Abstract
Purpose:
To evaluate diurnal variations in intraocular pressure (IOP), central corneal thickness (CCT), and macular and retinal nerve fiber layer (RNFL) thickness in diabetic patients and normal individuals.
Methods:
This study included 11 diabetic patients with macular edema and 11 healthy individuals. IOP, CCT, and macular and RNFL thickness were measured every 3 hours on a single day between 9 AM and 6 PM. Diurnal variations in IOP, CCT, total macular volume (TMV), central macular thickness (CMT), average macular thickness (AMT), and RNFL thickness were measured.
Results:
None of the parameters showed a significant absolute or relative change over the course of the day. However, the following non-significant changes were observed. In the control group, all parameters demonstrated the highest values at 9 AM. The lowest IOP, TMV and AMT occurred at 12 PM; lowest CCT and RNFL at 6 PM; and the lowest CMT at 3 PM. Diabetic subjects had the highest values of RNFL, CMT and TMV at 9 AM, and that for IOP, CCT and AMT at 6 PM. The lowest RNFL and CMT values occurred at 6 PM; lowest IOP at 12 PM; and the lowest CCT, TMV and AMT were observed at 3 PM. In the diabetic group, TMV, CMT, AMT and CCT were significantly higher and RNFL was significantly lower than the control group at all time points (all P- values < 0.05).
Conclusion:
While there were slight decreases in IOP, RNFL thickness and CMT during the day, these changes were not significant between 9 AM and 6 PM and probably do not affect the interpretation of measurements.
Keywords: Diurnal Variation, Retinal and Nerve Fiber Layer Thickness, Central Corneal Thickness, Optical Coherence Tomography
INTRODUCTION
Diurnal variations occur in many ocular parameters amongst which diurnal fluctuation of intraocular pressure (IOP) has been well studied.[1,2,3] Introduction and evolution of optical coherence tomography (OCT) has made the evaluation of diurnal retinal thickness changes possible. OCT is now commonly used for reliable in vivo measurements of retinal thickness both in research and clinical practice. The development of spectral domain OCT (SD-OCT) with higher speed and resolution has led to highly precise and reproducible images as compared to the previous generation of time domain OCT (TD-OCT).[4]
Several studies have demonstrated diurnal fluctuation in retinal thickness among normal individuals as well as diabetic patients using older generations of OCT (OCT1) and TD-OCT.[5,6,7,8] They reported higher retinal thickness in the morning which corresponds to lower vision in the early morning particularly in patients with diabetic macular edema (DME). However, there are few studies evaluating diurnal variations in retinal thickness using SD-OCT in health and disease.[9,10,11,12] These studies have not shown significant diurnal retinal thickness changes using SD-OCT contradicting the results of TD-OCT.
Central corneal thickness (CCT) measurement is commonly performed as a part of glaucoma management for which ultrasonic pachymetry is currently the gold standard. Studies have shown diurnal variations in CCT with higher values in the early morning.[13,14]
Diurnal fluctuations in ocular parameters may affect interpretation of a single measurement. The present study was conducted to evaluate diurnal variations in ocular parameters including CCT, IOP, and macular and retinal nerve fiber layer (RNFL) thickness using SD-OCT, in normal and diabetic patients.
METHODS
This prospective one-day observational study was conducted at the Department of Ophthalmology, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran. The study was approved by the ethics committee at mentioned university and its protocol adhered to the tenets of the Declaration of Helsinki. Informed consent was obtained from all study participants.
The study included 11 diabetic patients with diabetic macular edema (DME) involving the foveal center and 11 normal individuals. Inclusion criteria included retinal thickening at the center of fovea evident on slit lamp biomicroscopy and central macular thickness (CMT) of ≥250 µm on OCT. Exclusion criteria included media opacity precluding good quality OCT images, proliferative diabetic retinopathy (PDR), vitreous hemorrhage, previous ocular trauma or surgery, amblyopia, spherical equivalent of refractive error > 3 diopters (D), glaucoma, ocular hypertension, congestive heart failure, blood pressure higher than 180/110 mm Hg, diabetic nephropathy and the use of systemic medications which can affect IOP.
On the day of examination, all patients underwent a series of measurements at 9 AM, 12 PM, 3 PM and 6 PM including blood pressure (BP), best corrected visual acuity (BCVA), IOP using a calibrated Golmann applanation tonometer (GAT BQ 900, Haag-Streit, Konitz, Switzerland), CCT using an ultrasonic pachymeter (Pachymeter SP 3000, Tomey, Nagoya, Japan), and macular and peripapillary RNFL thickness using Topcon 3D OCT-1000 (Topcon Corp, Tokyo, Japan). OCT scans were performed using a raster scan pattern (512 × 128 axial A-scans) in a 6 × 6 mm2 area centered on the fovea or optic disc. Each measurement was repeated twice and the mean of the two readings for IOP, CMT, total macular volume (TMV), average macular thickness (AMT), and average RNFL thickness were used for further analysis. BCVA and fasting blood sugar were measured only once in the morning. We did not measure BCVA during the day because dilating the pupil and multiple measurements could affect vision.
Statistical analyses were performed using SPSS software version 17.0 (SPSS Inc, Chicago, IL, USA). To describe data, we used mean ± standard deviation (SD) and 95% confidence intervals (CIs). To compare values, absolute changes in any parameter as well as relative changes were calculated. To evaluate the reproducibility of the measurements, intraclass correlations (ICCs) were calculated. Mann-Whitney U test was used for comparing quantitative variables between the two groups. P-values less than 0.05 were considered as statistically significant.
RESULTS
Baseline characteristics of the study groups which included 11 diabetic patients with DME and 11 healthy individuals are detailed in Table 1. The control group were younger than the diabetics (P = 0.035). Table 2 summarizes the study parameters at each time point with the highest and lowest values highlighted. Figures 1–5 show the diurnal variation in the study parameters. None of the parameters showed a significant diurnal fluctuation in either group. (ANOVA, all P- values > 0.05, Table 2).
Table 1.
Baseline characteristics of the study groups

Table 2.
Measurements at four different hours on a single day in diabetics and normal subjects
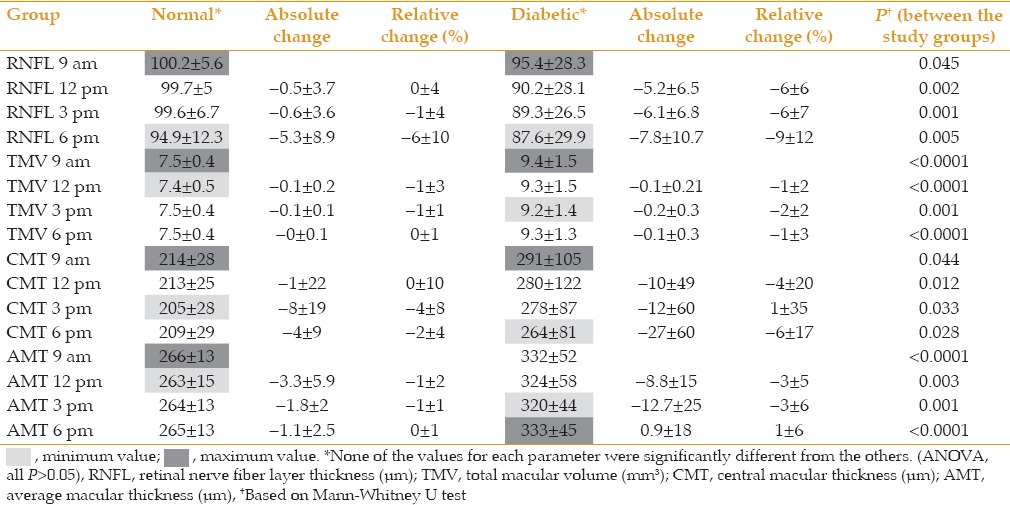
Figure 1.
Diurnal variations in central corneal thickness (CCT) in controls and diabetics were not significant. The diabetic group had consistently higher CCT.
Figure 5.
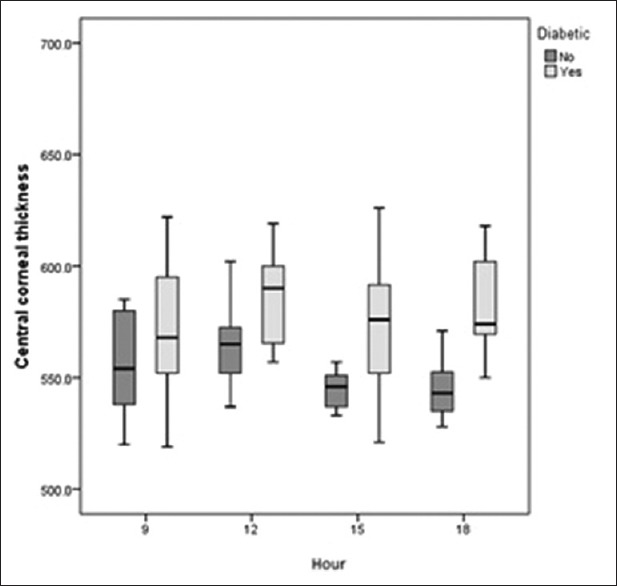
Diurnal variation in average macular thickness (AMT) in the control and diabetic groups. Neither group showed a significant diurnal variation; diabetics had significantly higher AMT.
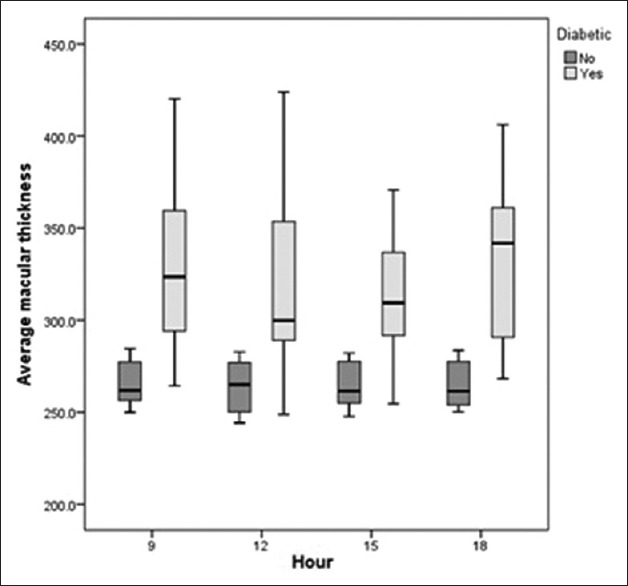
The range of IOP fluctuation was 2 mmHg in controls versus 1.6 mmHg in diabetic subjects (P = 0.374). In the control group, CCT was highest in the morning and decreased gradually during the course of the day to its lowest value at 6 PM. Diabetic patients had significantly higher CCT as compared to normal controls at all time points, and had the highest CCT at 6 PM and the lowest at 3 PM [Figure 1]. Diabetics had significantly lower RNFL thickness as compared to normal individuals. Both groups showed a similar diurnal pattern with the highest RNFL thickness in the morning and gradual decrease during the day [Table 2 and Figure 2]. In both groups, TMV and CMT were highest in the morning with non-significant decrease over the course of the day [Table 2, Figures 3 and 4]. AMT was highest in the morning in the control group and in the evening among diabetics [Table 2 and Figure 5]. ICCs were 0.98, 0.9, 0.83, and 0.76 for CMT, RNFL, AMT and TMV, respectively.
Figure 2.
Diurnal variations in retinal nerve fiber layer (RNFL) thickness in control and diabetic groups. Both groups showed a non-significant decrease during the course of the day. The control group had significantly higher RNFL thickness at all time points.
Figure 3.

Diurnal variations in total macular volume (TMV) in the control and diabetic groups. While diabetics showed significantly higher values, the diurnal changes were not significant in either group.
Figure 4.
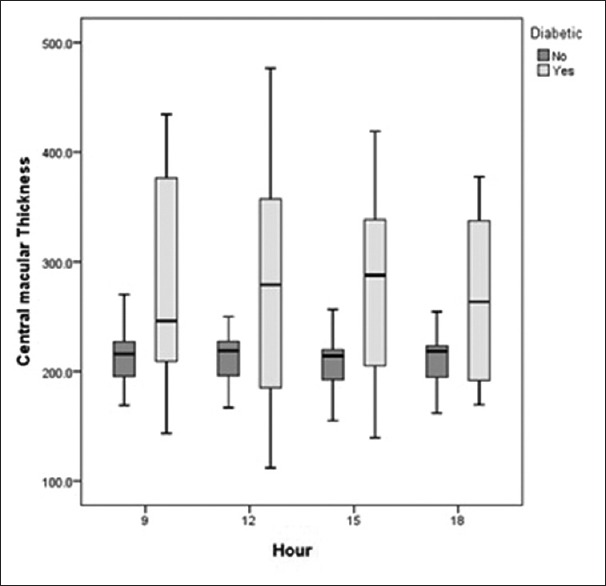
Diurnal variations in central macular thickness (CMT) in the control and diabetic groups were not significant in either group. Diabetics demonstrated significantly higher CMT; both groups showed the highest value at 9 AM.
DISCUSSION
The current study showed slight non-significant diurnal variations in retinal and corneal thickness both in diabetics and healthy individuals which seem to be clinically irrelevant.
The presence of diurnal IOP variations has been the subject of several studies in normal and glaucomatous eyes. Most studies are in agreement that IOP is higher in the early morning.[1,2] IOP fluctuation in normal eyes usually does not exceed 6 mmHg; however, fluctuations more than 10 mmHg are suggestive of glaucoma.[3] In the present study, normal eyes had the highest IOP at 9 AM and the lowest at 12 PM; diabetics had their corresponding values at 6 PM and 12 PM, respectively. The range of IOP fluctuation was 2 mmHg in controls and 1.6 mmHg in diabetics which was comparable. We had a small sample size and did not measure IOP immediately upon awakening. Thus, we might have missed higher IOPs. Nevertheless, the normal group showed a pattern of IOP fluctuations comparable to previous reports.
The cornea is believed to have its greatest thickness on awakening with a rapid decrease in thickness in a few hours. However, in a study on corneal thickness over a 48-hour period, it was shown that corneal thickness is highly variable during any 24-hour period and does not follow a regular pattern of overnight swelling and exponential decay.[13] Another study measuring CCT and IOP in healthy individuals over a 24-hour period revealed the highest values on awakening at 7 AM which dropped rapidly to baseline by 9 AM.[14] Our study showed a similar pattern in normal individuals. However, diabetics had the highest CCT at 6 PM and the lowest at 12 PM and also significantly higher CCTs as compared to the control group at all time points.
Normal physiological variations have been reported in retinal thickness along with most studies reporting greater macular thickness in the morning. Similarly, in patients with DME, a significant decrease has been reported in retinal thickness measurements by OCT over the course of the day.[5,6,7] Larsen et al evaluated overnight retinal thickness changes in patients with DME and normal individuals and observed no significant changes in normal individuals.[6] However, patients with DME showed significantly higher macular thickness and lower BCVA in the morning as compared to the evening. In these studies, OCT images were obtained using the first generation of OCT with lower resolution and reproducibility. In our study, however, we observed the lowest retinal thickness at 6 PM. In a study on DME and control eyes, Polito et al measured macular thickness by Stratus OCT at 4 time points on a single day.[7] No significant diurnal variation in retinal thickness was detected in control eyes and DME patients with CMT <300 μm. However, those with macular thickness ≥300 µm showed a significant decrease in thickness during the day. Moreover, it has been reported that foveal thickness decreases in both upright and recumbent positions.[12] In a report by Diabetic Retinopathy Clinical Research Network using Stratus OCT3 in 156 eyes with DME, a non-significant decrease in CMT was observed between 8 AM and 4 PM.[8]
Considering the continuous evolution of OCT technology from OCT1 to OCT3 and SD-OCT which has resulted in more reproducible and highly precise images, studies with older instruments should be interpreted cautiously. In one study comparing OCT1 and Stratus OCT3 in normal and DME eyes,[15] Stratus measurements were significantly higher than OCT1 readings, and artifacts were significantly more frequent with OCT1 and observed only in DME. Other studies using Stratus OCT3 showed a reproducibility of 10% in diabetic patients without macular edema[16] and 11% in subjects with DME.[17] It was concluded that changes more than 10-11% seem to be real and those less than these values are probably due to measurement errors.
Odell et al evaluated sources of error in macular thickness measurements by Cirrus SD-OCT and found that errant fixation has the greatest impact on the accuracy of OCT images specifically in macular diseases.[18] Fast scans and differences in axial length may also affect the accuracy and reproducibility of OCT images. Thus, it is possible that some of the significant changes reported in older studies were actually due to measurement errors especially in patients with DME and low vision. This may cause fixation errors resulting in measurements not to be performed exactly in previously measured area. SD-OCT has shown higher reproducibility for both macular and RNFL thicknesses.
It has also been suggested that hypoglycemia may play a role in the lack of diurnal CMT decrease in patients with DME.[19] In the present study, we were not able to evaluate such a correlation because only one single morning blood sugar measurement was obtained. Most studies investigating diurnal macular changes using SD-OCT have revealed non-significant changes in retinal thickness over the course of the day in normal eyes[9,10,11] and patients with DME. Furthermore, SD-OCT machines have shown more reproducible thickness measurements as compared to TD-OCT.[4,20,21] SD-OCT measurements have been reported to be significantly higher with more repeatability as compared to TD-OCT in DME patients[22] and healthy individuals.[23]
Although a few studies have evaluated diurnal variations of retinal thickness in patients with DME, we observed a nearly comparable pattern in normal and DME eyes. All macular parameters except for AMT in diabetics were highest at 9 AM with a subsequent decrease during the day. In our study, the highest reproducibility of 3D-OCT 1000 was for CMT measurements and the lowest was for TMV.
RNFL measurements have been reported to be less consistent as compared to macular thickness measurements.[20] Menke et al showed that reproducibility of RNFL measurements using Topcon 3D-OCT 1000 is 4% in normal individuals.[24] Pierro et al reported good reproducibility (ICC, 0.8) for RNFL thickness measurements by Topcon OCT 2000.[25]
In the current study, we observed a non-significant diurnal decrease in RNFL thickness in both groups with the highest RNFL thickness at 9 AM and the lowest at 6PM. The control group had significantly higher RNFL thickness at all time points as compared to the diabetic group. Reproducibility of RNFL measurements using Topcon 3D-OCT 1000 was high (ICC, 0.9) and comparable to other studies. However, there are some drawbacks to our study; the sample size was small, normal individuals were younger than diabetic subjects and measurements were not taken upon awakening.
In summary, the results of this study are in agreement with other studies performed using SD-OCT machines in which the amount of diurnal changes in macular and RNFL thicknesses is non-significant and negligible in clinical practice. Thus, OCT images can be interpreted irrespective of the time of acquisition between 9 AM and 6 PM. Previous reports on significant diurnal changes in these parameters using older generations of OCT are likely the result of lower reproducibility and not real tissue changes.
Financial Support and Sponsorship
Nil.
Conflicts of Interest
There are no conflicts of interest.
REFERENCES
- 1.David R, Zangwill L, Briscoe D, Dagan M, Yagev R, Yassur Y. Diurnal intraocular pressure variations: An analysis of 690 diurnal curves. Br J Ophthalmol. 1992;76:280–283. doi: 10.1136/bjo.76.5.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saccà SC, Rolando M, Marletta A, Macrí A, Cerqueti P, Ciurlo G. Fluctuations of intraocular pressure during the day in open-angle glaucoma, normal-tension glaucoma and normal subjects. Ophthalmologica. 1998;212:115–119. doi: 10.1159/000027290. [DOI] [PubMed] [Google Scholar]
- 3.Cioffi GA, Durcan FJ, Girkin CA, editors. Basic and Clinical Science Course: Glaucoma. San Francisco: American Academy of Ophthalmology; 2012-2013. p. 26. [Google Scholar]
- 4.Wolf-Schnurrbusch UE, Ceklic L, Brinkmann CK, Iliev ME, Frey M, Rothenbuehler SP, et al. Macular thickness measurements in healthy eyes using six different optical coherence tomography instruments. Invest Ophthalmol Vis Sci. 2009;50:3432–3437. doi: 10.1167/iovs.08-2970. [DOI] [PubMed] [Google Scholar]
- 5.Frank RN, Schulz L, Abe K, Iezzi R. Temporal variation in diabetic macular edema measured by optical coherence tomography. Ophthalmology. 2004;111:211–217. doi: 10.1016/j.ophtha.2003.05.031. [DOI] [PubMed] [Google Scholar]
- 6.Larsen M, Wang M, Sander B. Overnight thickness variation in diabetic macular edema. Invest Ophthalmol Vis Sci. 2005;46:2313–2316. doi: 10.1167/iovs.04-0893. [DOI] [PubMed] [Google Scholar]
- 7.Polito A, Del Borrello M, Polini G, Furlan F, Isola M, Bandello F. Diurnal variation in clinically significant diabetic macular edema measured by the Stratus OCT. Retina. 2006;26:14–20. doi: 10.1097/00006982-200601000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Diabetic Retinopathy Clinical Research Network. Danis RP, Glassman AR, Aiello LP, Antoszyk AN, Beck RW, et al. Diurnal variation in retinal thickening measurement by optical coherence tomography in center-involved diabetic macular edema. Arch Ophthalmol. 2006;124:1701–1707. doi: 10.1001/archopht.124.12.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Read SA, Collins MJ, Alonso-Caneiro D. Diurnal variation of retinal thickness with spectral domain OCT. Optom Vis Sci. 2012;89:611–619. doi: 10.1097/OPX.0b013e3182501917. [DOI] [PubMed] [Google Scholar]
- 10.Jo YJ, Heo DW, Shin YI, Kim JY. Diurnal variation of retina thickness measured with time domain and spectral domain optical coherence tomography in healthy subjects. Invest Ophthalmol Vis Sci. 2011;52:6497–6500. doi: 10.1167/iovs.11-7403. [DOI] [PubMed] [Google Scholar]
- 11.Tan CS, Ouyang Y, Ruiz H, Sadda SR. Diurnal variation of choroidal thickness in normal, healthy subjects measured by spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2012;53:261–266. doi: 10.1167/iovs.11-8782. [DOI] [PubMed] [Google Scholar]
- 12.Polito A, Polini G, Chiodini RG, Isola M, Soldano F, Bandello F. Effect of posture on the diurnal variation in clinically significant diabetic macular edema. Invest Ophthalmol Vis Sci. 2007;48:3318–3323. doi: 10.1167/iovs.06-1526. [DOI] [PubMed] [Google Scholar]
- 13.Harper CL, Boulton ME, Bennett D, Marcyniuk B, Jarvis-Evans JH, Tullo AB, et al. Diurnal variations in human corneal thickness. Br J Ophthalmol. 1996;80:1068–1072. doi: 10.1136/bjo.80.12.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamilton KE, Pye DC, Aggarwala S, Evian S, Khosla J, Perera R. Diurnal variation of central corneal thickness and Goldmann applanation tonometry estimates of intraocular pressure. J Glaucoma. 2007;16:29–35. doi: 10.1097/IJG.0b013e31802b350f. [DOI] [PubMed] [Google Scholar]
- 15.Pierre-Kahn V, Tadayoni R, Haouchine B, Massin P, Gaudric A. Comparison of optical coherence tomography models OCT1 and Stratus OCT for macular retinal thickness measurement. Br J Ophthalmol. 2005;89:1581–1585. doi: 10.1136/bjo.2005.069815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Browning DJ, Fraser CM, Propst BW. The variation in optical coherence tomography-measured macular thickness in diabetic eyes without clinical macular edema. Am J Ophthalmol. 2008;145:889–893. doi: 10.1016/j.ajo.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 17.Diabetic Retinopathy Clinical Research Network. Krzystolik MG, Strauber SF, Aiello LP, Beck RW, Berger BB, et al. Reproducibility of macular thickness and volume using Zeiss optical coherence tomography in patients with diabetic macular edema. Ophthalmology. 2007;114:1520–1525. doi: 10.1016/j.ophtha.2006.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Odell D, Dubis AM, Lever JF, Stepien KE, Carroll J. Assessing errors inherent in OCT-derived macular thickness maps. J Ophthalmol. 2011;2011:692574. doi: 10.1155/2011/692574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feldman-Billard S, Dupas B, Sedira N, Bitu J, Erginay A, Guillausseau PJ, et al. Hypoglycaemia is associated with the absence of a decrease in diurnal macular thickness in patients with diabetic macular oedema. Diabetes Metab. 2013;39:169–173. doi: 10.1016/j.diabet.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Huang J, Liu X, Wu Z, Guo X, Xu H, Dustin L, et al. Macular and retinal nerve fiber layer thickness measurements in normal eyes with the stratus OCT, the cirrus HD-OCT, and the topcon 3D OCT-1000. J Glaucoma. 2011;20:118–125. doi: 10.1097/IJG.0b013e3181d786f8. [DOI] [PubMed] [Google Scholar]
- 21.Pierro L, Giatsidis SM, Mantovani E, Gagliardi M. Macular thickness interoperator and intraoperator reproducibility in healthy eyes using 7 optical coherence tomography instruments. Am J Ophthalmol. 2010;150:199–204.e1. doi: 10.1016/j.ajo.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 22.Forooghian F, Cukras C, Meyerle CB, Chew EY, Wong WT. Evaluation of time domain and spectral domain optical coherence tomography in the measurement of diabetic macular edema. Invest Ophthalmol Vis Sci. 2008;49:4290–4296. doi: 10.1167/iovs.08-2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leung CK, Cheung CY, Weinreb RN, Lee G, Lin D, Pang CP, et al. Comparison of macular thickness measurements between time domain and spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2008;49:4893–4897. doi: 10.1167/iovs.07-1326. [DOI] [PubMed] [Google Scholar]
- 24.Menke MN, Knecht P, Sturm V, Dabov S, Funk J. Reproducibility of nerve fiber layer thickness measurements using 3D fourier-domain OCT. Invest Ophthalmol Vis Sci. 2008;49:5386–5391. doi: 10.1167/iovs.07-1435. [DOI] [PubMed] [Google Scholar]
- 25.Pierro L, Gagliardi M, Iuliano L, Ambrosi A, Bandello F. Retinal nerve fiber layer thickness reproducibility using seven different OCT instruments. Invest Ophthalmol Vis Sci. 2012;53:5912–5920. doi: 10.1167/iovs.11-8644. [DOI] [PubMed] [Google Scholar]


