Highlight
In family Chenopodiaceae, two unique forms of single cell C4 photosynthesis with different evolutionary origins show convergence in structural and biochemical expression of C4 traits during leaf ontogeny.
Key words: Bienertia sinuspersici, C4 plants, CO2 compensation point, development, immunolocalization, in situ hybridization, leaf anatomy, single cell C4 photosynthesis, Suaeda aralocaspica, ultrastructure.
Abstract
Temporal and spatial patterns of photosynthetic enzyme expression and structural maturation of chlorenchyma cells along longitudinal developmental gradients were characterized in young leaves of two single cell C4 species, Bienertia sinuspersici and Suaeda aralocaspica. Both species partition photosynthetic functions between distinct intracellular domains. In the C4-C domain, C4 acids are formed in the C4 cycle during capture of atmospheric CO2 by phosphoenolpyruvate carboxylase. In the C4-D domain, CO2 released in the C4 cycle via mitochondrial NAD-malic enzyme is refixed by Rubisco. Despite striking differences in origin and intracellular positioning of domains, these species show strong convergence in C4 developmental patterns. Both progress through a gradual developmental transition towards full C4 photosynthesis, with an associated increase in levels of photosynthetic enzymes. Analysis of longitudinal sections showed undeveloped domains at the leaf base, with Rubisco rbcL mRNA and protein contained within all chloroplasts. The two domains were first distinguishable in chlorenchyma cells at the leaf mid-regions, but still contained structurally similar chloroplasts with equivalent amounts of rbcL mRNA and protein; while mitochondria had become confined to just one domain (proto-C4-D). The C4 state was fully formed towards the leaf tips, Rubisco transcripts and protein were compartmentalized specifically to structurally distinct chloroplasts in the C4-D domains indicating selective regulation of Rubisco expression may occur by control of transcription or stability of rbcL mRNA. Determination of CO2 compensation points showed young leaves were not functionally C4, consistent with cytological observations of the developmental progression from C3 default to intermediate to C4 photosynthesis.
Introduction
Carbon gain through photosynthesis is limited in plants due to high levels of photorespiration, a metabolically wasteful process which is prominent in warm climates and under conditions of reduced water availability. Some plant species have evolved the distinct CO2 concentrating mechanism known as C4 photosynthesis, which suppresses photorespiration. The C4 pathway enhances the photosynthetic capacity of these plants relative to other plants that directly assimilate atmospheric CO2 via the C3 pathway (Edwards and Walker, 1983; Hatch, 1987). C4 photosynthesis has evolved independently more than 60 times across a wide range of monocot and dicot species (Edwards and Voznesenskaya, 2011; Sage et al., 2011). An important requirement for C4 function is spatial separation of two biochemical processes: the initial capture of atmospheric CO2 during the carboxylation phase of the C4 cycle occurs within one compartment (the carboxylation domain, C4-C), while the decarboxylation phase donates CO2 to ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) within a second compartment (the decarboxylation domain, C4-D). There are many structural and biochemical variations associated with anatomical development and function of the two domains (Edwards and Walker, 1983; Hatch, 1987; Edwards and Voznesenskaya, 2011; Sage et al., 2011). In its most common form, this separation is achieved by the dual-cell C4 system called Kranz anatomy. The leaves of Kranz species possess two biochemically and anatomically distinct photosynthetic cell types: an inner layer called bundle sheath (BS) or Kranz cells, and an outer layer of palisade mesophyll (M) cells. In the leaves of Kranz C4 plants, the C4-C domain is specifically compartmentalized to the M cells, while the C4-D domain is compartmentalized to BS cells (Edwards and Walker, 1983; Hatch, 1987; Kanai and Edwards, 1999).
Remarkably, a few terrestrial C4 species that lack Kranz anatomy have been shown to function by development of C4-C and C4-D domains within different parts of individual chlorenchyma cells (Edwards et al., 2004; Freitag and Stichler, 2000; Voznesenskaya et al., 2001, 2002; Akhani et al., 2005; Chuong et al., 2006; Edwards and Voznesenskaya, 2011). These unique species occur only in subfamily Suaedoideae (family Chenopodiaceae). Within this subfamily, independent evolutionary acquisition of C4 photosynthesis is considered to have occurred four times. This includes two origins of single cell C4 (SC-C4) with different forms of anatomy in genus Bienertia versus Suaeda aralocaspica (syn.=Borszczowia aralocaspica), as well as two separate origins of distinctive Kranz C4 anatomies between Suaeda sections Salsina sensu lato (s.l.) versus Schoberia (Schütze et al., 2003; Kapralov et al., 2006). All four lineages utilize a mitochondrial NAD-malic enzyme (NAD-ME) for the decarboxylation phase of the C4 cycle in the C4-D domain.
There are major structural differences between the two domains of S. aralocaspica and Bienertia species. In S. aralocaspica, biochemically dimorphic chloroplasts are partitioned between opposite ends of the elongated chlorenchyma cells; the distal end towards the exterior is the C4-C domain, and the proximal end towards the interior of the leaf is the C4-D domain. In contrast, in Bienertia species the dimorphic chloroplasts are partitioned between the peripheral cytoplasm (the peripheral compartment, PC) that functions as the C4-C domain and a ball-like central cytoplasmic compartment (CCC) in the center of the cell that functions as the C4-D domain, with the two compartments connected by cytoplasmic channels through the vacuole (Voznesenskaya et al., 2001, 2002; Edwards and Voznesenskaya, 2011).
In all forms of C4 that have been studied to date, during leaf development a progression occurs in structural and biochemical differentiation, which results in fully functional C4 photosynthesis. To understand the process of C4 development, it is essential to identify the regulatory processes and factors responsible for these transitional steps. Precise methods are needed for defining the sequence of these transitions and exactly where they occur in different types of C4. In Kranz-type C4 species, this is being approached through analyses of changes during development in mesophyll and bundle sheath cells along leaf longitudinal gradients to ultimately identify genetic components of C4 form and function; for a review, see Covshoff et al. (2014). This includes studies in representatives from families Poaceae (maize and Arundinella hirta), Cyperaceae (three anatomical types), Amaranthaceae (Amaranthus hypochondriacus), Chenopodiaceae (Suaeda taxifolia, S. eltonica) and Cleomaceae (Cleome angustifolia, Gynandropsis gynandra), which show some differences in development (Langdale et al., 1988; Wang et al., 1992; Dengler et al., 1995; Soros and Dengler, 2001; Wakayama et al., 2003; Li et al., 2010; Majeran et al., 2010; Koteyeva et al., 2011, 2014; Nelson, 2011; Pick et al., 2011; Aubry et al., 2014; Külahoglu et al., 2014).
In the current work, an analogous approach was used to analyze chlorenchyma development along longitudinal leaf gradients in two species, Bienertia sinuspersici and Suaeda aralocaspica, which have independent evolutionary origins and anatomical forms of SC-C4. Taken together, the integrated structural, biochemical, and functional analyses presented here reveal a stepwise progression in the development of C4 type chlorenchyma cells. This study provides new insights into processes responsible for the specialized photosynthetic characteristics of these unique plants. The findings highlight the dramatic differences in development of single-cell C4 compared to sister Kranz-type Suaeda species, and they suggest diversity exists in how regulatory factors control the evolution of different forms of C4.
Materials and methods
Plant material
The SC-C4 species Bienertia sinuspersici Akhani and Suaeda aralocaspica (Bunge) Freitag & Schütze (syn.=Borszczowia aralocaspica Bunge) were used in this study. These are classified as C4 structural forms called Bienertioid and Borszczowoid, respectively (Edwards and Voznesenskaya, 2011). Seeds of S. aralocaspica, originally collected in Kazakhstan, were germinated on moist paper towels in Petri dishes for 1–2 d at 22°C. After the radical appeared, seeds were transferred to a soil mixture of one part potting soil, two parts sand, 0.25 part gypsum, 0.5 part Perlite, and 0.5 part clay. B. sinuspersici Akhani (seeds originally from Kuwait) was propagated from cuttings in rooting MS media and transferred to potting soil according to the protocol of Smith et al. (2009).
Plants were grown in a growth chamber (model GC-16; Enconair Ecological Chambers Inc., Winnipeg, Canada) under a 14/10h 25/18°C day/night cycle under mid-day PPFD of ~400 μmol quanta m–2 s–1, and 50% relative humidity for ~2 months. Plants were fertilized once a week with Peter’s Professional (20:20:20; Scotts Miracle-Gro Co., Marysville, OH, USA) and watered once a week with 150mM NaCl.
For microscopy and biochemical analyses, leaf samples were taken from vegetative branches on ~2 month old plants. Mature leaves of B. sinuspersici are 2.5–3cm in length, and S. aralocaspica 1.5–2cm in length; for studies on transitions along a longitudinal gradient young leaves 0.5–0.7cm long were used (see Supplementary Fig. S1, available at JXB online, for a general view of mature and young leaves).
Voucher specimens are available at the Marion Ownbey Herbarium, Washington State University: Suaeda aralocaspica (E. Voznesenskaya 22), April 2006, WS369790 and Bienertia sinuspersici (E. Voznesenskaya 85), May 2013, WS386421.
Light and electron microscopy
Developmental studies were carried out on young expanding leaves and on mature leaves that were fully expanded. For structural studies, for each developmental stage sampled, three replicates were taken from three independent plants for each species (i.e. a total of nine samples for each species). Vegetative shoot apices with several leaf primordia (up to 0.3cm), and young leaves (0.5–0.7cm in length), were harvested and prepared for longitudinal and cross sectioning.
Sample preparation for light microscopy (LM) and transmission electron microscopy (TEM) was carried out according to Koteyeva et al. (2011). An Olympus BH-2 (Olympus Optical Co. Ltd) light microscope equipped with LM Digital Camera and Software (Jenoptik ProgRes Camera, C12plus, Jena, Germany) was used for observation and collection of images on LM level. Hitachi H-600 (Hitachi Scientific Instruments, Tokyo, Japan), and FEI Tecnai G2 (Field Emission Instruments Company, Hillsboro, OR, USA) equipped with Eagle FP 5271/82 4K HR200KV digital camera transmission electron microscopes were used for TEM studies.
Observations and image capture of vegetative shoot apices with the youngest primordia were obtained by scanning electron microscopy, using the low vacuum mode on an FEI SEM Quanta 200F (FEI Company, Field Emission Instruments, Hillsboro, OR, USA).
Observations of vascular development were obtained from leaves of different ages, from the youngest primordia (starting from ~0.3mm long) to fully expanded leaves (2.5–3cm for B. sinuspersici and 1.5–2cm for S. aralocaspica). These samples were cleared in 70% ethanol (v/v) until chlorophyll was removed, treated with 5% (w/v) NaOH overnight, and then rinsed three times in water. At least five vegetative shoot tips with different-aged leaves from two or three different plants were used. The leaves were mounted in water and examined under UV light (with DAPI filter) on a Fluorescence Microscope Leica DMFSA (Leica Microsystems Wetzlar GmbH, Germany) using autofluorescence of lignified tracheary elements of the xylem.
In situ immunolocalization
Sample preparation and immunolocalization by LM and TEM was carried out on longitudinal sections of leaves 0.5–0.7cm long according to the procedures in Koteyeva et al. (2011). Antibodies used (all polyclonal raised in rabbit) were anti-spinach Rubisco (rbcL) IgG (courtesy of B. McFadden), and commercially available anti-maize PEPC IgG (Chemicon, Temecula, CA, USA). The density of labeling was determined by counting the gold particles on digital electron micrographs using the UTHSCSA image analysis program and calculating the number per unit area (μm2). Additional details are available in Supplementary Methods S1 available at JXB online.
In situ localization of mRNAs encoding Rubisco large subunit protein
Sample preparation and in situ hybridization was carried out on longitudinal sections of leaves 0.5–0.7cm long according to the procedures in Koteyeva et al. (2011). Sense and antisense RNA probes for Rubisco rbcL were generated from pBlsl (which contains a 600-bp HindIII fragment from the central coding region of the amaranth rbcL gene) using a modification of procedures described in Wang et al. (1992). Sense and antisense transcripts were synthesized and labeled in vitro with Biotin-11-UTP (Roche) using T7 or T3 polymerase (Roche). Samples of young leaves were fixed in FAA (50% ethanol, 5% glacial acetic acid, 10% formalin) fixative at room temperature overnight. After ethanol and t-Butyl alcohol dehydration, samples were embedded in Paraplast Plus. The paraffin-embedded samples were sectioned (thickness 5–10 µm) using a rotary microtome; sections were mounted to poly-L-lysine-coated slides, dried, and stored at 4°C overnight. After deparaffinization by xylene, the sections were rehydrated through an ethanol series, incubated in 0.2M HCl for 20min at room temperature (RT), and rinsed in H2O. Slides were then incubated in 2×SSC (1×SSC is 0.15M NaCl, 0.15M sodium citrate) at 70°C for 30min and rinsed by H2O. Treatment of sections with proteinase K (1 µg ml−1 in TE: 100mM TRIS pH 8.0, 50mM EDTA) for 15min at 37°C was followed by a brief rinse in PBS (10mM phosphate buffer, 2.7mM KCl, 137mM NaCl, pH 7.4), blocking in glycine (2mg ml–1 in PBS) for 2min at RT, and then by fixation for 20min in 4% formaldehyde. The sections were incubated in 2×SSC for 10min at RT, and then placed into prehybridization medium [containing 1.25× in situ salts (10× salts: 3M NaCl, 0.1M Tris, 0.1M NaHPO4, 50mM EDTA, pH 6.8), 50% deionized formamide, 1mg ml−1 tRNA, 125mM DTT, 0.5mg ml−1 polyA] overnight at 50°C. The probes were first heated at 75°C for 30s and mixed with prehybridization medium with a final transcript concentration of 0.5 µg ml–1. Hybridizations with the Biotin-labelled transcripts were performed at 50°C overnight in a moist incubation chamber. The slides were then subjected to the following series of washes: prewarmed at 37°C in 2×SSC for 10min; 2×SSC for 1h at RT; 1×SSC for 1h at RT; 0.5×SSC for 30min at 42°C; 0.5×SSC for 30min at RT. Hybridized transcripts were detected by streptavidin–alkaline phosphate conjugate (NeutrAvidin; Pierce) using blocking buffer (100mM TRIS–HCl, pH 7.5, 150mM NaCl). The final detection step was carried out by nitroblue tetrazolium chloride and 5-bromo-4-chloro-3-indolyl phosphate (Sigma). Reactions were stopped by placing slides in 1×PBS and the sections were mounted in 50% glycerol in PBS. Observations and image capture were performed using an Olympus BH-2 light microscope equipped with LM Digital Camera and Software.
Western blot analysis
Analyses were made to determine accumulation of photosynthetic enzymes in samples of young leaves 0.5–0.7cm long divided into three sections (base, middle and tip) compared to mature leaves. Western blots were performed using anti-Amaranthus hypochondriacus NAD-malic enzyme (NAD-ME) IgG against the 65 KDa α subunit (Long and Berry, 1996) (1:5000), anti-Zea mays PEPC IgG (1:100 000), anti-Zea mays pyruvate,Pi dikinase (PPDK) IgG (courtesy of T. Sugiyama) (1:5000), anti-Amaranthus hypochondriacus Rubisco SSU IgG (courtesy of J. Berry) (1:5000); or anti- Spinacia oleracea Rubisco rbcL IgG (courtesy of B. McFadden) (1:10 000) overnight at 4°C. For details see Supplementary Methods S1, and Supplementary Fig. S2 for a loading control with protein samples (10 µg) separated by 10% (w/v) SDS-PAGE.
Mass spectrometric measurements
A membrane inlet mass spectrometer (DELTA V Plus; Thermo Scientific) connected to a closed leaf chamber via a membrane inlet was used to measure rates of isotopic CO2 exchange and CO2 compensation points (Γ), as described previously (Maxwell et al., 1998; Walker and Cousins, 2013). Leaves were detached from different branches and placed into the chamber at ambient CO2 and O2 partial pressures (Supplementary Fig. S1C–F). For analysis with B. sinuspersici, 3–4 mature leaves or 16–18 young leaves (length ~0.5cm) were used; for analysis with S. aralocaspica, 5–8 mature leaves or 30–40 young leaves (length ~0.6cm) were used. The leaf chamber was flushed with mixture of nitrogen and corresponding oxygen partial pressure and then sealed. After sitting for 5min in the dark, the leaves were illuminated with 1000 µmol quanta m–2 s–1, with a chamber temperature of 25°C controlled by a circulating water bath. Net CO2 assimilation was followed in the sealed chamber by measuring the change in CO2 concentration until the Γ was reached (i.e. when the amount of CO2 assimilated by photosynthesis was balanced with the amount of CO2 released by respiration and photorespiration, Г was recorded). The response of Γ to changes in O2 was measured at 10%, 20% and 40% (at partial pressures of 0.092, 0.184 and 0.369mbar O2, respectively). The rate of dark respiration was calculated by measuring the amount of CO2 release in the dark. The zero set point was recorded, before and after each measure, by flushing the chamber with a corresponding CO2-free nitrogen and O2 mixture.
Statistical analysis
Where indicated, standard errors were determined, and analysis of variance (ANOVA) was performed with Statistica 7.0 software (StatSoft, Inc.). Tukey’s HSD (honest significant difference) test were used to analyze differences between amounts of gold particles, intensities of bands in western blots and Г and dark respiration values at different stages of leaf development. All analyses were performed at the 95% significance level.
Results
Structure of shoot apex and early leaf development
Scanning electron microscopy and light microscopy were used to examine the morphology and anatomy of vegetative apical shoot meristems during active organogenesis of B. sinuspersici and S. aralocaspica in order to show the earliest stages of leaf initiation (Supplementary Fig. S2). The vegetative apices (apical meristems) of both species are very similar in shape and composition (Supplementary Fig. S2A–C, E, F). Leaf primordia are initiated alternately in both species forming the buds with more or less loosely packed leaves in B. sinuspersici and tightly packed leaves in S. aralocaspica (Supplementary Fig. S3 illustrates differences in branches and in leaf morphology of young versus mature leaves of the two species).
Observations on longitudinal sections of the youngest leaves (length 0.1–0.3mm) showed differences in the origin of chlorenchyma cells from the ground meristem. Periclinal divisions of subepidermal cells give rise to two layers of chlorenchyma in B. sinuspersici, versus a hypodermal and a chlorenchyma layer in S. aralocaspica (not shown).
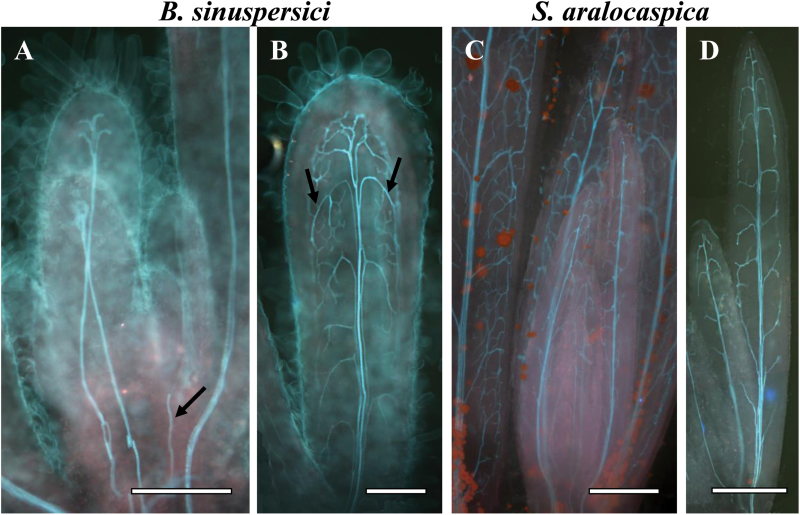
The development of vascular tissue was studied in young, cleared leaves of different lengths. The analysis showed that the central vein was formed acropetally from the base to tip in both species, beginning in leaves that were ~0.5mm long (a forming vein in B. sinuspersici is illustrated in Fig. 1A with an arrow). Peripheral veins were developed basipetally in both species (indicated by the label in Fig. 1B), starting from the tip of 0.7–1mm leaves with descending interconnecting loops (Fig. 1A, C). This was followed by dense reticulation that progressed along with continuing leaf growth (Fig. 1B, D).
Fig. 1.
Cleared young leaves of Bienertia sinuspersici (A, B) and Suaeda aralocaspica (C, D) viewed under UV light at different stages of vein initiation. There is acropetal formation of the central vein towards the leaf tip (illustrated by arrow in panel A) and basipetal direction of lateral vascular vein development from the tip to base of the leaf (illustrated by arrows in panel B). Scale bars: 250 μm for A, B; 500 μm for C, D.
Chlorenchyma developmental gradient along the leaf: light and transmission electron microscopy
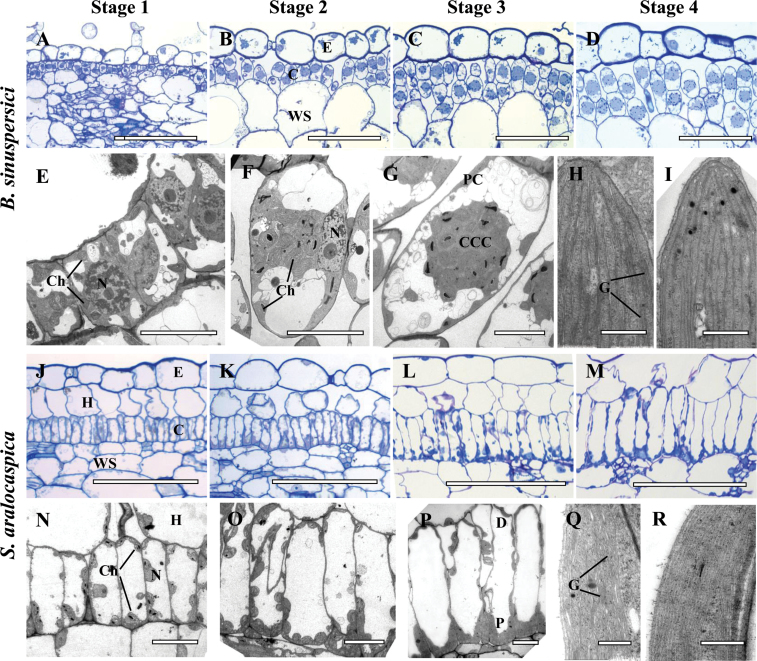
The structural differentiation of chlorenchyma cells was examined using longitudinal sections of young leaves (0.5–0.7cm long) that possess a basipetal developmental gradient with fully differentiated tissues at the tip (Fig. 2). Clear differences were observed in chlorenchyma cell differentiation between the tip and the base of leaves with a gradual developmental transition in structural specialization that was coupled with cell expansion. For comparison of the results the developmental progression was divided into four stages, with numbering 1 through 4 beginning at the basal region and towards the tip of the leaves (Fig. 2A–D and E–I for B. sinuspersici, J–M and N–R for S. aralocaspica).
Fig. 2.
Light and electron microscopy of Bienertia sinuspersici (A–I) and Suaeda aralocaspica (J–R) with longitudinal sections of young leaves. The sections show a basipetal developmental gradient with gradual structural differentiation of SC-C4 chlorenchyma cells with four stages along the longitudinal gradient: Stage 1 (A, E, J, N), Stage 2 (B, F, K, O), Stage 3 (C, G, L, P) and Stage 4 (D, H, I, M, Q, R). Panels A–D from B. sinuspersici and J–M from S. aralocaspica are light microscopy micrographs of longitudinal sections showing the development of chlorenchyma cell lineages from the base (A, J) to the tip (D, M) of young leaves, with the direction of maturation from left to right. Panels E–G from B. sinuspersici and N–P from S. aralocaspica, are TEM micrographs showing internal structural development within a single chlorenchyma cell along the longitudinal gradient from the base (E, N) to the middle region (G, P) of a young leaf. Panels H, I, show the ultrastructure of chloroplasts in the central cytoplasmic compartment, CCC (panel H) and the periphery (panel I) within a single chlorenchyma cell at the tip of a young B. sinuspersici leaf. Panels Q, R show ultrastructure of chloroplasts within the proximal (Q) and distal (R) regions of chlorenchyma cell at the tip of a young S. aralocaspica leaf. E, epidermis; C, chlorenchyma; CCC, central cytoplasmic compartment; D, distal end; G, grana; H, hypoderm; N, nucleus; P, proximal end; WS, water storage. Scale bars: 100 μm for A–D and J–M; 10 μm for E–G and N–P; 0.5μm for H, I, Q, R. (This figure is available in color at JXB online.)
The chlorenchyma cells near the leaf base (Stage 1) were found to be small and uniform. These cells undergo periclinal and anticlinal divisions in B. sinuspersici (Fig. 2A, E), and only anticlinal division in S. aralocaspica (Fig. 2J, N). The peripheral vascular tissues are immature at the leaf base, consisting of xylem elements in the process of differentiation, and undifferentiated phloem elements (not shown).
In B. sinuspersici, Stage 1 (at the leaf base) chlorenchyma cells have a dense cytoplasm, a centrally located large nucleus, and multiple small vacuoles. There are a few small chloroplasts, which are distributed throughout the cytoplasm (Fig. 2E). SC-C4 development initiates at Stage 2 (above the base towards the mid-section), where differentiation of the unique chlorenchyma cell morphology is first observed. At this stage, there is development of vacuoles at opposite poles of the cell, while chloroplasts and mitochondria continue to multiply and begin to aggregate at the center of the cell next to the nucleus, which is pressed against one side of the cell (Fig. 2F). In Stage 3 (mid-section of the leaf towards the tip, Fig. 2C, G) the following series of events in structural differentiation of SC-C4 were observed. First, is the formation of the CCC, which earlier in its development was connected to the PC on one side of the cell. The CCC is either attached to one of the radial cell walls and the nucleus (when visible it is usually adjacent to the CCC and the cell wall); or, as observed in Fig. 2G, the CCC is well delimited in the center of the cells. Examination by TEM shows that both PC and CCC chloroplasts have a similar structure, with small grana (not shown). In Stage 3, numerous mitochondria are selectively located within the CCC, but these are not fully differentiated. The peripheral vascular bundles at this stage have differentiated phloem and xylem elements (not shown). At Stage 4, near the leaf tip, development of the characteristic features of chlorenchyma cells that define SC-C4 in B. sinuspersici is complete (Fig. 2D). All of the CCCs are positioned at the central region of the cell, and are only connected with the PC by thin cytoplasmic channels. Structurally dimorphic chloroplasts that characterize this species are established in Stage 4, with well-developed grana in the CCC chloroplast (Fig. 2H), and a deficiency in grana in the peripheral chloroplasts (Fig. 2I). Also, in this stage the mitochondria have established a well-developed structure with clearly observable tubular cristae (not shown).
In S. aralocaspica, the basal Stage 1 chlorenchyma cells have a well-developed central vacuole that was observable from the earliest stages. The nucleus is located in the peripheral cytoplasm near the middle of the cell and chloroplasts are distributed along the cell periphery (Fig. 2J, N). Differentiation of these chlorenchyma cells, which is initiated in Stage 2, consists of cell elongation accompanied by the partitioning of organelles to distal and proximal ends, with multiplication of mitochondria and chloroplasts occurring at the proximal domain (Fig. 2K, O). At Stage 3, the cells were clearly more developmentally advanced, with an overall appearance that was mostly similar to the fully mature SC-C4 cells of Stage 4 (Fig. 2L, P). However, more detailed observations by TEM revealed that ultrastructural differentiation of chloroplasts and mitochondria is not completed at Stage 3 (not shown). Only in Stage 4 structurally distinct dimorphic chloroplasts were clearly present, with the proximal chloroplasts having well-developed grana (Fig. 2Q) compared to the distal chloroplasts (Fig. 2R). Also, mitochondria, which are localized to the proximal end of the cell, now show complete development, with a characteristic tubular structure (not shown).
In situ hybridization of rbcL mRNA and immunocytochemistry of rbcL protein at different stages of leaf development
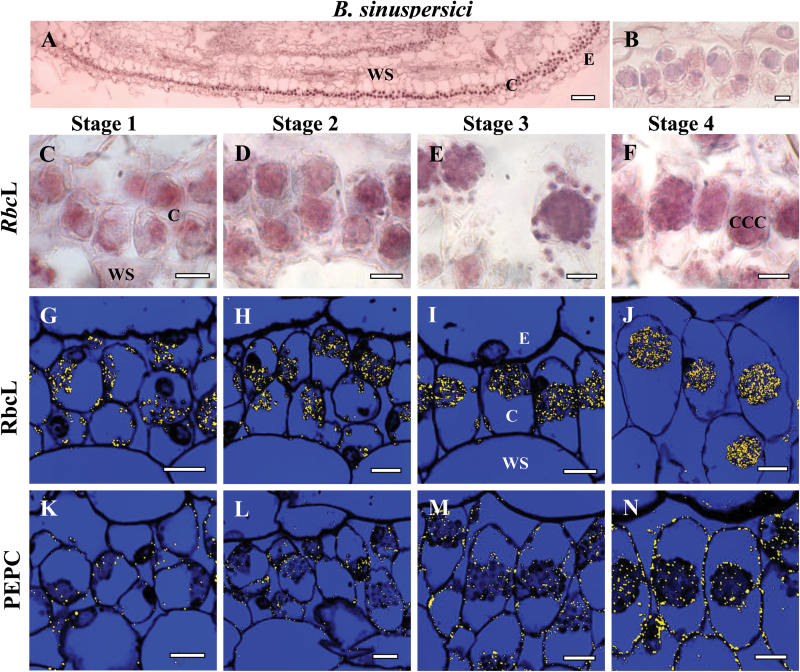
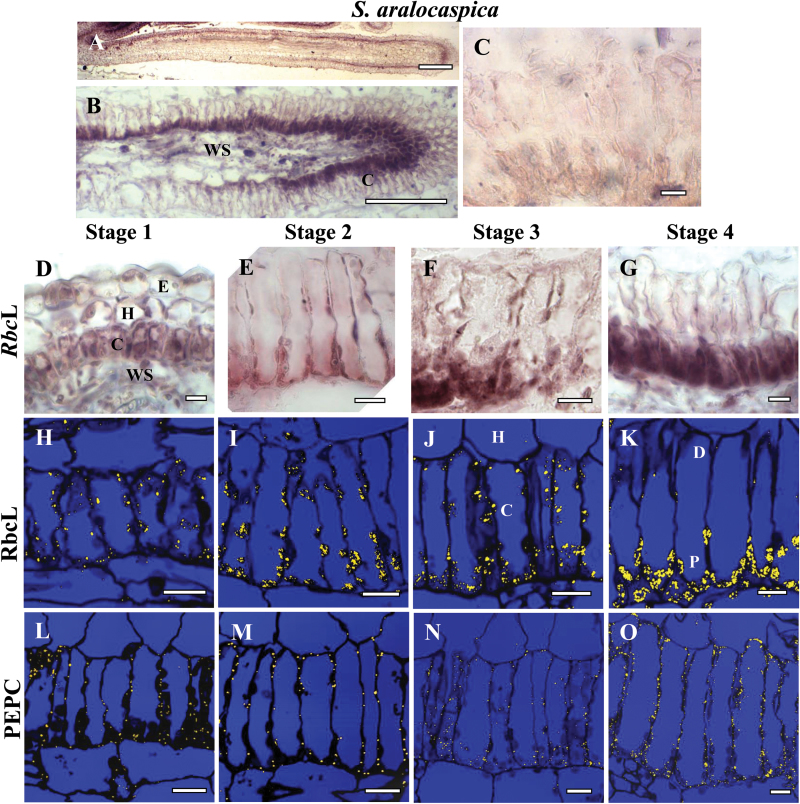
The temporal and spatial distribution of Rubisco rbcL mRNA was studied using in situ hybridization of biotin-11-UTP-labeled antisense RNA probes with longitudinal sections of young leaves (Fig. 3A, B. sinuspersici; Fig. 4A, S. aralocaspica). Specific hybridization was clearly visualized in chloroplasts as a dark purple color in B. sinuspersici (Fig. 3A, C–F) and S. aralocaspica (Fig. 4A, B, D–G). For a negative control, rbcL sense probes transcribed from the same plasmid in the opposite orientation showed little or no purple hybridization signal (Figs 3B, 4C). Antibody labeling of rbcL protein by immunolocalization with fluorescent confocal imaging appears in chloroplasts as yellow dots within the longitudinal sections (Figs 3G–J, 4H–K).
Fig. 3.
In situ hybridization of rbcL mRNA (A–F) and in situ immunolocalization of Rubisco rbcL (G–J) and PEPC (K–N) with longitudinal sections of young leaves from base to tip of Bienertia sinuspersici at four stages of development: Stage 1 (C, G, K), Stage 2 (D, H, L), Stage 3 (E, I, M) and Stage 4 (F, J, N). The dark purple signal indicates the specific hybridization to an antisense mRNA probe for rbcL mRNA (panels A, C–F). Yellow particles (panels G–N) indicate labeling with rbcL and PEPC antibodies. (A) Basipetal gradient of rbcL transcript accumulation from base (left) to tip (right). (B) Sense probe control showing the very low background staining that occurred in mRNA sense strand hybridization reactions. E, epidermis; CCC, central cytoplasmic compartment; C, chlorenchyma; WS, water storage. Scale bars: 100 μm for A; 10 μm for B–N.
Fig. 4.
In situ hybridization of rbcL mRNA (A–G) and in situ immunolocalization of Rubisco rbcL (H–K) and PEPC (L–O) with longitudinal leaf sections from base to tip of Suaeda aralocaspica at four stages of development: Stage 1 (D, H, L), Stage 2 (E, I, M), Stage 3 (F, J, N) and Stage 4 (G, K, O). The dark purple signal indicates the specific hybridization to an antisense mRNA probe for rbcL mRNA (A, B, D–G). Yellow particles indicate peptide antibody labeling (H–O). (A, B) Basipetal gradient of rbcL transcript accumulation from base (left) to tip (right). (C) Sense probe control showing the very low background staining that occurs in mRNA sense strand hybridization reactions. E, epidermis; C, chlorenchyma; H, hypoderm; WS, water storage. Scale bars: 200 μm for A, 100 μm for B; 10 μm for C–O.
In B. sinuspersici, in the Stage 1 leaf region a clear hybridization signal for rbcL mRNA was observed within all of the chlorenchyma cell chloroplasts (Fig. 3C), as well as in the chloroplasts present in the epidermal and water storage cells. Also in Stage 1, confocal imaging revealed considerable labeling for rbcL, by immunolocalization, within all chloroplasts (Fig. 3G). In Stage 2, there was a similar pattern of hybridization for rbcL mRNA and immunolocalization of rbcL protein in all chloroplasts, as the developmental progression of cell elongation and position of organelles was beginning to occur (Fig. 3D, H). At Stage 3, as positioning of chloroplasts into the two separate domains was occurring, the hybridization signal for rbcL became more enhanced, with labeling observed in all of the chloroplasts (Fig. 3E). Also, at this stage immunolocalization indicated that all of the chloroplasts contained rbcL protein (Fig. 3I). At Stage 4, the mature chlorenchyma cells clearly showed selective localization of both rbcL mRNA and rbcL protein specifically within the CCC chloroplasts, with little or no signal observed in the peripheral chloroplasts (Fig. 3F, J).
In Stage 1 in S. aralocaspica, in situ hybridization and immunolocalization showed labeling for rbcL mRNA (Fig. 4D) and rbcL protein (Fig. 4H) within all of the chloroplasts. At Stage 2 and 3, while a clear increase in cytoplasmic volume and chloroplast number was observed within the proximal domain of chlorenchyma cells, all chloroplasts, in both the proximal and distal ends of the cell showed rbcL mRNA hybridization (Fig. 4E, F) and immunolocalization of rbcL protein (Fig. 4I, J). It was only in the most developmentally advanced Stage 4 towards the leaf tip that rbcL mRNA and rbcL protein became specifically confined to chloroplasts located within the proximal domain of the cell, with no labeling observed in the distal end chloroplasts (Fig. 4G, K).
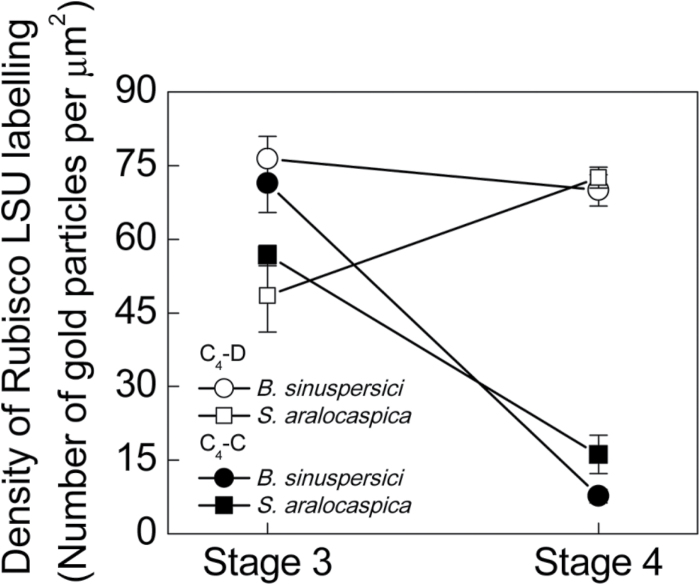
For a more precise quantitative evaluation of rbcL protein in the different cell regions, visualization of immunogold antibody labeling by TEM was applied with cross-sections of leaves at Stages 3 and 4 of development. Counting of gold particles within different subcellular compartments at Stage 3 in both B. sinuspersici and S. aralocaspica confirmed a high level of labeling in chloroplasts located in both domains (Fig. 5). In the mature Stage 4 chlorenchyma cells, quantitative evaluation confirmed a dramatic change, with selective localization of immunogold labeling within the CCC chloroplasts of B. sinuspersici, and within the chloroplasts in the proximal domain of S. aralocaspica. Chloroplasts at the cell periphery in B. sinuspersici and in the distal compartment in cells of S. aralocaspica showed a dramatic drop in the density of gold particles as development progressed from Stage 3 to Stage 4 (9.3-fold in B. sinuspersici and 3.5-fold in S. aralocaspica) (Fig. 5). Interestingly, at the same time the density of labeling was declining in the distal chloroplasts in S. aralocaspica from Stage 3 to 4, there was a 1.5-fold increase in density in the proximal chloroplasts, while in B. sinuspersici maximum density of labeling for rbcL in the CCC occurred earlier, by Stage 3. In these young leaves there was very strong selective localization of rbcL to one chloroplast type at Stage 4; but some rbcL was detected by TEM in the distal chloroplasts of S. aralocaspica (10% of that in the proximal chloroplasts) and in the peripheral chloroplasts of B. sinuspersici (20% of that in the CCC chloroplasts) (Fig. 5).
Fig. 5.
Quantitative graphical representation showing the density of immunolabeling for Rubisco rbcL in peripheral (●) versus CCC chloroplasts (○) in Bienertia sinuspersici, and in distal (■) versus proximal (□) chloroplasts of Suaeda aralocaspica at Stages 3 and 4 of development in young leaves. The y axis represents the number of gold particles per μm2 of chloroplast, and the x axis represents the developmental stages. 10–15 cell areas were used for counting in each cell type and for each stage of development.
Immunocytochemistry of PEP carboxylase protein at different stages of leaf development
For both B. sinuspersici and S. aralocaspica, immunolabeling of PEPC at the leaf base (Stage 1, Figs 3K, 4L) and during the first stages of special chloroplast positioning (Stage 2, Figs 3L, 4M) was very weak in chlorenchyma cells, and absent in all other tissues. At Stage 3 the accumulation of PEPC was more prominent (Figs 3M, 4N) and at Stage 4 chlorenchyma cells showed the highest levels of cytosolic PEPC protein, with even distribution throughout the cytoplasm (Figs 3N, 4O).
Western blot analysis of Rubisco and C4 pathway enzymes
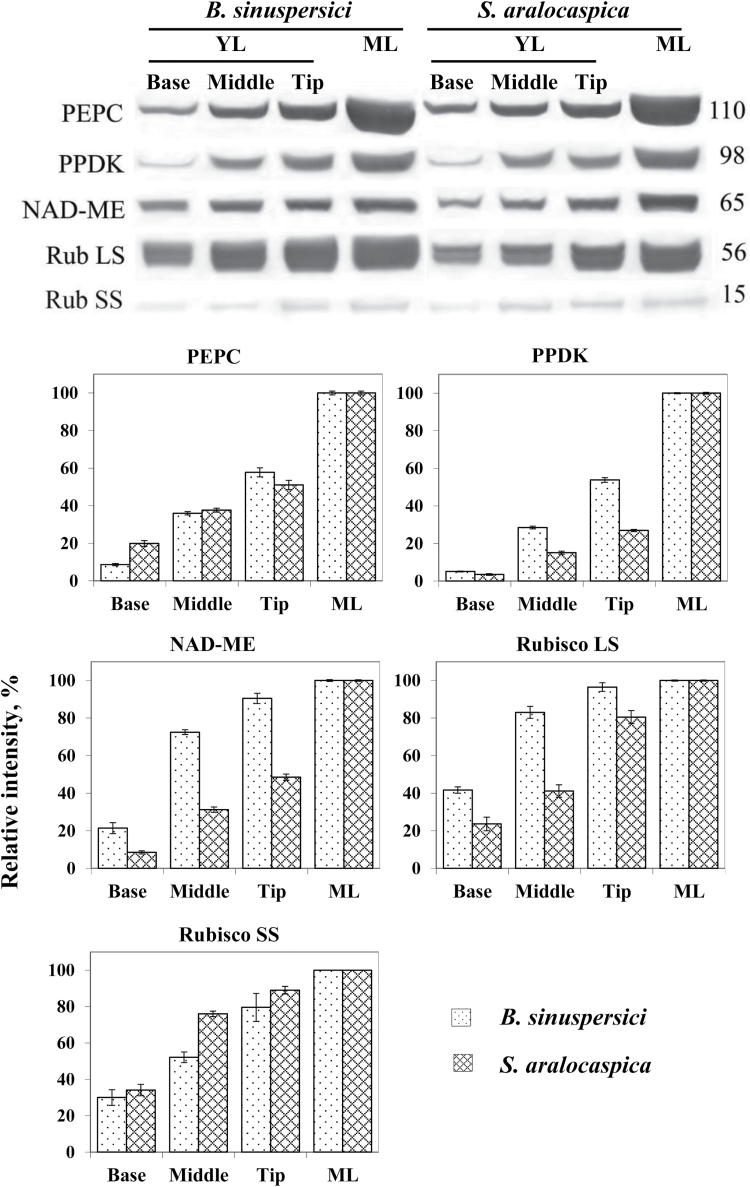
Accumulation of representative C4 pathway enzymes (PEPC, PPDK, NAD-ME) and Rubisco rbcL and rbcS, was analyzed by western blots during leaf development in B. sinuspersici and S. aralocaspica. Total soluble protein extracts prepared from the base (where most chlorenchyma cells are at Stage 1), middle (most chlorenchyma cells at Stages 2 and 3) and tip (chlorenchyma cells at Stage 4) of young leaves (length 0.5–0.7cm), and from mature leaves, were used for comparative developmental analysis (Fig. 6). All five proteins increased gradually as leaf development progressed from the base to the tip, with the highest levels of accumulation found in mature leaves. The rbcL content in B. sinuspersici at the base of the young leaf was 40% of that of mature leaves, increasing to 80% at the middle region of young leaves. In S. aralocaspica, rbcL was initially lower than in B. sinuspersici, with a more gradual increase in rbcL during development. The rbcS amount at the base of the young leaf was about 30% of mature leaf in both species and it increased towards the tip to 80 and 90% of mature leaf in B. sinuspersici and S. aralocaspica, respectively.
Fig. 6.
Western blot analysis showing accumulation of C4 enzymes, Rubisco rbcL and rbcS in protein extracts taken along the length of young leaf, and from mature leaves in Bienertia sinuspersici and Suaeda aralocaspica. Total soluble proteins were extracted from young leaves (0.5–0.7cm long) divided into three sections (base, middle, tip), and from mature fully expanded leaves. Blots were probed with antibodies raised against PEPC, PPDK, NAD-ME and Rubisco rbcL and rbcS, respectively. Top: Representative western blots showing detection of each protein with the antibody indicated. Numbers listed at the right indicate molecular mass in kilodaltons. Bottom panels: Quantitative representation of western blot data taking relative intensity of labeling of mature leaf as 100%. YL, young leaf; ML, mature leaf.
Among the three C4 cycle enzymes, levels of PPDK were found to be very low at the base of young leaves in both species (4–5% compared to mature leaves), with developmental increases lagging well behind those observed for Rubisco (Fig. 6). Both species showed initially low levels of PEPC at the base of the young leaves, with accumulation increasing along the gradient to maximum levels at the leaf tip, reaching up to ~50% of that in mature leaves. In B. sinuspersici, the levels of NAD-ME increased during development at a rate similar to rbcL, while its rate of increase in S. aralocaspica was notably slower.
Analysis of function: CO2 compensation points and dark respiration
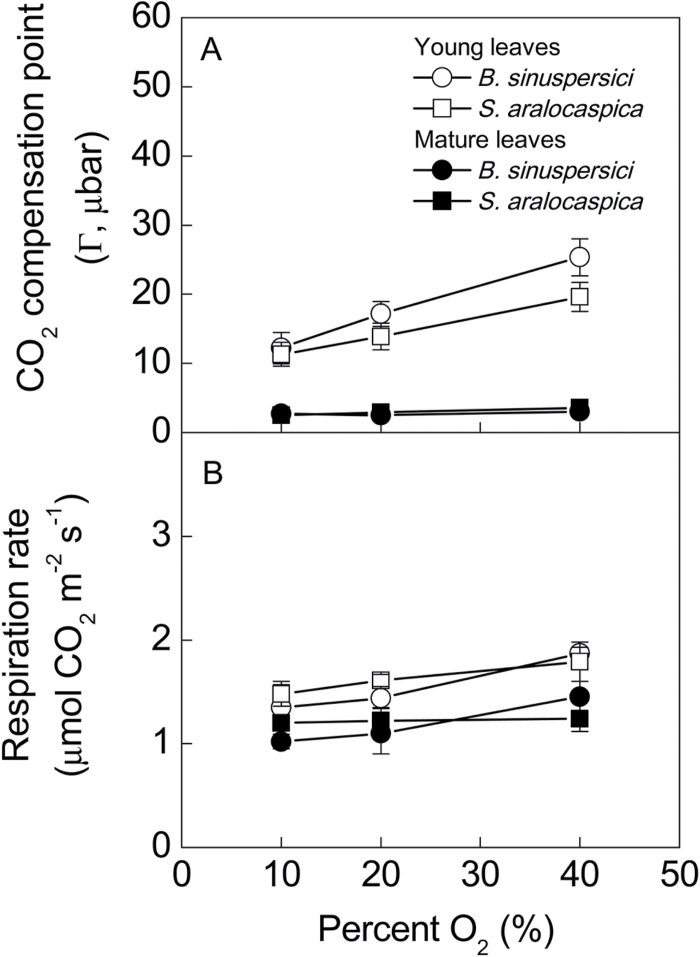
Mature leaves of B. sinuspersici and S. aralocaspica displayed low compensation point (Г) values (2.5 and 2.9 µbar) at 25ºC and current atmospheric levels of O2 (20%); and, these values were insensitive to changes in level of O2 from 10% to 20% to 40% (0.092, 0.184 and 0.369mbar O2) (Fig. 7A). However, in the young leaves of both species there was a linear increase in Г with increasing O2 from 10 to 40%. Additionally, at 25ºC and current ambient levels of O2 (20%), Г in young leaves (0.5–0.7cm) was ~7-fold higher than in mature B. sinuspersici leaves, and ~5-fold higher in S. aralocaspica. In comparing the two species, there was no significant difference (at P<0.05) between them for the value of Г in young leaves at 10 or 20% O2. However, Γ in B. sinuspersici appears to be more sensitive to increasing O2, and values at 40% O2 were significantly (at P<0.05) higher than in S. aralocaspica. With increasing O2, there was no significant increase (at P<0.05) in the rate of dark respiration for either species. In both species, at a given level of O2, rates appeared to be slightly higher in young than in mature leaves; however, there was no significant difference at P<0.1 (Fig. 7B).
Fig. 7.
CO2 compensation points (Г) measured in the light, and rates of dark respiration at variable oxygen levels for mature (filled symbols) and young (open symbols) leaves of Bienertia sinuspersici (circles) and Suaeda aralocaspica (squares).
Discussion
Structural and biochemical transitions towards development of SC-C4
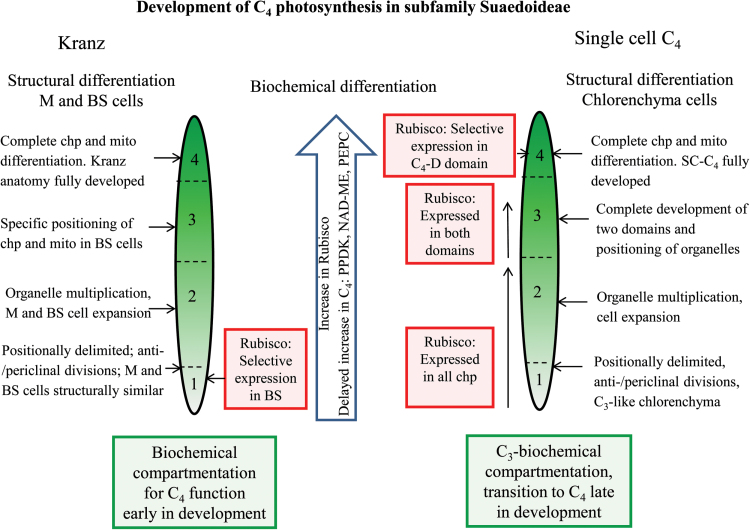
The development, maintenance, and function of both forms of SC-C4 require extensive structural differentiation to form two intracellular domains that strictly partition different sets of biochemical functions within a single cell. B. sinuspersici and S. aralocaspica have independent evolutionary origins and possess structurally different forms of SC-C4 chlorenchyma cells. In both species, the chlorenchyma cells originate from the ground meristem; however, in B. sinuspersici periclinal divisions at the base of leaves give rise to two layers of chlorenchyma, while in S. aralocaspica periclinal divisions give rise to a single chlorenchyma layer and a layer of hypodermal cells. Previous analyses of mid-sections of leaves of different ages showed that chlorenchyma cells in young leaves have not developed the structural and biochemical properties that indicate transitions in development of SC-C4 chlorenchyma in Bienertia (Voznesenskaya et al., 2005; Lara et al., 2008; Park et al., 2009) and S. aralocaspica (Voznesenskaya et al., 2003a). In this current study, detailed structural and biochemical analyses of these species along longitudinal gradients of young leaves demonstrate that, despite having independent origins, different leaf anatomy and distinct chlorenchyma cell structures, the leaves of both species undergo very similar base-to-tip transitions to form C4 type chlorenchyma cells. Four stages of chlorenchyma development were recognized and defined with respect to structural and biochemical changes. Figure 8 indicates the progression of development of SC-C4 in both species, in comparison to related Kranz-type C4 species in subfamily Suaedoideae.
Fig. 8.
Illustrations of major differences during development of C4 photosynthesis from the basal region to the tip of young leaves in SC-C4 species versus Kranz-type C4 species in subfamily Suaedoideae. Progressive changes in differentiation in two types of SC-C4 species (B. sinuspersici and S. aralocaspica) are summarized from the current study, and compared with two structural forms of Kranz-type Suaeda species (S. taxifolia and S. eltonica) described previously (Koteyeva et al., 2011). The numbers within the leaves refer to stages of chlorenchyma development along young leaves (length ~0.6cm). For comparison with SC-C4, the results with Kranz-type by Koteyeva et al. (2011) are summarized through four stages of development. The horizontal arrows point to specific defined changes that occur at each of the four stages. The vertical arrows indicate continual changes that occur along the longitudinal gradient. BS, bundle sheath; chp, chloroplasts; M, mesophyll; mito, mitochondria; SC-C4, single cell C4; C4-D domain, C4 cycle decarboxylation domain. (This figure is available in color at JXB online.)
In Stage 1, at the base of the leaf in the meristematic zone, chlorenchyma cells of B. sinuspersici and S. aralocaspica contain a few, ultrastructurally monomorphic, chloroplasts. There is no evidence for development of the two cytoplasmic zones in either species. Analogous to Stage 1 in the SC-C4 species, Kranz-type C4 Suaeda species possess M and BS precursor cells that contain ultrastructurally identical organelles (both chloroplasts and mitochondria) (Koteyeva et al., 2011).
At Stage 2 in both SC-C4 species and in Kranz-type Suaeda species, there is an increased expansion of photosynthetic cells. In B. sinuspersici the specialization of SC-C4 chlorenchyma cell structure continues with development of the CCC, together with enhanced chloroplast and mitochondria divisions around the nucleus. In S. aralocaspica, the chlorenchyma cells become increasingly elongated, accompanied by increasing cytoplasmic volume at the proximal end due to multiplication of chloroplasts and mitochondria. Kranz-type Suaeda species at this analogous stage of development are characterized by expansion of M cells with increase in vacuole volume and by multiplication of organelles in BS cells with initiation of their relocation to one side of the cell (Koteyeva et al., 2011).
At Stage 3, two intracellular domains are morphologically evident in both SC-C4 species. These are precursors to the complete formation of C4-C and C4-D compartments. At this stage, chloroplasts within both domains still have a similar ultrastructure, but the mitochondria are now localized to the C4-D domain. At the analogous developmental stage in the Suaeda Kranz-type C4 species, the chloroplasts and mitochondria in the BS cells have relocated from their original position around the periphery of the cell to either a centripetal position adjacent to the vascular bundles, or to a centrifugal position, depending on species. The chloroplasts in the BS and M cells have similar ultrastructure (Koteyeva et al., 2011), like chloroplasts in the two domains of Stage 3 SC-C4.
In both SC-C4 species, selective partitioning of chloroplasts and mitochondria into the two domains at Stage 3 is followed by later structural differentiation of these organelles in Stage 4. The C4-C domains contain chloroplasts with reduced grana formation, while chloroplasts in the C4-D domain have well developed grana. In NAD-ME type C4 species, including B. sinuspersici and S. aralocaspica, conversion of alanine and atmospheric CO2 to aspartate is considered to be the main flux in the carboxylation phase of the C4 pathway, a conversion that requires only ATP. The reduction in grana in the C4-C domain in NAD-ME type C4 species is considered to reflect a decreased requirement for PSII and NADPH production via linear electron flow (Hatch, 1987). Similarly, in the two structural forms of Kranz-type Suaeda species, complete differentiation to C4 chloroplasts and mitochondria also occurs during the final stage of leaf development (Koteyeva et al., 2011). Thus, in all four distinct forms of C4 that occur in subfamily Suaedoideae, a final step in organelle differentiation is the specialized ultrastructural development of mitochondria and dimorphic chloroplasts. It is likely that signals regulating photochemical energy production between the two types of chloroplasts start to be transmitted after C4 function has distributed different demands for energy assimilation between the two domains.
Quantitative analyses of protein accumulation from western blots indicate a basipetal developmental gradient for both SC-C4 species, with levels of photosynthetic enzymes increasing along the length of the young leaves (Fig. 8). rbcL and rbcS proteins are relatively abundant in young cells at the leaf base, indicating substantial levels of Rubisco during early Stage 1 development. In comparison, at the leaf base there is little expression of the representative C4 cycle enzymes PEPC, NAD-ME and especially PPDK, which could be functionally rate-limiting for the developing C4 cycle at this early stage. Similarly, previous studies showed earlier accumulation of Rubisco (rbcL and rbcS) than the C4 enzymes in very young leaves, with greatly increased accumulation of Rubisco and C4 enzymes in mature leaves of B. sinuspersici and B. cycloptera (Voznesenskaya et al., 2005; Lara et al., 2008) and S. aralocaspica (Voznesenskaya et al., 2003a). As with the SC-C4 species, photosynthetic enzyme levels in Kranz type Suaeda species increased along the leaf longitudinal gradient, with substantial Rubisco at the base of the leaf, and a lag in the accumulation of C4 enzymes, especially PPDK (Koteyeva et al., 2011).
Regulation of Rubisco expression in SC-C4
Mechanisms controlling the development of dimorphic chloroplasts in SC-C4 plants are not understood. Theories for selective targeting of nuclear encoded proteins to plastids in SC-C4 species (e.g. the small subunit of Rubisco, and PPDK) include selective import of required proteins into each plastid type, selective mRNA targeting from the nucleus to one domain and/or selective degradation of the protein following import (Offermann et al., 2011). In a proteomics analysis of B. sinuspersici by Offermann et al. (2015), 35 nuclear-encoded proteins were found to be enriched in chloroplasts located to either the C4-C or C4-D domain. In fact, both groups of chloroplast proteins had predicted plastid transit peptides with classical physiochemical properties found in other species. These groups were comprised of proteins with different functional properties, including C3 and C4 carbon fixation pathways as well as certain proteins in light reactions.
The selective localization of Rubisco (consisting of the nuclear-encoded rbcS and chloroplast-encoded rbcL) for function within the C4-D domain is one of the most definitive biochemical features of SC-C4. Transient expression studies using constructs expressing a GFP fusion with the B. sinuspersici rbcS plastid transit peptide in B. sinuspersici SC-C4 mesophyll protoplasts provided no evidence for selective import within these cells (Rosnow et al., 2014). In the current study, chloroplast-encoded Rubisco rbcL was used as an indicator of changes in gene expression that occurs during the differentiation of dimorphic chloroplast along the longitudinal gradient of SC-C4 development. rbcL mRNA, as well as its corresponding protein, was initially found within all chloroplasts of early chlorenchyma cells, both at the base (Stage 1) and middle (Stage 2, 3) regions of the young leaves. There were no quantitative differences in levels of Rubisco rbcL protein, mRNA or chloroplast structure that corresponded with the initial differentiation to form two domains in either of the SC-C4 species. Rather, selective partitioning of rbcL mRNA and its encoded protein to the C4-D domain only occurred later, during Stage 4 at the leaf tip. This localization was tightly coordinated with the establishment of chloroplast structural dimorphism. Thus, a ‘default’ C3-like pattern of rbcL mRNA and protein distribution appears to be maintained in chloroplasts during formation of the two cytoplasmic domains, with the more specialized SC-C4 distribution pattern becoming established only at the final developmental stage. Spatial and temporal accumulation patterns for Rubisco rbcL mRNA correlated with rbcL protein accumulation along the entire length of the longitudinal SC-C4 developmental gradient, indicating that transcript accumulation is a major determinant for Rubisco accumulation specifically within the C4-D domains of mature B. sinuspersici and S. aralocaspica leaves.
In contrast to SC-C4 species, two Kranz-type species of Suaeda (Koteyeva et al., 2011) showed selective compartmentalization of rbcL mRNA and protein to BS chloroplasts much earlier, at Stage 1 (Fig. 8). In this case, cell-specific control of rbcL mRNA accumulation (by transcription or mRNA stability) occurs prior to the structural dimorphism and positioning of BS chloroplasts. In contrast, in the SC-C4 species, plastid-specific localization was observed only later, after development of the two cytoplasmic domains and chloroplast structural differentiation was complete. Taken together, these findings suggest different ontogenetic programs for development of C4 biochemistry have evolved independently in Kranz and SC-C4 species within the subfamily Suaedoideae.
Like the Kranz-type Suaeda species, early development of C4 has also been observed in some other Kranz-type C4 species. Positional control of Rubisco expression occurs very early in BS precursor cells in leaf primordia of maize (Nelson and Langdale, 1992), in BS cells of Arundinella hirta (Wakayama et al., 2003), in BS cells at the base of young leaves in Atriplex rosea (Liu and Dengler, 1994; Dengler et al., 1995), and in C4 species in family Cleomaceae (Koteyeva et al., 2014). A similar pattern was shown also for three species of Cyperaceae (Soros and Dengler, 2001). However, in other Kranz-type species the pattern of Rubisco expression during leaf development was similar to that of SC-C4 species. Amaranthus hypochondriacus (Wang et al., 1992, 1993) and Salsola richteri (Voznesenskaya et al. 2003b) show a prolonged C3-like distribution in M and BS chloroplasts in the early stages of leaf development.
Recently it was shown that the rbcL-specific mRNA binding protein RLSB has a role in post-transcriptional rbcL expression and possibly selective plastid-specific accumulation of Rubisco in several C4 species, including SC-C4 B. sinuspersici as well as the C4 dicots Suaeda taxifolia and Flaveria bidentis, and the C4 monocots Zea mays and Setaria viridis (Bowman et al., 2013; Rosnow et al., 2014). In B. sinuspersici, RLSB is co-localized with Rubisco specifically within the central compartment chloroplasts (Rosnow et al., 2014), while in Kranz-type species it co-localizes with Rubisco only in BS chloroplasts. It has been proposed that RLSB and the differential redox status of the dimorphic chloroplasts might work together to selectively control synthesis of Rubisco in the C4-D domain in B. sinuspersici (Rosnow et al., 2014). The established changes in rbcL mRNA and protein at different stages of leaf development demonstrated in this current study will serve as a framework for analyses currently in progress to determine how RLSB and other regulatory factors determine plastid-specific Rubisco accumulation in SC-C4 plant species.
Functional analysis of young versus mature leaves
The leaves of B. sinuspersici and S. aralocaspica are too small to accommodate analysis of photosynthetic traits by traditional gas exchange methods from the individual defined regions along the longitudinal leaf gradient. Therefore, a functional analysis of photosynthesis on young compared to mature leaves was made by measuring the sensitivity of the CO2 compensation point (Г) to varying O2 concentrations using an inlet mass spectrometer. Typically at ~25°C, C4 species have a low Г between 1–5 µbar CO2, while in C3 plants the Г is 45–50 µbar CO2, and C3-C4 species have intermediate values in the range of 9–30 µbar CO2 (Krenzer et al., 1975; Ku et al., 1991; Vogan et al., 2007; Voznesenskaya et al., 2013). Increasing levels of O2 causes a linear increase in Γ in C3 plants (reflecting an increase in photorespiration), has little or no effect on Γ in C4 plants, but causes an intermediate, biphasic, response of Г in C3-C4 species (Ku et al., 1991). In mature leaves of both SC-C4 species, Г was insensitive to increasing O2 levels up to 40%, with values characteristic of other C4 plants (2.5 to 3 µbar). However, in young leaves of both species, the Г values at 20% O2 were intermediate to those of C3 and C4 plants. Also, values of Γ increase as O2 was raised from 10 to 40% (~1.7-fold increase in S. aralocaspica and 2-fold increase in B. sinuspersici), which is indicative of an increase in rates of photorespiration (Brooks and Farquhar, 1985). Since dark-type respiration was not affected by increasing O2 from 10 to 20%, it is not considered to contribute to the O2-dependent increase in Γ. The sensitivity of Γ to increasing O2 in young but not mature leaves provides additional support for transitions from C3, to intermediate, to a fully C4 state of photosynthesis along a longitudinal gradient in SC-C4 leaves.
C3 photosynthesis and photorespiration is expected at the base of young leaves where there is substantial Rubisco in all of the chloroplasts, with relatively low levels of other C4 enzymes. The two domains have not yet become established to support the C4 cycle, and are not yet capable of concentrating CO2 around Rubisco via dimorphic chloroplasts or for refixation of photorespired CO2 from mitochondria. However, at Stages 2 and 3, development of two separate domains is progressing with increased expression of both Rubisco and C4 enzymes. The partitioning between domains in the mid-section of young leaves is analogous to that of Kranz-like Type II C3-C4 intermediates. In C3-C4 intermediates Rubisco is localized in both M and BS chloroplasts. Intermediates reduce Γ and photorespiration by exclusive localization of glycine decarboxylase in BS mitochondria which allows photorespired CO2 to be partially refixed. Type II intermediates have in addition a partially functional C4 cycle (Edwards and Ku, 1987). In the mid-sections of young leaves of SC-C4 species, chloroplasts in both domains have Rubisco; however, the mitochondria, which are the sites of decarboxylation by glycine decarboxylase as a consequence of photorespiration and by NAD-ME in the C4 cycle, are localized only in the C4-D domain. Finally, towards the tip of young leaves, Rubisco is selectively compartmentalized within the C4-D domains of the chlorenchyma cells, and the SC-C4 anatomy and function have become fully developed with low photorespiration.
Concluding remarks
Two very different ontogenetic patterns of development exist between the SC-C4 and C4 Kranz-type members of the subfamily Suaedoideae (family Chenopodiaceae). This includes differences in structural, biochemical and functional differentiation in SC-C4 species compared to the Kranz-type species. The SC-C4 leaves show a clear longitudinal progression from a C3-default to intermediate to a full C4 state, while C4 traits develop very early in the Kranz-type species. This implies major differences between these groups at the molecular level along the leaf longitudinal gradient for regulatory factors associated with photosynthetic gene expression (such as the selective compartmentalization of rbcL expression), as well as regulatory factors required for the development of structural features (such as differentiation of the unique SC-C4 cellular domains and partitioning of organelles versus differentiation of different cell types in Kranz-type C4 Suaedoideae). This strong difference in C4 development between SC-C4 and Kranz forms of C4 in subfamily Suaedoideae has wider implications for theories regarding the evolution of the C4 pathway.
In the SC-C4 species B. sinuspersici and S. aralocaspica, the acquisition of full C4 capability along the longitudinal leaf gradient is associated with a developmental shift from monomorphic to dimorphic chloroplasts, in coordination with plastid-specific partitioning of Rubisco rbcL mRNA and protein accumulation. Despite differences in anatomy and independent origin of these two species, the strong similarity in their development of C4 suggests comparative studies to identify regulatory factors controlling the development of SC-C4 will be of interest. Future studies will focus on the identification and characterization of regulatory factors and signaling processes responsible for SC-C4 development and how these processes differ and/or overlap with those responsible for C4 development in Kranz-type C4 species.
Supplementary data
Supplementary data is available at JXB online.
Methods S1. Details of in situ immunolocalization and Western blot analysis.
Fig. S1. General views of the branches of Bienertia sinuspersici and Suaeda aralocaspica showing position of young leaves forming the vegetative buds, and excised mature and young leaves of B. sinuspersici and S. aralocaspica in the chamber for inlet mass-spectrometric measurements.
Fig. S2. Representative membranes stained with Ponceau S after transfer of proteins to nitrocellulose membrane and before immunoblotting.
Fig. S3. Structure of the vegetative shoot tip in Bienertia sinuspersici and Suaeda aralocaspica, consisting of the apical meristem and early leaf primordia.
Acknowledgements
This work is supported by the National Science Foundation under funds MCB #1146928 to GEE, USDA/NRI Grant 2008–01070 to JOB, partly by the Russian Foundation of Basic Research under Grant 08-04-00936 and a collaborative grant between the Russian Foundation of Basic Research, 10-04-95512 and the Civilian Research and Development Foundation, RUB1-2982-ST-10. We are grateful to the Core Facility Center ‘Cell and Molecular Technologies in Plant Science’ of the Komarov Botanical Institute (St Petersburg, Russia) and the Franceschi Microscopy and Imaging Center of Washington State University for use of their facilities and staff assistance, and to C. Cody for plant growth management.
Glossary
Abbreviations:
- BS
bundle sheath
- CCC
central cytoplasmic compartment
- C4-C
C4 cycle carboxylation domain
- C4-D
C4 cycle decarboxylation domain
- M
mesophyll
- NAD-ME
NAD-malic enzyme
- PC
peripheral compartment
- PEPC
phosphoenolpyruvate carboxylase
- PPDK
pyruvate,Pi dikinase
- SC-C4
single cell C4
- Г
CO2 compensation point.
References
- Akhani H, Barroca J, Koteeva N, Voznesenskaya E, Franceschi V, Edwards G, Ghaffari SM, Ziegler H. 2005. Bienertia sinuspersici (Chenopodiaceae): a new species from Southwest Asia and discovery of a third terrestrial C4 plant without Kranz anatomy. Systematic Botany 30, 290–301. [Google Scholar]
- Aubry S, Kelly S, Kümpers BMC, Smith-Unna RD, Hibberd JM. 2014. Deep evolutionary comparison of gene expression identifies parallel recruitment of trans-factors in two independent origins of C4 photosynthesis. PLOS Genetics 10, e1004365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman S, Patel M, Yerramsetty P, Mure C, Zielinski A, Bruenn J, Berry J. 2013. A novel RNA binding protein affects rbcL gene expression and is specific to bundle sheath chloroplasts in C4 plants. BMC Plant Biology 13, 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks A, Farquhar GD. 1985. Effect of temperature on the CO2/O2 specificity of ribulose-1,5-bisphosphate carboxylase/oxygenase and the rate of respiration in the light. Planta 165, 397–406. [DOI] [PubMed] [Google Scholar]
- Chuong SDX, Franceschi VR, Edwards GE. 2006. The cytoskeleton maintains organelle partitioning required for single-cell C4 photosynthesis in Chenopodiaceae species. The Plant Cell 18, 2207–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covshoff S, Burgess S, Kneřová J, Kümpers BC. 2014. Getting the most out of natural variation in C4 photosynthesis. Photosynthesis Research 119, 157–167. [DOI] [PubMed] [Google Scholar]
- Dengler NG, Dengler RE, Donnelly PM, Filosa MF. 1995. Expression of the C4 pattern of photosynthetic enzyme accumulation during leaf development in Atriplex rosea (Chenopodiaceae). American Journal of Botany 82, 318–327. [Google Scholar]
- Edwards GE, Franceschi VR, Voznesenskaya EV. 2004. Single cell C4 photosynthesis versus the dual-cell (Kranz) paradigm. Annual Review of Plant Physiology and Plant Molecular Biology 55, 173–196. [DOI] [PubMed] [Google Scholar]
- Edwards GE, Ku MSB. 1987. The biochemistry of C3-C4 intermediates. In: Hatch MD, Boardman NK, eds. The Biochemistry of Plants. Vol. 10. Photosynthesis. Academic Press, Inc: New York, 275–325. [Google Scholar]
- Edwards GE, Voznesenskaya EV. 2011. C4 photosynthesis: Kranz forms and single-cell C4 in terrestrial plants. In: Raghavendra AS, Sage RF, eds. C4 Photosynthesis and Related CO2 Concentrating Mechanisms. Springer: Dordrecht, The Netherlands, 29–61. [Google Scholar]
- Edwards GE, Walker DA. 1983. C3, C4: Mechanisms, and Cellular and Environmental Regulation, of Photosynthesis. Blackwell Scientific publications: Oxford. [Google Scholar]
- Freitag H, Stichler W. 2000. A remarkable new leaf type with unusual photosynthetic tissue in a Central Asiatic genus of Chenopodiaceae. Plant Biology 2, 154–160. [Google Scholar]
- Hatch MD. 1987. C4 photosynthesis: a unique blend of modified biochemistry, anatomy and ultrastructure. Biochimica et Biophysica Acta 895, 81–106. [Google Scholar]
- Kanai R, Edwards G. 1999. The biochemistry of C4 photosynthesis. In: Sage RF, Monson RK, eds. C4 Plant Biology. Physiological Ecology series. Academic Press: San Diego, 49–87. [Google Scholar]
- Kapralov MV, Akhani H, Voznesenskaya EV, Edwards G, Francheschi V, Roalson EH. 2006. Phylogenetic relationships in the Salicornioideae /Suaedoideae /Salsoloideae s.l. (Chenopodiaceae) clade and a clarification of the phylogenetic position of Bienertia and Alexandra using multiple DNA sequence datasets. Systematic Botany 31, 571–585. [Google Scholar]
- Koteyeva NK, Voznesenskaya EV, Berry JO, Chuong SDX, Franceschi VR, Edwards GE. 2011. Development of structural and biochemical characteristics of C4 photosynthesis in two types of Kranz anatomy in genus Suaeda (family Chenopodiaceae). Journal of Experimental Botany 62, 3197–3212. [DOI] [PubMed] [Google Scholar]
- Koteyeva NK, Voznesenskaya EV, Cousins AB, Edwards GE. 2014. Differentiation of C4 photosynthesis along a leaf developmental gradient in two Cleome species having different forms of Kranz anatomy. Journal of Experimental Botany 65, 3525–3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krenzer EG, Moss DN, Crookston RK. 1975. Carbon dioxide compensation points of flowering plants. Plant Physiology 56, 194–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku MS, Wu J, Dai Z, Scott RA, Chu C, Edwards GE. 1991. Photosynthetic and photorespiratory characteristics of Flaveria species. Plant Physiology 96, 518–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Külahoglu C, Denton AK, Sommer M, et al. 2014. Comparative transcriptome atlases reveal altered gene expression modules between two Cleomaceae C3 and C4 plant species. The Plant Cell 26, 3243–3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langdale JA, Rothermel BA, Nelson T. 1988. Cellular pattern of photosynthetic gene expression in developing maize leaves. Genes and Development 2, 106–115. [DOI] [PubMed] [Google Scholar]
- Lara MV, Offermann S, Smith M, Okita TW, Andreo CS, Edwards GE. 2008. Leaf development in the single-cell C4 system in Bienertia sinuspersici: expression of genes and peptide levels for C4 metabolism in relation to chlorenchyma structure under different light conditions. Plant Physiology 148, 593–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Ponnala L, Gandotra N, et al. 2010. The developmental dynamics of the maize leaf transcriptome. Nature Genetics 42, 1060–1067. [DOI] [PubMed] [Google Scholar]
- Liu Y, Dengler NG. 1994. Bundle sheath and mesophyll cell differentiation in the C4 dicotyledon Atriplex rosea: quantitative ultrastructure. Canadian Journal of Botany 72, 644–657. [Google Scholar]
- Long JJ, Berry JO. 1996. Tissue-specific and light-mediated expression of the C4 photosynthetic NAD-dependent malic enzyme of amaranth mitochondria. Plant Physiology 112, 473–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majeran W, Friso G, Ponnala L, et al. 2010. Structural and metabolic transitions of C4 leaf development and differentiation defined by microscopy and quantitative proteomics. The Plant Cell 22, 3509–3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell K, Badger MR, Osmond CB. 1998. A comparison of CO2 and O2 exchange patterns and the relationship with chlorophyll fluorescence during photosynthesis in C3 and CAM plants. Functional Plant Biology 25, 45–52. [Google Scholar]
- Nelson T. 2011. The grass leaf developmental gradient as a platform for a systems understanding of the anatomical specialization of C4 leaves. Journal of Experimental Botany 62, 3039–3048. [DOI] [PubMed] [Google Scholar]
- Nelson T, Langdale JA. 1992. Developmental genetics of C4 photosynthesis. Annual Review of Plant Physiology and Plant Molecular Biology 43, 25–47. [Google Scholar]
- Offermann S, Friso G, Doroshenk KA, Sun Q, Sharpe RM, Okita TW, Wimmer D, Edwards GE, van Wijk KJ. 2015. Developmental and subcellular organization of single-cell C4 photosynthesis in Bienertia sinuspersici determined by large-scale proteomics and cDNA assembly from 454 DNA sequencing. Journal of Proteome Research 14, 2090–2108. [DOI] [PubMed] [Google Scholar]
- Offermann S, Okita TW, Edwards GE. 2011. Resolving the compartmentation and function of C4 photosynthesis in the single-cell C4 species Bienertia sinuspersici . Plant Physiology 155, 1612–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Knoblauch M, Okita TW, Edwards GE. 2009. Structural changes in the vacuole and cytoskeleton are key to development of the two cytoplasmic domains supporting single-cell C4 photosynthesis in Bienertia sinuspersici . Planta 229, 369–382. [DOI] [PubMed] [Google Scholar]
- Pick TR, Bräutigam A, Schlüter U, et al. 2011. Systems analysis of a maize leaf developmental gradient redefines the current C4 model and provides candidates for regulation. The Plant Cell 23, 4208–4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosnow J, Yerramsetty P, Berry JO, Okita TW, Edwards GE. 2014. Exploring mechanisms linked to differentiation and function of dimorphic chloroplasts in the single cell C4 species Bienertia sinuspersici . BMC Plant Biology 14, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage RF, Christin P-A, Edwards EJ. 2011. The C4 plant lineages of planet Earth. Journal of Experimental Botany 62, 3155–3169. [DOI] [PubMed] [Google Scholar]
- Schütze P, Freitag H, Weising K. 2003. An integrated molecular and morphological study of the subfamily Suaedoideae Ulbr. (Chenopodiaceae). Plant Systematics and Evolution 239, 257–286. [Google Scholar]
- Smith ME, Koteyeva NK, Voznesenskaya EV, Okita TW, Edwards GE. 2009. Photosynthetic features of non-Kranz type C4 versus Kranz type C4 and C3 species in subfamily Suaedoideae (Chenopodiaceae). Functional Plant Biology 36, 770–782. [DOI] [PubMed] [Google Scholar]
- Soros CL, Dengler NG. 2001. Ontogenetic derivation and cell differentiation in photosynthetic tissues of C3 and C4 Cyperaceae. American Journal of Botany 88, 992–1005. [PubMed] [Google Scholar]
- Vogan PJ, Frohlich MW, Sage RF. 2007. The functional significance of C3–C4 intermediate traits in Heliotropium L. (Boraginaceae): gas exchange perspectives. Plant, Cell & Environment 30, 1337–1345. [DOI] [PubMed] [Google Scholar]
- Voznesenskaya EV, Edwards GE, Kiirats O, Artyusheva EG, Franceschi VR. 2003. a Development of biochemical specialization and organelle partitioning in the single celled C4 system in leaves of Borszczowia aralocaspica (Chenopodiaceae). American Journal of Botany 90, 1669–1680. [DOI] [PubMed] [Google Scholar]
- Voznesenskaya EV, Franceschi VR, Artyusheva EG, Black CC, Jr., Pyankov VI, Edwards GE. 2003. b Development of the C4 photosynthetic apparatus in cotyledons and leaves of Salsola richteri (Chenopodiaceae). International Journal of Plant Sciences 164, 471–487. [Google Scholar]
- Voznesenskaya EV, Franceschi VR, Kiirats O, Artyusheva EG, Freitag H, Edwards GE. 2002. Proof of C4 photosynthesis without Kranz anatomy in Bienertia cycloptera (Chenopodiaceae). The Plant Journal 31, 649–662. [DOI] [PubMed] [Google Scholar]
- Voznesenskaya EV, Franceschi VR, Kiirats O, Freitag H, Edwards GE. 2001. Kranz anatomy is not essential for terrestrial C4 plant photosynthesis. Nature 414, 543–546. [DOI] [PubMed] [Google Scholar]
- Voznesenskaya EV, Koteyeva NK, Akhani H, Roalson EH, Edwards GE. 2013. Structural and physiological analyses in Salsoleae (Chenopodiaceae) indicate multiple transitions among C3, intermediate, and C4 photosynthesis. Journal of Experimental Botany 64, 3583–3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voznesenskaya EV, Koteyeva NK, Chuong SDX, Edwards GE, Akhani H, Franceschi VR. 2005. Differentiation of cellular and biochemical features of the single cell C4 syndrome during leaf development in Bienertia cycloptera (Chenopodiaceae). American Journal of Botany 92, 1784–1795. [DOI] [PubMed] [Google Scholar]
- Wakayama M, Ueno O, Ohnishi J. 2003. Photosynthetic enzyme accumulation during leaf development of Arundinella hirta, a C4 grass having Kranz cells not associated with vascular tissues. Plant and Cell Physiology 44, 1330–1340. [DOI] [PubMed] [Google Scholar]
- Walker BJ, Cousins AB. 2013. Influence of temperature on measurements of the CO2 compensation point: differences between the Laisk and O2-exchange methods. Journal of Experimental Botany 64, 1893–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J-L, Turgeon R, Carr JP, Berry JO. 1993. Carbon sink-to-source transition is coordinated with establishment of cell-specific gene expression in a C4 plant. The Plant Cell 5, 289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JL, Klessig DF, Berry JO. 1992. Regulation of C4 gene expression in developing amaranth leaves. The Plant Cell 4, 173–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.