseven E, Morbelli H, Uceda-Montanes A, et al. Refractive and aberrometric outcomes of intracorneal ring segments for keratoconus: Mechanical versus femtosecond-assisted procedures. Ophthalmology. 2009;116:1675–1687. doi: 10.1016/j.ophtha.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 33.Colin J, Cochener B, Savary G, Malet F, Holmes-Higgin D. INTACS inserts for treating keratoconus: One-year results. Ophthalmology. 2001;108:1409–1414. doi: 10.1016/s0161-6420(01)00646-7. [DOI] [PubMed] [Google Scholar]
- 34.Hellstedt T, Mäkelä J, Uusitalo R, Emre S, Uusitalo R. Treating keratoconus with intacs corneal ring segments. J Refract Surg. 2005;21:236–246. doi: 10.3928/1081-597X-20050501-06. [DOI] [PubMed] [Google Scholar]
- 35.Kanellopoulos AJ, Pe LH, Perry HD, Donnenfeld ED. Modified intracorneal ring segment implantations (INTACS) for the management of moderate to advanced keratoconus: Efficacy and complications. Cornea. 2006;25:29–33. doi: 10.1097/01.ico.0000167883.63266.60. [DOI] [PubMed] [Google Scholar]
- 36.Alió JL, Shabayek MH, Artola A. Intracorneal ring segments for keratoconus correction: Long-term follow-up. J Cataract Refract Surg. 2006;32:978–985. doi: 10.1016/j.jcrs.2006.02.044. [DOI] [PubMed] [Google Scholar]
- 37.Shetty R, Kurian M, Anand D, Mhaske P, Narayana KM, Shetty BK. Intacs in advanced keratoconus. Cornea. 2008;27:1022–1029. doi: 10.1097/ICO.0b013e318172fc54. [DOI] [PubMed] [Google Scholar]
- 38.Alfonso JF, Lisa C, Merayo-Lloves J, Fernández-Vega Cueto L, Montés-Micó R. Intrastromal corneal ring segment implantation in paracentral keratoconus with coincident topographic and coma axis. J Cataract Refract Surg. 2012;38:1576–1582. doi: 10.1016/j.jcrs.2012.05.031. [DOI] [PubMed] [Google Scholar]


