Abstract
Purpose:
To determine the clinical features and risk factors of presumed ocular toxoplasmosis (POT) in patients affected with the condition at Irrua, Nigeria.
Methods:
The study included 69 patients with POT, and 69 age and sex matched subjects who served as the control group. Data was obtained using interviewer administered questionnaires. Examination included measurement of visual acuity (VA), intraocular pressure (IOP), slit lamp examination, gonioscopy and dilated fundus examination.
Results:
Mean age of cases and control subjects was 57.16 ± 18.69 and 56.09 ± 16.01 years respectively. The peak age group in patients with POT was 60 years and above. The most common presenting complaint was blurred vision occurring in 100% of cases. Drinking unfiltered water in 58 (84.1%) patients was the most common risk factor. Other risk factors included post cataract surgery status in 32 (46.4%) subjects, ingestion of poorly cooked meat in 30 (43.5%) cases and exposure to cats in 9 (13.0%) patients. All risk factors were more common in POT patients (P < 0.05). Out of 69 patients, 62 (89.9%) had unilateral while 7 (10.1%) had bilateral involvement. Out of 76 eyes with uveitis, 53 (69.7%) were blind. Active disease was significantly more common with increasing age (P < 0.05).
Conclusion:
Patients with POT were rather old and some risk factors were modifiable, therefore health education for preventing the transmission of toxoplasmosis and provision of sanitary water may help reduce the incidence of ocular toxoplasmosis.
Keywords: Clinical Features, Presumed Ocular Toxoplasmosis, Uveitis
INTRODUCTION
Ocular toxoplasmosis is a major cause of infectious uveitis.[1] Worldwide, it is the principal cause of posterior uveitis, a severe and life altering disease, and the most common infestation of the retina in the United States.[2,3] It is the predominant cause of posterior uveitis in immunocompetent individuals, and second only to cytomegalovirus infection in patients with human immunodeficiency virus (HIV) infection.[4,5,6]
The hallmark of ocular toxoplasmosis is the distinctive clinical picture. Diagnosis of the condition in Nigeria is usually clinical because it has over 90% sensitivity when compared to serology.[7] The condition is responsible for up to 10% of cases of visual loss and thus threatens employment and financial well-being.[8]
METHODS
This study included a total of 138 participants, including 69 patients with presumed ocular toxoplasmosis (POT) and 69 controls. The study was approved by the Ethics Committee of Irrua Specialist Teaching Hospital, Irrua, Edo State, Nigeria and the procedures were conducted according to the tenets of the Declaration of Helsinki. Written informed consent was obtained from all study participants.
Patients who were diagnosed with POT and received treatment over a period of three months, and age and sex-matched normal controls who attended the clinic within the study period were recruited. The clinical questionnaires designed for the study was administered to all study subjects.
A complete ophthalmologic examination was performed for all study subjects which included determination of best-corrected visual acuity (BCVA), slit lamp biomicroscopy, Goldmann applanation tonometry, gonioscopy and indirect ophthalmoscopy. The pupils were checked for shape, position, size and reaction to light. Slit lamp examination of both eyes was done to check for keratic precipitates, cells and flare in the anterior chamber, and vitreous cells, strands and floaters. Gonioscopy was performed to identify peripheral anterior synechiae (PAS), Berlin's nodules and other angle anomalies.
Fundus examination was performed using a direct ophthalmoscope followed by indirect ophthalmoscopy after dilating the pupil to identify peripheral lesions. A +90 D lens at the slit lamp was also used to visualize the fundus. Fundus photograph was taken in all cases where a chorioretinal lesion was visible. Clinical data included the location of inflammation (posterior uveitis or panuveitis), laterality (unilateral or bilateral involvement), uveitis activity (active, inactive or in remission) and uveitis severity as mild, moderate or severe using the criteria modified from Onal et al.[9]
The diagnosis of POT was established by the presence of a solitary inflammatory focus close to an old pigmented scar (satellite lesion), severe vitritis which may greatly impair visualization of the fundus although the inflammatory focus may still be discernable (headlight in the fog), retinal vasculitis, retinitis, cystoid macular edema and papillitis. The severity of the disease was categorized using the criteria modified from Onal et al[9] and was defined as mild if there was unilateral or bilateral involvement and VA of 6/18 or better in the involved eye. The condition was defined as moderate if there was unilateral posterior or panuveitis with unilateral loss of vision (<6/18), and severe if there was bilateral posterior or panuveitis, blindness or complications such as optic atrophy, macular scar or phthisis bulbi.
The disease was defined as active if there was a solitary inflammatory focus near an old pigmented scar (satellite lesion), or solitary/multiple inflammatory foci. It was described as inactive when there was no inflammatory focus. On the basis of the recommendations of the Standardization of Uveitis Nomenclature (SUN) working group, remission was defined as inactive disease for at least 3 months after discontinuation of treatment.[10]
Statistical Package for Social Sciences (SPSS) software version 16.0 was (SPSS Inc., Chicago, IL, USA) used for statistical analysis. P values less than 0.05 were regarded as statistically significant.
RESULTS
Out of 1,521 patients who presented to the hospital during the study period, 69 (100%) subjects who were diagnosed with POT and met the eligibility criteria were included. All eligible patients accepted to participate in the study (100% response).
There was an increase in the prevalence of POT with older age; the peak age group for POT was in the age-group of 60 years and above [Table 1]. There was no significant difference in age and gender between cases and control subjects (P > 0.05). Mean age of cases and controls was 57.16 ± 18.69 (range, 18-95) years and 56.09 ± 16.01 (range, 19-84) years, respectively. More female patients were present in both cases and controls; male to female ratio was 0.81 in cases and 0.86 in controls.
Table 1.
Age and gender distribution of 69 cases with presumed ocular toxoplasmosis and 69 controls
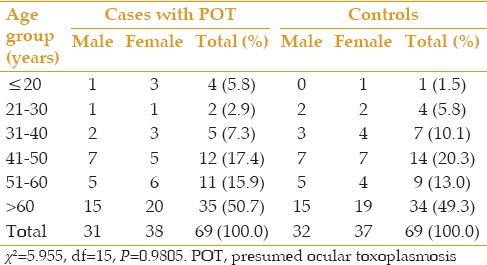
The majority of patients with POT and control subjects were married, and there was no statistically significant difference between the two groups (P > 0.05). In a similar fashion, educational status was comparable in patients with POT and control subjects (P > 0.05). Among patients with POT and formal education, 20 (29.0%) cases had primary education, 17 (24.6%) had secondary education and 11 (16.0%) had tertiary level education. Subjects in the control group with formal education included 19 (27.5%) subjects with primary education, 16 (23.2%) with secondary education and 16 (23.2%) with tertiary level education.
The study subjects were mainly peasant farmers 30 (43.5%) as well as petty traders and few civil servants most of whom were retired. There was also no significant difference in terms of occupation between patients with POT and control subjects (P > 0.05). The study subjects were mainly peasant farmers 30 (43.5%) as well as petty traders and few civil servants most of whom were retired. There was also no significant difference in terms of occupation between patients with POT and control subjects (P > 0.05). Farmers with POT were 30 (43.5%), farmers without POT were 27 (39.1%). Non farmers with POT were 39 (56.5%), non-farmers without POT were 42 (60.9%) (Fisher's Exact Test; P = 0.7297, Odd's ratio = 1.197, 95% CI = 0.6071-2.359). Those who were non-farmers among patients with POT included 11 (15.9%) petty traders, 10 (14.5%) artisans, 6 (8.7%) pensioners, 4 (5.8%) professionals, 4 (5.8%) students, and 4 (5.8%) civil servants. Those who were non farmers among controls included 20 (29.0%) petty traders, 4 (5.8%) artisans, 1 (1.5%) pensioners, 3 (4.3%) professionals, 6 (8.7%) students, and 8 (11.6%) civil servants.
The most common presenting complaint was blurred vision in 100% of cases. Less common complaints included photophobia in 53 (76.8%), deep ocular pain in 47 (68.1%), floaters in 41 (50.7%), redness in 35 (59.4%), itching in 10 (14.5%), tearing in 8 (11.6%), and dryness of the eyes in 5 (7.2%) cases. The mean duration of symptoms was 13.90 ± 20.4 (range, 1-72) months.
Exposure to cats (OR = 10.200; P < 0.05), consumption of poorly cooked meat (OR = 25.769; P < 0.0001) and drinking unfiltered water (OR = 67.491; P < 0.0001) were all significantly more common in cases as compared to controls [Table 2]. The most common presentation was active unilateral posterior uveitis [Table 3].
Table 2.
Risk factors for presumed ocular toxoplasmosis in 69 patients versus 69 controls
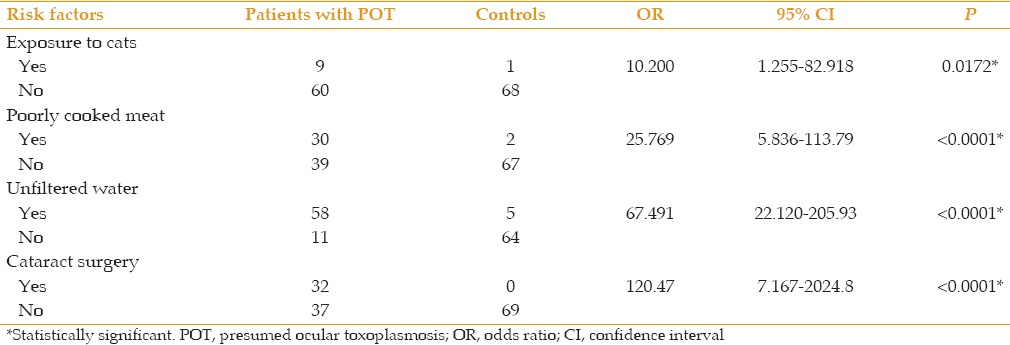
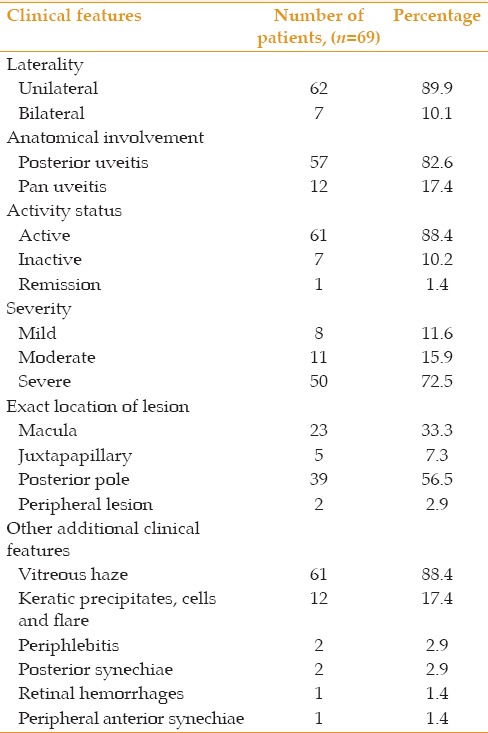
Table 3.
Clinical features of 69 patients with presumed ocular toxoplasmosis
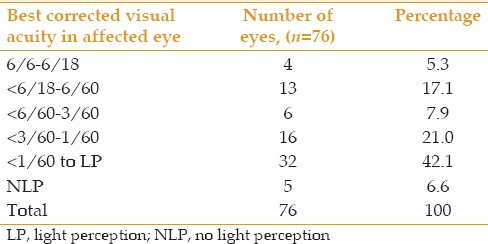
No significant difference was observed between the study groups in terms of age distribution, posterior uveitis or panuveitis (P > 0.05), and unilateral or bilateral disease (P > 0.05) [Table 4]. The number of patients with both types of POT increased with older age. There was also a significant difference regarding age in patients with active versus inactive disease [P > 0.05, Table 4]; active disease [Figure 1] was more common (34 out of 61, 55.7%) in patients over the age of 60 years while inactive disease was more common (5 out of 8, 62.5%) in patients younger than 40 years [Figure 2]. There was also a significant difference between age-groups regarding disease severity [P< 0.05, Table 4]. Severe disease was more common (30 out of 50 cases, 60.0%) among patients over the age of 60 years (P = 0.048). Out of 69 patients with POT, 62 had unilateral disease while 7 were bilateral cases, summing to a total of 76 eyes. Most affected eyes were visually impaired [Table 5] and 53 eyes (69.7%) were blind by WHO criteria of VA < 3/60.
Table 4.
Clinical types of presumed ocular toxoplasmosis in 69 patients in age groups
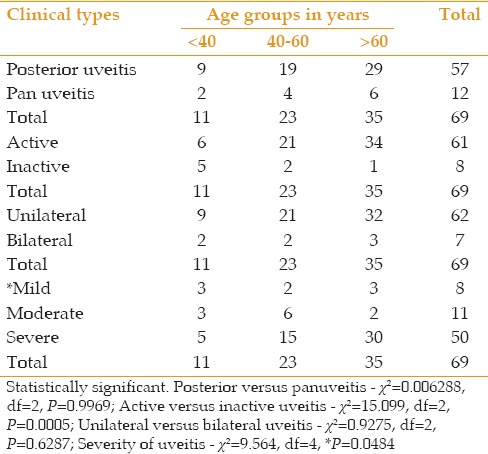
Figure 1.
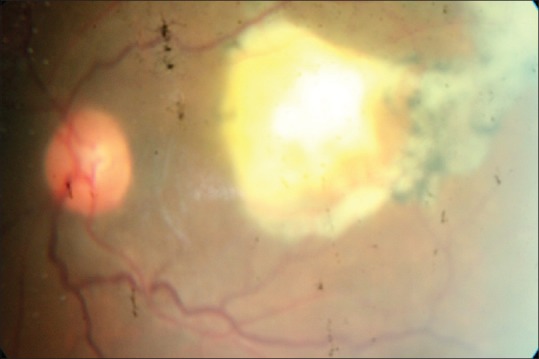
An active retinochoroidal lesion adjacent to a hyperpigmented scar with overlying vitritis causing the characteristic “headlight in the fog” appearance.
Figure 2.
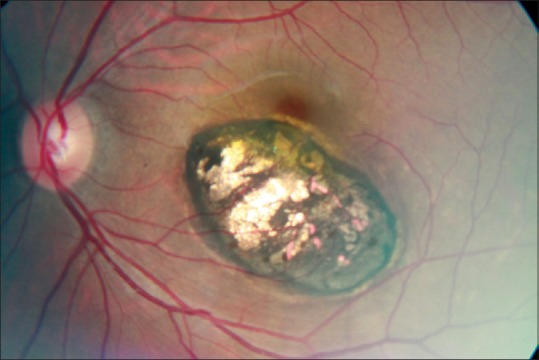
A punched out retinochoroidal scar in the macula.
Table 5.
Best corrected visual acuity in 76 affected eyes of 69 patients with presumed ocular toxoplasmosis
Recurrence was observed in 56 (73.7%) eyes of which 32 (57.1%) occurred following cataract surgery. These 32 eyes constituted 52.3% of the 61 eyes with active lesions. Of the 61 eyes with active lesions, the majority 49 (80.3%) had grade 2 + inflammation. IOP was significantly higher in eyes with POT than controls (P< 0.05). The mean IOP for patients with POT was 13.87 ± 4.649 with a range of 7-37 mmHg, while the mean IOP for control was 12.54 ± 2.055 with a range of 10-19 mmHg.
The most common ocular complication in our series was cataract in 12 eyes (15.8%) of the participants. Other complications included secondary glaucoma in 2 eyes (2.6%), cystoid macular edema (CME) in 2 eyes (2.6%), optic atrophy in 1 eye (1.3%), and tractional retinal detachment in 1 eye (1.3%).
DISCUSSION
The mean age of subjects with POT was 57.16 ± 18.69 and the peak age group was 60 years and above. Blurred vision was the most common complaint occuring in all cases. The risk factors were drinking unfiltered water in 58 (84.1%) patients, post cataract surgery in 32 (46.4%) subjects, ingestion of poorly cooked meat in 30 (43.5%) cases and exposure to cats in 9 (13.0%) patients. Of 69 patients, 62 (89.9%) had unilateral while 7 (10.1%) had bilateral involvement. Of 76 eyes with uveitis, 53 (69.7%) were blind. Active disease was significantly more common with increasing age (P< 0.05).
Mean age in the present study was different from the study by Abraham et al[11] which was also conducted in Nigeria and reported a mean age of 41.47 years. This may be due to differences in demographics between our study (which was performed in a suburban and rural population) versus the above mentioned report from Uyo (which is an urban area and a state capital). Furthermore, a large number of elderly post cataract surgical cases from a free eye camp were included in the current study. The peak age for POT in this study was ≥60 years which comprised 50.7% of patients which is in agreement with the finding that the prevalence of ocular toxoplasmosis increases with age.[12] Our peak age is higher than that reported by Ayanru[13] who reported a peak incidence in the second and third decades and Nkwogu et al[14] who found a peak age of 20-29 years. The reason for the observed difference between the peak age in this study versus others is that our study was conducted in a suburban to rural setting where a significant proportion of the population was elderly due to urban migration of the youth and return to the rural environment by the elderly. This may result in differences in population demographics as compared to the other studies in Nigeria, which were done in urban settings. Furthermore, a large number of elderly post cataract surgical cases were included in our study.
There was a female preponderance of 38 (55.1%) in this study. This is in agreement with the study by Abraham et al in Nigeria[11] and by de-la-Torre et al[15] in Columbia.
There was no significant difference in terms of marital status, education and occupation in patients with POT versus controls. The current study was performed in a suburban to rural setting which may account for the lower level of education in the majority of patients; there were 21 (30.4%) cases with no formal education. This may be tied to poverty and the poor socioeconomic status as well. We observed ocular toxoplasmosis to be common among farmers. Open disposal of feces and farmers' habit of using manure without pre-processing may be causes of the high incidence of the condition among farmers and breeders.
The most common presenting complaint was blurred vision which was present in all 69 (100%) patients involving all 76 affected eyes. The majority of cases, 61 (88.4%), were active while only 7 (10.2%) were inactive and only 1 (1.4%) was in remission. All inactive cases had either a macular or perimacular lesion accounting for reduced vision. Other complaints included photophobia, ocular pain, floaters and redness were comparable to presenting complaints seen in Kaduna, Nigeria, where reduced vision was the most common presenting complaint followed by photophobia, pain, irritation, redness and floaters.[14] Abraham et al[11] also reported diminution of vision as the chief complaint in the majority of their patients. Poor vision in POT may be due to intense vitritis; macular, papillomacular or optic nerve involvement; inflammation of the anterior segment, or complications of uveitis. Mean duration of symptoms in the current series was 13.90 ± 20.4 months which is rather long. The reason is that the study group included not only active cases, but also inactive and recurrent cases, and the duration of symptoms was considered from the onset of the initial episode of visual loss following posterior or pan-uveitis.
Drinking unfiltered water was the most common risk factor for disease transmission. This is probably because in Edo Central Senatorial zone, which includes most of the communities making up the catchment area of our study, pipe-borne water is not readily available. The major source of water is surface water such as shallow wells and streams, as well as rainfall, which is generally stored in a well which may be contaminated by cats which are pets in this area. Poorly cooked meat was the second most common mode of transmission, eaten either as improperly cooked bush-meat or “suya” (grilled pepper stick meat). Exposure to cats alone was the least common 273 risk factor identified. Cataract surgery was found to be a common risk factor (P< 0.0001) for reactivation of uveitis in this study. Cataract surgery was significantly more common among cases than controls. Ocular surgery is recognized as a trigger for POT and reactivation of toxoplasmosis after cataract surgery has been reported in a significant number of patients.[1,16] Bosch-Driessen et al[16] reported that reactivation of ocular toxoplasmosis following cataract extraction occurred in 5/14 patients (5/15 279 eyes), which was significantly higher than the incidence of recurrence in age-and sex-matched controls.
The impact of ocular toxoplasmosis on vision was marked; 53 eyes (69.7%) presented with VA less than 3/60 which is equivalent to blindness according to WHO criteria. There were 59 eyes (77.6%) with legal blindness which will definitely affect the quality of life in these patients. Our findings in terms of severe visual loss are in agreement with the study by Abraham et al[11] in Uyo in which presenting VA was 6/36 to hand movement (HM). Nkwogu et al[14] in Northern Nigeria reported that 22% of their patients had less than 6/60 vision, 32% had visual acuity between 6/24 and 6/60 while 46% had VA of 6/18 or better. The other study from Columbia reported that 11 patients (37.9%) were legally blind in at least one affected eye.[15]291 Recurrence in this study was seen in 56 (81.2%) patients including 32 eyes (57.1%) with reactivation following cataract surgery. This high number of cases of presumed reactivation may be related to the high number of cataract surgeries which were performed in eye camps and the hospital before and during the period of study. Nkwogu et al[14] also reported a history of recurrence in 42 patients in their study.
The majority of our cases had unilateral disease, accounting for 62 subjects (89.9%) while bilateral cases accounted for 7 cases (10.1%) which is comparable to other studies.[4,14,15] In our series, posterior uveitis was the most common presentation and the posterior pole was the most common area of involvement for ocular toxoplasmosis. Ayanru[13] also reported posterior choroidoretinal lesions in 40.9% and panuveitis in 15.1% of 1,987 patients. This is also consistent with the study by Nkwogu et al[14] which noted a high rate (46%) of posterior pole involvement, as well as Abraham et al[11] who reported such involvement in 48.4%. These reports support the fact that the organism has a clear preference for the posterior pole.[4] We observed vitreous haze in all active cases (88.4%), and keratic precipitates, cells and flare in all cases of pan-uveitis. The vitreous inflammation in the overwhelming majority of patients (95.0%) was grade 2 or less allowing adequate fundus visualization.
Cataract was the most common complication in our study in 15.8%. Arun et al[17] also reported cataract as a common complication in 11.6%. Other complications in the present study included secondary glaucoma, optic atrophy, cystoid macular edema and tractional retinal detachment. Secondary glaucoma occurs as a result of anterior segment involvement. In severe chronic cases, peripheral anterior synechiae and secondary glaucoma may occur.[18] Optic nerve involvement due to primary optic nerve involvement by toxoplasmosis or a juxtapapillary retinitis (Jensen choroiditis) may result in optic atrophy.[19] Tractional retinal detachment may be secondary to the organization of severe vitreous opacification.[19]
In summary, considering the high prevalence of preventable risk factors for POT in the current study, adequate structural facilities such as sanitary water supply and proper methods of waste disposal especially of cat waste, should be implemented to promote hygiene. Moreover, there is a necessity for health education especially in antenatal clinics and to the general public on proper handling and boiling of meat and meat products.
Financial Support and Sponsorship
Nil.
Conflicts of Interest
There are no conflicts of interest.
REFERENCES
- 1.Wakefield D, Cunningham ET, Jr, Pavesio C, Garweg JG, Zierhut M. Controversies in ocular toxoplasmosis. Ocul Immunol Inflamm. 2011;19:2–9. doi: 10.3109/09273948.2011.547157. [DOI] [PubMed] [Google Scholar]
- 2.Shobab L, Pleyer U, Johnsen J, Metzner S, James ER, Torun N, et al. Toxoplasma serotype is associated with development of ocular toxoplasmosis. J Infect Dis. 2013;208:1520–1528. doi: 10.1093/infdis/jit313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones JL, Holland GN. Annual burden of ocular toxoplasmosis in the US. Am J Trop Med Hyg. 2010;82:464–465. doi: 10.4269/ajtmh.2010.09-0664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonfioli AA, Orefice F. Toxoplasmosis. Semin Ophthalmol. 2005;20:129–141. doi: 10.1080/08820530500231961. [DOI] [PubMed] [Google Scholar]
- 5.London NJ, Shukla D, Heiden D, Rathinam SR, Arevalo JF, Cunningham ET., Jr HIV/AIDS in the developing world. Int Ophthalmol Clin. 2010;50:201–218. doi: 10.1097/IIO.0b013e3181d26fcf. [DOI] [PubMed] [Google Scholar]
- 6.Kestelyn PG, Cunningham ET., Jr HIV/AIDS and blindness. Bull World Health Organ. 2001;79:208–213. [PMC free article] [PubMed] [Google Scholar]
- 7.Uwakwem CA. Ocular Toxoplasmosis - The Nigerian Experience. National Eye Institute. Workshop on Ocular Toxoplasmosis. Lawton Chiles International House, Bethesda, Maryland. 2001. Apr, [Last accessed on 2013 Apr 20]. Available from: http://www.rarediseases.info.nih.gov/asp/html/conferences/conferences/ocular_toxo353_0401.html .
- 8.Suttorp-Schulten MS, Rothova A. The possible impact of uveitis in blindness: A literature survey. Br J Ophthalmol. 1996;80:844–848. doi: 10.1136/bjo.80.9.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Onal S, Savar F, Akman M, Kazokoglu H. Vision- and health-related quality of life in patients with Behçet uveitis. Arch Ophthalmol. 2010;128:1265–1271. doi: 10.1001/archophthalmol.2010.209. [DOI] [PubMed] [Google Scholar]
- 10.Jabs DA, Nussenblatt RB, Rosenbaum JT. Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005;140:509–516. doi: 10.1016/j.ajo.2005.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abraham EG, Umanah IN, Uwah AI, Edet E. Presumed ocular toxoplasmosis in University of Uyo Teaching Hospital, Uyo, Nigeria. J Dent Med Sci. 2013;5:24–26. [Google Scholar]
- 12.Holland GN. Ocular toxoplasmosis: The influence of patient age. Mem Inst Oswaldo Cruz. 2009;104:351–357. doi: 10.1590/s0074-02762009000200031. [DOI] [PubMed] [Google Scholar]
- 13.Ayanru JO. The problem of uveitis in Bendel State of Nigeria: Experience in Benin City. Br J Ophthalmol. 1977;61:655–659. doi: 10.1136/bjo.61.10.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nkwogu FU, Joss A, Dutton GN. Presumed toxoplasmosis retinochoroiditis as seen in Kaduna, Nigeria. Niger J Ophthalmol. 1994;2:25–34. [Google Scholar]
- 15.de-la-Torre A, González-López G, Montoya-Gutiérrez JM, Marín-Arango V, Gómez-Marín JE. Quality of life assessment in ocular toxoplasmosis in a Colombian population. Ocul Immunol Inflamm. 2011;19:262–266. doi: 10.3109/09273948.2011.582220. [DOI] [PubMed] [Google Scholar]
- 16.Bosch-Driessen LH, Plaisier MB, Stilma JS, Van der Lelij A, Rothova A. Reactivations of ocular toxoplasmosis after cataract extraction. Ophthalmology. 2002;109:41–45. doi: 10.1016/s0161-6420(01)00845-4. [DOI] [PubMed] [Google Scholar]
- 17.Arun V, Noble AG, Latkany P, Troia RN, Jalbrzikowski J, Kasza K, et al. Cataracts in congenital toxoplasmosis. J AAPOS. 2007;11:551–554. doi: 10.1016/j.jaapos.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tabbara KF. Ocular toxoplasmosis: Toxoplasmic retinochroiditis. Int Ophthalmol Clin. 1995;35:15–29. doi: 10.1097/00004397-199503520-00004. [DOI] [PubMed] [Google Scholar]
- 19.Kanski JJ. Clinical Ophthalmology: A Systemic Approach. 6th ed. Oxford: Elsevier Butterworth-Heinemann; 2007. Uveitis; pp. 468–472. [Google Scholar]


