Abstract
Purpose:
To characterize histopathologic and electroretinographic (ERG) changes in the retina of pigmented rats injected with sodium iodate in order to establish a model of retinal degeneration for future cell therapy studies.
Methods:
In 50 male pigmented rats weighing 250-300 grams, NaIO3 was injected into the left orbital venous plexus at 40 and 60 mg/kg doses (25 eyes in each group). Fourteen rats received phosphate buffered saline (PBS) injection in their left orbital plexus and were considered as the sham-control group. Histopathologic and ERG studies were performed at baseline and on days 1, 7, 14 and 28 after the injections.
Results:
Progressive retinal pigment epithelial (RPE) changes were observed from the first day of injection in both the 40 and 60 mg/kg study groups in a dose dependent manner. These changes manifested as loss of melanin pigment and accumulation of lipofuscin in RPE cells with subsequent cell death and patchy loss of RPE cells (in flat mounts), as well as thinning of the outer nuclear layer and later the inner nuclear layer in the succeeding days. ERG showed a progressive and significant decrease in a- and b- wave amplitudes in both case groups relative to baseline values and the controls (P < 0.05).
Conclusion:
NaIO3 injection into the retrobulbar venous plexus of pigmented rats can result in significant and progressive damage to the RPE and subsequently to the neuroretina of the injected eye, and may serve as a model of retinal degeneration.
Keywords: Pigmented Rat, Retinal Pigment Epithelium, Retro-orbital Sinus, Sodium Iodate
INTRODUCTION
The retinal pigment epithelium (RPE) performs highly specialized functions that are essential for retinal homeostasis, maintenance of the visual cycle, clearance of disc shedding, directional transport and optimization of ion concentrations in the surrounding tissues, plus other functions.[1,2] These cells also constitute the outer portion of the blood–retina barrier.[3] During aging, the RPE undergoes a number of well characterized structural changes including loss of melanin granules, accumulation of lipofuscin and drusen formation.[4,5] One of the retinal degenerative pathologies with primary and progressive involvement of the RPE is age related macular degeneration (AMD). Among individuals older than 65 years of age, AMD is regarded as the leading cause of blindness in the industrialized world.[6] The drusen develop as small and hard deposits that gradually increase with age.[7,8] Enlargement of these deposits may be associated with regional RPE loss and eventually degeneration of the overlying photoreceptors. These changes are funduscopically visible and their progression is used to classify lesions along the continuum of early to late dry AMD.[9] Another retinal dystrophic condition which also involves RPE cells with subsequent photoreceptor loss is retinitis pigmentosa (RP). As of yet, there is no efficient therapy for this group of retinal degenerative and dystrophic diseases; however, regenerative cell therapy has shed some light of hope. For this reason, a well characterized and cost effective animal model of retinal RPE and photoreceptor cell degeneration is very helpful to design studies on cell therapies.
It has been known that systemic injection of sodium iodate causes blindness.[10] Sodium iodate has been reported to be an effective chemical substance to induce RPE toxicity in a variety of animal species including rabbits, sheep and mice.[11,12,13,14,15] Melanin is considered as one of the possible intracellular particles that sensitize RPE cells to NaIO3.[16] However, some studies suggest that NaIO3 denatures retinal proteins which becomes manifest by changes in proteins containing a sulfhydryl group in the retina,[17] while others suggest that the retinotoxic effect of NaIO3 is due to inhibited activity of enzymes which contain the sulphydryl group.[6,18,19,20,21] Infliction of oxidative stress to the RPE by sodium iodate could be another possible reason for RPE changes.[22] One of the methods for induction of RPE cell loss in mice is by injecting sodium iodate into the retrobulbar sinus.[9,11,12,19,21] Studies on rats have shown induction of RPE cell loss by intravenous or intraperitoneal administration of sodium iodate.[23]
Electroretinography (ERG) is a tool to evaluate retinal function from RPE up to the most inner layer of retinal ganglion cells (RGC). ERG responses originate from RPE[24] cells as the c-wave which reflects RPE activity.[12] In contrast, a-and b-waves of the ERG originate from the neural retina.[21,25] Therefore, changes in the a-wave on the ERG are consistent with pathology of retinal photoreceptors, and changes in the b-wave indicate pathology in inner retinal layers, from bipolar cells to RGCs.[26]
The present study investigates the histologic and functional effects of unilateral injection of sodium iodate at two different doses into the orbital venous plexus on the ipsilateral RPE and neuroretina, as well as on the contralateral eye in pigmented rats. The results may establish a model for chemical RPE and neuroretinal degeneration in pigmented rats which can be used for evaluating cell based therapies.
METHODS
Animals
Sixty-four pigmented hooded male rats (Razi institute, Iran, Tehran) weighing 250 to 300 grams were used for this experiment, and were divided into three groups: 25 animals were allocated injection of sodium iodate 40 mg/kg, 25 others were assigned to injection of sodium iodate 60mg/kg, and 14 animals comprised the phosphate buffer saline injection (sham) group. The rats were housed under standard laboratory conditions with 12-hour light/dark cycles at 20°C. All experimental procedures were performed according to ARVO guidelines for the use of animals in ophthalmic and vision research, and the study was approved by the Ethics Committee at Tarbiat Modares University, Tehran, Iran.
RPE Injury Model
Sodium iodate (Sigma-Aldrich, CAS Number 7681-55-2, Linear Formula: NaIO3, M.W.:197.89, Steinheim, Germany) was diluted in sterile phosphate-buffered saline (PBS) to a concentration of 5%, filtered and PH adjusted to 7.4 and stored at 4°C. The left eyes of the rats in the two experimental groups were injected via the retro-bulbar venous plexus using two different NaIO3 doses: 60 mg/kg as the high dose and 40 mg/kg as the low dose. The control rats were injected with phosphate buffered saline (PBS) instead of NaIO3. The retrobulbar injection technique was applied as previously described.[27] Under anesthesia and on a warm pad (25°C), the animal was placed in lateral recumbent position with the eye to be injected facing up. A 30 gauge, one-inch insulin needle was inserted bevel up into the medial canthus of the eye at 45 degrees to the nose and deep into the vessels behind the eye. After applying gentle suction, a small flush of blood was observed into the syringe. The NaIO3 solution was gently injected into the retrobulbar vessels. After completion of injection, the needle was withdrawn and light pressure was applied on the globe to prevent bleeding.[27]
Histology
Rats (5 animals at each time point from both dose groups) were sacrificed 1, 7, 14, or 28 day (s) after the injection by cervical dislocation. The eyes were then enucleated and fixed in 4% paraformaldehyde at 4°C overnight. For preparation of tissue cross-sections, the eyes were embedded in paraffin, cut into 5-7 µm-thick sections, and stained with hematoxylin and eosin (Sigma-Aldrich, Steinheim, Germany). Morphological analyses including quantification of RPE cell numbers and outer nuclear layer thickness were performed at two different locations in each eye: (1) Approximately 100 μm from the optic nerve head for the central retina (posterior pole), and (2) approximately 500 μm from the ora serrata for the peripheral retina. The parafin embeded sections were stained with hematoxylin and eosin and counting was done on images from these sections. Counting of the nuclei in the outer nuclear layer was done according to Enzmann et al[19] The whole RPE flat mounts (FMs) were prepared by removing the anterior segment and the neurosensory retina from the eyeball, followed by making four radial relaxing incisions in the remaining sclera-choroid-RPE complex. The specimens (FMs) were mounted, cover slipped, and examined with fluorescence microscope (Olympus IX71, Olympus, Japan).
ERG Recordings
Scotopic and photopic full-field ERGs using Ganzfeld bowl light stimulation were recorded before and 1, 7, 14, and 28 day (s) after sodium iodate injection. The animals were kept for at least one hour in a dark room for dark adaptation,[28,29] thereafter the rats were anesthetized with an intraperitoneal injection of ketamine (40 mg/kg) and xylazine (4 mg/kg) (Alfasan Company, Woerden, the Netherlands). The cornea was anesthetized using Alcaine (Alcon, Fort Worth, TX, USA) and the pupils were dilated with 1% atropine (Sina, Tehran, Iran), in dim red light.
Scotopic recordings were performed in dark conditions under a dim red light at two light levels (3.0 and 10 candela.steradian per square meter [cd.s/m2]) to elicit maximal combined rod and cone responses. After light adaptation for 10 minutes, photopic ERGs were elicited using 3 cd.s/m2 light intensity on a white background of 30 cd/m2. For each recording, 5 separate responses were averaged by an ERG recording system (RETI port 21, version: 19-99-04-7.2E, Roland consult, electrophysiologic diagnostic system, D-14770 Brandenburg, Germany). The results were reported as a-wave and b-wave amplitudes in microvolts.
Data Analysis
Data was obtained and expressed in mean ± SE values from the experimental rats (5 rats at each time point with both NaIO3 doses of 40 and 60 mg/kg) and controls. The data were tested for normality using the Kolomogrov-Smirnov test. All data showed no departure from normal distribution. Then the data were analyzed by two-way ANOVA using post-hoc Tukey's test using SPSS software (version 16, SPSS Inc., Chicago, USA). P values less than 0.05 were considered as statistically significant.
RESULTS
Histology
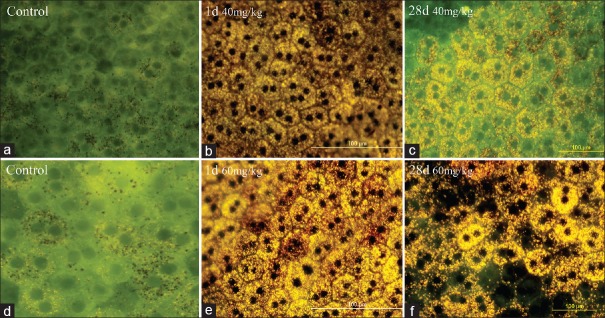
Whole-mount tissue preparation in the control groups and those from baseline samples, revealed melanin granules in an intact RPE layer [Figure 1a and d]. At one-day post-injection in animals treated with 40 mg/kg sodium iodate there was reduction of melanin granules along with accumulation of lipofuscin in RPE cells [Figure 1b]. The amount of lipofuscin granules increased in the animals which were sacrificed 28 days after injection, and the specimens also showed patchy loss of RPE cells [Figure 1c]. At the higher dose (60 mg/kg), the changes were more prominent and characterized by an increase in lipofuscin pigments on day one [Figure 1e], and patchy loss of RPE cells on day 28 post-injection [Figure 1f].
Figure 1.
Whole flat-mount images of the RPE layer by fluorescence microscope. Images a and d show control specimens before sodium iodate injection. b and e represent specimens prepared one day (24 hours) after injection of 40 and 60 mg/kg retro-bulbar sodium iodate, respectively. Yellow-brown pigment granules in RPE cells may be the result of accumulation of lipofuscin secondary to sodium iodate injection. c andfrepresent specimens prepared 28 days after injection of 40 and 60 mg/kg of retrobulbar sodium iodate, respectively. Patchy disappearance of RPE cells is evident in specimens after 60 mg/kg injection. (Scale bar: 100 µm).
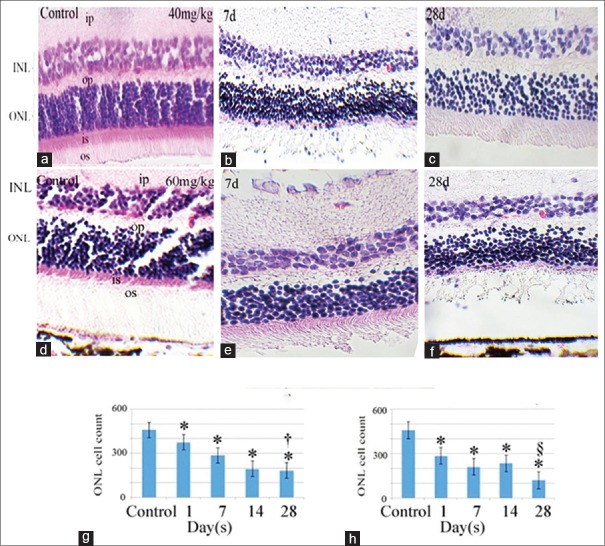
Cross sections of the retinas stained with hematoxylin and eosin are presented in Figure 2a–f, which show progressive reduction in the outer nuclear layer (ONL) and inner nuclear layer (INL) cell counts, and thus, reduction in thickness; however, the reduction was more prominent with the higher dose (60 mg/kg) in the animals sacrificed 28 days post-injection [Figure 2f]. These results are consistent with morphometry, where the ONL counts declined progressively at later days (28 days). Statistical analysis showed that the lowest nuclear count was noted in the 60 mg/kg dosage group at 28 days post-injection (P< 0.05, Figure 2g and h; 40 and 60 mg/kg, respectively).
Figure 2.
Histological cross section of the retina stained with hematoxylin and eosin. The upper panels (b and c) show specimens prepared 7 and 28 days after 40 mg/kg injection of retrobulbar sodium iodate. Lower panels (e and f) show specimens 7 and 28 days after 60 mg/kg of sodium iodate injection, respectively. a and d represent sections from normal retinas before any injection. Histograms (g and h, 40 and 60 mg/kg of sodium iodate, respectively) represent the morphometric evaluation of the outer nuclear layer (ONL cell count: Image J) in the control group and 1, 7, 14 and 28 days after injection. *This sign indicates a significant difference in ONL cell count between the control sections and sections of treated eyes from other time points. Indicates significantly lower ONL cell count in 28 days than the other time points except that of 14 days in 40 mg/kg group. § Indicates significantly lower ONL cell count than the other time points in 60 mg/kg group. OS, outer segment of photoreceptors; ONL, outer nuclear layer; OP, outer plexiform layer; INL, inner nuclear layer; IP, inner plexiform layer; Scale bar, A, B and D = 120 µm; C, E andF= 60 µm.
Electroretinography
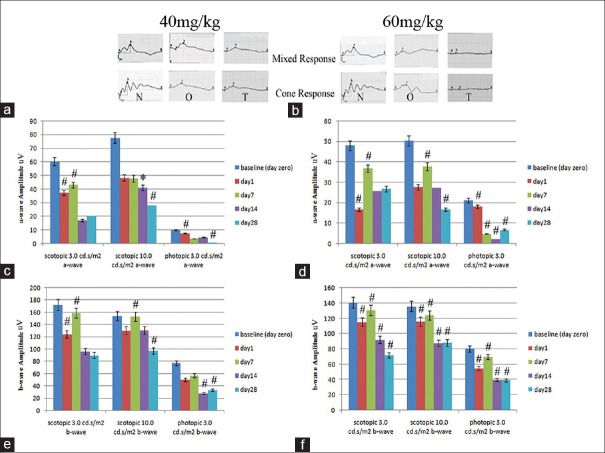
ERG was recorded in the three groups of animals at baseline (before injection, day 0), and on days 1, 7, 14 and 28. Figure 3 depicts representative recordings of maximal combined scotopic and photopic ERGs in the 40 and 60 mg/kg sodium iodate injection groups at one and 28 days respectively, which shows progressive reduction in a and b wave amplitudes [Figure 3a and b].
Figure 3.
a and b show representative electroretinogram waveforms of the scotopic maximum combined and photopic responses, respectively. N: Before NaIO3 injection, O: One day and T: 28 days after sodium iodate administration at doses of 40 and 60 mg/kg, respectively. c and d represent the histograms of mean amplitudes of the a-wave with 40 and 60 mg/kg sodium iodate, from day 0 (before injection) to 1, 7, 14 and 28 days after injection, repectively. e andfrepresent mean amplitudes of the b-wave with 40 and 60 mg/kg sodium iodate, from day 0 (before injection) to 1, 7, 14 and 28 days after injection, respectively. (*Indicates a significant reduction in mean amplitudes compared to mean amplitudes recorded before Sodium iodate injection).
Histograms of a-wave amplitudes plotted for the 40 and 60 mg/kg groups [Figures 3c and 4d] show progressive decline of a-wave amplitudes in both groups in 3 and 10 cd.s/m2 scotopic, and 3 cd.s/m2 photopic ERGs which were significantly lower than baseline values (P< 0.05). Significant reduction in a-wave amplitudes from baseline in both 3 and 10 cd.s/m2 scotopic ERGs were observed on day 14 which further decreased on day 28 (P< 0.05). For 3 cd.s/m2 photopic ERG, a similar pattern of reduction was observed earlier on days 7 and 28 (P< 0.05, Figure 3c).
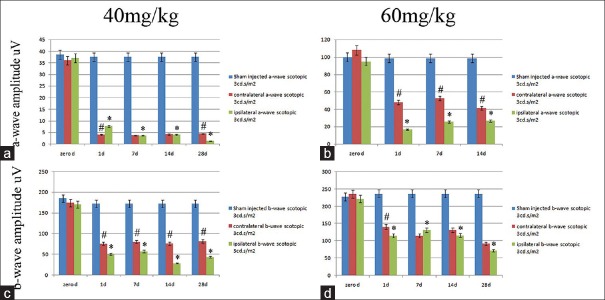
Figure 4.
Comparison of mean a-wave amplitudes (a and b) and b-wave amplitudes (c and d) of the scotopic maximal combined electroretinograms (3 cd.s/m2 intensity), for eyes from the sham group (blue columns), sodium iodate injection of the left or ipsilateral eyes (green columns) and the right or contralateral eyes (red columns), of the 40 mg/kg (a and c) and 60 mg/kg (b and d) dose groups, in consecutive time points from before injection to 1, 7, 14 and 28 days after injection, respectively. (*Asterisk indicates a significant difference between the sham treated and other time point values in treated animals (P< 0.05). (#Indicates a significant difference between the contralateral eyes or red columns and ipsilateral eyes or green columns,P< 0.05).
In the 60 mg/kg group, the amplitudes of a-waves showed abrupt and progressive decline in both 3 and 10 cd.s/m2 scotopic ERGs, as early as day one (P< 0.05). a-wave amplitude of 3 cd.s/m2 photopic ERG declined progressively from day one until day 14 (P< 0.05), which was the lowest point, and then non-significantly increased on day 28 [Figure 3d].
The amplitude of b-waves at the 40 mg/kg dose showed a significant decline in both 3 and 10 cd.s/m2 scotopic, and 3 cd.s/m2 photopic ERGs from day 14 to day 28 in comparison to baseline values. For unknown reasons, there was a slight and non-significant increase in b wave amplitudes on day 7 [Figure 3e].
With the 60 mg/kg dose, b-wave amplitudes declined significantly in 3 and 10 cd.s/m2 scotopic, and 3 cd.s/m2 photopic ERG recordings from day 14 to 28. There was no significant difference between day 7 and baseline values prior to injection, and the lowest amplitude occurred on day 28 (P< 0.05, Figure 3f).
Figure 4 shows the significant reduction in a- and b-wave amplitudes in 3 cd.s/m2 scotopic ERG of 40 mg/kg (figures 4a and 4c) and 60 mg/kg (figures 4b and 4d) of left or ipsilateral NaIO3 injected eyes (green columns) in comparison to the ERGs of the right or contralateral eyes (red columns) and sham injected eyes (blue columns) in different time points from before injection (zero day) to 1, 7, 14 and 28 days after injection, respectively (P< 0.05).
DISCUSSION
The current study showed structural and functional changes in the retina of rats exposed to two doses of sodium iodate (40 and 60 mg/kg) using histopathological and electrophysiological evaluations. Unilateral retrobulbar injection of sodium iodate caused histopathologic damage in the ipsilateral RPE and neuroretina in a dose- and time-dependent manner. The damage was more severe four weeks after the injections. The whole-mount tissue preparation from the RPE in animals treated with 40 and 60 mg/kg of sodium iodate showed an increase in yellow fluorescence on day one which suggests lipofuscin accumulation in these cells. Nishimura et al reported the same finding as early as 6 hours after intravenous injection of NaIO3, which also aggravated as time progressed.[30] Other researchers have reported breakdown of the blood retinal barrier and RPE damage along with RPE nuclear changes, 24 hours following intravenous sodium iodate delivery,[19] which subsequently resulted in lesions in the neurosensory retina and choriocapillaries.[11] Machalinska et al reported that marked RPE lesions were observed on the first day of NaIO3 delivery.[9] These findings are in agreement with the results of our study in which RPE changes were detected one day after injection. However, some researchers used different delivery routes and the pathologic changes in RPE layer were reported on days 3, 5 and 7 after injection of sodium iodate.[11,12,31] The decrease in melanin pigment and increase in lipofuscin with patchy loss of RPE cells on day 28 post-injection were similar to lesions in the aged human retina,[32] and are comparable with results of previous studies.[19] In contrast to the control group, a progressive increase in fluorescence from day one to 28 was observed with accumulation of lipofuscin granules in the injected eyes. Enzmann et al demonstrated that high dose sodium iodate caused progressive retinal damage with extensive RPE damage, while at lower doses, there were patches of RPE loss.[19] Our results also showed patchy RPE loss of sodium iodate treated animals as observed in the whole-mounted retina. In cross-section samples of the retina treated with sodium iodate, previous studies have shown reduction in ONL thickness at high dose in a time dependent manner.[19,33] Our morphometric study regarding the ONL showed that the thickness was reduced, and the reduction was more significant at 60 mg/kg dose than at 40 mg/kg which is consistent with the findings showed by Franco et al.[10] Similar results have also been reported by Machalinska et al.[9]
Full field ERG represents the summed activity of retinal neurons, starting from the outer retina (from RPE cells and photoreceptors) to the inner retinal layers of bipolar and amacrine cells excluding the RGCs.[18] Generally, after sodium iodate injection, both a- and b-wave amplitudes were decreased in scotopic maximal combined responses at two light intensities of 3 and 10 cd.s/m2. Such a decrease was also observed in the photopic responses. There was a significant depression on day 14 which further diminished on day 28.
Although a- and b-wave responses were depressed in both sodium iodate groups on day one, we observed a slight but non-significant temporary return of responses toward baseline values around day 7, which again significantly decreased on day 14. This observation was consistent in both scotopic and photopic responses and at both doses, and might have resulted from partial recovery of injured RPE cells.[25]
The reduction in b-wave amplitude following sodium iodate induced retinotoxicity has been reported in many studies being detectable from one to 3 day (s) after administration.[9,33] Carido et al reported that b-wave was affected on day 3 and decreased progressively until day 14.[33]
Although there was a significant reduction in ipsilateral scotopic maximal combined ERG responses from baseline to different time points in 3 cd.s/m2, we also observed the significant but less reduction in a and b wave amplitudes in 3 cd.s/m2 in contralateral eye [Figure 4]. In our model of injection of sodium iodate in the orbital plexus of rats, systemic absorption of the toxin may occur which causes similar but milder effects on the contralateral RPE cells and neuroretina. Harris et al reported that there was a significant difference in b-wave recordings between ipsilateral and contralateral sides 14 days after insult in mice.[34] The c-wave in rabbits receiving sodium iodate unilaterally was reduced on the contralateral side,[35] whereas no changes noticed in the contralateral retina RPE of primates following sodium iodate injection.[30] Therefore, the difference in the response of RPE layer in the rat retina may be attributed to species differences or the rate of delivery.
In summary, the current study shows significant histologic and electroretinographic evidence of degenerative effect by sodium iodate on the RPE cells and subsequently on the outer and inner layers of the neuro-retina in a dose and time dependent manner. The route of injection in our study was the retrobulbar orbital venous plexus on one side; however, there was evidence of retino-toxic effects on the contralateral retina with slightly less intensity, most probably due to systemic or direct distribution. Through injecting 60 mg/kg of sodium iodate unilaterally in the retrobulbar plexus of pigmented rats, we found no abrupt or florid destruction of retinal tissue, but progressive retinal degeneration. This model may be closer to the gradual progressive process which occurs in genetic dystrophic and also in degenerative retinal diseases. Further studies are needed to prove the applicability of this model for different fields of regenerative and cell therapy research.
Financial Support and Sponsorship
This project was funded by Shefa Neuroscience Research Center at Khatam Al-Anbia Hospital, Tehran, Iran (Grant# 86-N-105)..
Conflicts of Interest
There are no conflicts of interest.
Acknowledgement
The authors are also grateful for the support rendered by the Faculty of Medical Sciences, Tarbiat Modares University, Tehran, Iran.
REFERENCES
- 1.Guymer R, Luthert P, Bird A. Changes in Bruch's membrane and related structures with age. Prog Retin Eye Res. 1999;18:59–90. doi: 10.1016/s1350-9462(98)00012-3. [DOI] [PubMed] [Google Scholar]
- 2.Bok D. The retinal pigment epithelium: A versatile partner in vision. J Cell Sci Suppl. 1993;17:189–195. doi: 10.1242/jcs.1993.supplement_17.27. [DOI] [PubMed] [Google Scholar]
- 3.Marmor MF. Control of subretinal fluid: Experimental and clinical studies. Eye (Lond) 1990;4(Pt 2):340–344. doi: 10.1038/eye.1990.46. [DOI] [PubMed] [Google Scholar]
- 4.Gehrs KM, Anderson DH, Johnson LV, Hageman GS. Age-related macular degeneration – Emerging pathogenetic and therapeutic concepts. Ann Med. 2006;38:450–471. doi: 10.1080/07853890600946724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bird AC. Bruch's membrane change with age. Br J Ophthalmol. 1992;76:166–168. doi: 10.1136/bjo.76.3.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hageman GS, Luthert PJ, Victor Chong NH, Johnson LV, Anderson DH, Mullins RF. An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch's membrane interface in aging and age-related macular degeneration. Prog Retin Eye Res. 2001;20:705–732. doi: 10.1016/s1350-9462(01)00010-6. [DOI] [PubMed] [Google Scholar]
- 7.Davis MD, Gangnon RE, Lee LY, Hubbard LD, Klein BE, Klein R, et al. The age-related eye disease study severity scale for age-related macular degeneration: AREDS Report No 17. Arch Ophthalmol. 2005;123:1484–1498. doi: 10.1001/archopht.123.11.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sorsby A. Experimental pigmentary degeneration of the retina by sodium iodate. Br J Ophthalmol. 1941;25:58–62. doi: 10.1136/bjo.25.2.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Machalinska A, Lubinski W, Klos P, Kawa M, Baumert B, Penkala K, et al. Sodium iodate selectively injuries the posterior pole of the retina in a dose-dependent manner: Morphological and electrophysiological study. Neurochem Res. 2010;35:1819–1827. doi: 10.1007/s11064-010-0248-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franco LM, Zulliger R, Wolf-Schnurrbusch UE, Katagiri Y, Kaplan HJ, Wolf S, et al. Decreased visual function after patchy loss of retinal pigment epithelium induced by low-dose sodium iodate. Invest Ophthalmol Vis Sci. 2009;50:4004–4010. doi: 10.1167/iovs.08-2898. [DOI] [PubMed] [Google Scholar]
- 11.Korte GE, Gerszberg T, Pua F, Henkind P. Choriocapillaris atrophy after experimental destruction of the retinal pigment epithelium in the rat. A study in thin sections and vascular casts. Acta Anat (Basel) 1986;127:171–175. doi: 10.1159/000146277. [DOI] [PubMed] [Google Scholar]
- 12.Nilsson SE, Knave B, Persson HE. Changes in ultrastructure and function of the sheep pigment epithelium and retina induced by sodium iodate. III. Delayed effects. Acta Ophthalmol (Copenh) 1977;55:1027–1043. doi: 10.1111/j.1755-3768.1977.tb05683.x. [DOI] [PubMed] [Google Scholar]
- 13.Yoon YH, Marmor MF. Retinal pigment epithelium adhesion to Bruch's membrane is weakened by hemicholinium-3 and sodium iodate. Ophthalmic Res. 1993;25:386–392. doi: 10.1159/000267341. [DOI] [PubMed] [Google Scholar]
- 14.Baich A, Ziegler M. The effect of sodium iodate and melanin on the formation of glyoxylate. Pigment Cell Res. 1992;5:394–395. doi: 10.1111/j.1600-0749.1992.tb00568.x. [DOI] [PubMed] [Google Scholar]
- 15.Sorsby A, Reading HW. Experimental degeneration of the retina. XI. The effect of sodium iodate on retinal-SH levels. Vision Res. 1964;4:511–514. doi: 10.1016/0042-6989(64)90057-4. [DOI] [PubMed] [Google Scholar]
- 16.Jiang W, Chiou G. Effects of hydralazine on NaIO3-induced rat retinal pigment epithelium degeneration. Int J Ophthalmol. 2008;8:1504–1510. [Google Scholar]
- 17.Schaeppi U, Krinke G, Fink X, Hofer R, Duennenberger D. Electroretinography in rats. Agents Actions. 1988;24:395–402. doi: 10.1007/BF02028299. [DOI] [PubMed] [Google Scholar]
- 18.Gouras P. Electroretinography: Some basic principles. Invest Ophthalmol. 1970;9:557–569. [PubMed] [Google Scholar]
- 19.Enzmann V, Row BW, Yamauchi Y, Kheirandish L, Gozal D, Kaplan HJ, et al. Behavioral and anatomical abnormalities in a sodium iodate-induced model of retinal pigment epithelium degeneration. Exp Eye Res. 2006;82:441–448. doi: 10.1016/j.exer.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 20.Kinnunen K, Petrovski G, Moe MC, Berta A, Kaarniranta K. Molecular mechanisms of retinal pigment epithelium damage and development of age-related macular degeneration. Acta Ophthalmol. 2012;90:299–309. doi: 10.1111/j.1755-3768.2011.02179.x. [DOI] [PubMed] [Google Scholar]
- 21.Hawlina M, Jenkins HG, Ikeda H. Diurnal variations in the electroretinographic c-wave and retinal melatonin content in rats with inherited retinal dystrophy. Doc Ophthalmol. 1992;79:141–150. doi: 10.1007/BF00156573. [DOI] [PubMed] [Google Scholar]
- 22.Shen Y, Zhang WY, Chiou GC. Effect of naringenin on NaIO (3)-induced retinal pigment epithelium degeneration and laser-induced choroidal neovascularization in rats. Int J Ophthalmol. 2010;3:5–8. doi: 10.3980/j.issn.2222-3959.2010.01.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen Y, Zhuang P, Lin BQ, Zhang WY, Cy Chiou G. Effect of Tetramethylpyrazine on RPE degeneration, choroidal blood flow and oxidative stress of RPE cells. Int J Ophthalmol. 2010;3:205–210. doi: 10.3980/j.issn.2222-3959.2010.03.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hosoda L, Adachi-Usami E, Mizota A, Hanawa T, Kimura T. Early effects of sodium iodate injection on ERG in mice. Acta Ophthalmol (Copenh) 1993;71:616–622. doi: 10.1111/j.1755-3768.1993.tb04650.x. [DOI] [PubMed] [Google Scholar]
- 25.Adachi-Usami E, Mizota A, Ikeda H, Hanawa T, Kimura T. Transient increase of b-wave in the mouse retina after sodium iodate injection. Invest Ophthalmol Vis Sci. 1992;33:3109–3113. [PubMed] [Google Scholar]
- 26.Mizota A, Adachi-Usami E. Functional recovery of retina after sodium iodate injection in mice. Vision Res. 1997;37:1859–1865. doi: 10.1016/s0042-6989(97)00015-1. [DOI] [PubMed] [Google Scholar]
- 27.Li S, Li T, Luo Y, Yu H, Sun Y, Zhou H, et al. Retro-orbital injection of FITC-dextran is an effective and economical method for observing mouse retinal vessels. Mol Vis. 2011;17:3566–3573. [PMC free article] [PubMed] [Google Scholar]
- 28.Rösch S, Johnen S, Mazinani B, Müller F, Pfarrer C, Walter P. The effects of iodoacetic acid on the mouse retina. Graefes Arch Clin Exp Ophthalmol. 2015;253:25–35. doi: 10.1007/s00417-014-2652-0. [DOI] [PubMed] [Google Scholar]
- 29.Dreixler JC, Hagevik S, Hemmert JW, Shaikh AR, Rosenbaum DM, Roth S. Involvement of erythropoietin in retinal ischemic preconditioning. Anesthesiology. 2009;110:774–780. doi: 10.1097/ALN.0b013e31819c4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishimura T, Zhu ZR, Ryan SJ. Effects of sodium iodate on experimental subretinal neovascularization in the primate. Ophthalmologica. 1990;200:28–38. doi: 10.1159/000310074. [DOI] [PubMed] [Google Scholar]
- 31.Wang J, Iacovelli J, Spencer C, Saint-Geniez M. Direct effect of sodium iodate on neurosensory retina. Invest Ophthalmol Vis Sci. 2014;55:1941–1953. doi: 10.1167/iovs.13-13075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bonilha VL. Age and disease-related structural changes in the retinal pigment epithelium. Clin Ophthalmo l. 2008;2:413–424. doi: 10.2147/opth.s2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carido M, Zhu Y, Postel K, Benkner B, Cimalla P, Karl MO, et al. Characterization of a mouse model with complete RPE loss and its use for RPE cell transplantation. Invest Ophthalmol Vis Sci. 2014;55:5431–5444. doi: 10.1167/iovs.14-14325. [DOI] [PubMed] [Google Scholar]
- 34.Harris JR, Fisher R, Jorgensen M, Kaushal S, Scott EW. CD133 progenitor cells from the bone marrow contribute to retinal pigment epithelium repair. Stem Cells. 2009;27:457–466. doi: 10.1634/stemcells.2008-0836. [DOI] [PubMed] [Google Scholar]
- 35.Textorius O, Welinder E, Nilsson SE. Combined effects of DL-alpha-aminoadipic acid with sodium iodate, ethyl alcohol, or light stimulation on the ERG c-wave and on the standing potential of albino rabbit eyes. Doc Ophthalmol. 1985;60:393–400. doi: 10.1007/BF00158929. [DOI] [PubMed] [Google Scholar]