Abstract
Aims
We sought to determine characteristics which strengthen the association between markers of diabetic kidney disease and retinopathy.
Methods
Multivariate regression analyses of NHANES 2005–2008 assessed the association of retinopathy with renal insufficiency and albuminuria. Analyses were stratified to evaluate ethnicity/race, obesity, and use of renin–angiotensin–aldosterone system antagonists as effect modifiers of this relationship.
Results
Of 269 participants with renal insufficiency, 35% had no microalbuminuria and no retinopathy; 16.1% had retinopathy with no microalbuminuria; 27.1% had microalbuminuria and no retinopathy and 22% had both microalbuminuria and retinopathy. Stratified, multivariate logistic regression analyses demonstrated retinopathy to be significantly predictive of renal insufficiency only in nonHispanic Blacks (OR = 2.7; 95% CI 1.2, 6.1), obesity (OR = 2.6; 95% CI 1.3, 5.5) and in those participants not using renin–angiotensin–aldosterone blockers (OR = 2.5; 95% CI 1.1, 5.7). Analyses showed an independent relationship between retinopathy and albuminuria only when albuminuria was modeled continuously.
Conclusions
In older onset diabetes, the absence of albuminuria and retinopathy is common among individuals with renal insufficiency. The relationship between microvascular complications of the eye and kidney may vary according to ethnicity, obesity and use of renin– angiotensin–aldosterone antagonists. These findings need to be confirmed in other large, diverse cohorts.
Keywords: Retinopathy, Nephropathy, Albuminuria, Ethnicity, Obesity
1. Introduction
The rising prevalence of type 2 diabetes has increased the likelihood of microvascular complications [1,2]. In the United States (U.S.) alone, 24,000 people develop blindness and more than 45,000 people begin dialysis each year as the result of diabetes [2,3]. Yet, establishing a diagnosis of diabetic kidney disease often poses a clinical challenge among individuals with type 2 diabetes. Decreased glomerular filtration rate in the presence of systemic microvascular complications are commonly used to estimate diabetic nephropathy. While retinopathy and nephropathy are postulated to have similar pathogenic mechanisms [4], the correlation is stronger in type 1 than type 2 diabetes [5,6]. Up to one-third of individuals with type 2 diabetes and decreased estimated glomerular filtration rate (eGFR) have an absence of albuminuria [7,8]. This subgroup also tends to have lesser degrees of retinopathy than those with albuminuria, leading many to postulate that this population has a predominance of hypertensive and/or renal vascular disease rather than classical diabetic glomerulosclerosis [9].
In people with type 2 diabetes, the risk of developing microvascular complications is increased with obesity [10]. Ethnic minorities also have an increased risk of severe and progressive retinopathy and nephropathy [11–13]. Moreover, the strength of association between retinopathy and nephropathy has been noted to be greater in Latinos than nonHispanic Whites [14]. Examination of individual-level characteristics that modify the relationship between diabetic retinopathy and nephropathy, may assist in the clinical evaluation of diabetic kidney disease, as well as to further elucidate the underlying pathogenesis of microvascular complications. To the best of our knowledge the strength of this association has not been examined in nonHispanic Blacks and among individuals with significant obesity. It is also unknown whether antagonists of the renin–angiotensin–aldosterone system (RAAS) alters the renal–retinal relationship. The purpose of the present study is to characterize the effect of ethnicity, body mass index (BMI) and RAAS antagonists on the relationship between retinopathy and decreased eGFR or albuminuria in older onset diabetes, using a nationally representative sample of the U.S. population.
2. Subjects materials and methods
2.1. Study population
The National Health and Nutrition Examination Survey (NHANES) is a nationally representative sample of the total US civilian, non-institutionalized population. It uses a stratified, multistage probability design with planned oversampling of certain age and racial/ethnic groups. NHANES (2005–2008) included 7081 individuals with complete sociodemographic information and data from physical examination. The NHANES protocol was approved by a human subjects review board and written, informed consent was obtained from all participants.
2.2. Definition of type 2 diabetes
Diabetes was self-reported as being previously diagnosed by a physician (except during pregnancy) or as current or past use of insulin or oral agents. Diabetes type was not available. We excluded 66 individuals who reported the diagnosis of diabetes prior to age 30, as these individuals likely had type 1 diabetes. Adults who completed the physical examination and had fasted appropriately, had fasting serum glucose measured and underwent a 75 g oral glucose challenge test. Newly diagnosed diabetes was defined as a fasting glucose ≥6.99 mmol/L (126 mg/dL) or a 2 h post glucose challenge of ≥11.1 mmol/L (200 mg/dL).
2.3. Fundus photography
NHANES 2005–2008 used the Canon CR6-45NM ophthalmic digital imaging system and Canon EOS 10D digital camera (Canon, Tokyo, Japan) to take 2 digital images per eye through a nonpharmacologically dilated pupil. Participants were seated in a windowless room with the lights turned off to allow for nonpharmacological pupillary dilation. One retinal image was centered on the macula and the second image was centered on the optic disc. The digital images were graded using a modification of the Airlie House classification scheme of diabetic retinopathy at the University of Wisconsin Ocular Epidemiologic Reading Center (Madison, WI). Capture and grading of digital images and quality of control by the Wisconsin group have been described in detail previously [15]. In brief, for each eye the maximum grade in any of the seven standard photographic fields was determined for each of the lesions. If the retinopathy severity could not be graded in an eye, it was considered to have a score equivalent to that in the other eye. Retinopathy severity was categorized into four levels: none, mild nonproliferative diabetic retinopathy, moderate to severe nonproliferative diabetic retinopathy and proliferative retinopathy. The retinopathy grade based on the more advanced eye was used for analyses [16].
2.4. Albuminuria measurements
A single, random urine collection was obtained for urine albumin:creatinine ratio (UACR). Urinary albumin was quantified using solid-phase fluorescent immunoassay. Urinary creatinine was measured with the Jaffe rate reaction. Micro-albuminuria was defined as a UACR of 3.4–33.8 mg/mmol (30–299 µg/mg) and macroalbuminuria as ≥33.9 mg/mmol (300ug/ mg). Where albuminuria is analyzed as a bivariate variable, 3.4 mg/mmol (30 µg/mg) was used as a threshold cut-point.
2.5. Estimation of GFR
GFR was estimated using the 6-variable Modification of Diet in Renal Disease (MDRD) equation, as follows:
Serum creatinine was measured using the modified kinetic reaction of Jaffe. We defined low eGFR as less than 60 ml/min/1.73 m2.
2.6. Covariate measurements
Age was defined as the age at the time of the examination. Race and ethnicity were self reported as Non-Hispanic White, Non-Hispanic Black, Mexican American and all others were grouped as ‘Other’. The latter group was excluded from the stratified analyses by ethnicity due to the very low number of participants in this category. Blood pressure was defined as the mean of six separate readings. BMI was calculated from the weight and height measurements taken during the physical examination. Hemoglobin A1c (HbA1c) was measured by a high performance liquid chromatographic assay. Individuals taking an angiotensin converting enzyme inhibitor or angiotensin receptor blocker or aldosterone receptor blocker were categorized as being on a RAAS antagonist.
2.7. Statistical methods
All analyses were performed using Stata (version 11), and SPSS (version 16) statistical software. Two-sided hypotheses and tests were adopted for all statistical inference. NHANES is a complex probability sample, so we used appropriate sample weights to estimate mean values and standard errors [17]. To combine data from two 2-year cycles of the continuous NHANES (2005–2006 and 2007–2008), we created new sample weights.
We used univariate logistic regression models between independent variables and low eGFR to calculate odds ratios and 95% confidence intervals. The same analyses were performed with albuminuria as a bivariate dependent variable as well as an ordinal variable (normal–micro–macro). Multivariate regression analyses were performed to further assess the relationship between retinopathy and low eGFR as well as between retinopathy and albuminuria. Covariates included age, gender, HbA1c, systolic and diastolic blood pressures. Univariate logistic regression was used to evaluate the significance of the interactions: retinopathy × ethnicity and retinopathy × BMI and retinopathy × RAAS antagonist. Significance of the interaction terms led to stratified analyses. Significance was defined by a p-value < 0.05.
3. Results
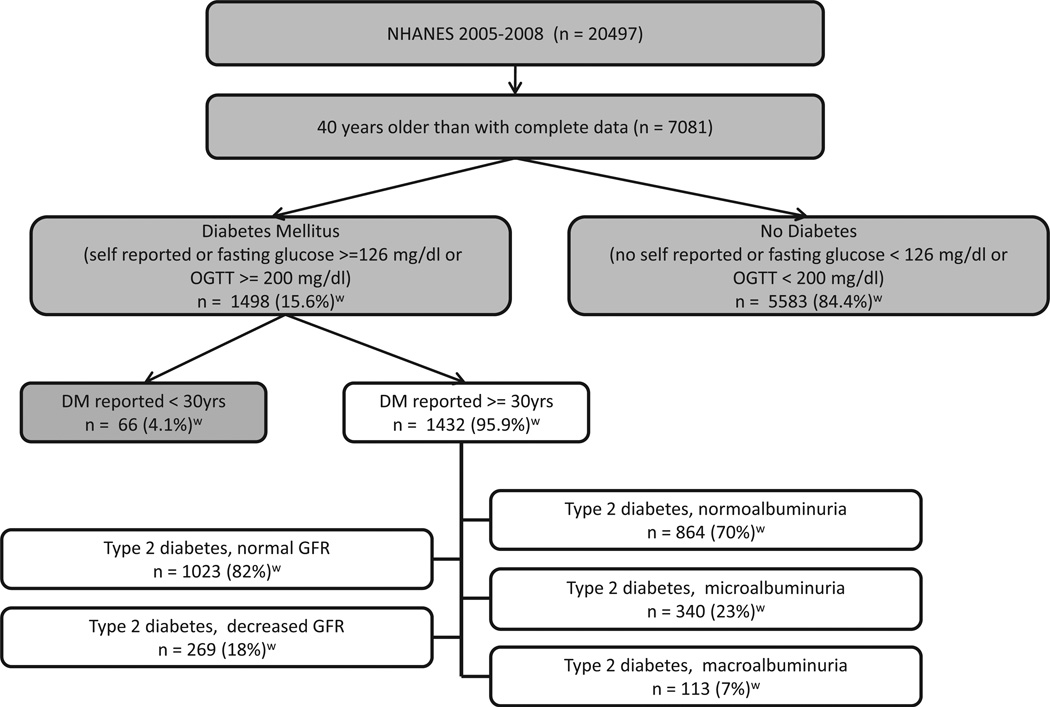
The participants from NHANES 2005–2008 with data available for analysis are depicted in Fig. 1. There were 1108 participants who self-reported being previously diagnosed with diabetes, 512 participants with a fasting glucose ≥6.99 mmol/L (126 mg/ dl) and 248 participants who had an OGTT ≥11.1 mmol/L (200 mg/dl). Individuals reporting diabetes diagnosed prior to the age of 30 years were felt likely to have type 1 diabetes and hence were excluded (n = 66). An additional 140 individuals were excluded from eGFR analyses due to lack of available serum creatinine data. In analyses of albuminuria, 115 individuals were excluded due to lack of available UACR data. In total, there were 1292 participants with older onset diabetes available for eGFR analyses and 1317 for albuminuria analyses.
Fig. 1.
Flow Chart of selection criteria for analysis of retinopathy and markers of kidney disease in type 2 diabetics: NHANES 2005–2008. Percentages are weighted for the distribution of the U.S. population.
Decreased eGFR, defined as less than 60 ml/min/1.73 m2, was present in 18%; microalbuminuria in 26% and macro-albuminuria in 9% of participants with adult onset diabetes. Of 269 participants with decreased eGFR and available UACR data, 35% had no microalbuminuria and no retinopathy; 16.1% had retinopathy with no microalbuminuria; 27.1% had microalbuminuria and no retinopathy and 22% had both microalbuminuria and retinopathy. Fig. 1 displays the weighted U.S. population estimates for these statistics.
Characteristics of participants with later onset diabetes with decreased versus ‘normal’ eGFR are displayed in Table 1. Those with decreased eGFR tended to be older, had a higher pulse pressure and more commonly had a history of retinopathy and/or cardiovascular disease compared to those with normal eGFR. The presence of any retinopathy was associated with a two-fold increased odds ratio of decreased eGFR. The presence of proliferative retinopathy was associated with a 7-fold increased odds ratio of low eGFR. The frequency of decreased eGFR was similar in nonHispanic whites and nonHispanic Blacks but was lower in Latinos.
Table 1.
Clinical characteristics of older onset diabetic participants according to estimated glomerular filtration rate (eGFR) ≥ or < 60 ml/min/1.73 m2: NHANES 2005–2008.
| Characteristics | eGFR ≥60 ml/min/1.73 m2 (95% CI) n = 1023 |
eGFR < 60 ml/min/1.73 m2 (95% CI) n = 269 |
Odds ratio (95% CI) |
|---|---|---|---|
| Age (years) | 60.4 (59.2–61.6) | 72 (70–73) | 1.1 (1.07–1.1)* |
| Female gender (%) | 49 (45–54) | 60 (53–67) | 1.5 (1.1–2.2) |
| Non-hispanic white (%) | 66 (59–74) | 77 (70–84) | 1.0 |
| Non-hispanic black (%) | 15 (11–19) | 14 (9–19) | 0.8 (0.6–1.2) |
| Latino (%) | 13 (9–17) | 6 (3–9) | 0.4 (0.3–0.6)* |
| Other ethnicity (%) | 6 (3–8) | 3 (1–5) | 0.4 (0.2–1.1) |
| Retinopathy any (%) | 24 (20–27) | 39 (29–48) | 2.0 (1.3–3.1) |
| None (%) | 76 (73–80) | 61 (52–71) | 1.0 |
| Mild (%) | 18 (15–21) | 26 (19–33) | 1.8 (1.1–2.8) |
| Moderate–severe (%) | 5 (3–7) | 9 (2–15) | 2.1 (0.96–4.8) |
| Proliferative (%) | 0.7 (0.3–1.0) | 4 (3–5) | 7.1 (3.8–13.0)* |
| History of CV event (%) | 24 (21–28) | 52 (45–59) | 3.4 (2.5–4.5)* |
| Body mass index (kg/m2) | 32 (32–33) | 32.6 (31–34) | 1.0 (1.0–1.0) |
| BMI ≥30 (%) | 57 | 57 | 1.0 (1.0–1.0) |
| HbA1C (%) (mmol/mol) |
6.9 (6.8–7.0) | 6.8 (6.5–7.0) | 0.9 (0.8–1.1) |
| 52 (51–53) | 51 (48–53) | ||
| SBP (mmHg) | 131 (129–133) | 140 (134–143) | 1.0 (1.0–1.0)* |
| DBP (mmHg) | 70 (69–71) | 61.3 (59–63) | 1.0 (1.0–1.0)* |
| Pulse pressure (mmHg) | 61 (59–63) | 79 (76–81) | 1.0 (1.0–1.0)* |
| Use of RAAS blockers (%) | 42 (36–48) | 58 (52–64) | 1.4 (1.5–2.9) |
CV = cardiovascular; SBP = systolic blood pressure; DBP = diastolic blood pressure; RAAS = renin–angiotensin–aldosterone system.
p < 0.001.
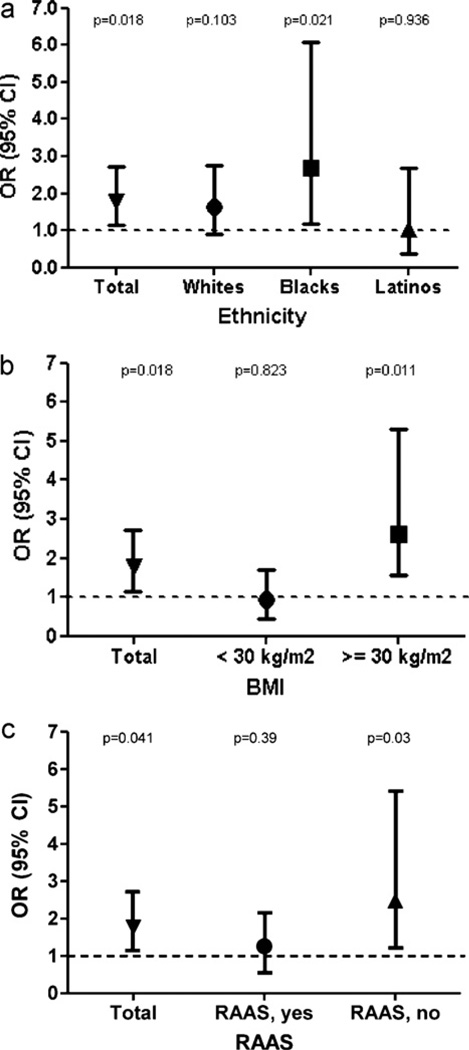
The univariate interaction terms, retinopathy × ethnicity, retinopathy × obesity and retinopathy × RAAS antagonist were all significant (p < 0.05). Multivariate logistic regression analyses stratified by ethnicity showed a significant association of retinopathy with decreased eGFR in nonHispanic Blacks but not nonHispanic Whites or Latinos (Fig. 2a). Multivariate logistic regression analyses stratified by BMI ≥30 kg/m2 versus <30 kg/m2 showed retinopathy to be significantly associated with decreased eGFR in obese but not nonobese individuals (Fig. 2b). Similarly, the stratified analyses by use of a RAAS antagonist demonstrated a significant relationship between retinopathy and low eGFR only in those who were not on a RAAS inhibitor (Fig. 2c). We explored the possibility that ethnicity and BMI could confound one another by including both terms in the analyses and the results were not changed (data not shown).
Fig. 2.
Multivariate logistic regression analysis of the relationship between retinopathy and decreased eGFR < 60 ml/min/1.73 m2 stratified by (a) ethnicity and (b) body mass index and (c) use of RAAS antagonist. Analyses were adjusted for the following covariates: age, gender, HbA1C, systolic blood pressure and diastolic blood pressure.
Similar analyses of albuminuria categorized as normal, micro- or macro- did not reveal any differences with regard to covariates including age, ethnicity, retinopathy, BMI, HbA1c or systolic/diastolic blood pressure (Table 2). Univariate, ordinal logistic regression demonstrated a 1.8-fold risk of albuminuria with any retinopathy and a 2.7-fold risk for mod-severe retinopathy. There was also an increased risk of albuminuria with a history of cardiovascular events (OR = 2.4 and 95% CI 1.6, 3.5). Multivariate logistic regression analyses of retinopathy and albuminuria (both bivariate and ordinal) were not significant. Recategorization of retinopathy by severity did not change these results. Modeling of albuminuria in a continuous fashion did detect a significant association (p < 0.001). Univariate analysis of interaction between retinopathy and ethnicity, obesity and RAAS inhibitor use were all nonsignificant.
Table 2.
Clinical characteristics of older onset diabetic participants according to status of albuminuria: NHANES 2005–2008.
| Characteristics | Normoalbuminuria (95% CI) n = 864 |
Microalbuminuriaa (95% CI) n = 340 |
Macroalbuminuriab (95% CI) n = 113 |
Odds ratio (95% CI) |
|---|---|---|---|---|
| Age (years) | 60.5 (59.2–61.9) | 63.7 (61.4–66.0) | 63.2 (59.5–66.8) | 1.0 (1.0–1.0) |
| Female gender (%) | 51 (47–56) | 53 (45–60) | 49 (35–63) | 1.01 (0.8–1.4) |
| Non-hispanic white (%) | 68 (61–76) | 66 (58–75) | 53 (36–69) | 1.0 |
| Non-hispanic black (%) | 15 (10–19) | 15 (10–20) | 24 (15–33) | 1.3 (0.9–1.9) |
| Latino (%) | 12 (8–16) | 14 (9–20) | 11 (4–18) | 1.2 (0.9–1.7) |
| Other ethnicity (%) | 5 (2–8) | 4 (1–8) | 12 (3–21) | 1.5 (0.7–3.3) |
| Retinopathy any (%) | 23 (19–27) | 27 (20–34) | 54 (42–66) | 1.8 (1.1–2.8) |
| None (%) | 77 (73–81) | 73 (66–80) | 46 (34–58) | 1.0 |
| Mild (%) | 18 (15–22) | 20 (14–26) | 33 (18–48) | 1.5 (0.9–2.5) |
| Moderate–severe (%) | 4 (2–6) | 6 (2–10) | 17 (7–28) | 2.7 (1.4–5.5)§ |
| Proliferative, (%) | 1 (0–2) | 0 (0–2) | 4 (0–7) | 3.1 (0.7–12.9) |
| History of CV event (%) | 23 (19–27) | 39 (32–47) | 46 (34–59) | 2.4 (1.6–3.5)§ |
| Body mass index (kg/m2) | 32 (32–33) | 32 (30–33) | 33 (31–35) | 1.0 (1.0–1.0) |
| HbA1C (%) (mmol/mol) |
6.8 (6.6–7.0) | 7.0 (6.8–7.3) | 7.4 (7–7.9) | 1.1 (1.0–1.3) |
| 51 (49–53) | 53 (51–56) | 57 (53–63) | ||
| SBP (mmHg) | 129 (127–130) | 138 (135–140) | 150 (145–156) | 1.0 (1.0–1.0)§ |
| DBP (mmHg) | 69 (68–70) | 68 (66–71) | 70 (63–78) | 1.0 (1.0–1.0) |
| Pulse pressure (mmHg) | 60 (58–62) | 69 (66–73) | 80 (72–87) | 1.0 (1.0–1.0)§ |
| RAAS blockers (%) | 41 (36–46) | 48 (43–53) | 53 (42–64) | 1.4 (1.0–1.9) |
CV = cardiovascular; SBP = systolic blood pressure; DBP = diastolic blood pressure; RAAS = renin–angiotensin–aldosterone system.
Microalbuminuria defined as urine albumin:creatinine ratio (UACR) 3.4–33.8 mg/mmol (30–299 µg/mg).
Macroalbuminuria defined as UACR ≥33.9 mg/mmol (300 µg/mg).
p < 0.001.
4. Discussion
In this analysis of a nationally representative U.S. population of people with older onset diabetes, we confirmed the independent association between retinopathy and decreased eGFR but not albuminuria [5,6,18]. The association between retinopathy and low eGFR < 60 ml/min/1.73 m2 was stronger in nonHispanic black, obese populations and individuals who are not taking a RAAS antagonist. Approximately one-third of individuals with older onset diabetes and decreased eGFR had neither albuminuria nor retinopathy.
We examined ethnicity, obesity and RAAS antagonist use as effect modifiers of the renal–retinal relationship because we hypothesized that these populations were more likely to have ‘typical’ diabetic glomerulosclerosis. Obesity was examined as an effect modifier due to the fact that the risk of diabetic microvascular disease is increased in those with concurrent metabolic syndrome [10,19]. Moreover, obesity and metabolic syndrome are known to result in increased inflammatory mediators known to be relevant to microangiopathies [20,21].
Ethnic minorities have a significantly higher risk of both diabetic retinopathy and nephropathy, likely due to increased risk factors and possibly an underlying genetic susceptibility [11,13,22,23]. We did not find an increased association in Latino individuals as has been cited by others [14], likely due to the small sample size of this ethnic group. The heavily overlapping confidence intervals between nonHispanic Whites and Blacks underscore the possibility that the discrepancy between these ethnic groups could be due to mediation of the effect by covariates included in the analysis. Alternatively, our finding could be due to chance. However, it is equally possible that the discrepancy between ethnicities could be even greater than that found in this analysis, especially as the confidence interval in the nonHispanic Black population are skewed toward higher odds ratios. The wide confidence intervals in the nonHispanic Black stratum were partly due to sample size limitations that could not be avoided. Our finding of a discrepancy in the strength of association between retinopathy and low eGFR is merely suggestive, but does warrant further investigation.
We detected an association between retinopathy and albuminuria only when UACR was modeled in a continuous fashion, likely due to increased power of this approach. Historically, categorical and ordinal classification of albuminuria has yielded an association with retinopathy in diabetes [18,24,25]. The reason for our disparate results is unclear, but could be due to a number of factors including sample size, population characteristics or a shift away from albuminuria in this age of maximal blockade of the RAAS. Our findings are particularly relevant, given that epidemiologic studies have demonstrated over the last decade that microalbuminuria does not carry the same prognostic value in diabetic kidney disease as once thought. Albuminuria regresses more often than it progresses, and is often absent despite a decline in eGFR [7,8,26,27].
The strength of association between retinopathy and diabetic kidney disease likely varies depending on the duration of disease and whether nephropathy is being analyzed with respect to histologic measurements versus albuminuria versus eGFR. In a Caucasian population with type 1 diabetes and normoalbuminuria and normal or elevated eGFR, there was a strong association of retinopathy severity and histologic indicators of basement membrane thickness and mesangial expansion [16]. Nephropathologic study of type 2 diabetics with nephrotic range proteinuria show those with Kimmelstiel Wilson nodules tend to have significantly worse retinopathy than those with solely mesangioproliferative lesions [28].
Analysis of an Italian population with type 2 diabetes and microalbuminuria found more severe retinopathy in those with ‘classical’ diabetic glomerulosclerosis versus those with mostly tubulointerstitial lesions and vasculopathy [29]. We hypothesize that the stronger association between retinopathy and nephropathy occurs in those with ‘typical’ diabetic glomerulosclerosis. The emergence of nonproteinuric diabetic kidney disease and reduced association between retinopathy and kidney disease in type 2 diabetes could be due to a rise in the prevalence of renal vascular disease. The role of kidney biopsy may grow in importance to accurately define histopathology in people with type 2 diabetes. Furthermore, the histologic phenotype of diabetic kidney disease could aide in the design of future clinical trials where investigational drugs for diabetic kidney disease would be targeted to patient populations with a predominance of classical or other histologic phenotype.
Limitations to the present study include its exclusion of institutionalized individuals who likely have higher rates of diabetic complications, inability to definitively distinguish between type 1 and type 2 diabetes, and exclusion of a substantial number of individuals who did not have available serum creatinine, UACR or retinal fundus photographs. Survey participants who had no light perception or severe visual impairment in both eyes, or a severe infection in 1 or both eyes were also excluded. Albuminuria was assessed using a single random urine collection which tends to be less robust than first morning urine specimen. A true diagnosis of microalbuminuria requires two out of three positive specimens and hence we cannot rule out the possibility of misclassification. We did not include duration of diabetes in multivariate analyses because this information was missing in the majority of participants.
It is noted that use of odds ratios has the potential to overestimate the effect size, especially in the setting of relatively common outcomes and in small to moderate sample sizes [30]. With respect to the interaction between ethnicity and retinopathy, there is a notable overlap in the 95% confidence intervals between blacks and whites. It is possible that the finding of this interaction is due to chance. We feel this is unlikely, however, given our a priori hypothesis about ethnicity as a modifier based on the existing evidence for more severe disease in Blacks [11–13,23]. It is likely that the overlap in confidence intervals is due to the limited sample size and use of self-report to categorize ethnicity.
Strengths of the study include the ethnically diverse, population-based national sample and assessment of diabetic retinopathy with bilateral digital retinal images using a standardized grading scheme. We used unbiased diagnostic criteria for diabetes, including those who were previously undiagnosed. Lastly, we examined the association between diabetic retinopathy and kidney disease using both eGFR and albuminuria measurements.
In conclusion, we found an increased association of diabetic retinopathy and decreased eGFR in nonHispanic black and obese populations and in those who were not on a RAAS blocking agent. We hypothesize that this may be due to a higher prevalence of true diabetic glomerulosclerosis in these populations as opposed to diabetic renal vascular disease. We suggest that research protocol kidney biopsies may help to elucidate the spectrum of renal microvascular disease in people with type 2 diabetes in this era of optimal diabetes and blood pressure control.
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
REFERENCES
- 1.de Boer IH, Rue TC, Hall YN, Heagerty PJ, Weiss NS, Himmelfarb J. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA. 2011 Jun 24;305:2532–2539. doi: 10.1001/jama.2011.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Engelgau MM, Geiss LS, Saaddine JB, Boyle JP, Benjamin SM, Gregg EW, et al. The evolving diabetes burden in the United States. Ann Intern Med. 2004 Jun 11;140:945–950. doi: 10.7326/0003-4819-140-11-200406010-00035. [DOI] [PubMed] [Google Scholar]
- 3.U S Renal Data System. USRDS 2010 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2010. [Google Scholar]
- 4.Sheetz MJ, King GL. Molecular understanding of hyperglycemia’s adverse effects for diabetic complications. JAMA. 2002 Nov 20;288:2579–2588. doi: 10.1001/jama.288.20.2579. [DOI] [PubMed] [Google Scholar]
- 5.Pedro RA, Ramon SA, Marc BB, Juan FB, Isabel MM. Prevalence and relationship between diabetic retinopathy and nephropathy, and its risk factors in the North-East of Spain, a population-based study. Ophthalmic Epidemiol. 2010 Aug 4;17:251–265. doi: 10.3109/09286586.2010.498661. [DOI] [PubMed] [Google Scholar]
- 6.Wolf G, Muller N, Mandecka A, Muller UA. Association of diabetic retinopathy and renal function in patients with types 1 and 2 diabetes mellitus. Clin Nephrol. 2007 Aug 2;68:81–86. doi: 10.5414/cnp68081. [DOI] [PubMed] [Google Scholar]
- 7.Kramer HJ, Nguyen QD, Curhan G, Hsu CY. Renal insufficiency in the absence of albuminuria and retinopathy among adults with type 2 diabetes mellitus. JAMA. 2003 Jun 24;289:3273–3277. doi: 10.1001/jama.289.24.3273. [DOI] [PubMed] [Google Scholar]
- 8.Bash LD, Selvin E, Steffes M, Coresh J, Astor BC. Poor glycemic control in diabetes and the risk of incident chronic kidney disease even in the absence of albuminuria and retinopathy: atherosclerosis Risk in Communities (ARIC) Study. Arch Intern Med. 2008 Dec 22;168:2440–2447. doi: 10.1001/archinte.168.22.2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MacIsaac RJ, Tsalamandris C, Panagiotopoulos S, Smith TJ, McNeil KJ, Jerums G. Nonalbuminuric renal insufficiency in type 2 diabetes. Diabetes Care. 2004 Jan 1;27:195–200. doi: 10.2337/diacare.27.1.195. [DOI] [PubMed] [Google Scholar]
- 10.Costa LA, Canani LH, Lisboa HR, Tres GS, Gross JL. Aggregation of features of the metabolic syndrome is associated with increased prevalence of chronic complications in Type 2 diabetes. Diabet Med. 2004 Mar 3;21:252–255. doi: 10.1111/j.1464-5491.2004.01124.x. [DOI] [PubMed] [Google Scholar]
- 11.Karter AJ, Ferrara A, Liu JY, Moffet HH, Ackerson LM, Selby JV. Ethnic disparities in diabetic complications in an insured population. JAMA. 2002 May 19;287:2519–2527. doi: 10.1001/jama.287.19.2519. [DOI] [PubMed] [Google Scholar]
- 12.Young BA, Maynard C, Boyko EJ. Racial differences in diabetic nephropathy, cardiovascular disease and mortality in a national population of veterans. Diabetes Care. 2003 Aug 8;26:2392–2399. doi: 10.2337/diacare.26.8.2392. [DOI] [PubMed] [Google Scholar]
- 13.Wong TY, Klein R, Islam FM, Cotch MF, Folsom AR, Klein BE, et al. Diabetic retinopathy in a multi-ethnic cohort in the United States. Am J Ophthalmol. 2006 Mar 3;141:446–455. doi: 10.1016/j.ajo.2005.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Estacio RO, McFarling E, Biggerstaff S, Jeffers BW, Johnson D, Schrier RW. Overt albuminuria predicts diabetic retinopathy in hispanics with NIDDM. Am J Kidney Dis. 1998 Jun 6;31:947–953. doi: 10.1053/ajkd.1998.v31.pm9631838. [DOI] [PubMed] [Google Scholar]
- 15.Klein R, Klein BE, Magli YL, Brothers RJ, Meuer SM, Moss SE, et al. An alternative method of grading diabetic retinopathy. Ophthalmology. 1986 Sep 9;93:1183–1187. doi: 10.1016/s0161-6420(86)33606-6. [DOI] [PubMed] [Google Scholar]
- 16.Klein R, Zinman B, Gardiner R, Suissa S, Donnelly SM, Sinaiko AR, et al. The relationship of diabetic retinopathy to preclinical diabetic glomerulopathy lesions in type 1 diabetic patients: the renin-angiotensin system study. Diabetes. 2005 Feb 2;54:527–533. doi: 10.2337/diabetes.54.2.527. [DOI] [PubMed] [Google Scholar]
- 17.National Center for Health Statistics. Analytic and Reporting Guidelines: The National Health and Nutrition Examination Survey (NHANES) Hyattsville, MD: Centers for Disease Control and Prevention; 2006. [Google Scholar]
- 18.Ballone E, Colagrande V, Di Nicola M, Di Mascio R, Di Mascio C, Capani F. Probabilistic approach to developing nephropathy in diabetic patients with retinopathy. Stat Med. 2003 Dec 24;22:3889–3897. doi: 10.1002/sim.1644. [DOI] [PubMed] [Google Scholar]
- 19.Ejerblad E, Fored CM, Lindblad P, Fryzek J, McLaughlin JK, Nyren O. Obesity and risk for chronic renal failure. J Am Soc Nephrol. 2006 Jun 6;17:1695–1702. doi: 10.1681/ASN.2005060638. [DOI] [PubMed] [Google Scholar]
- 20.Ramos LF, Shintani A, Ikizler TA, Himmelfarb J. Oxidative stress and inflammation are associated with adiposity in moderate to severe CKD. J Am Soc Nephrol. 2008 Mar 3;19:593–599. doi: 10.1681/ASN.2007030355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldberg RB. Cytokine and cytokine-like inflammation markers, endothelial dysfunction and imbalanced coagulation in development of diabetes and its complications. J Clin Endocrinol Metab. 2009 Sep 9;94:3171–3182. doi: 10.1210/jc.2008-2534. [DOI] [PubMed] [Google Scholar]
- 22.Bryson CL, Ross HJ, Boyko EJ, Young BA. Racial and ethnic variations in albuminuria in the US Third National Health and Nutrition Examination Survey (NHANES III) population: associations with diabetes and level of CKD. Am J Kidney Dis. 2006 Nov 5;48:720–726. doi: 10.1053/j.ajkd.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 23.Emanuele N, Sacks J, Klein R, Reda D, Anderson R, Duckworth W, et al. Ethnicity, race, and baseline retinopathy correlates in the veterans affairs diabetes trial. Diabetes Care. 2005 Aug 8;28:1954–1958. doi: 10.2337/diacare.28.8.1954. [DOI] [PubMed] [Google Scholar]
- 24.Cruickshanks KJ, Ritter LL, Klein R, Moss SE. The association of microalbuminuria with diabetic retinopathy. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. Ophthalmology. 1993 Jun 6;100:862–867. doi: 10.1016/s0161-6420(93)31562-9. [DOI] [PubMed] [Google Scholar]
- 25.Klein R, Klein BE, Moss SE, Cruickshanks KJ. Ten-year incidence of gross proteinuria in people with diabetes. Diabetes. 1995 Aug 8;44:916–923. doi: 10.2337/diab.44.8.916. [DOI] [PubMed] [Google Scholar]
- 26.Perkins BA, Ficociello LH, Ostrander BE, Silva KH, Weinberg J, Warram JH, et al. Microalbuminuria and the risk for early progressive renal function decline in type 1 diabetes. J Am Soc Nephrol. 2007 Apr 4;18:1353–1361. doi: 10.1681/ASN.2006080872. [DOI] [PubMed] [Google Scholar]
- 27.Perkins BA, Ficociello LH, Silva KH, Finkelstein DM, Warram JH, Krolewski AS. Regression of microalbuminuria in type 1 diabetes. N Engl J Med. 2003 Jun 23;48:2285–2293. doi: 10.1056/NEJMoa021835. [DOI] [PubMed] [Google Scholar]
- 28.Schwartz MM, Lewis EJ, Leonard-Martin T, Lewis JB, Batlle D. Renal pathology patterns in type II diabetes mellitus: relationship with retinopathy. The Collaborative Study Group. Nephrol Dial Transplant. 1998 Oct 10;13:2547–2552. doi: 10.1093/ndt/13.10.2547. [DOI] [PubMed] [Google Scholar]
- 29.Fioretto P, Mauer M, Brocco E, Velussi M, Frigato F, Muollo B, et al. Patterns of renal injury in NIDDM patients with microalbuminuria. Diabetologia. 1996 Dec 12;39:1569–1576. doi: 10.1007/s001250050616. [DOI] [PubMed] [Google Scholar]
- 30.Nemes S, Jonasson JM, Genell A, Steineck G. Bias in odds ratios by logistic regression modelling and sample size. BMC Med Res Methodol. 2009;9:56. doi: 10.1186/1471-2288-9-56. [DOI] [PMC free article] [PubMed] [Google Scholar]