Abstract
Background
Early-life physical fitness rarely has been examined in relation to type 2 diabetes mellitus (T2DM) in adulthood, because of the lengthy follow-up required. Elucidation of modifiable risk factors at young ages may help facilitate earlier and more effective interventions.
Objective
We examined aerobic capacity and muscular strength at age 18 in relation to T2DM risk in adulthood.
Design
National cohort study.
Setting
Sweden.
Participants
All 1,534,425 military conscripts during 1969-1997 (97-98% of all 18-year-old males nationwide) without prior T2DM.
Measurements
Aerobic capacity and muscular strength measured in Watts and Newtons per kg of body weight, respectively, were examined in relation to T2DM identified from outpatient and inpatient diagnoses during 1987-2012 (maximum age 62 years).
Results
34,008 men were diagnosed with T2DM in 39.4 million person-years of follow-up. Low aerobic capacity and low muscular strength were independently associated with increased risk of T2DM. Comparing lowest vs. highest tertiles of both aerobic capacity and muscular strength, the absolute difference in cumulative incidence of T2DM was 0.22% at 20 years of follow-up (95% CI, 0.20-0.25), 0.76% at 30 years (0.71-0.81), and 3.97% at 40 years (3.87-4.06). Overall, the combination of low aerobic capacity and low muscular strength was associated with a 3-fold risk of T2DM (adjusted hazard ratio, 3.07; 95% CI, 2.88-3.27; P<0.001), with a positive additive interaction (P<0.001). These associations were observed even among men with normal BMI.
Limitations
This cohort did not include women or physical fitness measurements at older ages.
Conclusions
In this large cohort of Swedish male military conscripts, low aerobic capacity and low muscular strength at age 18 were associated with increased long-term risk of T2DM, even among those with normal BMI.
Primary funding source
NIH.
INTRODUCTION
Type 2 diabetes mellitus (T2DM) affects more than 300 million people worldwide and has more than doubled in prevalence over the past 3 decades, concurrently with increasing rates of obesity and sedentary lifestyle (1). US economic costs of T2DM and its complications exceed $200 billion annually (2). Although physical inactivity is a well-established risk factor, few studies have examined objective measurements of physical fitness in relation to T2DM. Physical fitness (which includes both aerobic capacity and muscular strength) may be a more informative risk factor, because it can be measured more objectively and is a better indicator of habitual physical activity than self-reported activity (3). Most studies of physical fitness have examined aerobic but not muscular fitness, and have focused on adults but lacked data at younger ages with sufficient follow-up to examine the long-term risk of T2DM. As a result, the relative effects of aerobic capacity and muscular strength, and their effects at younger ages on long-term T2DM risk, are still unknown. Elucidation of these risk factors at young ages may help facilitate earlier and more effective preventive interventions.
We analyzed data from a national cohort of military conscripts to examine aerobic capacity and muscular strength at age 18 years in relation to T2DM risk in adulthood. Aerobic capacity and muscular strength were assessed using standardized tests in ~1.5 million male military conscripts in Sweden who were followed up to a maximum age of 62 years. Our aims were to examine whether low aerobic capacity and low muscular strength at age 18 are associated with long-term risk of T2DM in this cohort.
METHODS
Study Population
We identified 1,547,478 men (age 18 years) who underwent a military conscription examination in Sweden during 1969-1997. This examination was compulsory for all 18-year-old men nationally each year except for 2-3% who either were incarcerated or had severe chronic medical conditions or disabilities documented by a physician. We excluded all 13,053 (0.8%) men who had a prior inpatient or outpatient diagnosis of diabetes. A total of 1,534,425 (99.2% of the original cohort) remained for inclusion in the study.
Physical Fitness Ascertainment
Aerobic capacity and muscular strength measurements were obtained using the Swedish Military Conscription Registry, which contains information from a 2-day standardized physical and psychological examination required for all conscripts starting in 1969. Aerobic capacity was measured as the maximal aerobic workload in Watts, using a standard well-validated electrically-braked stationary bicycle ergometer test, as previously described (4). Maximal aerobic workload is highly correlated with maximal oxygen uptake (VO2 max; correlation ~0.9) (5), and its measurement using this bicycle ergometer test is highly reproducible, with a test-retest correlation of 0.95 (6). Muscular strength was measured as the weighted sum of maximal knee extension (weighted × 1.3), elbow flexion (weighted × 0.8), and hand grip (weighted × 1.7), each measured in Newtons, using standard well-validated isometric dynamometer tests (7). Each dynamometer test was performed three times and the maximum value recorded for analysis, except when the last value was highest, in which case testing was repeated until strength values stopped increasing. All testing equipment was calibrated daily (7). In the present study, aerobic capacity and muscular strength were standardized per kg of body weight, and were examined alternatively as continuous linear variables, categorical variables in tertiles (aerobic capacity in Watts per kg of body weight: low [<3.58], medium [3.58-4.18], high [≥4.18]; muscular strength in Newtons per kg of body weight: low [<28.23], medium [28.23-32.13], high [≥32.13]), and using cubic spline curves.
T2DM Ascertainment
The study cohort was followed up for T2DM from the time of the military conscription examination through December 31, 2012. T2DM was identified using International Classification of Diseases (ICD) diagnosis codes in the Swedish Hospital and Outpatient Registries. The Swedish Hospital Registry contains all primary and secondary hospital discharge diagnoses from six populous counties in southern Sweden starting in 1964, and with nationwide coverage starting in 1987; and the Swedish Outpatient Registry contains outpatient diagnoses nationwide starting in 2001. Diagnoses in the Hospital Registry are currently >99% complete and have a reported positive predictive value of 85-95% (8). Because earlier ICD versions did not distinguish between type 1 and type 2 diabetes, we ascertained T2DM using ICD-9 code 250 (excluding codes 250.X1 and 250.X3) during 1987-1996, and ICD-10 code E11 during 1997-2012. A sensitivity analysis was performed which further included all diabetes diagnoses during 1969-1986 using ICD-8 code 250 from hospital discharge records (before outpatient data were available). Among men without T2DM diagnoses during 1987-2012, 542 were diagnosed with diabetes during 1969-1986, of which the majority would be expected to be type 2 (e.g., among men with the same age distribution, 75% of inpatient diabetes diagnoses during 1987-2012 were type 2).
Adjustment Variables
Other variables that may be associated with T2DM were obtained from the Swedish Military Conscription Registry and national census data, which were linked using an anonymous personal identification number. The following were used as adjustment variables: year of the military conscription examination (modeled simultaneously as a continuous and categorical [1969-1979, 1980-1989, 1990-1997] variable); body mass index (BMI = [weight in kg]/[height in m]2; modeled simultaneously as a continuous and categorical variable using Centers for Disease Control and Prevention [CDC] definitions for children and adolescents aged 2 to 19 years: “overweight or obesity” is defined as ≥85th percentile on the CDC's 2000 sex-specific BMI-for-age growth charts, which corresponds to BMI ≥25.6 for 18-year-old males (9)); family history of diabetes in a parent or sibling (yes or no, identified from diagnoses in the Swedish Hospital Registry during 1964-2012 and the Swedish Outpatient Registry during 2001-2012, not self-reported, thus enabling unbiased ascertainment); highest education level attained during the study period (<12, 12-14, ≥15 years); and neighborhood socioeconomic status at baseline (SES, included because neighborhood SES characteristics have been associated with T2DM (10, 11) and with physical activity and BMI (12); comprised of an index that includes low education level, low income, unemployment, and social welfare receipt, as previously described (13), and categorized as low [<−1 SD from the mean], medium [≥−1 SD and ≤1 SD], or high [>1 SD]). As alternatives to BMI, we also examined height and weight in a separate model, which were modeled simultaneously as continuous and categorical (height: <175, 175-184, ≥185 cm; weight: <60, 60-79, ≥80 kg) variables (9).
Missing data for each variable were imputed using a standard multiple imputation procedure based on the variable's relationship with all other covariates (14). Missing data were relatively infrequent for aerobic capacity (5.7%), muscular strength (5.0%), height (7.2%), weight (7.3%), education level (0.4%), and neighborhood SES (9.1%). As an alternative to multiple imputation, sensitivity analyses were performed after restricting to individuals with complete data for all variables (N=1,361,083; 88.0%).
Statistical Analysis
Absolute time-to-event measures were calculated using the cumulative incidence function for T2DM. Covariate-standardized cumulative incidence curves for T2DM were generated using the method of Simon and Makuch (15). We also used Cox proportional hazards regression to estimate the relative hazard of T2DM for different levels of aerobic capacity and muscular strength. The Cox model time scale was elapsed time since the military conscription examination (which also corresponds to attained age because baseline age was the same [18 years] for all conscripts). Individuals were censored at emigration (n=112,158; 7.3%) or death (n=58,835; 3.8%). The proportional hazards assumption was evaluated by graphical assessment of log-log plots and was met in all models. Interactions between aerobic capacity and muscular strength were examined on either the additive or multiplicative scale. Additive interactions were assessed using the “relative excess risk due to interaction” (RERI), which is computed for binary variables as: RERIHR = HR11 – HR10 – HR01 + 1 (16, 17). Multiplicative interactions were assessed using the ratio of HRs: HR11 / (HR10 HR01). We also examined interactions graphically using cubic spline curves.
Sensitivity analyses were performed that included only men with at least 30 years of follow-up (N=686,964; 44.8%), or that evaluated the effect of unmeasured confounders (e.g., smoking) using external adjustment (18). In the analysis of smoking, we performed 10,000 model simulations assuming two uniform independent distributions for smoking prevalences among exposed and unexposed between 0.2 and 0.4 (19), and a lognormal distribution for the smoking-T2DM relative hazard that implies a mean relative hazard of 1.5 and SD of 0.4 (20). All statistical tests were 2-sided and used an α-level of 0.05. All analyses were conducted using Stata version 13.0 (21).
Role of the Funding Source
This work was supported by the National Heart, Lung, and Blood Institute at the National Institutes of Health (R01 HL116381); the Swedish Research Council; and ALF project grant, Region Skåne/Lund University, Sweden. The funding agencies had no role in the study design, conduct, or reporting. This study was approved by the Regional Ethics Committee of Lund University.
RESULTS
Among the 1,534,425 men in this cohort, 34,008 (2.2%) were subsequently diagnosed with T2DM in 39.4 million person-years of follow-up (mean follow-up, 25.7 years). The median age at the end of follow-up was 46.1 years (mean 45.9, SD 8.9, range 19.0 to 62.0), and at T2DM diagnosis was 46.8 years (mean 44.7, SD 9.9, range 18.0 to 62.0). Table 1 shows aerobic capacity, muscular strength, and other characteristics among 18-year-old men who did or did not subsequently develop T2DM.
Table 1.
Physical fitness and other characteristics among 18-year-old men who did or did not develop type 2 diabetes.
| Type 2 Diabetes | ||
|---|---|---|
| Yes (N=34,008) |
No (N=1,500,417) |
|
| Aerobic capacity per kg body weight (tertiles) | ||
| Low, n (%) | 22,878 (67.3) | 489,539 (32.6) |
| Medium, n (%) | 7,604 (22.4) | 499,672 (33.3) |
| High, n (%) | 3,526 (10.4) | 511,206 (34.1) |
| Mean (SD) | 3.3 (0.7) | 3.9 (0.7) |
| Muscular strength per kg body weight (tertiles) | ||
| Low, n (%) | 15,887 (46.7) | 498,406 (33.2) |
| Medium, n (%) | 10,751 (31.6) | 501,151 (33.4) |
| High, n (%) | 7,370 (21.7) | 500,860 (33.4) |
| Mean (SD) | 28.4 (5.0) | 29.2 (6.5) |
| Body mass index | ||
| Normal, n (%) | 25,918 (76.2) | 1,388,856 (92.6) |
| Overweight or obese, n (%) | 8,090 (23.8) | 111,561 (7.4) |
| Mean (SD) | 23.3 (4.3) | 21.6 (2.8) |
| Height (cm) | ||
| <175, n (%) | 9,552 (28.1) | 336,657 (22.4) |
| 175-184, n (%) | 18,684 (54.9) | 883,191 (58.9) |
| ≥185, n (%) | 5,772 (17.0) | 280,569 (18.7) |
| Mean (SD) | 177.6 (7.4) | 178.0 (7.5) |
| Weight (kg) | ||
| <60, n (%) | 3,694 (10.9) | 187,755 (12.5) |
| 60-79, n (%) | 20,543 (60.4) | 1,115,921 (74.4) |
| ≥80, n (%) | 9,771 (28.7) | 196,741 (13.1) |
| Mean (SD) | 73.5 (14.3) | 68.8 (10.3) |
| Family history of diabetes | ||
| No, n (%) | 18,251 (53.7) | 1,164,670 (77.6) |
| Yes, n (%) | 15,757 (46.3) | 335,747 (22.4) |
| Education (years) | ||
| <12, n (%) | 8,247 (24.2) | 225,154 (15.0) |
| 12-14, n (%) | 16,177 (47.6) | 662,094 (44.1) |
| ≥15, n (%) | 9,584 (28.2) | 613,169 (40.9) |
| Neighborhood socioeconomic status | ||
| Low, n (%) | 7,864 (23.1) | 231,265 (15.4) |
| Medium, n (%) | 21,166 (62.2) | 988,613 (65.9) |
| High, n (%) | 4,978 (23.2) | 280,539 (18.7) |
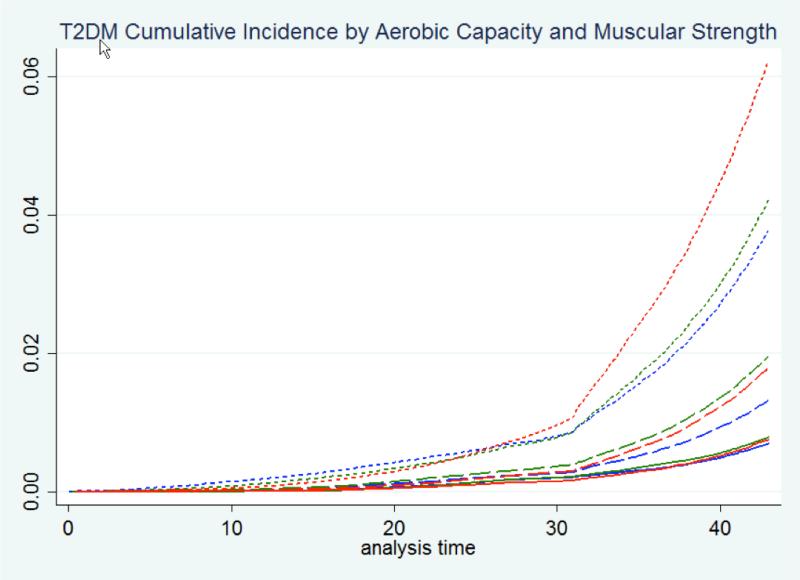
Table 2 shows the covariate-standardized cumulative incidence of T2DM at 10, 20, 30, and 40 years of follow-up by aerobic capacity and muscular strength at age 18. Low aerobic capacity was associated with significantly increased cumulative incidence at each of these follow-up times, irrespective of muscular strength level. Low muscular strength was associated with increased cumulative incidence after 40 years of follow-up among men with low or medium (but not high) aerobic capacity. The combination of low aerobic capacity and low muscular strength was associated with highest cumulative incidence, which reached 4.45% at 40 years of follow-up (risk difference relative to high aerobic capacity and high muscular strength, 3.97%; 95% CI, 3.87-4.06; P<0.001). Cumulative incidence curves for T2DM by aerobic capacity and muscular strength are shown in Figure 1.
Table 2.
Cumulative incidence of type 2 diabetes by aerobic capacity and muscular strength in 18-year-old men.
| Follow-up time | Muscular strength (tertiles) | Aerobic capacity (tertiles) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| High | Medium | Low | ||||||||
| Cumulative incidence (%) | Risk difference | P value | Cumulative incidence (%) | Ris k difference | P value | Cumulative incidence (%) | Ris k difference | P value | ||
| 10 years | High | 0.01 (0.00, 0.01) | Reference | -- | 0.01 (0.01, 0.02) | 0.01 (0.00, 0.01) | 0.05 | 0.14 (0.12, 0.17) | 0.14 (0.11, 0.16) | <0.001 |
| Medium | 0.00 (0.00, 0.01) | 0.00 (−0.01, 0.00) | 0.06 | 0.02 (0.01, 0.03) | 0.01 (0.01, 0.02) | <0.001 | 0.08 (0.07, 0.09) | 0.07 (0.06, 0.09) | <0.001 | |
| Low | 0.00 (0.00, 0.01) | 0.00 (−0.01, 0.00) | 0.53 | 0.01 (0.00, 0.01) | 0.00 (0.00, 0.01) | 0.46 | 0.05 (0.04, 0.05) | 0.04 (0.03, 0.05) | <0.001 | |
| 20 years | High | 0.06 (0.05, 0.07) | Reference | -- | 0.11 (0.10, 0.12) | 0.05 (0.03, 0.07) | <0.001 | 0.41 (0.37, 0.45) | 0.35 (0.31, 0.39) | <0.001 |
| Medium | 0.05 (0.04, 0.06) | −0.01 (−0.03, 0.00) | 0.15 | 0.14 (0.12, 0.16) | 0.08 (0.06, 0.10) | <0.001 | 0.33 (0.31, 0.36) | 0.27 (0.24, 0.30) | <0.001 | |
| Low | 0.04 (0.03, 0.06) | −0.02 (−0.03, 0.00) | 0.03 | 0.09 (0.07, 0.11) | 0.03 (0.01, 0.05) | 0.003 | 0.28 (0.26, 0.31) | 0.22 (0.20, 0.25) | <0.001 | |
| 30 years | High | 0.19 (0.18, 0.22) | Reference | -- | 0.26 (0.24, 0.28) | 0.06 (0.03, 0.09) | <0.001 | 0.80 (0.75, 0.85) | 0.61 (0.55, 0.66) | <0.001 |
| Medium | 0.20 (0.18, 0.22) | 0.01 (−0.02, 0.03) | 0.70 | 0.36 (0.33, 0.39) | 0.16 (0.13, 0.20) | <0.001 | 0.78 (0.74, 0.82) | 0.58 (0.53, 0.63) | <0.001 | |
| Low | 0.15 (0.13, 0.17) | −0.05 (−0.08, −0.02) | 0.001 | 0.28 (0.25, 0.31) | 0.08 (0.05, 0.12) | <0.001 | 0.96 (0.92, 1.00) | 0.76 (0.71, 0.81) | <0.001 | |
| 40 years | High | 0.48 (0.45, 0.52) | Reference | -- | 0.92 (0.88, 0.97) | 0.43 (0.37, 0.48) | <0.001 | 2.69 (2.59, 2.79) | 2.21 (2.10, 2.31) | <0.001 |
| Medium | 0.55 (0.52, 0.59) | 0.07 (0.02, 0.11) | 0.006 | 1.35 (1.29, 1.41) | 0.88 (0.81, 0.95) | <0.001 | 2.98 (2.89, 3.06) | 2.50 (2.41, 2.59) | <0.001 | |
| Low | 0.51 (0.48, 0.55) | 0.03 (−0.02, 0.08) | 0.39 | 1.22 (1.16, 1.28) | 0.74 (0.67, 0.80) | <0.001 | 4.45 (4.36, 4.54) | 3.97 (3.87, 4.06) | <0.001 | |
Figure 1.
Cumulative incidence of type 2 diabetes by aerobic capacity and muscular strength in 18-year-old men with maximum follow-up of 44 years (aerobic capacity, tertiles: solid line = high, long dash = medium, short dash = low; muscular strength, tertiles: blue = high, green = medium, red = low).
Table 3 summarizes the adjusted relative hazards of T2DM across the entire follow-up period by aerobic capacity and muscular strength at age 18. Low aerobic capacity and low muscular strength were independently associated with higher risk of T2DM, although low aerobic capacity was the stronger risk factor (Pheterogeneity<0.001). The combination of low aerobic capacity and low muscular strength was associated with highest T2DM risk (HR, 3.07; 95% CI, 2.88-3.27; P<0.001). Comparing lowest vs. highest tertiles, aerobic capacity and muscular strength had a positive interaction on the additive (Pinteraction<0.001) but not multiplicative (Pinteraction=0.62) scale. The same additive interaction was found when examined at 30 or 40 years of follow-up (Pinteraction<0.001), but not at earlier times (Pinteraction>0.05).
Table 3.
Interactions between aerobic capacity and muscular strength among 18-year-old men in relation to subsequent risk of type 2 diabetes.a
| Aerobic capacity (tertiles) | HRs for medium aerobic capacity within strata of muscular strength | HRs for low aerobic capacity within strata of muscular strength | ||||||
|---|---|---|---|---|---|---|---|---|
| High | Medium | Low | ||||||
| No. cases/total | HR (95% CI) | No. cases/total | HR (95% CI) | No. cases/total | HR (95% CI) | |||
| Muscular strength (tertiles) | ||||||||
| High | 1,252/195,299 | 1.00 | 2,371/202,217 | 1.08 (1.01, 1.16); P=0.028 | 3,747/110,714 | 1.90 (1.77, 2.03); P<0.001 | 1.08 (1.01, 1.16); P=0.028 | 1.90 (1.77, 2.03); P<0.001 |
| Medium | 1,268/173,644 | 1.19 (1.10, 1.29); P<0.001 | 2,893/162,087 | 1.60 (1.49, 1.71); P<0.001 | 6,590/176,171 | 2.25 (2.11, 2.39); P<0.001 | 1.35 (1.25, 1.44); P<0.001 | 1.89 (1.77, 2.01); P<0.001 |
| Low | 1,006/145,789 | 1.58 (1.45, 1.72); P<0.001 | 2,340/142,972 | 1.92 (1.79, 2.06); P<0.001 | 12,541/225,532 | 3.07 (2.88, 3.27); P<0.001 | 1.22 (1.13, 1.31); P<0.001 | 1.94 (1.81, 2.08); P<0.001 |
| HRs (95% CI) for medium muscular strength within strata of aerobic capacity | 1.19 (1.10, 1.29); P<0.001 | 1.48 (1.39, 1.56); P<0.001 | 1.19 (1.14, 1.23); P<0.001 | |||||
| HRs (95% CI) for low muscular strength within strata of aerobic capacity | 1.58 (1.45, 1.72); P<0.001 | 1.78 (1.67, 1.88); P<0.001 | 1.62 (1.55, 1.68); P<0.001 | |||||
| Interaction on additive scale, lowest vs. highest tertiles: RERI (95% CI) | 0.59 (0.45, 0.73); P<0.001 | |||||||
| Interaction on multiplicative scale, lowest vs. highest tertiles: Ratio of HRs (95% CI) | 1.02 (0.93, 1.12); P=0.62 | |||||||
HRs are adjusted for year of the military conscription exam, body mass index, family history of diabetes, education, and neighborhood socioeconomic status.
HR = hazard ratio, RERI = relative excess risk due to interaction
The univariate effects of aerobic capacity, muscular strength, BMI, and other variables in association with T2DM are shown in Supplemental Table 1. In secondary analyses, we found positive additive (but not multiplicative) interactions between either low aerobic capacity or low muscular strength and high BMI in relation to T2DM (P<0.001; Supplemental Tables 2 and 3). Low aerobic capacity and low muscular strength were associated with higher risk of T2DM even among men with normal BMI. In sensitivity analyses that included diabetes diagnoses from 1969-1986 (for which type 1 and type 2 could not be distinguished), that were restricted to men with no missing data, or that included only men with at least 30 years of follow-up, all risk estimates were very similar to the main results (data not shown). External adjustment for smoking yielded risk estimates for association between low aerobic capacity or low muscular strength and T2DM that were 9% lower and remained highly significant (P<0.001), suggesting that unmeasured confounding had little influence on our main findings.
DISCUSSION
In this large national cohort study, low aerobic capacity and low muscular strength in 18-year-old men were associated with higher risk of developing T2DM in adulthood, independent of BMI, family history, or socioeconomic factors. A combination of low aerobic capacity and low muscular strength was associated with highest risk, although aerobic capacity had the stronger influence. Furthermore, both of these factors were associated with increased risk of T2DM even among men with normal BMI. Positive additive interactions were found between low aerobic capacity and low muscular strength, suggesting that, if the associations are causal, interventions to improve aerobic capacity would have the greatest public health impact on T2DM among men with low muscular strength.
Most previous studies have examined physical fitness only in adulthood (22-30). The largest of these was a US study of 46,979 middle-aged adults with median follow-up of 5 years, which reported that higher physical fitness based on a treadmill stress test was independently protective against diabetes (Ptrend<0.001) (27). Fewer studies have examined physical fitness early in life, and none examined physical fitness in adolescence in relation to the long-term risk of T2DM. Our findings suggest that low aerobic capacity at age 18 is strongly associated with higher risk of developing T2DM later in life, irrespective of baseline muscular strength or BMI, after follow-up to a maximum age of 62 years.
We also found that low muscular strength was an independent risk factor for T2DM later in life, although was less influential than aerobic capacity. These findings are broadly consistent with previously reported associations between muscular strength among adults and reduced risk of metabolic syndrome (31, 32), between resistance training among adults and reduced risk of T2DM (33, 34), and between resistance training and improved glycemic control among adults with T2DM (35). The overall evidence to date suggests that high muscular strength or resistance training improves glycemic control and is protective against T2DM, independent of aerobic capacity. However, the combination of high muscular strength and high aerobic capacity is associated with the greatest protective benefit (33, 34).
Obesity is a well-established strong risk factor for T2DM (36-38). Importantly, we found that low aerobic capacity and low muscular strength were long-term risk factors for T2DM even among men with normal BMI. Other cohort studies have reported that low aerobic capacity is associated with T2DM even among non-obese adults, without examining muscular strength (22, 27, 30). Overall, these findings suggest that physical fitness has important health benefits for all, even those who are not overweight or obese.
There are several mechanisms by which aerobic and muscular fitness may enhance glycemic control (39, 40). Aerobic exercise is known to increase mitochrondrial density and oxidative enzyme activity, which promotes fatty acid oxidation and insulin sensitivity (41). Strength training augments type II muscle fiber growth, increasing glucose use capacity (41), and may up-regulate proteins in the insulin-signaling cascade, increasing insulin activity and further enhancing glucose utilization (42). Both aerobic exercise and strength training help reduce adiposity, a known risk factor for T2DM (43).
Strengths of the present study include its large national cohort design with prospective ascertainment of aerobic capacity, muscular strength, BMI, and T2DM. The national cohort design prevented selection bias, and the use of registry data with prospectively measured exposures prevented bias that may result from self-reporting. We examined objective, well-validated measures of aerobic capacity and muscular strength, which are likely better indicators of habitual physical activity than self-reported activity (3). We were able to adjust for other strong risk factors for T2DM, including BMI, family history, and socioeconomic factors, which also were prospectively ascertained and not self-reported.
Limitations include the measurement of physical fitness and BMI at only one age (18 years), and hence we were unable to examine changes in these factors over time. Because this study was based on Swedish military conscripts, the cohort consisted entirely of men. Other studies have reported similar associations between low physical fitness and risk of T2DM among women (22, 29). Outpatient diagnoses in the present study were available only starting in 2001, and hence T2DM prior to this period was underreported. This underreporting is expected to be non-differential with respect to physical fitness and therefore to influence results toward the null hypothesis. In addition, diagnoses prior to 1987 were excluded from the main analyses because they did not distinguish between type 1 and type 2 diabetes. However, sensitivity analyses that included all diabetes diagnoses prior to 1987 (of which most are expected to be type 2) yielded very similar results as our main findings. Last, this was a relatively young cohort in Sweden. Additional studies will be needed in other populations, diverse ethnic groups, and with follow-up to older ages.
In summary, we found that low aerobic capacity and low muscular strength at age 18 were independently associated with higher risk of developing T2DM in adulthood, among men with either normal or high BMI. These findings suggest that interventions to improve aerobic and muscular fitness early in life could help reduce T2DM risk in adulthood. Additional studies with longitudinal measurements of fitness will be needed to delineate the most important windows of susceptibility and further inform preventive interventions.
Supplementary Material
ACKNOWLEDGMENTS
GRANT SUPPORT
This work was supported by the National Heart, Lung, and Blood Institute at the National Institutes of Health (R01 HL116381); the Swedish Research Council; and ALF project grant, Region Skåne/Lund University, Sweden. The funding agencies had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
Footnotes
Author Contributions: Dr. Jan Sundquist had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Crump, J. Sundquist, Winkleby, Sieh, K. Sundquist.
Acquisition of data: J. Sundquist, K. Sundquist.
Analysis and interpretation of data: Crump, J. Sundquist, Winkleby, Sieh, K. Sundquist.
Drafting of the manuscript: Crump.
Critical revision of the manuscript for important intellectual content: Crump, J. Sundquist, Winkleby, Sieh, K. Sundquist.
Statistical analysis: Crump, J. Sundquist.
Obtained funding: J. Sundquist, K. Sundquist.
REPRODUCIBLE RESEARCH STATEMENT
Protocol, statistical code, and data: Not available
REFERENCES
- 1.Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet. 2011;378(9785):31–40. doi: 10.1016/S0140-6736(11)60679-X. [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association Economic costs of diabetes in the U.S. in 2012. Diabetes Care. 2013;36(4):1033–46. doi: 10.2337/dc12-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Swift DL, Lavie CJ, Johannsen NM, Arena R, Earnest CP, O'Keefe JH, et al. Physical activity, cardiorespiratory fitness, and exercise training in primary and secondary coronary prevention. Circ J. 2013;77(2):281–92. doi: 10.1253/circj.cj-13-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nordesjo L, Schele R. Validity of an ergometer cycle test and measures of isometric muscle strength when prediction some aspects of military performance. Swedish J Defence Med. 1974;10:11–23. [Google Scholar]
- 5.Patton JF, Vogel JA, Mello RP. Evaluation of a maximal predictive cycle ergometer test of aerobic power. Eur J Appl Physiol Occup Physiol. 1982;49(1):131–40. doi: 10.1007/BF00428971. [DOI] [PubMed] [Google Scholar]
- 6.Andersen LB. A maximal cycle exercise protocol to predict maximal oxygen uptake. Scand J Med Sci Sports. 1995;5(3):143–6. doi: 10.1111/j.1600-0838.1995.tb00027.x. [DOI] [PubMed] [Google Scholar]
- 7.Hook O, Tornvall G. Apparatus and method for determination of isometric muscle strength in man. Scand J Rehabil Med. 1969;1:139–42. [PubMed] [Google Scholar]
- 8.Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C, et al. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450. doi: 10.1186/1471-2458-11-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ogden CL, Flegal KM. Changes in terminology for childhood overweight and obesity. Natl Health Stat Report. 2010;(25):1–5. [PubMed] [Google Scholar]
- 10.Mezuk B, Chaikiat A, Li X, Sundquist J, Kendler KS, Sundquist K. Depression, neighborhood deprivation and risk of type 2 diabetes. Health Place. 2013;23:63–9. doi: 10.1016/j.healthplace.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sundquist K, Eriksson U, Mezuk B, Ohlsson H. Neighborhood walkability, deprivation and incidence of type 2 diabetes: a population-based study on 512,061 Swedish adults. Health Place. 2015;31:24–30. doi: 10.1016/j.healthplace.2014.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stoddard PJ, Laraia BA, Warton EM, Moffet HH, Adler NE, Schillinger D, et al. Neighborhood deprivation and change in BMI among adults with type 2 diabetes: the Diabetes Study of Northern California (DISTANCE). Diabetes Care. 2013;36(5):1200–8. doi: 10.2337/dc11-1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crump C, Sundquist K, Sundquist J, Winkleby MA. Neighborhood deprivation and psychiatric medication prescription: a Swedish national multilevel study. Ann Epidemiol. 2011;21(4):231–7. doi: 10.1016/j.annepidem.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rubin DB. Multiple Imputation for Nonresponse in Surveys. Wiley; New York: 1987. [Google Scholar]
- 15.Simon R, Makuch RW. A non-parametric graphical representation of the relationship between survival and the occurrence of an event: application to responder versus non-responder bias. Stat Med. 1984;3(1):35–44. doi: 10.1002/sim.4780030106. [DOI] [PubMed] [Google Scholar]
- 16.Li R, Chambless L. Test for additive interaction in proportional hazards models. Ann Epidemiol. 2007;17(3):227–36. doi: 10.1016/j.annepidem.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 17.VanderWeele TJ, Knol MJ. A tutorial on interaction. Epidemiol. Methods. 2014;3(1):33–72. [Google Scholar]
- 18.Orsini N, Bellocco R, Bottai M, Wolk A, Greenland S. A tool for deterministic and probabilistic sensitivity analysis of epidemiologic studies. The Stata Journal. 2008;8(1):29–48. [Google Scholar]
- 19.Furberg H, Lichtenstein P, Pedersen NL, Bulik C, Sullivan PF. Cigarettes and oral snuff use in Sweden: Prevalence and transitions. Addiction. 2006;101(10):1509–15. doi: 10.1111/j.1360-0443.2006.01550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Willi C, Bodenmann P, Ghali WA, Faris PD, Cornuz J. Active smoking and the risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2007;298(22):2654–64. doi: 10.1001/jama.298.22.2654. [DOI] [PubMed] [Google Scholar]
- 21.StataCorp . Stata Statistical Software: Release 13. StataCorp LP; College Station, TX: 2013. [Google Scholar]
- 22.Carnethon MR, Gidding SS, Nehgme R, Sidney S, Jacobs DR, Jr., Liu K. Cardiorespiratory fitness in young adulthood and the development of cardiovascular disease risk factors. JAMA. 2003;290(23):3092–100. doi: 10.1001/jama.290.23.3092. [DOI] [PubMed] [Google Scholar]
- 23.Eriksson KF, Lindgarde F. Poor physical fitness, and impaired early insulin response but late hyperinsulinaemia, as predictors of NIDDM in middle-aged Swedish men. Diabetologia. 1996;39(5):573–9. doi: 10.1007/BF00403304. [DOI] [PubMed] [Google Scholar]
- 24.Lynch J, Helmrich SP, Lakka TA, Kaplan GA, Cohen RD, Salonen R, et al. Moderately intense physical activities and high levels of cardiorespiratory fitness reduce the risk of non-insulin-dependent diabetes mellitus in middle-aged men. Arch Intern Med. 1996;156(12):1307–14. [PubMed] [Google Scholar]
- 25.Sawada SS, Lee IM, Muto T, Matuszaki K, Blair SN. Cardiorespiratory fitness and the incidence of type 2 diabetes: prospective study of Japanese men. Diabetes Care. 2003;26(10):2918–22. doi: 10.2337/diacare.26.10.2918. [DOI] [PubMed] [Google Scholar]
- 26.Wei M, Gibbons LW, Mitchell TL, Kampert JB, Lee CD, Blair SN. The association between cardiorespiratory fitness and impaired fasting glucose and type 2 diabetes mellitus in men. Ann Intern Med. 1999;130(2):89–96. doi: 10.7326/0003-4819-130-2-199901190-00002. [DOI] [PubMed] [Google Scholar]
- 27.Juraschek SP, Blaha MJ, Blumenthal RS, Brawner C, Qureshi W, Keteyian SJ, et al. Cardiorespiratory fitness and incident diabetes: the FIT (Henry Ford ExercIse Testing) project. Diabetes Care. 2015;38(6):1075–81. doi: 10.2337/dc14-2714. [DOI] [PubMed] [Google Scholar]
- 28.Katzmarzyk PT, Craig CL, Gauvin L. Adiposity, physical fitness and incident diabetes: the physical activity longitudinal study. Diabetologia. 2007;50(3):538–44. doi: 10.1007/s00125-006-0554-3. [DOI] [PubMed] [Google Scholar]
- 29.Sui X, Hooker SP, Lee IM, Church TS, Colabianchi N, Lee CD, et al. A prospective study of cardiorespiratory fitness and risk of type 2 diabetes in women. Diabetes Care. 2008;31(3):550–5. doi: 10.2337/dc07-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee DC, Sui X, Church TS, Lee IM, Blair SN. Associations of cardiorespiratory fitness and obesity with risks of impaired fasting glucose and type 2 diabetes in men. Diabetes Care. 2009;32(2):257–62. doi: 10.2337/dc08-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jurca R, Lamonte MJ, Barlow CE, Kampert JB, Church TS, Blair SN. Association of muscular strength with incidence of metabolic syndrome in men. Med Sci Sports Exerc. 2005;37(11):1849–55. doi: 10.1249/01.mss.0000175865.17614.74. [DOI] [PubMed] [Google Scholar]
- 32.Wijndaele K, Duvigneaud N, Matton L, Duquet W, Thomis M, Beunen G, et al. Muscular strength, aerobic fitness, and metabolic syndrome risk in Flemish adults. Med Sci Sports Exerc. 2007;39(2):233–40. doi: 10.1249/01.mss.0000247003.32589.a6. [DOI] [PubMed] [Google Scholar]
- 33.Grontved A, Rimm EB, Willett WC, Andersen LB, Hu FB. A prospective study of weight training and risk of type 2 diabetes mellitus in men. Arch Intern Med. 2012;172(17):1306–12. doi: 10.1001/archinternmed.2012.3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grontved A, Pan A, Mekary RA, Stampfer M, Willett WC, Manson JE, et al. Muscle-strengthening and conditioning activities and risk of type 2 diabetes: a prospective study in two cohorts of US women. PLoS Med. 2014;11(1):e1001587. doi: 10.1371/journal.pmed.1001587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Umpierre D, Ribeiro PA, Kramer CK, Leitao CB, Zucatti AT, Azevedo MJ, et al. Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2011;305(17):1790–9. doi: 10.1001/jama.2011.576. [DOI] [PubMed] [Google Scholar]
- 36.Ganz ML, Wintfeld N, Li Q, Alas V, Langer J, Hammer M. The association of body mass index with the risk of type 2 diabetes: a case-control study nested in an electronic health records system in the United States. Diabetol Metab Syndr. 2014;6(1):50. doi: 10.1186/1758-5996-6-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carey VJ, Walters EE, Colditz GA, Solomon CG, Willett WC, Rosner BA, et al. Body fat distribution and risk of non-insulin-dependent diabetes mellitus in women. The Nurses' Health Study. Am J Epidemiol. 1997;145(7):614–9. doi: 10.1093/oxfordjournals.aje.a009158. [DOI] [PubMed] [Google Scholar]
- 38.Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289(1):76–9. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 39.Hawley JA. Molecular responses to strength and endurance training: are they incompatible? Appl Physiol Nutr Metab. 2009;34(3):355–61. doi: 10.1139/H09-023. [DOI] [PubMed] [Google Scholar]
- 40.Fyfe JJ, Bishop DJ, Stepto NK. Interference between concurrent resistance and endurance exercise: molecular bases and the role of individual training variables. Sports Med. 2014;44(6):743–62. doi: 10.1007/s40279-014-0162-1. [DOI] [PubMed] [Google Scholar]
- 41.LeBrasseur NK, Walsh K, Arany Z. Metabolic benefits of resistance training and fast glycolytic skeletal muscle. Am J Physiol Endocrinol Metab. 2011;300(1):E3–10. doi: 10.1152/ajpendo.00512.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Holten MK, Zacho M, Gaster M, Juel C, Wojtaszewski JF, Dela F. Strength training increases insulin-mediated glucose uptake, GLUT4 content, and insulin signaling in skeletal muscle in patients with type 2 diabetes. Diabetes. 2004;53(2):294–305. doi: 10.2337/diabetes.53.2.294. [DOI] [PubMed] [Google Scholar]
- 43.Church T. Exercise in obesity, metabolic syndrome, and diabetes. Prog Cardiovasc Dis. 2011;53(6):412–8. doi: 10.1016/j.pcad.2011.03.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.