Highlight
Bundle-sheath conductance, g bs , is commonly assumed to be independent of temperature. We report that temperature response of maize g bs followed a peaked or non-peaked Arrhenius equation, triggering further investigations on this response.
Key words: Diffusive resistance, maximum PEPc activity, maximum Rubisco activity, modelling, warming effect, Zea mays.
Abstract
A small bundle-sheath conductance (g bs) is essential for the C4 CO2-concentrating mechanism to suppress photorespiration effectively. To predict the productivity of C4 crops accurately under global warming, it is necessary to examine whether and how g bs responds to temperature. We investigated the temperature response of g bs in maize by fitting a C4 photosynthesis model to combined gas exchange and chlorophyll fluorescence measurements of irradiance and CO2 response curves at 21% and 2% O2 within the range of 13.5–39 °C. The analysis was based on reported kinetic constants of C4 Rubisco and phosphoenolpyruvate carboxylase and temperature responses of C3 mesophyll conductance (g m). The estimates of g bs varied greatly with leaf temperature. The temperature response of g bs was well described by the peaked Arrhenius equation, with the optimum temperature being ~34 °C. The assumed temperature responses of g m had only a slight impact on the temperature response of g bs. In contrast, using extreme values of some enzyme kinetic constants changed the shape of the response, from the peaked optimum response to the non-peaked Arrhenius pattern. Further studies are needed to confirm such an Arrhenius response pattern from independent measurement techniques and to assess whether it is common across C4 species.
Introduction
C4 crop species have the CO2-concentrating mechanism (CCM) in photosynthesis, which raises the partial pressure of CO2 in bundle-sheath cells to a very high level, thereby minimizing the oxygenation activity of Rubisco and the loss by photorespiration (Hatch et al., 1995). This explains why C4 food crops such as maize (Zea mays L.) generally have higher productivities than their C3 counterparts (Ort and Long, 2014), at least under relatively warm conditions, and why C4 species are preferred as a major source of sustainable bioenergy production (Heaton et al., 2008; Slattery and Ort, 2015).
The CCM mechanism in C4 crops requires a number of biochemical, physical, and structural adaptations, especially the Kranz anatomy including the specialization of the mesophyll and bundle-sheath cells (Hatch et al., 1995; Leegood, 2002; Kromdijk et al., 2014). In C4 photosynthesis, CO2 is first fixed via phosphoenolpyruvate carboxylase (PEPc) in mesophyll cells into C4 acids, which are then transported into bundle-sheath cells where the C4 acids are decarboxylated and the released CO2 is refixed by Rubisco. An efficient CCM would require that (i) PEPc has higher CO2 affinity and carboxylation capacity than Rubisco; and (ii) the rate of CO2 leakage from bundle-sheath cells back into mesophyll cells (L) is low.
This CO2 leakage rate L depends on both the bundle-sheath conductance for CO2 (g bs) and the gradient between the CO2 concentration in mesophyll cells (C m) and that around the carboxylation sites in bundle-sheath cells (C c): L=g bs(C c –C m) (von Caemmerer and Furbankm 1999; Kromdijk et al., 2014). Therefore, g bs is an important parameter for the effectiveness of CCM. We have previously analysed how g bs responds to nitrogen supply, and showed that g bs increases with increasing leaf nitrogen content (Yin et al., 2011)—a trend similar to that found for mesophyll conductance (g m) in response to leaf photosynthetic capacity in C3 photosynthesis (e.g. Loreto et al., 1992). It has been well established that g m in C3 photosynthesis responds to leaf temperature (Bernacchi et al., 2002; Warren and Dreyer, 2006; Yamori et al., 2006; Scafaro et al., 2011; Evans and von Caemmerer, 2013; Walker et al., 2013), although the effects differ greatly among species (von Caemmerer and Evans, 2015). Massad et al. (2007) have parameterized temperature dependence of some C4 parameters such as the maximum rates of PEPc carboxylation (V pmax), of Rubisco carboxylation (V cmax), and of electron transport. However, there is hardly any information in the literature on whether and how g bs responds to leaf temperature. Reports of Kubien et al. (2003) and von Caemmerer et al. (2014) on the temperature effect on leakiness (ϕ; which is defined as L/V p, where V p is the PEPc carboxylation rate) indirectly suggest that g bs, among many C4 parameters, may (co-)vary with temperature. To the best of our knowledge, only one study (Kiirats et al., 2002) has directly reported on the temperature response of g bs: g bs increased almost linearly with increasing leaf temperature within the range of 16–35 °C. That study used a PEPc mutant of Amaranthus edulis with a defective C4 cycle. However, concerns have been raised about potential alterations of Rubisco kinetic constants, gas diffusion resistance, and bundle-sheath cell structure by the PEPc mutation (He and Edwards, 1996).
Given the effect of elevating atmospheric CO2 and global warming, it is necessary to assess the production potential of C4 species, as well as whether their relative advantages over C3 species vary, under climate change. Like the widely used C3 photosynthesis model of Farquhar et al. (1980), the C4 biochemical models (Berry and Farquhar, 1978; von Caemmerer and Furbank, 1999) or their variants (Collatz et al., 1992; Chen et al., 1994; Yin and Struik, 2009a) are now increasingly coupled with stomatal conductance models and applied to a general ecosystem or crop simulation framework. However, these modelling studies all assume that g bs does not vary with temperature, even when applied to a wide range of natural field environments. To apply the biochemical C4 photosynthesis model to assess the (relative) production potentials and their response to climate change variables, information on the effects of temperature on g bs is urgently needed. The objectives of this study are to assess whether or not g bs in leaves of a maize cultivar responds to leaf temperature and, if so, to quantify the magnitude of this effect over a wide range of temperatures. To that end, we use the method of Yin et al. (2011) that can estimate g bs by fitting a C4 photosynthesis model to a wide range of data covering different amounts of photorespiration.
Materials and methods
Experimental set-up
An experiment was conducted in a glasshouse at Wageningen University, using maize cv. ‘Atrium’. To spread out the measurement work in time, a weekly staggered sowing was carried out on 28 August and 4, 10, and 18 September 2014, respectively, to grow plants for four replicate measurements. Plants were transplanted 7 d after sowing to pots of 12 litres, with one plant per pot. The initial soil nutrient contents were: 1.23g of nitrogen, 0.69g of phosphate, and 2.49g of potassium per pot. Extra nutrients came from 10.3g per pot of a slow-release fertilizer ‘Osmocote Pro’ (which contained 17% N, 11% P2O5, 10% K2O, 2% MgO plus trace elements). Temperature in the glasshouse was 27±2 °C for daytime and 21±1 °C for night-time . Photoperiod was maintained at 12h d−1 (8:00–20:00h), and relative humidity ranged between 60% and 70%. Of the photosynthetically active radiation incident on the glasshouse, 60% was transmitted to plant level. During daytime, supplemental light from 600W HPS Hortilux Schréder lamps (Monster, The Netherlands; 0.4 lamps m−2) was switched on automatically as soon as the global solar radiation incident on the glasshouse dropped below 400W m−2, and then switched off if it exceeded 500W m−2. The supplementary light largely ensured that, despite the staggered sowing, plants for measurements grew under similar light intensities.
Photosynthesis measurements
After growth in the glasshouse for ~6 weeks, plants were moved to a climate room illuminated by cool-white fluorescent tubes (~350 μmol m−2 s−1 at leaf level), where all measurements were undertaken. We used an LI-6400XT open gas exchange system with an integrated fluorescence chamber head, enclosing 2cm2 areas, for combined gas exchange and chlorophyll fluorescence measurements, which were done on fully expanded leaves at the seventh leaf layer counted from below. The CO2 response curves were taken under both 21% and 2% O2 conditions, and the ambient CO2 (C a) steps were: 370, 200, 100, 85, 70, 55, 370, 370, 370, 500, 700, and 1500 μmol mol−1 (~4min per step) while keeping incident irradiance (I inc) at 1500 μmol m−2 s−1 (while measurements were done four times at 370 μmol mol−1, data of only the first and fourth times were included for the analysis). For light response curves, I inc was of the order of 2000, 1500, 1000, 500, 200, 100, 80, 60, 40, and 20 μmol m−2 s−1 (~8min per step), while keeping C a either at 250 μmol mol−1 for 21% O2 or at 1000 μmol mol−1 for 2% O2 conditions; this was done to induce different levels of photorespiration. Gas from a cylinder containing a mixture of N2 and required O2 was humidified and supplied via an overflow tube to the air inlet of the LI-6400XT where CO2 was blended with the gas, and the IRGA calibration was adjusted for O2 composition of the gas mixture according to the manufacturer’s instructions.
Each curve was made at six set-point leaf temperatures (13.5, 18, 25, 30, 34, and 39 °C), of which extreme temperatures (13.5, 34, and 39 °C) were achieved not only by setting the temperature in the LI-6400XT measuring head but also by adjusting the temperature of the climate room. Measurement of one replicate took 5 d and was done on the same leaf for all temperatures. Any influence of different measuring days was minimized by randomizing temperatures and measuring days among the replicates. In total, 48 (i.e. 6 temperatures×4 replicates×2 O2 levels) light response curves and 48 CO2 response curves were generated. Leaf-to-air vapour pressure difference increased with leaf temperature, but was always within the range of 0.5–2.0 kPa, within which little impact of vapour pressure difference on stomatal conductance is expected (Bernacchi et al., 2002). The measurement flow rate was 400 μmol s−1. CO2 exchange rates were corrected for CO2 leakage into and out of the leaf cuvette, based on measurements at specific temperatures using the same flow rate on boiled leaves across a range of CO2 levels, and intercellular CO2 levels (C i) were then re-calculated.
For each step of light or CO2 response, when the CO2 exchange rate reached steady state, steady-state fluorescence (F s) was measured. Dwyer et al. (2007) have shown that for C4 leaves, the multiple-flash method is more reliable than the traditional single-flash method in measuring the maximum fluorescence (F m′). The F m′ was therefore obtained from using the multiphase flashes: the flash intensity was ~8000 µmol m−2 s−1 during phase 1 for a duration of 300ms, was attenuated by 35% during phase 2 for ~300ms, and was back to ~8000 µmol m−2 s−1 for phase 3 of 300ms. The intercept of the linear regression of fluorescence yields against the inverse of the flash intensity during phase 2 gives the estimate of F m′ (Loriaux et al. 2013). The apparent operating efficiency of photosystem II (PSII) e− transport was obtained as: ∆F/F m′=(F m′–F s)/F m′ (Genty et al., 1989; Schreiber et al., 1995).
The portions of the leaf used for above measurements were excised afterwards, with a punch that produced a disc of ~5cm2 per leaf portion. The leaf discs were then weighed after drying at 70 °C to constant weight, and total N content was analysed using an element C/N analyser (Flash 2000, Thermo Scientific) based on the Micro-Dumas combustion method.
Modelling
The model of von Caemmerer and Furbank (1999), as modified by Yin et al. (2011), was used to estimate g bs (see Supplementary appendix A at JXB online). The first modification was to include all four combinations of rate limitations to describe CO2 and light response curves of the C4 leaf CO2 assimilation rate (A) more smoothly:
| (1) |
where A EE is the net CO2 assimilation rate when both C4 and C3 cycles are limited by enzyme activity, A ET is the net rate when the C4 cycle is limited by enzyme activity and the C3 cycle is limited by e− transport, A TE is the rate when the C4 cycle is limited by e- transport and the C3 cycle is limited by enzyme activity, and A TT is the rate when both C4 and C3 cycles are limited by e− transport. The formulation of von Caemmerer and Furbank (1999) used only two combinations, namely A=min(A EE,A TT). The second modification was to consider mesophyll conductance (g m) explicitly so that A is modelled using C i (rather than mesophyll CO2 level C m) as input, as C m is not measured. The model considering g m becomes more complicated, and Yin et al. (2011) presented an analytical solution for each of the four limitations (see Supplementary appendix A). The third modification was to use the potential rate of ATP production (J atp), instead of the potential rate of e− transport rate (J), because energy is partitioned between C4 and C3 cycles ultimately in terms of ATP (rather than in terms of electron) requirement. von Caemmerer and Furbank (1999) implicitly assumed that J atp=J, whereas the analysis of Yin and Struik (2012) showed that J atp may not equal J.
We estimated the value of J atp empirically from chlorophyll fluorescence measurements, according to Yin et al. (2011):
| (2) |
where x is the fraction of ATP partitioned to the C4 cycle (set to 0.4; von Caemmerer and Furbank 1999), and s′ is a lumped parameter resulting as the slope from the linear regression of A measured under low irradiances (≤500 µmol m−2 s−1) against I inc(∆F/F m′)/3 using only the data at 2% O2 combined with high CO2, at which photorespiration is suppressed. The intercept of the same linear regression will give the estimate of day respiration (R d) (Yin et al., 2011). It is worth noting the importance of only using an e− transport-limited range of data for estimating s′ which calibrates for the impact of any basal alternative e− transport. Impacts of any additional alternative e− transport, such as under high I inc or low C i conditions, arising from the higher J atp than required for C4 and C3 cycles, are accounted for by Equation 1 via assigning to enzyme activity-limited rates.
The model of von Caemmerer and Furbank (1999) was proposed for the reference temperature 25 °C. To be applied for a range of varying temperatures, the potential variation of relative O2/CO2 diffusivities and solubilities with temperature needs to be quantified. This is presented in Supplementary appendix B, based on data in the literature (e.g. Frank et al., 1996; Han and Bartels, 1996).
Pre-determination of some photosynthetic parameters
To use the model to estimate g bs, a number of other input parameter values are required (see Table 1 for parameter definitions). The Rubisco kinetic parameters (V cmax, γ*, K mC, and K mO), the PEPc Michaelis–Menten constant (K p), and R d are expected to increase with temperature, and this is conventionally described by an Arrhenius function normalized at 25 °C:
Table 1.
Model input parameters, with their default values as derived from the literature or in this study
| Symbol | Definition | Value | Source |
|---|---|---|---|
| α | Fraction of PSII activity in the bundle sheath | 0.1 | Chapman et al. (1980) |
| R m | Mitochondrial respiration in the mesophyll | 0.5R d | von Caemmerer and Furbank (1999) |
| x | Fraction of ATP allocated to the C4 cycle | 0.4 | von Caemmerer and Furbank (1999) |
| K p25 | Michaelis–Menten constant of PEPc for CO2 at 25 °C | 40 μbar | Leegood and von Caemmerer (1989); Pfeffer and Peisker (1995) |
| K mC25 | Michaelis–Menten constant of Rubisco for CO2 at 25 °C | 485 μbar | Cousins et al. (2010) |
| K mO25 | Michaelis–Menten constant of Rubisco for O2 at 25 °C | 146 000 μbar | Cousins et al. (2010) |
| γ*25 | Half the reciprocal of Rubisco specificity at 25 °C | 0.0001747 | Cousins et al. (2010) |
| E Vcmax | Activation energy for V cmax (maximum Rubisco activity) | 53.4 kJ mol−1 | Sage (2002); Kubien et al. (2003); Perdomo et al. (2015) |
| E γ* | Activation energy for γ* | 27.4 kJ mol−1 | Derived from data of Jordan and Ogren (1984) |
| E KmC | Activation energy for K mC | 35.6 kJ mol−1 | Perdomo et al. (2015) |
| E KmO | Activation energy for K mO | 15.1 kJ mol−1 | Derived from results of Perdomo et al. (2015) |
| E Vpmax | Activation energy for V pmax (maximum PEPc activity) | 37.0 kJ mol−1 | Derived from data of Chinthapalli et al. (2003) |
| D Vpmax | Deactivation energy for V pmax | 214.5 kJ mol−1 | Derived from data of Chinthapalli et al. (2003) |
| S Vpmax | Entropy term for V pmax | 0.663 kJ K−1 mol−1 | Derived from data of Chinthapalli et al. (2003) |
| E Kp | Activation energy for K p | 68.1 kJ mol−1 | Estimated in our report (see text) |
| E gm | Activation energy for g m (mesophyll conductance) | 49.6 kJ mol−1 | Bernacchi et al. (2002) |
| D gm | Deactivation energy for g m | 437.4 kJ mol−1 | Bernacchi et al. (2002) |
| S gm | Entropy term for g m | 1.4 kJ K−1 mol−1 | Bernacchi et al. (2002) |
PEPc, phosphoenolpyruvate carboxylase; PSII, photosystem II.
| (3) |
where R is the universal gas constant (0.008314 kJ K−1 mol−1) and E is the activation energy (kJ mol−1) for the parameter. It is impossible to derive a complete set of in vivo kinetic constants of C4 photosynthesis, and we mostly used in vitro values as reported in the literature. Values of γ*, K mC, K mO, and K p at the reference temperature 25 °C (Table 1) were the same as those we used previously (Yin et al., 2011), based on data for maize (e.g. Cousins et al., 2010). Other parameter values are hardly available for maize, and the values used are summarized below.
(i) Sage (2002), Kubien et al. (2003), and Perdomo et al. (2015) reported the activation energy E of V cmax. Sage′s data on E Vcmax for seven C4 species range from 50.1 kJ mol−1 for Cynodon dactylon and 53.5 kJ mol−1 for Flaveria trinervia to 68.0 kJ mol−1 for Amaranthus retroflexus. Kubien et al. (2013) reported 56.1 kJ mol−1 within 18–42 °C for Flaveria bidentis. E Vcmax values reported by Perdomo et al. (2015) for Flaveria bidentis and Flaveria trinervia were 47.6 kJ mol−1 and 48.8 kJ mol−1, respectively. Despite the variation of E Vcmax even for the same species, E Vcmax for C4 species did not differ greatly from that for C3 species (Sage, 2002; Perdomo et al., 2015). The average E Vcmax of the three reports for C4 species was used here (Table 1).
(ii) Jordan and Ogren (1984) were the first to report on Rubisco specificity from 5 °C to 35 °C of a C4 species Amaranthus hybridus, from which we derived the activation energy for γ* (Table 1). This estimate is quite similar to the value of Bernacchi et al. (2002) for C3 species, and the report of Boyd et al. (2015) for a C4 species Setaria viridis.
(iii) The activation energy for K mC of C4 Rubisco was based on the recent report of Perdomo et al. (2015) on two Flaveria C4 species (Table 1).
(iv) Little is known for the activation energy for K mO of C4 Rubisco, and Table 1 gives its value that we derived from data of Perdomo et al. (2015) on activation energies for specificity (S c/o) and K mC, using the formula K mO=S c/o K mC(V omax/V cmax) (where V omax is the maximum oxygenation rate of Rubisco) and assuming that the V omax:V cmax ratio does not vary with temperature (i.e. activation energy for this ratio=0). The latter assumption was based on reports that the activation energy for V omax:V cmax of C3 Rubisco is either small (Bernacchi et al., 2001) or inconsistent (either positive or negative) across species (von Caemmerer and Quick, 2000; Walker et al., 2013). This is in line with the C3 photosynthesis modelling (Farquhar et al., 1980) that V omax:V cmax is set to be independent of temperature. The derived activation energy for K mO (15.1 kJ mol−1) is only slightly higher than 10.5 (±4.8) kJ mol−1, the value that Boyd et al. (2015) published for S. viridis while we were revising our paper. Our E KmO corresponds to a Q 10 factor of ~1.23, very close to the value 1.20 that Chen et al. (1994) used. Sensitivity analysis showed that the temperature response of g bs was least sensitive to E KmO (see the Results).
-
(v) V pmax follows an optimum response to temperature (Chinthapalli et al., 2003; Massad et al., 2007; Boyd et al., 2015). This optimum response can be described by the peaked Arrhenius function (Medlyn et al. 2002):
(4) where S is an entropy term (kJ K−1 mol−1), and E and D are energies of activation and deactivation (kJ mol−1), respectively. Differentiating Equation 4 gives the optimum temperature T opt (°C) as:(5) We used the in vitro data of Chinthapalli et al. (2003) for Amaranthus hypochondriacus, which cover a very wide range of temperatures from 15 °C to 50 °C, to fit Equation 4 to derive values for S, E, and D of V pmax (Table 1). These estimates resulted in an estimate of T opt=44.4 °C.
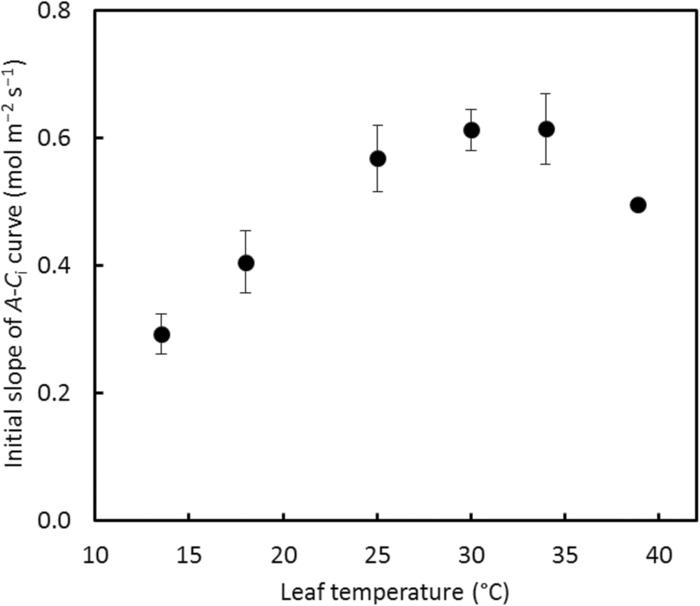
(vi) Little is known about the activation energy of K p. We examined the initial slope of A–C i curves at 2% O2, since A at low C i is limited by the PEPc activity (Sage and Kubien, 2007) and can be approximated to C i V pmax/(C i+K p)–R m (von Caemmerer and Furbank, 1999). The first-order derivative of this equation, dA/dC i, is K p V pmax/(C i+K p)2, and was set to equal the slope value of the initial linear part of the A–C i curve. The initial linear slope of the A–C i curve followed an optimum response to temperature (see the Results) and this response is expected to result from temperature responses of both K p and V pmax. Using the pre-estimated temperature response parameters of V pmax, we then derived the activation energy for K p by fitting combined Equation 3 and dA/dC i=K p V pmax/(C i+K p)2 to data on the initial linear slope of the A–C i curves over the six temperatures. We will confirm our estimate on E Kp from fitting a full model to data of the initial part of A–C i curves (see the Results).
(vii) Mesophyll conductance (g m) may be a significant limiting factor for C4 photosynthesis (Pfeffer and Peisker, 1998), has an impact on estimation of leakiness (von Caemmerer et al., 2014), and its role in estimating g bs has yet to be quantified. However, g m for C4 photosynthesis is hard to estimate (Pfeffer and Peisker, 1998; Barbour et al., 2016), let alone its temperature response parameters. We took the widely used values of Bernacchi et al. (2002) for g m in C3 photosynthesis, which include E, D, and S as quantified in Equation 4 (Table 1). This approach assumes that C3 and C4 photosynthesis have a similar relative response of g m to temperature, although g m in C4 does not involve chloroplast-related resistance components. Our assumption for the same relative response of the overall g m to temperature for C3 and C4 leaves will be tested through a sensitivity analysis.
Curve fitting and sensitivity analysis
With all these parameters pre-determined, we estimated g bs of the six temperatures as well as g m25, V cmax25, V pmax25 (i.e. g m, V cmax, V pmax at 25 °C, respectively), by the non-linear curve-fitting using Equation 1 in combination with solutions in Supplementary appendix A. We used a dummy variable approach (Yin et al., 2009), in which we introduced six dummy variables to correspond to six temperatures, allowing us to estimate treatment-specific parameters (i.e. g bs at six temperatures) and common parameters (i.e. g m25, V cmax25, and V pmax25) simultaneously. The statistical fitting algorithms, implemented in SAS, autoassigned the range of data points to each of the four limitations as defined by Equation 1. The g bs estimates when plotted against leaf temperature followed an optimum response (see the Results), and parameters characterizing this response were derived from fitting the estimated g bs to Equation 4. All required curve fitting was carried out using the least-squares non-linear regression with the GAUSS method in PROC NLIN of SAS (SAS Institute Inc., Cary, NC, USA). The SAS codes for estimating g bs parameters are available from the corresponding author upon request.
Regardless of the technique used, estimates for kinetic constants of Rubisco are full of uncertainties (Kubien et al., 2008; Cousins et al., 2010), so are the constants of PEPc (Pfeffer and Peisker, 1998) and of g m (Silim et al., 2010; Walker et al., 2013). Also, the input values of some constants were not determined exclusively for maize. Therefore, a full sensitivity analysis on the g bs estimates was conducted with respect to these input parameters in Table 1, except for x and α. The value of x is expected to be very invariant in terms of the ATP requirement between C4 and C3 cycles (von Caemmerer and Furbank, 1999), and a previous analysis (Yin et al., 2011) showed little sensitivity of g bs to parameter α.
Results
Overall response curves of A and ∆F/F m′ to CO2 and irradiance
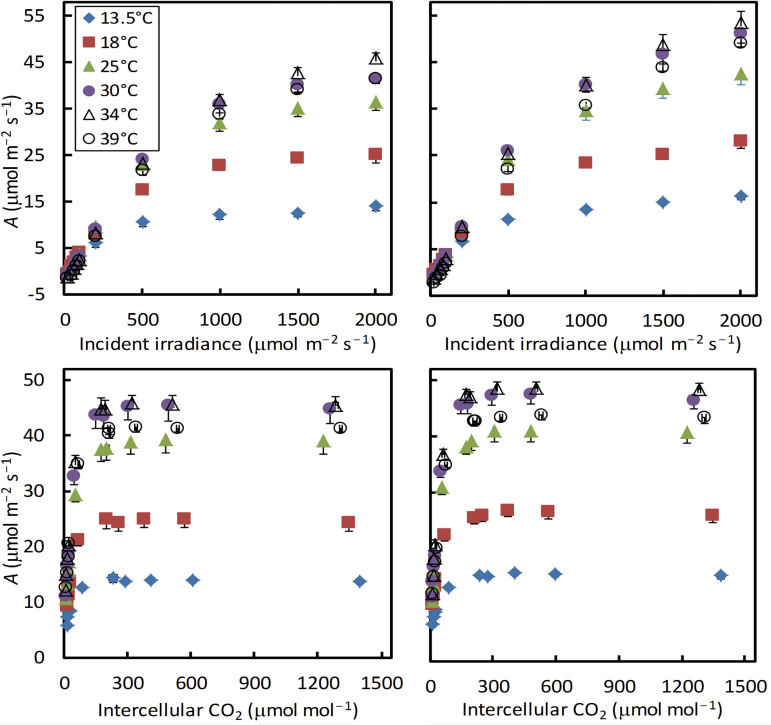
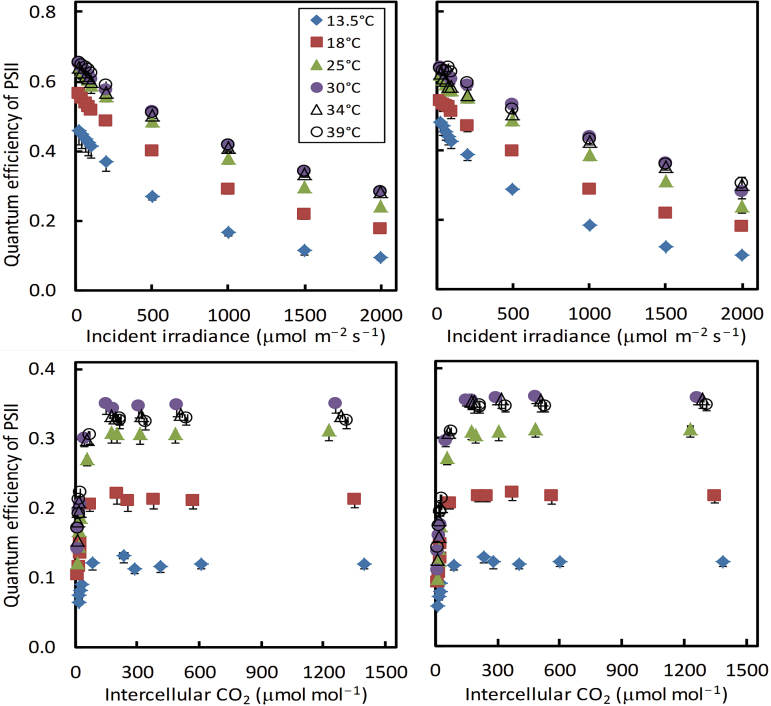
Our experimental results at the six temperatures showing typical irradiance and CO2 responses of the C4 photosynthesis rate were obtained (Fig. 1). The non-photorespiratory condition (2% O2 combined with 1000 μmol mol−1 C a) had a moderate positive effect on the irradiance response curves, compared with the curves of 21% O2 combined with 250 μmol mol−1 C a. Temperature strongly affected both irradiance and CO2 response curves, and its effect was more significant from 13.5 °C to 25 °C than from 25 °C to 39 °C. The maximum photosynthesis was observed at ~34 °C. The effect of temperature on photosynthesis was reflected by the data for temperature effect on ∆F/F m′, the apparent operating efficiency of PSII e− transport (Fig. 2).
Fig. 1.
Net CO2 assimilation rate A at six leaf temperatures in response to incident irradiance or to intercellular CO2 levels under 21% (left panels) or 2% (right panels) O2 conditions. Each symbol represents the mean of four replicated leaves (SEMs are visible in bars if larger than the symbols).(This figure is available in colour at JXB online.)
Fig. 2.
Apparent operating quantum efficiency of photosystem II (PSII) electron transport (Φ2 or ∆F/F m′) at six leaf temperatures in response to incident irradiance or to intercellular CO2 levels under 21% (left panels) or 2% (right panels) O2 conditions. Each symbol represents the mean of four replicated leaves (SEMs are visible if larger than the symbols). (This figure is available in colour at JXB online.)
Estimates of s′ and R d
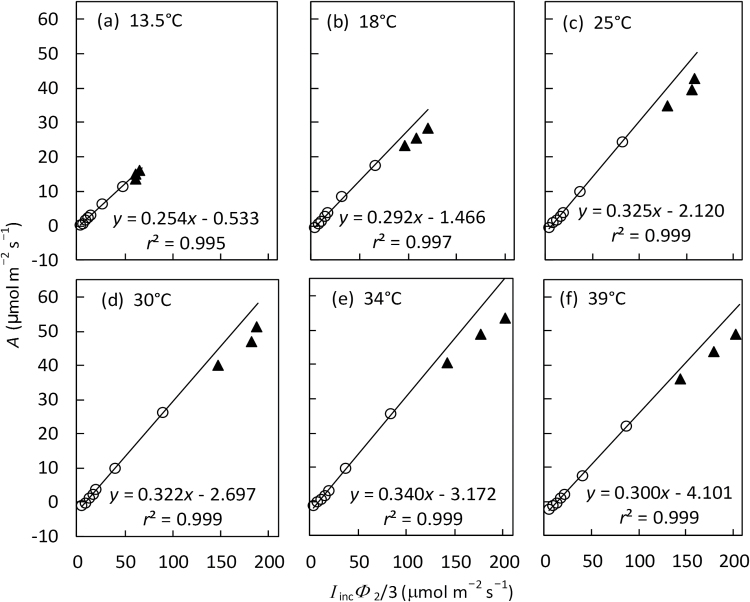
The relationship between A and I inc(∆F/F m′)/3 measured at low irradiances (≤500 µmol m−2 s−1) under non-photorespiratory conditions was linear for all temperatures (Fig. 3). The slope of this linear relationship gives the estimate for s′, a lumped parameter for calculating J atp (see Equation 2), and its intercept gives the estimate of R d (day respiration). Data points of higher irradiances (>500 µmol m−2 s−1) lay below the linear trend for all temperatures (Fig. 3), indicating that A was limited by A EE or A TE (see Equation 1) at high irradiances.
Fig. 3.
The relationship between net CO2 assimilation rate A and the lumped variable I incΦ2/3 (mean of four replicates) from irradiance response curves under non-photorespiratory conditions (i.e. at 2% O2 combined with high CO2) at six leaf temperatures. Open circles are for I inc ≤500 μmol m−2 s−1 and filled triangles come from the three levels of I inc >500 μmol m−2 s−1. The lines represent linear regression based on data with I inc ≤500 μmol m−2 s−1, in which the slope gives the estimate of calibration factor s′ and the intercept gives the estimate of day respiration R d (see the text).
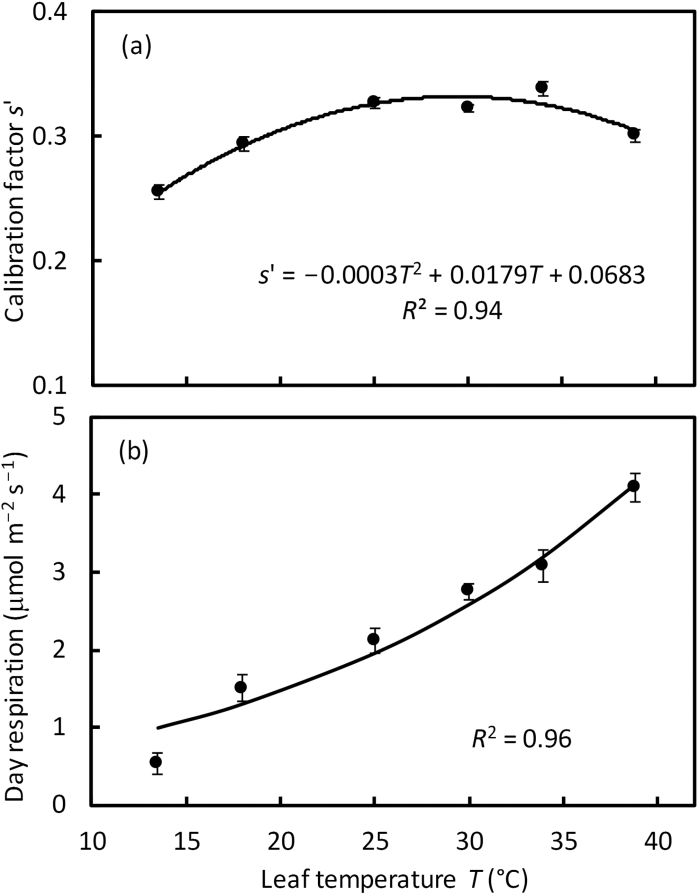
The estimated calibration factor s′ varied from 0.25 to 0.34, and its response to temperature can be empirically described by a quadratic equation with the optimum temperature at ~30 °C (Fig. 4a). As expected, the estimated values of R d increased with increasing temperature (Fig. 4b). This response was well described by the Arrhenius equation, Equation 3, with the estimated R d at 25 °C being 1.95 μmol m−2 s−1 and the activation energy being 41.9 kJ mol−1 (Table 2).
Fig. 4.
(a) Temperature response of the estimated calibration factor s′, and (b) temperature response of the estimated day respiration R d. Values of s′ and R d were estimated as the slope and the intercept, respectively, of linear regression in Fig. 3. In (b), the curve represents the Arrhenius plot of Equation 3 with estimated parameter values in Table 2. Bars in (a) and (b) represent SEs of the estimates.
Table 2.
Values (standard errors of the estimates in parentheses) of parameters at the reference temperature 25 °C, activation energy E in Equation 3 for day respiration (R d), as well as activation energy E, deactivation energy D, and entropy term S of Equation 4 for bundle-sheath conductance (g bs) and its optimum temperature T opt calculated from Equation 5, of maize leaves, as estimated from data in the present study
| Parameter | Estimate at 25 °C | E (kJ mol−1) | D (kJ mol−1) | S (kJ K−1 mol−1) | T opt (°C) |
|---|---|---|---|---|---|
| R d | 1.95(0.14) μmol m−2 s−1 | 41.85 (5.32) | NA | NA | NA |
| g m | 1.33(0.06) mol m−2 s−1 | – | – | – | – |
| V cmax | 49.0(0.9)μmol m−2 s−1 | – | NA | NA | NA |
| V pmax | 119.2(4.1)μmol m−2 s−1 | – | – | – | – |
| g bs | 2.87(0.31) mmol m−2 s−1 | 116.77 (30.39) | 264.60 (51.96) | 0.86 (0.16) | 33.9 |
NA, not applicable; –, not estimated from data of the present study, and most of them are given in Table 1, based on data in the literature, and were used as input to our present model analysis.
Initial slope of A– C i curves
The A–C i curves at 2% O2 for the first three (for 13.5 °C) or four (for the remaining temperatures) points were essentially linear (Supplementary Fig. S1). The slope of this initial linear section of A–C i curves at six temperatures followed an optimum response to temperature (Fig. 5), and this response is expected to result from temperature responses of both K p and V pmax. Using the pre-estimated temperature response parameter values of V pmax (Table 1), we estimated the activation energy for K p by fitting combined Equation 3 and dA/dC i=K p V pmax/(C i+K p)2 to data in Fig. 5 for the initial slope of A–C i curves at the six temperatures. The estimated E Kp, the activation energy for K p, was 68.1 (SE 6.8) kJ mol−1 (Table 1).
Fig. 5.
Temperature response of the initial slope of the A–C i curve at 2% O2. The error bar of each point indicates ±SEM of four replicated leaves. The error bar of the last point is smaller than the symbol.
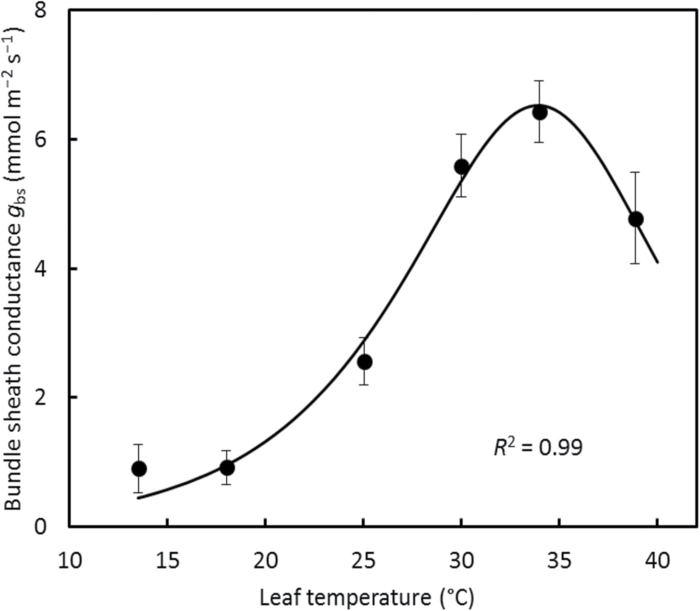
Estimated response of g bs to temperature
With s′, R d, and E Kp pre-fixed as presented above and other input parameters available (Table 1), we were able to estimate g m25, V cmax25, V pmax25, and g bs of the six temperatures by fitting our model (Equation 1 combined with solutions given in the Supplementary data) to all data collected in the experiment. The model described the whole data set across A–C i and A–I inc curves at 2% and 21% O2, with R 2=0.98 and relative root-mean-square error rRMSE (RMSE×100/the mean of measured A)=12.0%, and a plot comparing modelled and measured A–I inc and A–C i curves is given in Supplementary Fig. S2 for 21% O2. The obtained g m25 was 1.33mol m−2 s−1, V cmax25 was 49.0 μmol m−2 s−1, and V pmax25 was 119.2 μmol m−2 s−1 (Table 2), meaning that the V pmax25:V cmax25 ratio is 2.43. We measured leaf N content, which was 1.10 (SE 0.05) g m−2. Assuming the base leaf nitrogen content for photosynthesis is 0.24g m−2 (Yin et al. 2011), the slope of V cmax and V pmax versus leaf N is 57.7 μmol and 140.3 μmol (g N)−1 s−1, respectively.
The estimated g bs clearly varied with leaf temperature, and this response can be well described by the peaked Arrhenius equation, Equation 4 (Fig. 6). The obtained parameters of Equation 4 are: g bs25=2.87 mmol m−2 s−1, E=116.7 kJ mol−1, D=264.6 kJ mol−1, and S=0.86 kJ K−1 mol−1 (Table 2). These parameter values of the peaked Arrhenius equation predicted, according to Equation 5, 34 °C as the optimum temperature for g bs.
Fig. 6.
Temperature response of estimated bundle-sheath conductance g bs in maize leaves. The error bar at each point represents ±SE of the estimate. The curve represents the peaked Arrhenius fit of Equation 4 with estimated values of the parameters in Table 2.
If we set g bs independent of temperature, the obtained estimates from fitting to our data would be: g m25=4.37mol m−2 s−1, g bs=4.53 mmol m−2 s−1, V cmax25=52.7 μmol m−2 s−1, and V pmax25=80.0 μmol m−2 s−1, with R 2=0.98 and rRMSE=13.3%. The obtained g m25 increased by >3-fold, V pmax25 decreased by 33%, and, therefore, the V pmax25:V cmax25 ratio dropped to 1.5 and may have been underestimated (see the Discussion). Furthermore, this g bs temperature-insensitive model statistically decreased the goodness of fit (P<0.001 based on the F-test).
If we use the original model of von Caemmerer and Furbank (1999) which predicts A as the minimum of two limiting A TT and A EE, we obtained the estimates: g m25=1.53mol m−2 s−1, V cmax25=41.9 μmol m−2 s−1, and V pmax25=119.8 μmol m−2 s−1, with g bs being 1.18, 0.72, 3.39, 10.60, 9.35, and 5.07 mmol m−2 s−1 at 13.5, 18, 25, 30, 34, and 39 °C, respectively. The model described the data (R 2=0.97 and rRMSE=14.3%) somewhat less adequately than our four-rates model. Also, the estimates of g bs at 30 °C and 34 °C became higher, resulting in different parameter estimates for g bs temperature response: g bs25=3.54 mmol m−2 s−1, E=264.9 kJ mol−1, D=385.7 kJ mol−1, and S=1.27 kJ K−1 mol−1. This gives T opt=31.3 °C, ~2.6 °C lower than T opt resulting from the four-rates model. The difference stemmed from the fact that many data points were determined by A TE (results not shown), which is excluded in the two-rates model.
Sensitivity of estimated g bs–temperature relationships to input parameters
In Table 1, the value of E Kp was derived from our own data using an approximate model for describing the initial slope of A–C i curves (see earlier). This procedure may be criticized because (i) the approximate model assumes an infinite g m; (ii) the procedure requires that the initial section of A–C i curves is exactly linear; and (iii) the required estimates for temperature response parameters of V pmax, which we derived from Chinthapalli et al. (2003), may actually be uncertain. To examine the uncertainties in our estimated E Kp, we used a more complete model (Supplementary appendix C) combined with two other reports (Massad et al., 2007; Boyd et al., 2015) on temperature response parameters of V pmax. We fitted the model to the initial section of A–C i curves where A is expected to be limited by the PEPc activity, and the obtained E Kp estimate was 66.3, 79.5, and 73.3 kJ mol−1 if temperature response parameters of V pmax came from Chinthapalli et al. (2003), Massad et al. (2007), and Boyd et al. (2015), respectively (Supplementary Table S1). When the E Kp estimate was combined with their corresponding temperature response parameter values of V pmax from the three studies, the resulting estimates of g bs at six temperatures and of V cmax25 were hardly affected by the use of these different sets of input for E Kp and temperature response of V pmax (Supplementary Table S1).
The temperature responses of g m do not yet exist for C4 species, and temperature responses for C3 species differed greatly among reports for tobacco (Bernacchi et al., 2002; Evans and von Caemmerer, 2013; Walker et al., 2013), and among species (von Caemmerer and Evans, 2015), in particular between tobacco and Arabidopsis (Walker et al., 2013). The responses for tobacco ranged from the peaked Arrhenius response (Bernacchi et al., 2002; Walker et al., 2013) to a linear pattern (Evans and von Caemmerer, 2013), whereas data of Walker et al. (2013) for Arabidopsis showed virtually no effect of temperature on g m. Despite such a contrast in the temperature response of g m used as input, the overall response of g bs to temperature remained similar, following a peaked Arrhenius pattern (Supplementary Fig. S3). The obtained V cmax25 varied little, from 48.5 μmol m−2 s−1 to 51.4 μmol m−2 s−1. The obtained V pmax25 varied to a greater extent, from 75.3 μmol m−2 s−1 (from using the Arabidopsis response) to 155.6 μmol m−2 s−1 (from using the tobacco response of Walker et al., 2013). The obtained g m25 was mostly between 1.25mol m−2 s−1 and 1.45mol m−2 s−1, but using the Arabidopsis response gave an infinite estimate of g m25.
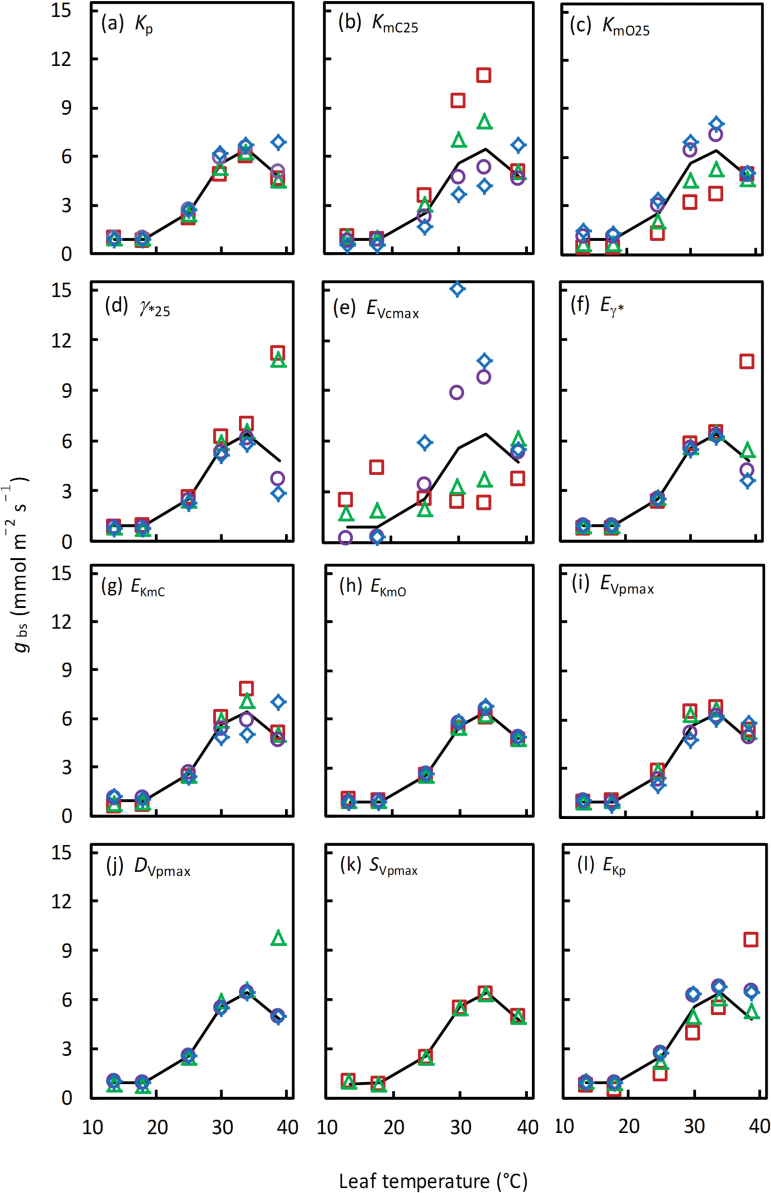
For sensitivity analyses of the estimated g bs temperature response to 12 other parameters, the input parameters were varied by ±25% and 50% of their default values (Fig. 7). The estimated temperature response of g bs was least sensitive to changes in K p (Fig. 7a), E KmO (Fig. 7h), and E Vpmax (Fig. 7i). Overall, the sensitivity depended on the level of the parameter changes. Using extreme values of γ*25, E Vcmax, E γ*, E KmC, D Vpmax, and E Kp changed the shape of the response, from the peaked optimum response to the non-peaked Arrhenius pattern. The −50% change in D Vpmax and +25% and +50% changes in S Vpmax resulted in a biologically unrealistic (negative) estimate of g bs, so their resulting response pattern is not given (Fig. 7j, k), suggesting that these changes may have reached beyond biologically realistic scopes of the two parameters. Note that Equation 5 suggests a co-determination of T opt by E, D, and S and a much higher sensitivity to D Vpmax and S Vpmax than to E Vpmax in determining T opt of V pmax.
Fig. 7.
Sensitivity of bundle-sheath conductance, g bs, temperature response to changes in 12 input parameters as shown in (a–l). The input parameters and their default values are defined in Table 1. The changes were made to be 0.50 (open squares), 0.75 (open triangles), 1.25 (open circles), and 1.50 (open diamonds) times their default value. The temperature response of g bs using the default set of input parameter values is given by the solid curve of each panel. One or two types of symbols are missing in (j) and (k) because extreme values of either D Vpmax or S Vpmax resulted in a biologically unrealistic negative estimate of g bs. (This figure is available in colour at JXB online.)
The ±25% and 50% changes of the 12 parameters also resulted in changes in estimated g m25, V cmax25, and V pmax25 (results not shown). Overall, the estimated V cmax25 varied least (from 45.7 μmol m−2 s−1 to 58.4 μmol m−2 s−1), and its relative change, defined as the difference between its maximum and minimum divided by its mean, was 26%, whereas V pmax varied most (from 67.6 μmol m−2 s−1 to 322.6 μmol m−2 s−1), and its relative change was 195%. The relative change of the estimated g m25 was 92% (from 0.93mol m−2 s−1 to 2.05mol m−2 s−1).
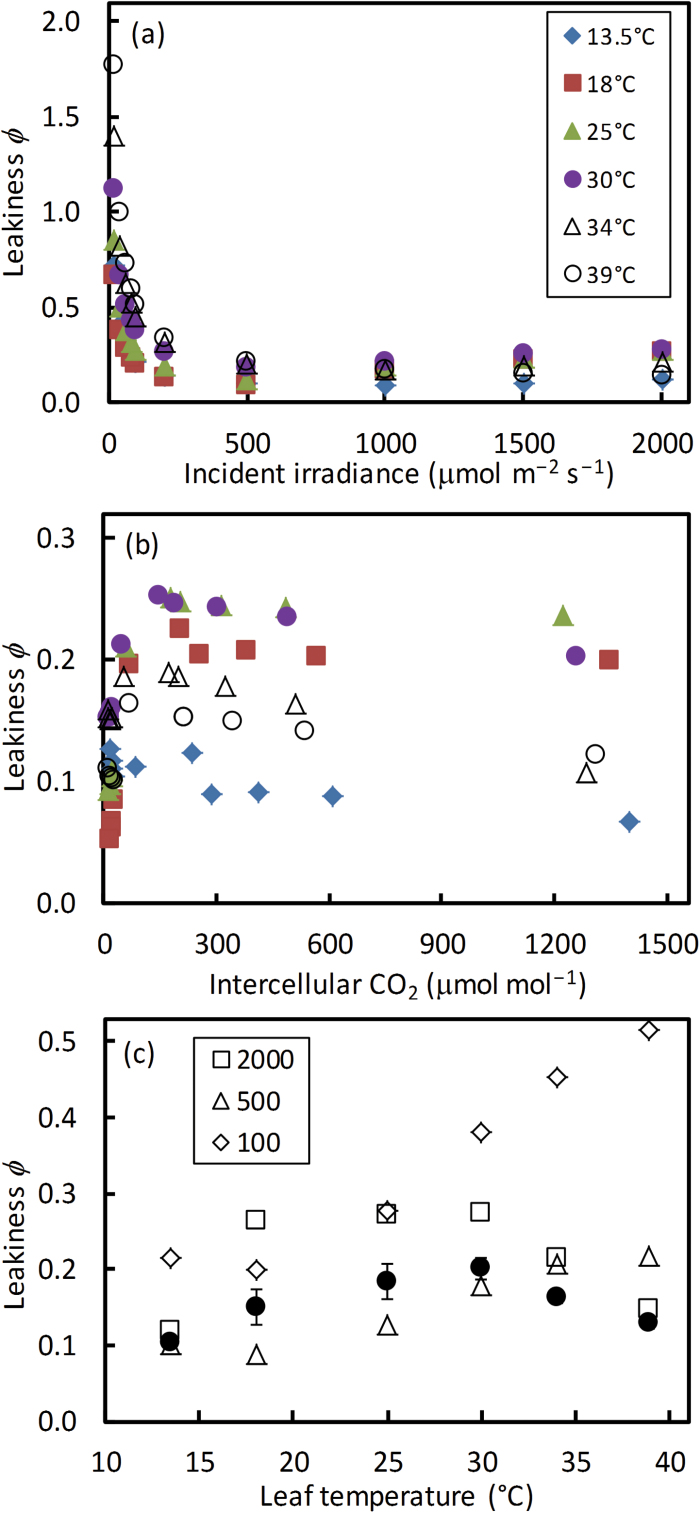
Estimated leakiness, and V o:V c and V c:V p ratios
When model parameters are estimated, one can solve for leakiness ϕ (=L/V p), V o:V c, and V c:V p ratios, using Equations A1–A6 in Supplementary appendix A. The calculated ϕ declined sharply with increasing irradiance (Fig. 8a), and increased initially and then saturated with increasing C i (Fig. 8b). At low irradiances, ϕ values were very high, even exceeding 1.0 when the temperature was >30 °C (Fig. 8a). The temperature response of ϕ did not vary with CO2 levels but depended strongly on the irradiance levels (Fig. 8c). The estimated ϕ at high irradiance (2000 μmol m−2 s−1) and the average ϕ of various CO2 levels showed a peaked response to temperature (Fig. 8c).
Fig. 8.
Calculated CO2 leakiness ϕ as a function of irradiance (a) and of intercellular CO2 level (b) at six temperatures. The values of leakiness ϕ from (a) for three contrasting irradiance levels of 100, 500, and 2000 μmol m−2 s−1 (open symbols) and the mean ϕ (SEM in bars) across all CO2 levels from (b) (filled circles) are shown as a function of temperature (c). The O2 level was at 21%.(This figure is available in colour at JXB online.)
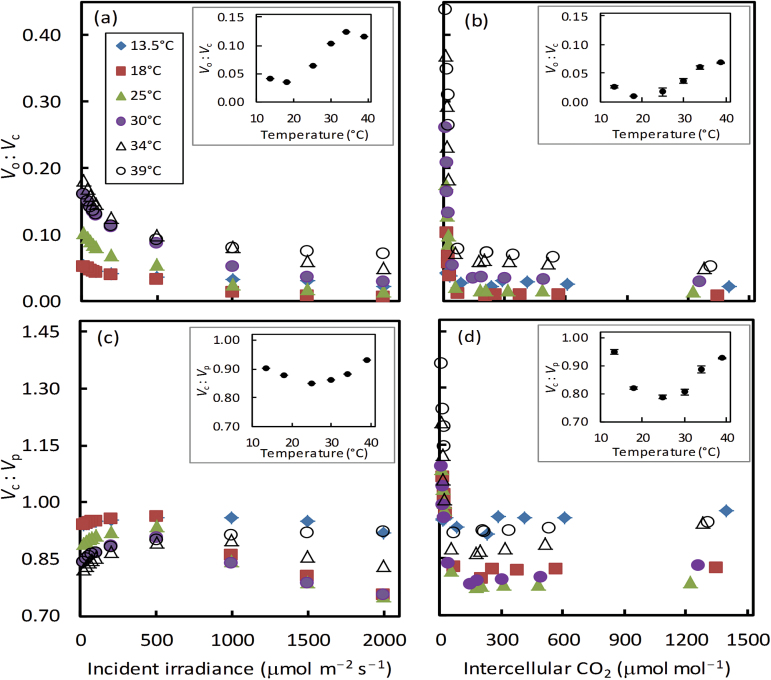
The calculated V o:V c ratio varied slightly with irradiance (Fig. 9a), and initially declined sharply and then became stable with increasing CO2 levels (Fig. 9b). In accordance with this pattern, the V c:V p ratio responded to irradiance and CO2 levels (Fig. 9c, d). The V c:V p ratio was <1.0 across irradiances (Fig. 9c), and it was also <1.0 for most CO2 levels but became >1.0 at low C i of 10–30 μmol mol−1, especially at high temperatures (Fig. 9d). Excluding the four low CO2 levels, the average ratios were calculated to show how these ratios under normal irradiance and CO2 conditions responded to temperature (inset in each panel of Fig. 9). Overall, the V o:V c ratio increased with temperature (insets in Fig. 9a, b), and the V c:V p ratio had a non-linear response to temperature (insets in Fig. 9c, d).
Fig. 9.
Calculated V o:V c ratios (a, b) and V c:V p ratios (c, d) as a function of irradiance (a, c) and of intercellular CO2 level (b, d) at six temperatures. The mean ratios (SEM in bars if larger than symbols) across all irradiance levels and across CO2 levels with C a ≥200 μmol mol−1 against temperature during measurements are shown in the inset of each panel. The O2 level was 21%.(This figure is available in colour at JXB online.)
Discussion
To estimate g bs, we used the model method of Yin et al. (2011), which is based on the combined measurements of gas exchange and chlorophyll fluorescence on leaves. There are some concerns about this technique as the distribution of e− transport between mesophyll and bundle-sheath cells on C4 leaves is uncertain (von Caemmerer, 2013; Kromdijk et al., 2014). The mesophyll and bundle-sheath cells have different chloroplast populations which could result in a complex relationship between ∆F/F m′ and the quantum yield of CO2 fixation. However, despite a few exceptions (e.g. Fryer et al., 1998; Dwyer et al., 2007), most studies (e.g. Krall and Edwards, 1990; Edwards and Baker, 1993; Oberhuber and Edwards, 1993; Oberhuber et al., 1993; Peterson, 1994; Earl and Tollenaar, 1998; Laisk and Edwards, 1998; Siebke et al., 2003; Naidu and Long, 2004; Loriaux et al., 2013; Bellasio and Griffiths, 2014) have reported a good linear relationship for C4 species between (quantum yields of) PSII e− transport from fluorescence analysis and CO2 fixation from gas exchange data over a wide range of conditions. This empirical evidence suggests that combined gas exchange and chlorophyll fluorescence measurements, commonly applied to estimate C3 photosynthesis parameters, can be similarly applied for C4 photosynthesis, as implemented by Yin et al. (2011), Bellasio and Griffiths (2013, 2014), and Bellasio et al. (2016). The way we conducted calibrations using non-photorespiratory measurements to derive s′ for calculating J atp may also have reduced the uncertainty of applying a chlorophyll fluorescence technique to C4 leaves.
In fact, the estimate of s′ is not just a calibration factor, but has physiological meanings and integrates a number of hard to determine parameters (Yin et al., 2011):
| (6) |
where β is absorptance by leaf photosynthetic pigments, ρ2 is the fraction of absorbed irradiance partitioned to PSII, z is the factor of converting PSII e− flux into ATP flux, and ξ is the ratio of true PSII efficiency to fluorescence-measured apparent PSII efficiency. Theoretically, ρ2 and z can be written as (Yin and Struik. 2012):
| (7) |
| (8) |
where f cyc is the fraction of e− flux at PSI that follows cyclic transport, f Q is the fraction of e− flux at reduced plastoquinone that follows the Q cycle, h is the H+:ATP ratio, and Φ2LL/Φ1LL is the PSII:PSI e− transport efficiency ratio. Given recent quantitative estimation that Φ2LL/Φ1LL= ~0.825, f Q=1, f cyc= ~0.45, and h=4 (Yin and Struik, 2012), and assuming that x=0.4, β=0.9, and ξ=1, the value of s′ must be ~0.25. This theoretical value is close to our estimates for s′, 0.25–0.34 (Figs 3, 4a). Combining Equations 6–8 with the equation of Yin and Struik (2012) for the condition that the produced NADPH and ATP from e− transport match the metabolic requirements (their equation 5), s′ can also be expressed as:
| (9) |
where ϕ is leakiness, f pseudo is the fraction of e− flux at PSI that follows the basal pseudocyclic transport (e.g. nitrate reduction, and malate export from chloroplasts), and the term [1−f pseudo/(1−f cyc)] as a whole refers to the fraction of the PSII e− flux that is used for supporting the Calvin circle and any photorespiration (Yin and Struik, 2012). Therefore, our calibration factor s′ takes into account only the part of the fluorescence signal dedicated to e− sinks represented by the Calvin cycle, any photorespiration, and some energy loss due to CO2 leakage. Our calibration procedure has excluded the effect of possible basal alternative e− sinks. For example, the calibration factor s′ was found to vary with temperature (Fig. 4a), and one possible reason for this variation is that the extent of any basal alternative e− transport may depend on temperature.
Kromdijk et al. (2010) and Ubierna et al. (2013) estimated g bs by fitting the model of von Caemmerer and Furbank (1999) to carbon isotope discrimination data that were measured simultaneously with gas exchange, thereby providing an independent method to estimate g bs. As this isotopic method has more assumptions than the fluorescence-based method (Kromdijk et al., 2014) and does not estimate J atp and R d, Bellasio and Griffiths (2013) compared the two methods in estimating g bs by using J atp and R d estimated along the lines of our method. It is noteworthy that the fluorescence method used by Bellasio and Griffiths (2013) slightly differs from our method in that they estimated g bs by minimizing the difference between modelled and measured J atp, whereas our fitting method is to minimize the difference between modelled and measured A. Yin and Struik (2009b ) have shown for C3 photosynthesis that the two minimizing targets can result in slightly different estimates of g m, and we prefer our method because it is generally A, rather than J atp, that is to be predicted from the general use of photosynthesis models. Nevertheless, Bellasio and Griffiths (2013) found that, compared with g bs estimated by the isotopic method, g bs estimated by the fluorescence method by fitting to J atp was similar for maize leaves grown under low light conditions but was much lower for leaves from high light conditions; and the reasons for the difference are unresolved (Kromdijk et al., 2014). Bellasio and Griffiths (2013) discussed several advantages of the fluorescence method compared with the isotopic method (e.g. lower noise/signal ratio). However, since the isotopic method does not have the same problem as the fluorescence method in dealing with the two cell types, it is necessary to study further whether the relative temperature response of g bs we obtained here (Figs 6, 7) can be confirmed using the independent isotopic method.
Our model considered only two major enzymes (i.e. PEPc for the C4 cycle and Rubisco for the C3 cycle). Other enzymes [e.g. pyruvate orthophosphate dikinase, C4-acid decarboxylase, and carbonic anhydrase (CA)] are also important. The fact that detailed kinetic constants of these enzymes are rare and uncertain forces us to consider only two enzymes as in most applications of the C4 model of von Caemmerer and Furbank (1999). Even for these two enzymes, only in vitro estimates of kinetic constants were used here as their in vivo estimates are practically impossible to obtain, and, when estimated, possibly confounded by the assumptions made on other parameters. For example, Massad et al. (2007) assumed that g bs and γ* are independent of temperature, and obtained in vivo estimates of the peaked Arrhenius temperature response (i.e. Equation 4) for V cmax and V pmax, with T opt=32.5°C and 43.3 °C, respectively. Their T opt estimate for V pmax is similar to our estimate of 44.4 °C based on in vitro data of Chinthapalli et al. (2003). However, the peaked temperature response for V cmax has seldom been observed in vitro, for both C3 and C4 Rubisco, even when the temperature is up to 35–40 °C (e.g. Badger and Collatz, 1977; Jordan and Ogren, 1984; Sage, 2002; Kubien et al., 2003; Walker et al., 2013; Boyd et al., 2015; Perdomo et al., 2015). We believe that the low T opt for V cmax obtained by Massad et al. (2007) could simply be because they ignored the temperature sensitivity of g bs and γ* when estimating V cmax.
Our analysis showed the necessity of accounting for temperature response of g bs. The shape of our temperature response of g bs using default parameters (Fig. 6) followed an optimum temperature response pattern of light- and CO2-saturated photosynthesis rates (A max), similar to that Bernacchi et al. (2002) and others obtained for g m in C3 leaves. The similar temperature response of g m and A max is expected as g m positively affects A max. The similar temperature response of g bs and A max found here is unexpected, as g bs negatively affects A max in C4 leaves. Nevertheless, given the uncertainties in all input parameters we used, our result on the temperature response of g bs (Fig. 6) should be considered as tentative, and, as already stated, needs confirmation by other independent techniques. Our sensitivity analysis showed that the estimated temperature response of g bs depended only slightly on the assumed temperature response of g m (Supplementary Fig. S3), but more on Rubisco and PEPc kinetic parameters (Fig. 7). Using extreme values of some kinetic parameters occasionally caused a change from an optimum response to an accelerating response to temperature (Fig. 7). The g bs temperature response rarely depends on g m parameters but more on Rubisco and PEPc kinetic parameters; this is expected from the model of von Caemmerer and Furbank (1999), because the calculation of C c is minimally affected by g m through C m, and V c depends strongly on g bs and V cmax. However, those kinetics parameters to which the estimated temperature response of g bs is very sensitive were either measured for maize (e.g. K mC25, K mO25,and γ*25, reported by Cousins et al., 2010) or were relatively conserved among C4 species (E Vcmax and E γ*; see above). Also uncertainties related to a set of PEPc kinetics parameters as a whole had little impact on g bs estimates (Supplementary Table S1), although individual parameters such as D Vpmax and S Vpmax—when varied independently—strongly influenced the estimated g bs (Fig. 7). Despite these uncertainties of the input parameters, the obtained g bs–T leaf relationship between 13.5 °C and 39.0 °C followed either a peaked or non-peaked Arrhenius pattern (Figs 6, 7). Below 35 °C, the estimated g bs almost exclusively increased monotonously with increasing temperature (Fig. 7). The activation energy estimate based on Equation 3 for our estimates of g bs in Fig. 6 within 35 °C was ~74.45 kJ mol−1 for our study, and its corresponding Q 10 value was ~2.74.
The Q 10 factor for diffusion of CO2 in water is ~1.25 (Bernacchi et al., 2002). Our higher Q 10 value for g bs suggests the possibility that some proteins/enzymes are involved in inter- and intracellular CO2 diffusion in C4 leaves. One candidate is CA, which facilitates the CO2 diffusion rate (Badger and Price, 1994). CA has often been considered to play a role in mediating g m in C3 species (e.g. Bernacchi et al., 2002). Its role for g m in C4 leaves is justified since in C4 plants CA is mainly found in the cytosol alongside PEPc (Ludwig et al., 2011), and CA activity is temperature dependent (Boyd et al., 2015). This actually gives indirect support to the use of the modified Arrhenius response for the temperature effect on g m in our analysis. The bundle-sheath cells may contain some amount of CA isoforms (Ludwig et al., 2011), seemingly in support of its potential mediating role for g bs as well. The other candidate may be an aquaporin that increases the CO2 permeability of the cell membrane (Terashima and Ono, 2002). Genetic manipulation of specific aquaporins has been used to vary g m in C3 species (e.g. Hanba et al., 2004). Brautigam et al. (2010) showed that a 20-fold up-regulation in the abundance of an mRNA coding for an aquaporin was registered in a C4 species. Other temperature-dependent activities may also shape our observed g bs–temperature relationship. For example, the current model simply assumes that the rate of decarboxylation equals V p. Recently it has been suggested that maize leaves have mixed decarboxylation pathways (Furbank, 2011). Theoretical modelling by Wang et al. (2014) showed that engaging the mixed pathways could decrease the need to maintain a high concentration gradient of metabolites between mesophyll and bundle-sheath cells, reconciling an earlier analysis of Sowinski et al. (2008) that simple diffusion-driven transport of metabolites is not adequate to explain the metabolite exchange during C4 photosynthesis. The mixed decarboxylation pathways and associated cell to cell exchange of metabolites suggest it likely that PEPc carboxylation and the overall decarboxylation rates are not the same and have different temperature responses. In that case, such a difference will have been lumped with our estimated parameters for temperature response of g bs.
The temperature response of g bs, together with the temperature responses of J atp, Rubisco, and PEPc kinetic parameters, co-determined the temperature responses of leakiness ϕ (Fig. 8), and V o:V c and V c:V p ratios (Fig. 9). In line with the trends shown by theoretical modelling (von Caemmerer and Furbank, 1999) and experimental calculation (Kromdijk et al., 2010; Pengelly et al., 2010; Yin et al., 2011; Ubierna et al., 2013; Bellasio and Griffiths, 2014), the calculated ϕ declined sharply with increasing irradiance (Fig. 8a). However, ϕ at low irradiances even exceeded 1.0 when the temperature was above 30 °C (Fig. 8a). The extreme ϕ values >1 at strictly limiting irradiances and high temperatures resulted from relatively high mitochondrial respiration in bundle-sheath cells combined with relatively high photorespiratory rates (as indicated by relatively high V o:V c ratios at low irradiances; Fig. 9a). As a result, the temperature response of ϕ did not vary with CO2 levels but depended strongly on the irradiance levels (Fig. 8c). The estimated ϕ at high irradiances and the average ϕ of various CO2 levels showed a flat peaked response to temperature (Fig. 8c), in line with the trend shown by von Caemmerer et al. (2014) with the carbon isotope method.
For an effective CCM, the V c:V p ratio is expected to be <1.0. However, the V c:V p ratio went up to >1.0 at low CO2, especially at high temperatures (Fig. 9d). The model of von Caemmerer and Furbank (1999) (see Equations A1 and A4 in Supplementary appendix A) predicts that the V c:V p ratio is ≥1.0 only if L≤0.5V o+(R d–R m). This condition was met at our four lowest CO2 levels (C i of 10–30 μmol mol−1) where V o was high. The average ratios under normal irradiance and CO2 conditions (calculated by excluding those four low CO2 levels) responded to temperature. Overall, the V o:V c ratio increased with temperature (insets in Fig. 9a, b), because increasing temperature favoured the RuBP oxygenation relative to carboxylation. However, this general temperature response of the V o:V c ratio seemed to be modified by the temperature response of g bs. The temperature response of g bs and its associated temperature response of leakiness (Fig. 8c) required V p to vary accordingly, resulting in a non-linear response of the V c:V p ratio to temperature (insets in Fig. 9c, d).
Our parameter estimates can be compared with previous literature reports. Our estimate of activation energy for R d, 41.9 kJ mol−1 (Table 2), is within the range of reports for R d in C3 species (24.2–65.2 kJ mol−1; see review by Yin et al., 2014) as well as the range reported for C4 species (28.2–57.8 kJ mol−1; Dwyer et al. 2007). Our estimate for g m25,1.33mol m−2 s−1 (Table 2), is within the range of the earlier estimated or suggested values for maize (Pfeffer and Peisker, 1998; Kromdijk et al., 2010; Yin et al., 2011; Barbour et al., 2016), and is higher than that for C3 leaves (for which the maximum g m25 is ~0.6mol m−2 s−1; e.g. Loreto et al., 1992). Our estimated V pmax25:V cmax25 ratio, 2.43, agrees with our earlier estimate 2.5 (Yin et al., 2011). Biochemical measurements on this ratio were 2.1–2.5 (Pengelly et al., 2010), 3.1 (Kubien et al., 2003), or higher (Sage et al., 1987). The leaf N content in our experiment was on average 1.1g N m−2. Our estimated g bs25, 2.87 mmol m−2 s−1 (Table 2), is within the values reported for maize, 1.5 mmol m−2 s−1 (Ubierna et al., 2013), 0.37–2.35 mmol m−2 s−1 (Kromdijk et al., 2010), and 0.82–4.64 mmol m−2 s−1 (Bellasio and Griffiths, 2014), and also agrees with our previous estimate for this leaf nitrogen level (Yin et al., 2011). However, our estimates for the slope of V cmax25 and V pmax25 versus leaf nitrogen, 57.7 μmol and 140.3 μmol (g N)−1 s−1, respectively, are lower than the previous estimates [96.0 μmol and 242.2 μmol (g N)−1 s−1, respectively] by Yin et al. (2011). The difference between the two studies in glasshouse environments (i.e. ~1 month later in the present study than in the previous study of Yin et al., 2011), cultivars used (‘Atrium’ versus ‘2-02R10074’), and leaf ranks for measurements (the seventh versus the eighth to ninth) might have caused this disparity. It has been shown that acclimation to growing light intensities affected photosynthesis parameters in maize (Kromdijk et al., 2010; Bellasio and Griffiths, 2013) and other C4 species (Ubierna et al., 2011).
Previously only one study (Kiirats et al., 2002) has reported the temperature response of g bs; that is, g bs increases almost linearly with increasing leaf temperature, in contrast to our result for the Arrhenius response. Their temperature was only up to 35 °C. If normalized to 25 °C, the response within temperatures up to 35 °C was still different between their study and ours (Supplementary Fig. S4): the activation energy estimate based on Equation 3 was ~24.92 kJ mol−1 for their study, lower than 74.45 kJ mol−1 for our study (see above). This may highlight species differences in temperature response of g bs, although the impact of methodological differences between the two studies and/or the impact of uncertainty in input parameter values for our study cannot be ruled out. Temperature response of g m for C3 photosynthesis has recently been reported to vary greatly with plant species (Walker et al., 2013; von Caemmerer and Evans, 2015). There is a need to investigate further whether or not species diversity exists with regards to temperature response of g bs for C4 photosynthesis. To that end, comprehensive investigations on enzyme kinetic constants may need to be carried out across contrasting C4 species.
In short, our study demonstrates that in contrast to existing modelling assumptions, g bs does vary with leaf temperature. Although the presented temperature response curve still needs to be confirmed by other independent techniques, our results provide a step forward to more accurate modelling of C4 photosynthesis and productivity, especially for maize, under changing climatic environments.
Supplementary data
Supplementary data are available at JXB online.
Appendix A. Basic equations in the C4 photosynthesis model of von Caemmerer and Furbank (1999) and analytical solutions of the model as given by Yin et al. (2011).
Appendix B. Quantifying temperature dependence of diffusivities and solubilities of CO2 and O2 in water.
Appendix C. Model and data for describing PEPc-limited rates of photosynthesis within the initial section of A–C i curves.
Table S1. Estimated values of E Kp, V cmax25, and g bs at six temperatures when using three sets of V pmax parameters.
Figure S1. The initial linear section of A–C i curves of 2% O2 at six measurement temperatures.
Figure S2. Comparison between modelled and measured A–I inc and A–C i curves at six leaf temperatures under the condition of 21% O2.
Figure S3. Temperature response of bundle-sheath conductance, estimated using four contrasting temperature responses of mesophyll conductance.
Figure S4. Comparison of temperature response of bundle-sheath conductance normalized to 1.0 at 25 °C between Kiirats et al. (2002) for Amaranthus edulis and our study for maize.
Acknowledgements
This research is financed in part by the BioSolar Cells open innovation consortium, supported by the Dutch Ministry of Economic Affairs, Agriculture and Innovation.
References
- Badger MR, Collatz GJ. 1977. Studies on the kinetic mechanism of ribulose-1,5-biophosphate carboxylase and oxygenase reactions, with particular reference to the effect of temperature on kinetic parameters. Carnegie Instute of Washington Yearbook 76, 355–361. [Google Scholar]
- Badger MR, Price GD. 1994. The role of carbonic anhydrase in photosynthesis. Annual Review of Plant Physiology and Plant Molecular Biology 45, 369–392. [Google Scholar]
- Barbour MM, Evans JR, Simonin KA, von Caemmerer S. 2016. Online CO2 and H2O oxygen isotope fractionation allows estimation of mesophyll conductance in C4 plants, and reveals that mesophyll conductance decreases as leaves age in both C4 and C3 plants. New Phytologist (in press). [DOI] [PubMed] [Google Scholar]
- Bellasio C, Griffiths H. 2013. Acclimation to low light by C4 maize: implications for bundle sheath leakiness. Plant, Cell and Environment 37, 1046–1058. [DOI] [PubMed] [Google Scholar]
- Bellasio C, Griffiths H. 2014. Acclimation of C4 metabolism to low light in mature maize leaves could limit energetic losses during progressive shading in a crop canopy. Journal of Experimental Botany 65, 3725–3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellasio C, Beerling DJ, Griffiths H. 2016. Deriving C4 photosynthetic parameters from combined gas exchange and chlorophyll fluorescence using an Excel tool: theory and practice. Plant, Cell and Environmen t (in press). [DOI] [PubMed] [Google Scholar]
- Bernacchi CJ, Portis AR, Nakano H, von Caemmerer S, Long SP. 2002. Temperature response of mesophyll conductance. Implication for the determination of Rubisco enzyme kinetics and for limitations to photosynthesis in vivo . Plant Physiology 130, 1992–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernacchi CJ, Singsaas EL, Pimentel C, Portis AR, Jr, Long SP. 2001. Improved temperature response functions for models of Rubisco-limited photosynthesis. Plant, Cell and Environment 24, 253–259. [Google Scholar]
- Berry JA, Farquhar GD. 1978. The CO2-concentrating function of C4 photosynthesis. A biochemical model. In Hall DO, Coombs J, Goodwin TW, eds, Proceedings of the 4th International Congress on Photosynthesis. London: Biochemical Society, 119–131. [Google Scholar]
- Boyd RA, Gandin A, Cousins AB. 2015. Temperature response of C4 photosynthesis: biochemical analysis of Rubisco, phosphoenolpyruvate carboxylase and carbonic anhydrase in Setaria viridis . Plant Physiology 169, 1850–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brautigam A, Kajala K, Wullenweber J, et al. 2010. An mRNA blueprint for C4 photosynthesis derived from comparative transcriptomics of closely related C3 and C4 species. Plant Physiology 155, 142–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman KSR, Berry JA, Hatch MD. 1980. Photosynthetic metabolism in bundle sheath cells of the C4 species Zea mays: sources of ATP and NADPH and the contribution of Photosystem II. Archives of Biochemistry and Biophysics 202, 330–341. [DOI] [PubMed] [Google Scholar]
- Chen D-X, Coughenour MB, Knapp AK, Owensby CE. 1994. Mathematical simulation of C4 grass photosynthesis in ambient and elevated CO2 . Ecological Modelling 73, 63–80. [Google Scholar]
- Chinthapalli B, Murmu J, Raghavendra AS. 2003. Dramatic difference in the response of phosphoenolpyruvate carboxylase to temperature in leaves of C3 and C4 plants. Journal of Experimental Botany 54, 707–714. [DOI] [PubMed] [Google Scholar]
- Collatz GJ, Ribas-Carbo M, Berry JA. 1992. Coupled photosynthesis–stomatal conductance model for leaves of C4 plants. Australian Journal of Plant Physiology 19, 519–538. [Google Scholar]
- Cousins AB, Ghannoum O, von Caemmerer S, Badger MR. 2010. Simultaneous determination of Rubisco carboxylase and oxygenase kinetic parameters in Triticum aestivum and Zea mays using membrane inlet mass spectrometry. Plant, Cell and Environment 33, 444–452. [DOI] [PubMed] [Google Scholar]
- Dwyer SA, Ghannoum O, Nicotra A, von Caemmerer S. 2007. High temperature acclimation of C4 photosynthesis is linked to changes in photosynthetic biochemistry. Plant, Cell and Environment 30, 53–66. [DOI] [PubMed] [Google Scholar]
- Earl H, Tollenaar M. 1998. Relationship between thylakoid electron transport and photosynthetic CO2 uptake in leaves of three maize (Zea mays L.) hybrids. Photosynthesis Research 58, 245–257. [Google Scholar]
- Edwards GE, Baker NR. 1993. Can assimilation in maize leaves be predicted accurately from chlorophyll fluorescence analysis? Photosynthesis Research 37, 89–102. [DOI] [PubMed] [Google Scholar]
- Evans JR, von Caemmerer S. 2013. Temperature response of carbon isotope discrimination and mesophyll conductance in tobacco. Plant, Cell and Environment 36, 745–756. [DOI] [PubMed] [Google Scholar]
- Farquhar GD, von Caemmerer S, Berry JA. 1980. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149, 78–90. [DOI] [PubMed] [Google Scholar]
- Frank MJW, Kuipers JAM, Vanswaaij WPM. 1996. Diffusion coefficients and viscosities of CO2 + H2O, CO2 + CH3OH, NH3 + H2O, and NH3 + CH3OH liquid mixtures. Journal of Chemical and Engineering Data 41, 297–302. [Google Scholar]
- Fryer MJ, Andrews JR, Oxborough K, Blowers DA, Baker NR. 1998. Relationship between CO2 assimilation, photosynthetic electron transport, and active O2 metabolism in leaves of maize in the field during periods of low temperature. Plant Physiology 116, 571–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furbank RT. 2011. Evolution of the C4 photosynthetic mechanism: are there really three C4 acid decarboxylation types? Journal of Experimental Botany 62, 3103–3208. [DOI] [PubMed] [Google Scholar]
- Genty B, Briantais J-M, Baker N. 1989. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochimica et Biophysica Acta 990, 87–92. [Google Scholar]
- Han P, Bartels DM. 1996. Temperature dependence of oxygen diffusion in H2O and D2O. Journal of Physical Chemistry 100, 5597–5602. [Google Scholar]
- Hanba YT, Shibasaka M, Hayashi Y, Hayakawa T, Kasamo K, Terashima I, Katsuhara M. 2004. Overexpression of the barley aquaporin HvPIP2:1 increases internal CO2 conductance and CO2 assimilation in the leaves of transgenic rice plants. Plant and Cell Physiology 45, 521–529. [DOI] [PubMed] [Google Scholar]
- Hatch MD, Agostino A, Jenkins CLD. 1995. Measurement of the leakage of CO2 from bundle-sheath cells of leaves during C4 photosynthesis. Plant Physiology 108, 173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He D, Edwards GE. 1996. Estimation of diffusive resistance of bundle sheath cells to CO2 from modeling of C4 photosynthesis. Photosynthesis Research 49, 195–208. [DOI] [PubMed] [Google Scholar]
- Heaton EA, Dohleman FG, Long SP. 2008. Meeting US biofuel goals with less land: the potential of Miscanthus. Global Change Biology 14, 2000–2014. [Google Scholar]
- Jordan DB, Ogren WL. 1984. The CO2/O2 specificity of ribulose 1,5-biphosphate carboxylase/oxygenase: dependence on ribulose bisphosphate concentration, pH and temperature. Planta 161, 308–313. [DOI] [PubMed] [Google Scholar]
- Kiirats O, Lea PJ, Franceschi VR, Edwards GE. 2002. Bundle sheath diffusive resistance to CO2 and effectiveness of C4 photosynthesis and refixation of photorespired CO2 in a C4 cycle mutant and wild-type Amaranthus edulis . Plant Physiology 130, 964–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krall JK, Edwards GE. 1990. Quantum yields of photosystem II electron transport and carbon fixation in C4 plants. Australian Journal of Plant Physiology 17, 579–588. [Google Scholar]
- Kromdijk J, Griffiths H, Schepers HE. 2010. Can the progressive increase of C4 bundle sheath leakiness at low PFD be explained by incomplete suppression of photorespiration? Plant, Cell and Environment 33, 1935–1948. [DOI] [PubMed] [Google Scholar]
- Kromdijk J, Ubierna N, Cousins AB, Griffiths H. 2014. Bundle-sheath leakiness in C4 photosynthesis: a careful balancing act between CO2 concentration and assimilation. Journal of Experimental Botany 65, 3443–3457. [DOI] [PubMed] [Google Scholar]
- Kubien DS, von Caemmerer S, Furbank RT, Sage RF. 2003. C4 photosynthesis at low temperature. A study using transgenic plants with reduced amounts of Rubisco. Plant Physiology 132, 1577–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laisk A, Edwards GE. 1998. Oxygen and electron flow in C4 photosynthesis: Mehler reaction, photorespiration and CO2 concentration in the bundle sheath. Planta 205, 632–645. [Google Scholar]
- Leegood RC. 2002. C4 photosynthesis: principles of CO2 concentration and prospects for its introduction into C3 plants. Journal of Experimental Botany 53, 581–590. [DOI] [PubMed] [Google Scholar]
- Leegood RC, von Caemmerer S. 1989. Some relationships between contents of photosynthetic intermediates and the rate of photosynthetic carbon assimilation in leaves of Zea mays L. Planta 178, 258–266. [DOI] [PubMed] [Google Scholar]
- Loreto F, Harley PC, Di Marco G, Sharkey TD. 1992. Estimation of mesophyll conductance to CO2 flux by three different methods. Plant Physiology 98, 1437–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loriaux SD, Avenson TJ, Wells JM, McDermitt DK, Eckles RD, Riensche B, Genty B. 2013. Closing in on maximum yield of chlorophyll fluorescence using a single multiphase flash of sub-saturating intensity. Plant, Cell and Environment 36, 1755–1770. [DOI] [PubMed] [Google Scholar]
- Ludwig M. 2011. The molecular evolution of β-carbonic anhydrase in Flaveria. Journal of Experimental Botany 62, 3071–3081. [DOI] [PubMed] [Google Scholar]
- Massad R-S, Tuzet A, Bethenod O. 2007. The effect of temperature on C4-type leaf photosynthesis parameters. Plant, Cell and Environment 30, 1191–1204. [DOI] [PubMed] [Google Scholar]
- Medlyn BE, Dreyer E, Ellsworth D, et al. 2002. Temperature response of parameters of a biochemically based model of photosynthesis. II. A review of experimental data. Plant, Cell and Environment 25, 1167–1179. [Google Scholar]
- Naidu SL, Long SP. 2004. Potential mechanisms of low-temperature tolerance of C4 photosynthesis in Miscanthus × giganteus: an in vivo analysis. Planta 220, 145–155. [DOI] [PubMed] [Google Scholar]
- Oberhuber W, Dai Z-Y, Edwards GE. 1993. Light dependence of quantum yields of photosystem II and CO2 fixation in C3 and C4 plants. Photosynthesis Research 35, 265–274. [DOI] [PubMed] [Google Scholar]
- Oberhuber W, Edwards GE. 1993. Temperature dependence of the linkage of quantum yield of photosystem II to CO2 fixation in C4 and C3 plants. Plant Physiology 101, 507–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ort DR, Long SP. 2014. Limits on yields in the corn belt. Science 344, 484–485. [DOI] [PubMed] [Google Scholar]
- Pedomo JA, Cavanagh AP, Kubien DS, Galmes J. 2015. Temperature dependence of in vitro Rubisco kinetics in species of Flaveria with different photosynthetic mechanisms. Photosynthesis Research 124, 67–75. [DOI] [PubMed] [Google Scholar]
- Pengelly JJL, Sirault XRR, Tazoe Y, Evans JR, Furbank RT, von Caemmerer S. 2010. Growth of the C4 dicot Flaveria bidentis: photosynthetic acclimation to low light through shifts in leaf anatomy and biochemistry. Journal of Experimental Botany 61, 4109–4122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson RB. 1994. Regulation of electron transport in photosystems I and II in C3, C3–C4, and C4 species of Panicum in response to changing irradiance and O2 levels. Plant Physiology 105, 349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer M, Peisker M. 1995. In vivo K m for CO2 (K p) of phosphoenolpyruvate carboxylase (PEPC) and mesophyll CO2 transport resistance (r m) in leaves of Zea mays L. In: Mathis P, ed. Photosynthesis: from light to biosphere , Vol. V Dordrecht, The Netherlands: Kluwer Academic Publishers, 547–550. [Google Scholar]
- Pfeffer M, Peisker M. 1998. CO2 gas exchange and phosphoenolpyruvate carboxylase activity in leaves of Zea mays L. Photosynthesis Research 58, 281–291. [Google Scholar]
- Sage RF. 2002. Variation in the k cat of Rubisco in C3 and C4 plants and some implications for photosynthetic performance at high and low temperature. Journal of Experimental Botany 53, 609–620. [DOI] [PubMed] [Google Scholar]
- Sage RF, Kubien DS. 2007. The temperature response of C3 and C4 photosynthesis. Plant, Cell and Environment 30, 1086–1106. [DOI] [PubMed] [Google Scholar]
- Sage RF, Pearcy RW, Seamann JR. 1987. The nitrogen use efficiency of C3 and C4 plants. III. Leaf nitrogen effects on the activity of carboxylating enzymes in Chenopodium album (L.) and Amaranthus retroflexus (L.). Plant Physiology 85, 355–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scafaro AP, von Caemmerer S, Evans JR, Atwell BJ. 2011. Temperature response of mesophyll conductance in cultivated and wild Oryza species with contrasting mesophyll cell wall thickness. Plant, Cell and Environment 34, 1999–2008. [DOI] [PubMed] [Google Scholar]
- Schreiber U, Hormann H, Neubauer C, Klughammer C. 1995. Assessment of photosystem II photochemical quantum yield by chlorophyll fluorescence quenching analysis. Australian Journal of Plant Physiology 22, 209–220. [Google Scholar]
- Siebke K, Ghannoum O, Conroy JP, Badger MR, von Caemmerer S. 2003. Photosynthetic oxygen exchange in C4 grasses: the role of oxygen as electron acceptor. Plant, Cell and Environment 26, 1963–1972. [Google Scholar]
- Silim SN, Ryan N, Kubien DS. 2010. Temperature response of photosynthesis and respiration in Populus balsamifera L.: acclimation versus adaptation. Photosynthesis Research 104, 19–30. [DOI] [PubMed] [Google Scholar]
- Slattery RA, Ort DR. 2015. Photosynthetic energy conversion efficiency: setting a baseline for gauging future improvements in important food and biofuel crops. Plant Physiology 168, 383–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowinski P, Szczepanik J, Minchin PEH. 2008. On the mechanism of C4 photosynthetic intermediate exchange between Kranz mesophyll and bundle sheath cells in grasses. Journal of Experimental Botany 59, 1137–1147. [DOI] [PubMed] [Google Scholar]
- Terashima I, Ono K. 2002. Effects of HgCl2 on CO2 dependence of leaf photosynthesis: evidence indicating involvement of aquaporins in CO2 diffusion across the plasma membrane. Plant and Cell Physiology 43, 70–78. [DOI] [PubMed] [Google Scholar]
- Ubierna N, Sun W, Cousins AB. 2011. The efficiency of C4 photosynthesis under low light conditions: assumptions and calculations with CO2 isotope discrimination. Journal of Experimental Botany 62, 3119–3134. [DOI] [PubMed] [Google Scholar]
- Ubierna N, Sun W, Kramer DM, Cousins AB. 2013. The efficiency of C4 photosynthesis under low light conditions in Zea mays, Miscanthus × giganteus and Flaveria bidentis . Plant, Cell and Environment 36, 365–381. [DOI] [PubMed] [Google Scholar]
- von Caemmerer S. 2013. Steady-state models of photosynthesis. Plant, Cell and Environment 36, 1617–1630. [DOI] [PubMed] [Google Scholar]
- von Caemmerer S, Evans JR. 2015. Temperature responses of mesophyll conductance differ greatly between species. Plant, Cell and Environment 38, 629–637. [DOI] [PubMed] [Google Scholar]
- von Caemmerer S, Furbank RT. 1999. Modeling C4 photosynthesis. In: Sage RF, Monson RK, eds. C4 plant biology . Toronto: Academic Press, 173–211. [Google Scholar]
- von Caemmerer S, Ghannoum O, Pengelly JJL, Cousins AB. 2014. Carbon isotope discrimination as a tool to explore C4 photosynthesis. Journal of Experimental Botany 65, 3459–3470. [DOI] [PubMed] [Google Scholar]
- von Caemmerer S, Quick WP. 2000. Rubisco: physiology in vivo . In: Loogood RC, Sharkey TD, von Caemmerer S, eds. Photosynthesis: physiology and metabolism . Kluwer Academic Publishers; Dordrecht, The Netherlands, 85–113. [Google Scholar]
- Walker BJ, Ariza LS, Kaines S, Badger MR, Cousins AB. 2013. Temperature response of in vivo Rubisco kinetics and mesophyll conductance in Arabidopsis thaliana: comparisons to Nicotiana tabacum . Plant, Cell and Environment 36, 2108–2119. [DOI] [PubMed] [Google Scholar]
- Wang Y, Brautigam A, Weber APM, Zhu X-G. 2014. Three distinct biochemical subtypes of C4 photosynthesis? A modelling analysis. Journal of Experimental Botany 65, 3567–3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren CR, Dreyer E. 2006. Temperature response of photosynthesis and internal conductance to CO2: results from two independent approaches. Journal of Experimental Botany 57, 3057–3067. [DOI] [PubMed] [Google Scholar]
- Yamori W, Noguchi K, Hanba YT, Terashima I. 2006. Effects of internal conductance on the temperature dependence of the photosynthetic rate in spinach leaves from contrasting growth temperatures. Plant and Cell Physiology 47, 1069–1080. [DOI] [PubMed] [Google Scholar]
- Yin X, Struik PC. 2009. a C3 and C4 photosynthesis models: an overview from the perspective of crop modelling. NJAS-Wageningen Journal of Life Sciences 57, 27–38. [Google Scholar]
- Yin X, Struik PC. 2009. b Theoretical reconsiderations when estimating the mesophyll conductance to CO2 diffusion in leaves of C3 plants by analysis of combined gas exchange and chlorophyll fluorescence measurements. Plant, Cell and Environment 32, 1513–1524. [DOI] [PubMed] [Google Scholar]
- Yin X, Struik PC. 2012. Mathematical review of the energy transduction stoichiometries of C4 leaf photosynthesis under limiting light. Plant, Cell and Environment 35, 1299–1312. [DOI] [PubMed] [Google Scholar]
- Yin X, Belay DW, van der Putten PEL, Struik PC. 2014. Accounting for the decrease of photosystem photochemical efficiency with increasing irradiance to estimate quantum yield of leaf photosynthesis. Photosynthesis Research 122, 323–335. [DOI] [PubMed] [Google Scholar]
- Yin X, Struik PC, Romero P, Harbinson J, Evers JB, van der Putten PEL, Vos J. 2009. Using combined measurements of gas exchange and chlorophyll fluorescence to estimate parameters of a biochemical C3 photosynthesis model: a critical appraisal and a new integrated approach applied to leaves in a wheat (Triticum aestivum) canopy. Plant, Cell and Environment 32, 448–464. [DOI] [PubMed] [Google Scholar]
- Yin X, Sun Z, Struik PC, van der Putten PEL, van Ieperen W, Harbinson J. 2011. Using a biochemical C4-photosynthesis model and combined gas exchange and chlorophyll fluorescence measurements to estimate bundle-sheath conductance of maize leaves differing in age and nitrogen content. Plant, Cell and Environment 34, 2183–2199. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.