Abstract
Background
We aimed to investigate the effect of levosimendan on biomarkers of myocardial injury and systemic hemodynamics in patients with septic shock.
Material/Methods
After achieving normovolemia and a mean arterial pressure of at least 65 mmHg, 38 septic shock patients with low cardiac output (left ventricular ejective fraction), LEVF ≤45%) were randomly divided into two groups: levosimendan dobutamine. Patients in the levosimendan and dobutamine groups were maintained with intravenous infusion of levosimendan (0.2 μg/kg/minute) and dobutamine (5 μg/kg/minute) for 24 hours respectively. During treatment we monitored hemodynamics and LVEF, and measured levels of heart-type fatty acid binding protein (HFABP), troponin I (TNI), and brain natriuretic peptide(BNP). In addition, the length of mechanical ventilation, intensive care unit (ICU) stay, hospital stay, and 28-day mortality were compared between the two groups.
Results
The levosimendan group and the dobutamine group were well matched with respect to age (years, 55.4±1 7.5 versus 50.2±13.6) and gender (males, 68.4% versus 57.9%). Levosimendan-treated patients had higher stroke volume index (SVI), cardiac index (CI), LVEF, and left ventricular stroke work index (LVSWI), and lower extravascular lung water index (EVLWI) compared to dobutamine-treated patients (p<0.05). HFABP, TNI, and BNP in the levosimendan group were less than in the dobutamine group (p<0.05). There was no difference in the mechanical ventilation time, length of stay in ICU and hospital, and 28-day mortality between the two groups.
Conclusions
Compared with dobutamine, levosimendan reduces biomarkers of myocardial injury and improves systemic hemodynamics in patients with septic shock. However, it does not reduce the days on mechanical ventilation, length of stay in ICU and hospital, or 28-day mortality.
MeSH Keywords: Cardiomyopathies; Fatty Acid-Binding Proteins; Hemodynamics; Shock, Septic
Background
The latest several decades have witnessed the progress in the treatment of severe sepsis and septic shock, acute organ dysfunction and consequential multiple organ dysfunction [1], however, cardiovascular dysfunction due to severe infection is still a major contributor to sepsis related morbidity and mortality [2].
Cardiovascular dysfunction induced by severe sepsis and septic shock is characterized by signs of distributive shock and septic cardiomyopathy consisting of bi-ventricular myocardial contractility impairment and diastolic dysfunction [3]. The characteristics of septic cardiomyopathy include left ventricular dilatation, depressed ejection fraction and recovery during 7–10 days. In severe sepsis and septic shock, myocardial depression is the manifestation of septic cardiomyopathy and may attribute to the overwhelming production of inflammatory cytokines, mitochondrial dysfunction, and decreased myofibrillar sensitivity to calcium [4,5].
Rivers et al. suggested in 2001 that early goal-directed therapy (EGDT) was effective for severe sepsis management [6], however, several recent studies, including the ProCESS [7] and ARISE [8] trials in 2014 and the ProMiSe trial [9] in 2015 indicated that EGDT did not improve outcomes compared to usual care.
International sepsis guidelines have been adopted worldwide, and it is widely accepted that the standard treatment for sepsis should concentrate on infection control and optimization of hemodynamic parameters by fluid resuscitation and vasopressor therapy including noradrenaline and vasopressin [10]. These standard treatment also apply to septic cardiomyopathy. In addition, using dobutamine to increase the cardiac index is recommended by international sepsis guidelines [10]. However, several studies have demonstrated that the use of dobutamine to increase cardiac output did not improve microcirculation, peripheral perfusion, or the outcome of septic shock patients [11], and even increased the 90-day mortality rate [12].
Another inotropic agent is levosimendan, a Ca2+ sensitizer and inodilator, which has been used successfully in the management of acute heart failure. Levosimendan not only has inotropic and vasodilator effects, but also has anti-inflammatory and anti-apoptotic effects [13]. In addition, meta-analysis has shown that levosimendan reduced mortality in critically ill patients and chronic advanced heart failure patients [14,15]. However, levosimendan is not widely used in intensive care units (ICUs).
Therefore, the aim of the present study was to compare the effects of levosimendan and dobutamine on biomarkers of myocardial injury and systemic hemodynamics in patients with septic shock in the ICU.
Material and Methods
Ethics statement
This pilot study was conducted in the medical-surgical ICU at Tongde Hospital of Zhejiang Province in Hangzhou, China between March 2014 and January 2016. It was conducted in strict accordance with the protocol approved by the Ethics Committee of Tongde Hospital of Zhejiang Province(Hangzhou, China). All participants were recruited by Tongde Hospital of Zhejiang Province and they all (or their guardians) signed informed consents prior to enrollment.
Study population
Patients with low cardiac output (left ventricular ejective fraction (LVEF ≤45%) were enrolled within the first 24 hours from the onset of septic shock after having established normovolemia (CVP=12 to 15 mmHg) [10] and mean arterial pressure (MAP) of at least 65 mmHg using norepinephrine, if needed. Red blood cells were transfused when hemoglobin concentrations decreased to below 7 g/dL [10] to elevate systemic oxygen supply.
Inclusion criteria were: (1) all participants were diagnosed with septic shock and established normovolemia, and used norepinephrine to maintain MAP of at least 65 mmHg [10]; (2) all participants had LVEF ≤45% after fluid resuscitation and vasopressor therapy. Exclusion criteria included: (1) onset of septic shock >24 hours; (2) patients younger than 18 years of age; (3) LVEF ≤45% before fluid resuscitation; (4) patients with pre-existing cardiomyopathy, valvular heart disease, or heart failure; (5) present or suspected acute coronary syndrome within recent two weeks; (6) pregnancy; and (7) ventricular outflow tract obstruction.
Experimental procedure
All patients received mechanical ventilation using a volume-controlled mode with a tidal volume of 6 to 8 mL/kg of predicted body weight, and were sedated with midazolam and fentanil. According to the international sepsis guidelines [10], for septic shock treatment we used norepinephrine to maintain a mean arterial pressure (MAP) of at least 65 mmHg despite quantitative fluid resuscitation (the variation of CVP from 12 to 15 mmHg) within the first 24 hours from the onset of septic shock. After these goals were achieved, cardiac ultrasound scans were used to measure LVEF immediately. Finally, 38 patients with low cardiac output (LVEF ≤45%) were enrolled and randomized (by the use of a table of random numbers) into two groups: a group receiving 0.2 μg/kg/minute (without a loading bolus dose) of levosimendan (levosimendan group, n=19), and a group receiving 5 μg/kg/minute of dobutamine (dobutamine group, n=19). During the 24-hour drug intervention period, all patients also received fluid therapy and norepinephrine to maintain normovolemia and MAP of more than 65 mmHg. After this period, levosimendan and dobutamine were discontinued and the attending ICU physicians decided whether dobutamine should be started based on the hemodynamic status of the patients. In addition, in order to avoid the interference of depurative extracorporeal circulation on biomarkers of myocardial injury, all patients did not receive continuous blood purification (CBP) before or within 48 hour after enrollment. The treating physicians and echocardiographers were not blinded to the experimental procedure or the echocardiographic and laboratory results during the study.
Standard echocardiographic examination
Transthoracic echocardiography was performed before and again after fluid resuscitation and vasopressor therapy, and 24 hours after inclusion. All echocardiograms were performed by an expert echocardiographer not involved in patient care, using a Vivid E9 ultrasound scanner and acquiring LVEF (modified Simpson’s rule).
Hemodynamics monitoring and lactate
All patients were monitored using a pulse-indicated continuous cardiac output (PiCCO) system (Pulsion Medical System, Munich, Germany). They were required to have both a left femoral artery catheter and a right central venous catheter. The correct placement of the catheter insertion was further confirmed by chest radiography.
A 5-French thermistor-tipped catheter (PV2013L16, Pulsion Medical System, German) was inserted into the femoral artery and a central venous catheter (CS-277202-E, ARROW, USA) was placed into a central vein (jugular or subclavian vein); both were connected to the PiCCO system. Thermodilution parameters and pulse contour parameter were obtained with the PiCCO monitor, based on triplicate injections of 15 mL of cold isotonic saline 0.9% (<8°C) via the central venous catheter, and were recorded as the average of the three measurements. The corresponding ventilator function and perfusion parameters were observed and kept constant during the 6-hour period preceding the measurements. The patients were kept in a horizontal position.
Blood gas samples, including lactate samples, were obtained from the arterial catheters via 3 mL heparinized syringes (PL67BP; BD Diagnostics, Plymouth, UK) anaerobically and analyzed on blood gas bedside machines (ABL800: Radiometer, Copenhagen, Denmark).
Outcome measures
The changes in heart rate (HR), CVP, MAP, stroke volume index (SVI), cardiac index (CI), left ventricular stroke work index (LVSWI), systemic vascular resistance index (SVRI), intrathoracic blood volume index (ITBVI), global end diastolic volume index (GEDI), extravascular lung water index (EVLWI), oxygen delivery index (DO2I), oxygen consumption index (VO2I), LVEF, lactate, positive end-expiratory pressure(PEEP), and norepinephrine dose were recorded at baseline and 24 hours after randomization.
We collected 3 mL venous blood samples from the two groups of patients at baseline and 24 hours after randomization and sent samples immediately to Department of Biochemistry at Tongde Hospital of Zhejiang Province to measure plasma levels of heart-type fatty acid binding protein (HFABP), troponin I (TNI), and brain natriuretic peptide (BNP).
Statistical analysis
PASS software (version 11; NCSS, LLC) was used to calculate sample size. Sample size was determined by two-sample t-test power analysis using preliminary data obtained in our laboratory with the following assumptions: α of 0.05 (two-tailed), power of 80%, differences in the mean of HFABP between patients in the levosimendan and the dobutamine groups of −3.4 ng/mL, and a standard deviation of 1.4 ng/mL. Therefore, we calculated that a sample size of 17 would provide 80% power of detecting a difference at a 0.05 level of significance.
The multivariate liner regression analysis was used to assess the effect of levosimendan on biomarkers of myocardial injury (HFABP, TNI, and BNP) and heart function (LEVF and CI) after controlling for age, gender, APACHEII scores, SOFA scores, and baseline values in the septic shock patients. Statistically significant levels were two-tailed and set at p<0.05.
Data were expressed as mean ± standard deviation of the mean (SDM) for quantitative variables, and as count and percentages for qualitative values. Distributions of the discrete variables were compared between the two treatment groups with either the chi-square test or Fisher exact tests. Two sample t-test was used to compared between the two groups; and paired t-test was used for continuous variables before and after treatment. SPSS software (version 16; SPSS Inc., Chicago, IL, USA) was used for statistical analysis; all tests were 2-tailed and p<0.05 was considered to be statistically significant.
Results
Demographic and clinical characteristics of the study population
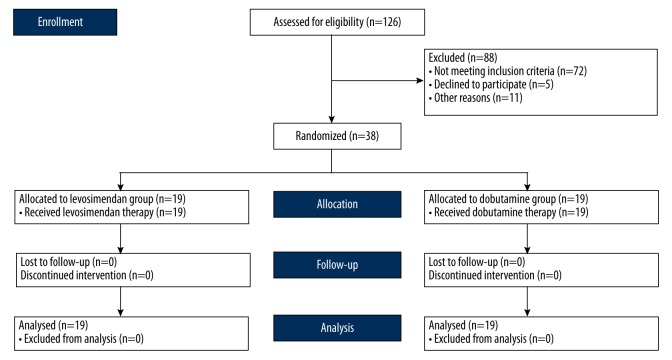
Of the 126 cases assessed for eligibility, 38 subjects were enrolled. Figure 1 shows a flow diagram that quantifies progress through the trial. Of the 72 patients that did not meet the inclusion criteria, five patients declined to participate, 11 patients were excluded from the study for other reasons (six because of an onset of septic shock >24 hours, three because of LVEF ≤45% before fluid resuscitation, and two due to acute coronary syndrome within the recent two weeks).
Figure 1.
Flow diagram.
The demographic data of patients are summarized in Table 1. Age, gender, APACHE II scores, SOFA scores, and type of infection were balanced between the two groups at admission. There were no difference in the duration of mechanical ventilation, length of stay in ICU and hospital, or 28-day mortality between the two groups (all p>0.05).
Table 1.
Demographic variables at baseline in each group.
| Characteristics | Levosimendan (n=19) | Dobutamine (n=19) | P-value |
|---|---|---|---|
| Age(years) | 55.4±17.5 | 50.2±13.6 | 0.313 |
| Male (n,%) | 13 (68.4) | 11 (57.9) | 0.501 |
| APCHEII | 18.4±4.5 | 19.5±4.3 | 0.446 |
| SOFA | 4.2±1.8 | 4.3±2.6 | 0.891 |
| Type of infection (n,%) | |||
| Pneumonia | 8 (42.1.%) | 10 (52.6%) | 0.516 |
| Peritonitis | 5 (26.3%) | 4 (21.1%) | 0.703 |
| CRBSI | 4 (21.1%) | 2 (10.5%) | 0.374 |
| Urinary tract infection | 2 (10.5%) | 3 (15.8%) | 0.631 |
| Days on MV | 6.9±5.5 | 7.2±5.3 | 0.865 |
| Length of stay in ICU(days) | 12.6±10.1 | 13.3±10.5 | 0.835 |
| Length of stay in hospital(days) | 20.4±21.5 | 22.5±23.1 | 0.773 |
| 28-day mortality% (n) | 31.6% (6) | 36.8% (7) | 0.732 |
yr – years; APACHE II – acute physiology and chronic health evaluation; SOFA – sequential organ failure assessment; CRBSI – catheter related bloodstream infection; MV – mechanical ventilation; ICU – intensive care unit.
The changes of hemodynamics and lactate in the different groups
As shown in Table 2, no significant differences were observed in HR, CVP, MAP, SVRI, ITBVI, GEDI, PEEP, and norepinephrine dose (all p>0.05). Compared to dobutamine, levosimendan increased CI (p=0.001), SVI (p=0.030), LVSWI (p=0.002), and LVEF (p=0.018), and decreased EVLWI and lactate (p=0.012 and p=0.022, respectively) after 24 hours of intervention. In addition, levosimendan improved DO2 and VO2 of systemic tissues (p=0.040 and p=0.031, respectively).
Table 2.
Hemodynamic data at different points in each group.
| Levosimendan (n=19) | Dobutamine (n=19) | P-value | ||
|---|---|---|---|---|
| HR (beats/min) | Baseline | 116.1±7.5 | 113.8±6.9 | 0.332 |
| 24 hours | 111.6±6.8 | 110.3±6.5 | 0.551 | |
| CVP (mmHg) | Baseline | 13.2±1.1 | 13.6±1.4 | 0.334 |
| 24 hours | 13.1±0.9 | 13.7±1.2 | 0.090 | |
| MAP (mmHg) | Baseline | 67.6±2.0 | 67.4±2.1 | 0.765 |
| 24 hours | 68.1±1.8 | 67.9±1.9 | 0.741 | |
| SVI (ml/m2) | Baseline | 33.5±8.9 | 32.5±10.3 | 0.750 |
| 24 hours | 40.5±9.1 | 33.6±9.7 | 0.030 | |
| CI (L/min/m2) | Baseline | 3.0±0.2 | 2.9±0.3 | 0.235 |
| 24 hours | 3.5±0.3 | 3.1±0.4 | 0.001 | |
| LVSWI (kg/min/m2) | Baseline | 31.5±1.8 | 32.6±3.2 | 0.200 |
| 24 hours | 36.9±2.7 | 33.8±2.9 | 0.002 | |
| SVRI (kPa·s·L/m2) | Baseline | 1185±109 | 1236±121 | 0.181 |
| 24 hours | 1257±117 | 1198±99 | 0.102 | |
| LVEF (%) | Baseline | 36.2±5.1 | 37.2±7.2 | 0.624 |
| 24 hours | 45.6±7.6 | 39.1±8.5 | 0.018 | |
| ITBVI (ml/m2) | Baseline | 889.8±124.9 | 850.3±162.2 | 0.406 |
| 24 hours | 873.6±134.8 | 860.5±122.9 | 0.756 | |
| GEDI (ml/m2) | Baseline | 709.7±97.6 | 683.3±130.6 | 0.484 |
| 24 hours | 693.4±101.6 | 685.0±95.2 | 0.794 | |
| EVLW I(ml/kg) | Baseline | 9.5±3.6 | 9.3±3.8 | 0.869 |
| 24 hours | 6.4±2.8 | 8.9±3.0 | 0.012 | |
| DO2I (kg·min/·m2) | Baseline | 716.8±56.2 | 725.5±58.7 | 0.644 |
| 24 hours | 755.0±52.1 | 719.8±49.6 | 0.040 | |
| VO2I (kg·min/·m2) | Baseline | 123.2±16.9 | 125.6±13.4 | 0.631 |
| 24 hours | 139.5±18.3 | 127.4±14.8 | 0.031 | |
| Lactate (mmol/L) | Baseline | 5.1±1.2 | 4.7±1.1 | 0.291 |
| 24 hours | 3.6±0.8 | 4.3±1.0 | 0.022 | |
| PEEP (cmH2O) | Baseline | 5.68±2.05 | 6.05±2.24 | 0.633 |
| 24 hours | 5.26±1.63 | 5.47±1.54 | 0.823 | |
| Norepinephrine dose (ug/kg/min) | Baseline | 0.42±0.13 | 0.40±0.11 | 0.619 |
| 24 hours | 0.36±0.11 | 0.37±0.09 | 0.761 |
HR – heart rate; CVP – central venous pressure; MAP – mean arterial pressure; SVI – stroke volume index; CI – cardiac index; LVSWI – left ventricular stroke work index; SVRI – systemic vascular resistance index; ITBVI – intrathoracic blood volume index; GEDI – global end diastolic volume index; EVLWI – extravascular lung water index; DO2I – oxygen delivery index; VO2I – oxygen consumption index; LVEF – left ventriculus ejective fraction; PEEP – positive end-expiratory pressure.
Levosimendan decreased the plasm levels of biomarkers of cardiac injury induced by septic shock
Compared with the dobutamine group, concentrations of HFABP, TNI, and BNP were significantly decreased at 24 hours after intervention in the levosimendan group (p<0.001, p=0.025, and p<0.001, respectively, see Figures 2–4.)
Figure 2.
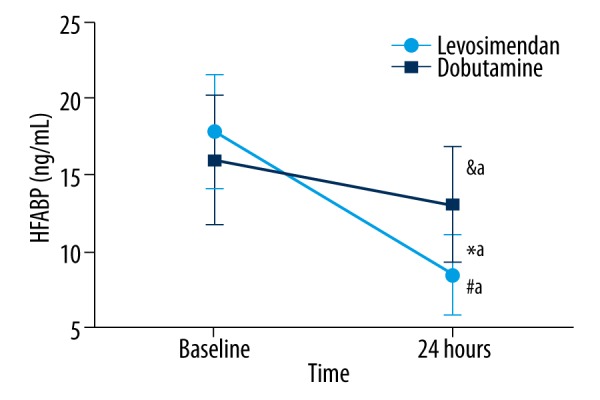
The change of HFABP in the different groups. No significant differences were observed at baseline. It was decreased after 24 hours of treatment compared to baseline values in the dobutamine group (&ap=0.032), and significantly reduced after 24 hours of treatment compared to baseline values in levosimendan group (#ap<0.001). HFABP was lower in the levosimendan group than in the dobutamine group (*ap<0.001). HFABP – heart-type fatty acid binding.
Figure 3.
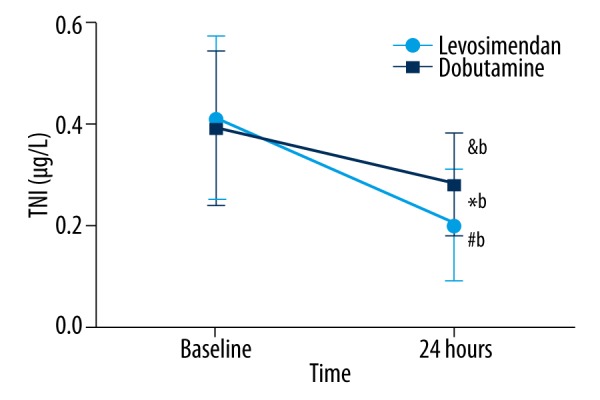
The change of TNI in the different groups. No significant differences were observed at baseline. It was decreased after 24 hours of treatment compared to baseline values in the dobutamine group (&bp=0.011), and significantly reduced after 24 hours of treatment compared to baseline values in the levosimendan group (#bp<0.001). TNI was lower in the levosimendan group than in the dobutamine group (*bp=0.025). TNI – troponin I.
Figure 4.
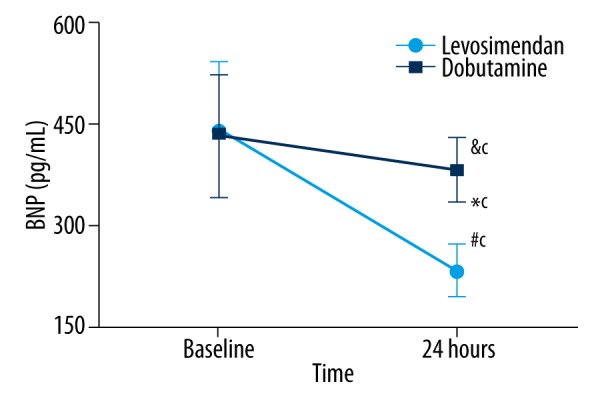
The change of BNP in the different groups. No significant differences were observed at baseline. It was decreased after 24 hours of treatment compared to baseline values in the dobutamine group (&cp=0.037), and significantly reduced after 24 hours of treatment compared to baseline values in the levosimendan group (#cp<0.001). BNP was lower in the levosimendan group than in the dobutamine group (*cp<0.001). BNP – brain natriuretic peptide.
Multivariate regression analysis showed that compared to the dobutamine group, after adjusting for the patients’ age, gender, APACHE II, SOFA, and baseline of HFABP, TNI, BNP, LVEF, and CI, the levosimendan group had significantly decreased levels of HFABP, TNI, and BNP (p<0.001, p=0.03 and p<0.001, respectively, Tables 3–5), and increased LVEF and CI (p=0.03 and p<0.001, respectively, see Tables 6, 7)
Table 3.
Multivariate regression analysis for the effect of levosimendan on the HFABP.
| Variables | Paramenter estimate | Standardized estimate | Standard error | t-value | p-value |
|---|---|---|---|---|---|
| Intercept | 1.53 | 0.00 | 5.81 | 0.26 | 0.79 |
| Age | −0.01 | −0.03 | 0.04 | −0.22 | 0.83 |
| Gender | −1.73 | −0.21 | 1.26 | −1.37 | 0.18 |
| APACHEII | 0.01 | 0.02 | 0.15 | 0.09 | 0.93 |
| SOFA | 0.21 | 0.12 | 0.27 | 0.78 | 0.44 |
| Baseline HFABP | 0.19 | 0.20 | 0.17 | 4.40 | 0.25 |
| Levosimendan | −5.05 | −0.64 | 1.15 | 1.16 | <0.001 |
APACHEII – acute physiology and chronic health evaluation; SOFA – sequential organ failure assessment; HFABP – heart-type fatty acid binding protein.
Table 4.
Multivariate regression analysis for the effect of levosimendan on the TNI.
| Variables | Paramenter estimate | Standardized estimate | Standard error | t-value | p-value |
|---|---|---|---|---|---|
| Intercept | 0.23 | 0.00 | 0.12 | 1.94 | 0.06 |
| Age | −0.0002 | −0.04 | 0.001 | −0.26 | 0.80 |
| Gender | −0.06 | −0.26 | 0.04 | −1.57 | 0.13 |
| APACHEII | 0.001 | 0.04 | 0.01 | 0.26 | 0.79 |
| SOFA | 0.01 | 0.20 | 0.01 | 1.19 | 0.24 |
| Baseline TNI | −0.18 | −0.27 | 0.11 | −1.62 | 0.12 |
| Levosimendan | −0.08 | −0.37 | 0.03 | −2.34 | 0.03 |
APACHEII – acute physiology and chronic health evaluation; SOFA – sequential organ failure assessment; TNI – troponin I.
Table 5.
Multivariate regression analysis for the effect of levosimendan on the BNP.
| Variables | Paramenter estimate | Standardized estimate | Standard error | t-value | p-value |
|---|---|---|---|---|---|
| Intercept | 189.81 | 0.00 | 59.86 | 3.17 | 0.003 |
| Age | −0.73 | −0.13 | 0.44 | −1.66 | 0.11 |
| Gender | 34.67 | 0.19 | 14.48 | 2.39 | 0.02 |
| APACHEII | −1.41 | −0.07 | 1.62 | −0.87 | 0.39 |
| SOFA | −1.35 | −0.03 | 3.23 | −0.42 | 0.68 |
| Baseline BNP | −0.16 | −0.18 | 0.08 | −2.14 | 0.04 |
| Levosimendan | −142.05 | −0.83 | 13.46 | −10.55 | <0.001 |
APACHEI – acute physiology and chronic health evaluation; SOFA – sequential organ failure assessment; BNP – brain natriuretic peptide.
Table 6.
Multivariate regression analysis for the effect of levosimendan on the LVEF.
| Variables | Paramenter estimate | Standardized estimate | Standard error | t-value | p-value |
|---|---|---|---|---|---|
| Intercept | 43.20 | 0.00 | 11.55 | 3.74 | <0.001 |
| Age | 0.08 | 0.14 | 0.09 | 0.88 | 0.38 |
| Gender | 3.90 | 0.22 | 2.98 | 1.31 | 0.20 |
| APACHEII | −0.003 | −0.002 | 0.33 | −0.01 | 0.99 |
| SOFA | −0.016 | −0.004 | 0.64 | −0.02 | 0.98 |
| Baseline LVEF | −0.016 | −0.01 | 0.22 | −0.07 | 0.94 |
| Levosimendan | 6.41 | 0.38 | 2.78 | 2.31 | 0.03 |
APACHEII – acute physiology and chronic health evaluation; SOFA – sequential organ failure assessment; LEVF– left ventricular ejective fraction.
Table 7.
Multivariate regression analysis for the effect of levosimendan on the CI.
| Variables | Paramenter estimate | Standardized estimate | Standard error | t-value | p-value |
|---|---|---|---|---|---|
| Intercept | 5.13 | 0.00 | 0.82 | 6.29 | <0.001 |
| Age | −0.003 | −0.13 | 0.004 | −0.87 | 0.39 |
| Gender | −0.05 | −0.07 | 0.12 | −0.44 | 0.66 |
| APACHEII | −0.01 | −0.05 | 0.01 | −0.34 | 0.74 |
| SOFA | 0.04 | 0.21 | 0.03 | 1.46 | 0.15 |
| Baseline CI | −0.35 | −0.23 | 0.23 | −1.53 | 0.14 |
| Levosimendan | 0.43 | 0.55 | 0.11 | 3.81 | <0.001 |
APACHEII – acute physiology and chronic health evaluation; SOFA – sequential organ failure assessment; CI – cardiac index.
Discussion
Septic cardiomyopathy, a kind of cardiovascular dysfunction induced by severe sepsis and septic shock, is manifested by low cardiac output and is closely related to higher mortality in septic shock patients [16–18]. Inotropic agents including dobutamine and levosimendan may show benefits in the treatment of cardiovascular dysfunction induced by sepsis [10,19]. The major findings of the present study were that levosimendan increased LVSWI and LVEF, decreased EVLWI and lactate, and improved tissue perfusion, hemodynamics, and cardiac function. However, levosimendan did not reduce the days on mechanical ventilation, lengths of stay in ICU and hospital, or 28-day mortality.
Elevated concentrations of TNI and BNP are frequently observed in patients with severe sepsis and septic shock even in the absence of an acute coronary syndrome (ACS) [20,21]. Kandil et al. demonstrated the relationship between elevated BNP level and severity of sepsis regardless of congestive heart failure; their findings also supported the utility of BNP level as a marker for mortality in septic shock [22]. Studies have shown that TNI was sensitive and superior to creatinine kinase-MB (CK-MB) for the detection of myocardial injury in septic shock and after coronary angioplasty with or without stenting [20,23]. Hence, BNP and TNI have been identified as cardiac biomarkers and predictors of cardiac dysfunction and death in septic patients and successfully used in ICUs. However, it is difficult to distinguish septic patients with or without cardiac dysfunction related to septic shock via utilization of BNP alone [24]. Furthermore, Tiruvoipati et al. suggested TNI was related to lower ejection fraction and higher need for inotropic/vasopressor support, but did not independently predict mortality in critically ill patients with severe sepsis [25].
HFABP is a more sensitive and specific biomarker of myocardial injury than TNI and BNP, which make it superior to TNI and BNP for the assessment of recurrent or persistent myocardial injury [26,27]. Zhang et al. found that serum HFABP was frequently elevated among patients with severe sepsis and was associated with sepsis-related myocardial dysfunction, and elevated HFABP independently predicted 28-day mortality in severe sepsis [28].Therefore, HFABP, TNI, and BNP were utilized as biomarkers of myocardial injury for septic shock patients in the present study.
This study demonstrated that both dobutamine and levosimendan reduced the concentrations of biomarkers of myocardial injury in critically ill patients with septic shock. But HFABP, TNI, and BNP were lower in the levosimendan group than in the dobutamine group, which suggested that levosimendan could play a more important role in attenuating septic myocardial injury. Wu et al. [29] conducted a study in patients with acute myocardial infarction (AMI) who received emergency percutaneous coronary intervention (PCI) and found that the TNI concentration decreased much more in the levosimendan group than in the placebo group, suggesting that the myocardium was suffering less damage. In myocardium injury and stunning, the activation of the Ca2+-dependent protease results in degradation of TNI partially and selectively during reperfusion [30]. Because levosimendan has the ability to increase responsiveness to calcium while keeping the cytosolic Ca2+ concentration unchanged, levosimendan prevented the degradation of TNI, which could prevent the contractile dysfunction of myocardium stunning and damage [29].
Studies found that BNP levels were elevated in severe sepsis and septic shock patients and elevated BNP were related to myocardial dysfunction, global tissue hypoxia, and mortality [22,31]. Several mechanisms probably account for the elevated BNP levels in sepsis, including neuro-hormonal activation, volume resuscitation, and sepsis-induced biventricular dilation [32], as well as stimulation of lipopolysaccharide or pro-inflammatory cytokines, acute lung injury, and acute respiratory distress syndrome induced sepsis [33]. Levosimendan was found to have significantly decreased BNP levels at Day 1 and Day 3 in patients with decompensated heart failure (DHF) and renal dysfunction [34]. Feola et al. demonstrated that BNP concentrations decreased more significantly in a levosimendan group than a furosemide group [35]. Kyrzopoulos et al. suggested that the decreasing effect of levosimendan treatment on BNP was associated with its decreasing effect on end-diastolic cardiac wall tension [36]. Immunomodulatory and anti-inflammatory properties of levosimendan might also contribute to its effect on decreased BNP [37,38]. These probably mechanisms of decreased TNI and BNP concentrations could explain the changes of TNI and BNP in our study.
HFABP, a stable low molecular weight protein found in the cytoplasm of myocardial cells, is a sensitive and specific biomarker of myocardial injury. It is frequently elevated in patients with severe sepsis and septic shock and is associated with sepsis-related myocardial dysfunction. Elevated HFABP independently predicts 28-day mortality in severe sepsis and septic shock cases [28,39]. The probably mechanisms leading to elevated HFABP concentrations in sepsis and septic shock cases may be related to sepsis-induced myocardial dysfunction and elevated levels of free fatty acids due to an increased catabolism of glycogen and lipids during multiple organ dysfunction syndrome (MODS) associated inflammatory responses [40]. In addition, decreased renal function also contributes to the elevation of HFABP [41].
In this study, compared to dobutamine, levosimendan significantly decreased HFABP levels in patients with cardiomyopathy associated with severe sepsis and septic shock.
To date, however, the mechanisms of decreased HFABP concentrations remains unclear. There are several probably mechanisms. First, levosimendan improves inotropic function due to binding to the Ca2+ saturated troponin C of myocardial thin filament and diastolic function because it does not promote calcium flux into the cell [42]. The improvement in systolic and diastolic function and myocardial oxygen supply [43,44] atttributed to levosimendan may decrease HFABP concentration via preventing myocardial dysfunction associated sepsis. Second, the anti-inflammatory and anti-apoptotic effects [13,45] enable levosimendan to reduce catabolism of glycogen and lipids via mediating inflammatory responses and decreasing the level of HFABP during sepsis and septic shock. Finally, levosimendan decreases HFABP through reducing renal injury and improving renal function [34] and because levosimendan leads to augmentation of renal perfusion [46], increases in renal blood flow [47] and increases glomerular capillary surface area and glomerular filtration rate (GFR) [48].
The present study demonstrated that levosimendan had no adverse effects on hemodynamics, on the contrary, it elevated LVEF, SVI, and CI and did not increase the dose of norepinephrine needed. The absence of adverse effects on hemodynamics is in harmony with previous studies [49,50]. In addition, decreased EVLWI and lactate, and increased DO2I and VO2I in patients with myocardial dysfunction suggests that levosimendan improved pulmonary vascular permeability and tissue perfusion via elevated cardiac output, and the driving pressure of blood flow entering into microcirculation [51]. In our study, however, although there were no significant difference in mechanical ventilation time, ICU length of stay, hospital length of stay, or 28-day mortality between the two groups, all of these were still shorter in the levosimendan group than the dobutamine group.
Limitations of the study
There are several limitations in our study. First, we chose change in HFABP as the primary endpoint of this study. The number of septic shock patients investigated in our study was small and the study period was relative brief, therefore, the risk of positive results in a study with numerous secondary variables has to be taken into account.
Second, our study did not show a statistically significant difference in 28-day mortality between the two groups and the difference was 5.2%, suggesting that the effect size was much lower than that assumed in the sample size calculation. Post hoc sample size calculations showed that ≥1,305 patients per group would be required to show a statistically significant difference in 28-day mortality between the two groups. It is impossible to achieve a study like this in a single center; a multi-centers investigation would be required in the future.
Finally, it is also indispensable to make clear the molecular biologic mechanisms of decreased HFABP in septic shock patients treated by levosimendan.
Conclusions
Compared with dobutamine, this study demonstrated levosimendan reduced the level of biomarkers of myocardial injury including HFABP, TNI, and BNP, increased LVEF, strengthened systolic function, and improved systemic hemodynamics significantly in patients with septic shock; but there were no significant difference in duration of mechanical ventilation, length of stay in ICU, or 28-day mortality between the two treatment groups.
In conclusion, although levosimendan improved the biomarkers of myocardial injury and hemodynamic parameters, our study failed to show any improvement in clinically relevant outcomes like length of ICU stay, duration of mechanical ventilation, and 28-day mortality.
Therefore, further investigations are required to elucidate the fundamental mechanisms underlying these processes.
Acknowledgements
The authors thank Wenjie Liang, MD, and Conghua Ji, MD, MPH, for their critical revision and statistical analysis.
Footnotes
Source of support: Financial support for this research was provided by the Science and Technology Department of Zhejiang Province, China (2013C33198
References
- 1.Tsiotou AG, Sakorafas GH, Anagnostopoulos G, et al. Septic shock; Current pathogenetic concepts from a clinical perspective. Med Sci Monit. 2005;11(3):RA76–85. [PubMed] [Google Scholar]
- 2.Parrillo JE. Myocardial depression during septic shock in humans. Crit Care Med. 1990;18:1183–84. doi: 10.1097/00003246-199010000-00028. [DOI] [PubMed] [Google Scholar]
- 3.Charalampos P, Dimitrios V, Federico F, et al. Levosimendan in critical illness: A literature review. J Clin Med Res. 2014;6:75–85. doi: 10.14740/jocmr1702w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ryota S, Michitaka N. A review of sepsis-induced cardiomyopathy. J Intensive Care. 2015;3:48. doi: 10.1186/s40560-015-0112-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tavernier B, Mebazaa A, Mateo P, et al. Phosphorylation-dependent alteration in Ca2+ sensitivity but normal mitochondrial function in septic heart. Am J Respir Crit Care Med. 2001;163:362–67. doi: 10.1164/ajrccm.163.2.2002128. [DOI] [PubMed] [Google Scholar]
- 6.Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N En J Med. 2001;345:1368–77. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 7.ProCESS Investigators. Yearly DM, Kellum JA, Huang DT, et al. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;370:1683–93. doi: 10.1056/NEJMoa1401602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.ARISE Investigators, ANZICS Clinical trial Group. Peake SL, Delaney A, Balley M, et al. Goal-directed resuscitation for patients with early septic shock. N Engl J Med. 2014;371:1496–506. doi: 10.1056/NEJMoa1404380. [DOI] [PubMed] [Google Scholar]
- 9.Mouncey PR, Osborn TM, Power GS, et al. Trial of early, goal-directed resuscitation for septic shock. N Engl J Med. 2015;372:1301–11. doi: 10.1056/NEJMoa1500896. [DOI] [PubMed] [Google Scholar]
- 10.Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41:580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 11.Hernandez G, Bruhn A, Luengo C, et al. Effects of dobutamine on systemic, regional and microcirculatory perfusion parameters in septic shock: A randomized, placebo-controlled, double-blind, crossover study. Intensive Care Med. 2013;39:1435–43. doi: 10.1007/s00134-013-2982-0. [DOI] [PubMed] [Google Scholar]
- 12.Wilkman E, Kaukonen KM, Pettila V, et al. Association between inotrope treatment and 90-day mortality in patients with septic shock. Acta Anaesthesiol Scand. 2013;57:431–42. doi: 10.1111/aas.12056. [DOI] [PubMed] [Google Scholar]
- 13.Parissis JT, Adamopoulos S, Antoniades C, et al. Effects of levosimendan on circulating pro-inflammatory cytokines and soluble apoptosis mediators in patients with decompensated advanced heart failure. Am J Cardiol. 2004;93:1309–12. doi: 10.1016/j.amjcard.2004.01.073. [DOI] [PubMed] [Google Scholar]
- 14.Landoni G, Mizzi A, Biondi-Zoccai G, et al. Levosimendan reduces mortality in critically ill patients. A meta-analysis of randomized controlled studies. Minerva Anestesiol. 2010;76:276–86. [PubMed] [Google Scholar]
- 15.Yi GY, Li JX, Zhang J, et al. Repetitive infusion of levosimendan in patients with chronic heart failure: A meta-analysis. Med Sci Monit. 2015;21:895–901. doi: 10.12659/MSM.893736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spies C, Haude V, Fitzner R, et al. Serumcardiac troponin T as a prognostic marker in early sepsis. Chest. 1998;113:1055–63. doi: 10.1378/chest.113.4.1055. [DOI] [PubMed] [Google Scholar]
- 17.Rudiger A, Singer M. Mechanisms of sepsis-induced cardiac dysfunction. Crit Care Med. 2007;35:1599–608. doi: 10.1097/01.CCM.0000266683.64081.02. [DOI] [PubMed] [Google Scholar]
- 18.Charpentier J, Luyt CE, Fulla Y, et al. Brain natriuretic peptide: Amarker ofmyocardial dysfunction and prognosis during severe sepsis. Crit Care Med. 2004;32:660–65. doi: 10.1097/01.ccm.0000114827.93410.d8. [DOI] [PubMed] [Google Scholar]
- 19.Barraud D, Faivre V, Damy T, et al. Levosimendan restores both systolic and diastolic cardiac performance in lipopolysaccharide-treated rabbits: Comparison with dobutamine and milrinone. Crit Care Med. 2007;35:1376–82. doi: 10.1097/01.CCM.0000261889.18102.84. [DOI] [PubMed] [Google Scholar]
- 20.Ammann P, Fehr T, Minder EI, et al. Elevation of troponin I in sepsis and septic shock. Intensive Care Med. 2001;27:965–69. doi: 10.1007/s001340100920. [DOI] [PubMed] [Google Scholar]
- 21.Witthaut R, Busch C, Fraunberger P, et al. Plasma atrial natriuretic peptide and brain natriuretic peptide are increased in septic shock: Impact of interleukin-6 and sepsis-associated left ventricular dysfunction. Intensive Care Med. 2003;29:1696–702. doi: 10.1007/s00134-003-1910-0. [DOI] [PubMed] [Google Scholar]
- 22.Kandil E, Burack J, Sawas A, et al. B-type natriuretic peptide: A biomarker for the diagnosis and risk stratification of patients with septic shock. Arch Surg. 2008;143:242–46. doi: 10.1001/archsurg.2007.69. [DOI] [PubMed] [Google Scholar]
- 23.Saadeddin SM, Habbab MA, Sobki SH, Ferns SG. Detection of minor myocardial injury after successful percutaneous transluminal coronary angioplasty with or without stenting. Med Sci Monit. 2000;6:708–71. [PubMed] [Google Scholar]
- 24.McLean AS, Huang SJ, Hyams S, et al. Prognostic values of B-type natriuretic peptide in severe sepsis and septic shock. Crit Care Med. 2007;35:1019–26. doi: 10.1097/01.CCM.0000259469.24364.31. [DOI] [PubMed] [Google Scholar]
- 25.Tiruvoipati R, Sultana N, Lewis D. Cardiac troponin I does not independently predict mortality in critically ill patients with severe sepsis. Emerg Med Australas. 2012;24:151–58. doi: 10.1111/j.1742-6723.2011.01530.x. [DOI] [PubMed] [Google Scholar]
- 26.Dekker MS, Mosterd A, van ‘t Hof AW, et al. Novel biochemical markers in suspected acute coronary syndrome: Systematic review and critical appraisal. Heart. 2010;96:1001–10. doi: 10.1136/hrt.2009.189886. [DOI] [PubMed] [Google Scholar]
- 27.Lackner KJ. Laboratory diagnostics of myocardial infarction-troponins and beyond. Clin Chem Lab Med. 2013;51:83–90. doi: 10.1515/cclm-2012-0572. [DOI] [PubMed] [Google Scholar]
- 28.Zhang ZC, Dai HY, Yu YH, et al. Usefulness of heart-type fatty acid binding protein in patients with severe sepsis. J Crit Care. 2012;27:415.e13–18. doi: 10.1016/j.jcrc.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 29.Wu XU, Wu J, Yan XF, Zhang YZ. Enhancement of myocardial function and reduction of injury with levosimendan after percutaneous coronary intervention for acute myocardial infarction: A pilot study. Cardiology. 2014;128:202–8. doi: 10.1159/000360933. [DOI] [PubMed] [Google Scholar]
- 30.Gao WD, Atar D, Liu Y, et al. Role of troponin I proteolysis in the pathogenesis of stunned myocardium. Circ Res. 1997;80:393–99. [PubMed] [Google Scholar]
- 31.Rivers EP, McCord J, Otero R, et al. Clinical utility of B-type natriuretic peptide in early severe sepsis and septic shock. J Intensive Care Med. 2007;22:363–73. doi: 10.1177/0885066607307523. [DOI] [PubMed] [Google Scholar]
- 32.Court O, Kumar A, Parrillo JE, Kumar A. Clinical review: Myocardial depression in sepsis and septic shock. Crit Care. 2002;6:500–8. doi: 10.1186/cc1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maeder M, Fehr T, Rickli H, Ammann P. Sepsis-associated myocardial dysfunction: Diagnostic and prognostic impact of cardiac troponins and natriuretic peptides. Chest. 2006;129:1349–66. doi: 10.1378/chest.129.5.1349. [DOI] [PubMed] [Google Scholar]
- 34.Hou ZQ, Sun ZX, Su CY, et al. Effect of levosimendan on estimated glomerular filtration rate in hospitalized patients with decompensated heart failure and renal dysfunction. Cardiovasc Ther. 2013;31:108–14. doi: 10.1111/1755-5922.12001. [DOI] [PubMed] [Google Scholar]
- 35.Feola M, Lombardo E, Taglieri E, et al. Effects of levosimendan/furosemide infusion on plasma brain natriuretic peptide, echocardiographic parameters and cardiac output in end-stage heart failure patients. Med Sci Monit. 2011;17(3):PI17–13. doi: 10.12659/MSM.881433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kyrzopoulos S, Adamopoulos S, Parissis JT, et al. Levosimendan reduces plasma B-type natriuretic peptide and interleukin 6, and improves central hemodynamics in severe heart failure patients. Int J Cardiol. 2005;99:409–13. doi: 10.1016/j.ijcard.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 37.Erbuyun K, Vatansever S, Tok D, et al. Effects of levosimendan and dobutamine on experimental acute lung injury in rats. Acta Histochem. 2009;111:404–14. doi: 10.1016/j.acthis.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 38.Hasslacher J, Bijuklic K, Bertocchi C, et al. Levosimendan inhibits release of reactive oxygen species in polymorphonuclear leukocytes in vitro and in patients with acute heart failure and septic shock: A prospective observational study. Crit Care. 2011;15:R166. doi: 10.1186/cc10307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jo YH, Kim K, Lee JH, et al. Heart-type fatty acid-binding protein as a prognostic factor in patients with severe sepsis and septic shock. Am J Emerg Med. 2012;30:1749–55. doi: 10.1016/j.ajem.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 40.Yan GT, Lin J, Hao XH, et al. Heart-type fatty acid-binding protein is a useful marker for organ dysfunction and leptin alleviates sepsisinduced organ injuries by restraining its tissue levels. Eur J Pharmacol. 2009;616:244–50. doi: 10.1016/j.ejphar.2009.06.039. [DOI] [PubMed] [Google Scholar]
- 41.Tanaka T, Hirota Y, Sohmiya K, et al. Serum and urinary human heart fatty acid-binding protein in acute myocardial infarction. Clin Biochem. 1991;24:195–201. doi: 10.1016/0009-9120(91)90571-u. [DOI] [PubMed] [Google Scholar]
- 42.Haikala H, Nissinen E, Etemadzadeh E, et al. Troponin C-mediated calcium sensitization induced by levosimendan does not impair relaxation. J Cardiovasc Pharmacol. 1995;25:794–801. doi: 10.1097/00005344-199505000-00016. [DOI] [PubMed] [Google Scholar]
- 43.Ikonomidis I, Parissis JT, Paraskevaidis I, et al. Effects of levosimendan on coronary artery flow and cardiac performance in patients with advanced heart failure. Eur J Heart Fail. 2007;9:1172–77. doi: 10.1016/j.ejheart.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 44.Michaels AD, McKeown B, Kostal M, et al. Effects of intravenous levosimendan on human coronary vasomotor regulation, left ventricular wall stress, and myocardial oxygen uptake. Circulation. 2005;111:1504–9. doi: 10.1161/01.CIR.0000159252.82444.22. [DOI] [PubMed] [Google Scholar]
- 45.Latva-Hirvela J, Kyto V, Saraste A, et al. Effects of levosimendan in experimental acute coxsackievirus myocarditis. Eur J Clin Invest. 2009;39:876–82. doi: 10.1111/j.1365-2362.2009.02202.x. [DOI] [PubMed] [Google Scholar]
- 46.Yilmaz MB, Yalta K, Yontar C, et al. Levosimendan improves renal function in patients with acute decompensated heart failure: Comparison with dobutamine. Cardiovasc Drugs Ther. 2007;21:431–35. doi: 10.1007/s10557-007-6066-7. [DOI] [PubMed] [Google Scholar]
- 47.Fotbolcu H, Duman D. A promising new inotrope: Levosimendan. Anadolu Kardiyol Derg. 2010;10:176–82. doi: 10.5152/akd.2010.045. [DOI] [PubMed] [Google Scholar]
- 48.Zager RA, Johnson AC, Lund S, et al. Levosimendan protects against experimental endotoxemic acute renal failure. Am J Physiol Renal Physiol. 2006;290:F1453–62. doi: 10.1152/ajprenal.00485.2005. [DOI] [PubMed] [Google Scholar]
- 49.Morelli A, De Castro S, Teboul JL, et al. Effects of levosimendan on systemic and regional hemodynamics in septic myocardial depression. Intensive Care Med. 2005;31:638–44. doi: 10.1007/s00134-005-2619-z. [DOI] [PubMed] [Google Scholar]
- 50.Matejovic M, Krouzecky A, Radej J, Novak I. Successful reversal of resistent hypodynamic septic shock with levosimendan. Acta Anaesthesiol Scand. 2005;49:127–28. doi: 10.1111/j.1399-6576.2005.00541.x. [DOI] [PubMed] [Google Scholar]
- 51.Morelli A, Donati A, Ertmer C, et al. Levosimendan for resuscitating the microcirculation in patients with septic shock: A randomized controlled study. Critical Care. 2010;14:R232. doi: 10.1186/cc9387. [DOI] [PMC free article] [PubMed] [Google Scholar]