Abstract
Treatment options for stroke remain limited. Neuroprotective therapies, in particular, have invariably failed to yield the expected benefit in stroke patients, despite robust theoretical and mechanistic background and promising animal data. Insulin and insulin-like growth factor 1 (IGF-1) play a pivotal role in critical brain functions, such as energy homeostasis, neuronal growth, and differentiation. They may exhibit neuroprotective properties in acute ischemic stroke based upon their vasodilatory, anti-inflammatory and antithrombotic effects, as well as improvements of functional connectivity, neuronal metabolism, neurotransmitter regulation, and remyelination. Intranasally administered insulin has demonstrated a benefit for prevention of cognitive decline in older people, and IGF-1 has shown potential benefit to improve functional outcomes in animal models of acute ischemic stroke. The intranasal route presents a feasible, tolerable, safe, and particularly effective administration route, bypassing the blood–brain barrier and maximizing distribution to the central nervous system (CNS), without the disadvantages of systemic side effects and first-pass metabolism. This review summarizes the neuroprotective potential of intranasally administered insulin and IGF-1 in stroke patients. We present the theoretical background and pathophysiologic mechanisms, animal and human studies of intranasal insulin and IGF-1, and the safety and feasibility of intranasal route for medication administration to the CNS.
Keywords: Intranasal insulin, Intranasal insulin-like growth factor 1, Intranasal IGF-1, Stroke, Acute ischemic stroke, Neuroprotection
Introduction
Stroke is the 4th leading cause of death in the USA and the leading cause of long-term disability, affecting 795,000 people in the country [1]. It has significant global impact, being the 2nd most common cause of death and the 3rd leading cause of disability worldwide [2, 3]. Despite significant advances in the care of stroke patients in the last two decades, the only intervention with proven benefit in the acute phase is intravenous tissue plasminogen activator [4]. Catheter-based endovascular interventions have shown promise, and although prior randomized controlled trials failed to yield the expected benefit [5–7], four very recently published multicenter randomized trials have yielded significantly positive breakthrough results, adding endovascular management to our armamentarium for acute stroke management in select patients [8–10] (and SWIF T-PRIME, NCT01657461, results announced by Dr Jeffrey Saver at the International Stroke Conference, Nashville, TN, February 2015).
In addition to reperfusion-oriented therapies, neuroprotection has attracted significant attention in acute ischemic stroke (AIS). Neuroprotection in AIS refers to mechanisms, strategies, and interventions aiming to limit the extent of neuronal injury that ensues after AIS and results in attenuating the detrimental effect of stroke, reducing mortality, and improving functional outcome for stroke victims.
AIS consists of the irreversibly damaged neuronal tissue (referred to as “core infarct”) and an additional “at-risk” area, known as the ischemic penumbra. The penumbra is defined as a brain area with decreased blood flow that is at risk for permanent damage [11, 12]. Maximizing penumbral salvage is thought to lead to improved outcome. Therefore, many neuroprotective therapies target different deleterious mechanisms such as inflammation [13–16], excitotoxicity [14, 17], and apoptosis [18–21] that may contribute to neuronal death after an ischemic insult. Despite robust theoretical and mechanistic background and promising animal data, neuroprotective therapies have invariably failed to yield the expected benefit in human subjects: studies of N-methyl-D-aspartate (NMDA) receptor non-competitive [22] and competitive [23–26] antagonists, calcium channel blockers [27], free radical scavengers [28–31], cellular membrane stabilizers (citicoline) [32, 33], and monoclonal antibodies blocking intercellular adhesion molecule-1 [34] either did not result in functional improvement or were prematurely terminated due to adverse events and safety concerns.
Brief Overview of Cellular and Molecular Mechanisms of Neuronal Death in Ischemic Stroke
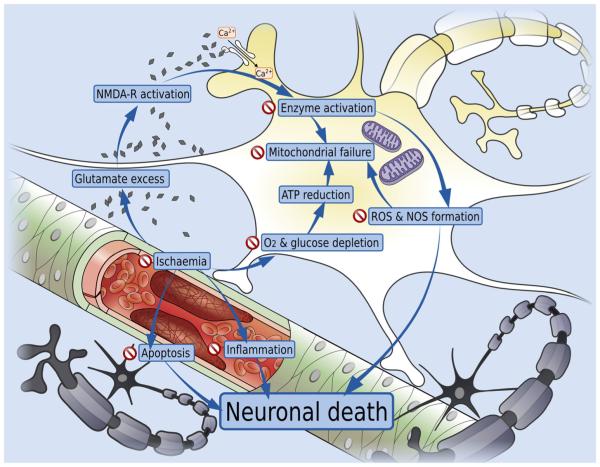
Brain ischemia and oligemia trigger a cascade of events eventually leading to neuronal death: oxygen and glucose depletion causes ATP reduction, production and release of reactive oxygen species (ROS), and eventually catastrophic energy failure [35]. Excitotoxic mechanisms play a central role: NMDA receptor-mediated calcium entry into the neurons is increased, leading to ROS and reactive nitrogen species (RNS) production, protease activation, and mitochondrial dysfunction [36]. Inflammation is a significant secondary mechanism, and the deleterious cascade is further fuelled by the release of ROS and other cytotoxic cytokines by the microglia [37–39]. In addition to immediate cytotoxicity and neuronal death, brain ischemia triggers apoptotic mechanisms resulting in further neuronal damage [19–21, 40]. Insulin’s pleiotropic effects in the CNS can affect all the aforementioned mechanisms. Figure 1 depicts a summary of the mechanisms implicated in the ischemic cascade and potential targets for insulin in response to acute ischemia.
Fig. 1.
Mechanisms implicated in the ischemic cascade and potential targets for insulin in response to acute ischemia. “Stop” signs indicate the steps of the ischemic cascade where insulin can intervene to limit the extent of ischemic damage. Notice that its impact can be exerted along several different steps and mechanisms, indicative of its pleiotropic effect
In this review, we summarize the neuroprotective potential of intranasally administered insulin and insulin-like growth factor 1 (IGF-1) in AIS patients. We present the theoretical background and pathophysiologic mechanisms of AIS that can be modulated by insulin and IGF-1, summarize the results of studies of intranasal insulin and IGF-1 administration in animals and humans with central nervous system (CNS) conditions, and provide a brief synopsis of the safety and feasibility of intranasal route for medication administration to the CNS.
Insulin, a Key Neuromodulator in the Brain
Insulin Transport, Localization of Insulin Receptors in the CNS
Insulin and IGF-1 belong to a superfamily of structurally related proteins [41]. Insulin’s role in the brain differs from its peripheral actions. Insulin in the adult CNS is primarily derived from pancreatic β-cells and is dependent upon transport from the periphery through the blood–brain barrier (BBB) [42, 43], via transporter-mediated and saturable transport [44]. De novo insulin synthesis in the brain has also been proposed as an alternative source of insulin in the CNS [45]. Insulin receptors are abundant throughout the CNS, mostly in neurons, whereas the IGF-1 receptors are detected in both neurons and glia [46–48]. They are expressed in numerous brain regions, namely in the olfactory bulb, hypothalamus, cerebral cortex, cerebellum, and hippocampus [44, 47, 48]. Even wider insulin receptor distribution overlaps with expression of downstream proteins and isoforms in insulin-related pathways [44, 49]. The insulin/IGF-1-mediated signaling pathways play a central role in several critical processes including cognition [50, 51], energy homeostasis [52, 53], food intake [54], neuron-astrocyte signaling [55, 56], synapse formation, and neuronal survival [50].
Cellular and Molecular Mechanisms—Targets of Insulin
Anti-Inflammatory Effect, Moderator of Oxidative Stress
Insulin has been shown to suppress the pro-inflammatory transcription factors, such as nuclear factor κB early growth response-1 and activator protein-1 and their regulated gene products, indicated by a decrease in plasma concentration of matrix metalloproteinase-9 (MMP-9), vascular endothelial growth factor (VEGF), tissue factor (TF), and plasminogen activator inhibitor-1 (PAI-1) [57–59]. The suppressing effects on MMP-9 and VEGF by insulin may decrease the disruption of the blood–brain barrier, preventing cerebral edema, leakage of plasma proteins, and inflammatory cells during ischemia [60] thereby attenuating the detrimental effect of the inflammatory cascade.
Antithrombotic and Vasodilatory Effects
Insulin-mediated decrease in plasma TF and PAI-1 levels may inhibit thrombosis and promote fibrinolysis during acute ischemia, producing an anticoagulant effect [60]. Moreover, insulin has also been shown to suppress ROS generation [57], increasing the release of endothelial nitric oxide (NO) and the expression of NO synthase in the endothelial cells [61]. Insulin receptors are widely distributed within the neurons, capillaries, and small vessel walls, modulating signaling within the neurovascular unit to regulate regional perfusion and neuronal activity [46, 47]. In the setting of cerebral ischemia, insulin administration increased phosphorylation of protein kinase B (PKB; also known as Akt) and endothelial NO synthase protein that are responsible for initiating several cellular effects, including improvement of synaptic plasticity and neuronal survival following ischemic brain injury [62, 63]. Insulin-related activation of endothelial Akt and NO production led to reduction of sympathetic nerve activity, activation of ATP-dependent K+ channels and release of vasodilator adenosine, resulting in vasodilation [62, 64], thereby increasing blood flow and cell survival [62, 64, 65]. As a result, cerebral infarct size and neurologic functional deficit both can be decreased [62]. These vasodilatory and antithrombotic effects could enhance the collateral vessel network and improve cerebral perfusion in the oligemic (ischemic penumbra) area, resulting in smaller final infarct volume and better long-term functional outcomes [62].
Antiapoptotic Effect
Activation of neuronal insulin-receptor-mediated signaling pathways (phosphatidylinositol 3-kinase (PI3K)/Akt/glycogen synthase kinase-3β (GSK-3β), and the Src homology-2 domain-containing (SHC)/extracellular signal-regulated kinases (ERK) 1/2) signaling cascades by insulin has been shown to have a neuroprotectant effect by preventing neuronal apoptosis following oxidative stress, and stimulate the synthesis of proteins involved in neuronal antioxidant [45, 66].
Modulator of Energy Use—Prevention of Energy Failure
Previous studies reported the downregulation of cerebral glucose transporters (GLUTs) after ischemic injury [67]. Insulin is known to increase the glucose uptake in the brain, stimulating global cerebral glucose metabolism especially in the human brain cortex [53]. Insulin also inhibits the neuronal nor-epinephrine uptake, with subsequent activation of glial β-adrenoreceptors, converting the glycogen stores in astrocytes to glucose [41, 45] and providing additional energy for neurons. However, a recent study did not demonstrate increase of membranous GLUT 1 protein level and glucose uptake after insulin treatment in diabetic rats subjected to cerebral ischemia [62], suggesting the beneficial action of insulin upon ischemic stroke might be independent of cerebral glucose uptake.
Long-Term Regeneration Effects
Other proposed mechanisms of insulin in the brain include the increase of neurite outgrowth [68], regeneration of small myelinated fibers [41, 45], survival of sympathetic and sensory neurons [50], enhancement of neurotransmission [69], and improvement of cognitive functions by increasing perfusion [70, 71] and resting state functional connectivity of the brain [72].
The Intranasal Route—Feasibility for Insulin
The blood–brain barrier (BBB) protects the brain by allowing the entrance of only specific molecules and proteins, but at the same time, it presents an important obstacle for the delivery of medications to the CNS. The presence of tight junctions and a characteristic low rate of pinocytosis create a seal between opposing endothelial membranes, resulting in a very low permeability that favors passage of only very small lipophilic molecules [73], significantly limiting brain penetration of systemically administered medications. As a result, intranasal (IN) delivery has attracted the attention of researchers and clinicians as an alternative, non-invasive, and effective route for medication delivery. IN drug administration presents remarkable advantages: it is painless, non-invasive, easy, and does not require sterile preparation [74]. In addition, it allows BBB bypass, systemic absorption reduction, and a decrease in first-pass metabolism, reducing potential side effects [75].
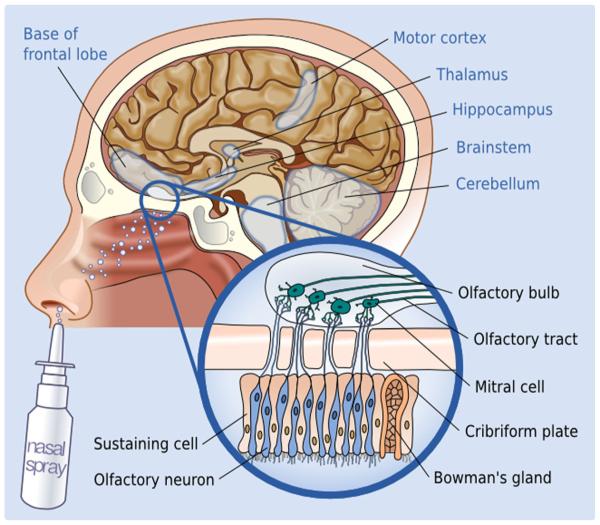
The nasal cavity is unique, as it brings the CNS in direct contact with the external environment via the olfactory and trigeminal neural pathways [76, 77]. Bipolar olfactory sensory neurons project axons that form aggregates, which enter the CNS across the cribriform plate; these axons synapse with mitral and tufted cells in the olfactory bulb of the brain [76] (Fig. 2).
Fig. 2.
The intranasal route of molecule transport to the CNS. Paracellular and axonal transport along the olfactory and trigeminal neurons results in increased concentrations in the highlighted brain areas (although not limited to those)
It was initially proposed that IN drug absorption occurs through the nasal epithelium, with subsequent brain delivery via blood vessels [78, 79]. However, recent research suggests that paracellular transport, or endocytosis by nerve processes or by olfactory sensory neurons, accounts for the majority of drug absorption [73]. Given the rapidity of molecule transmission (detected within 1 h from administration), paracellular transport is likely the dominant mechanism; endocytosis would result in slower transmission [75]. Areas of maximum brain concentration following intranasal administration suggest a rostral–caudal direction of transmission [75], with the olfactory and trigeminal pathways being the main vectors of transport [75]. Following IN administration of radiolabeled IGF-I in rats, nanomolar range concentrations of the substance could be identified in the olfactory bulb, frontal cortex, hippocampal formation, cerebellum, brainstem, and trigeminal nucleus [80] (Fig. 2). Though the concentration seems to be higher in these areas, it is not restricted to them, as there is evidence of more diffuse spread in other cerebral regions [75].
Besides bypassing the systemic circulation and eliminating the side effects, IN administration offers significantly higher CNS penetration: administering radiolabeled IGF-1 intranasally and intravenously, IN administration resulted in over 100-fold higher brain concentration compared to intravenous administration [80], a feature of paramount importance in clinical practice, given the fact that poor CNS penetration is the key disadvantage limiting the clinical efficacy of many medications targeting CNS processes (e.g., antibiotics, antiretrovirals).
Several medications that are currently being investigated for IN delivery for CNS indications have shown encouraging results: IN interferon-beta has been studied as a non-invasive treatment for multiple sclerosis, targeting the CNS and cervical lymph nodes [81]. IN insulin administration was first evaluated as an alternative to subcutaneous insulin injections in patients with diabetes mellitus, but research demonstrated a low bioavailability and a high rate of therapeutic failure [82]. Instead, current research supports the use of IN insulin as a potential treatment to improve memory and cognition not only in healthy participants [83] but also in patients with Alzheimer’s disease (AD) [71, 84] and diabetes [70].
Human and Animal Studies of Intranasal Insulin to the CNS
Given its pleiotropic neuromodulatory effects, insulin has been tested in several human and animal studies; the majority of which focus on cognitive performance in cognitively intact healthy or diabetic subjects, as well as patients with previously established cognitive impairment [51, 83, 85–95]. Despite its promising potential as neuroprotectant in older people at risk for cognitive decline, there is a marked lack of both animal and human researches in acute stroke. We therefore present a synopsis of studies with primary focus on cognition. Although not directly applicable, those can be particularly helpful for designing future stroke studies by providing useful information regarding dosing, safety, tolerability, and efficacy for a CNS indication. It should also be noted that vascular risk factors (diabetes, hypertension, atherosclerosis, hyperlipidemia) associated with AD [96, 97] are also major risk factors of cerebrovascular disease, and besides resulting in AIS and intracerebral hemorrhage, they also cause cerebral dysfunction in more overt ways, such as subclinical decreased cerebral blood flow [98] and silent strokes [96].
Animal Studies
AD is characterized by a series of changes in brain microstructure including depositions of β-amyloid peptides, abnormal hyperphosphorylation of tau protein and glycogen synthase kinase-3β, which are considered to be the pathologic substrate and biomarkers of neurodegeneration and are linked to most of the cognitive deficits found in AD patients [99–102]. In a study of AD rat models comparing IN with subcutaneous insulin administration for 4 weeks, the level of protein kinase B and glycogen synthase kinase-3β normalized and those of hyperphosphorylated tau were significantly reduced in the IN group [103].
Human Studies
Beneficial effects of insulin on cognition and brain function are summarized in Table 1.
Table 1.
Summary of effects of intranasal insulin on cognition and brain function
| Variable | Acute/chronic | Effect | Population |
|---|---|---|---|
| Cognition [49, 81, 83–91] | Acute | Improved visuospatial memory, verbal learning [49] | Healthy young, old, MCI, AD, type 2 DM |
| Chronic | Delayed recall memory [69], attention, posttraumatic stress disorder, other CNS disorders |
Healthy, young, old, MCI, mild AD, bipolar disorder | |
| Brain blood flow [92, 104, 105] | Acute | Increased perfusion in the insular cortex, putamen, caudate [106] | Healthy young, older, type 2 DM |
| fMRI functional connectivity [105] | Default mode | Prefrontal, orbitofrontal and anterior cingulate cortex, hypothalamus, hippocampus, putamen [107] |
Healthy young, old, type 2 DM |
| fMRI-blood flow-neuronal activity [107] | fALFF | Increased activity reward circuits; orbitofrontal, prefrontal, anterior cingulate cortex, hypothalamus |
Healthy young women |
| Brain energy | Acute | Increased ATP and phosphocreatine (PCr) in the motor cortex [101] | Healthy young men |
| PET-FDG | Chronic | Slower progression of hypometabolism [69] | MCI, mild AD |
| AD markers | Chronic | Cognitive response correlates with lower amyloid beta-42 and tau protein in the cerebrospinal fluid and APoE4—phenotype [49] |
MCI, mild AD |
| Reduced tau hyperphosphorylation in rats [93, 108] | |||
| Food intake [109, 110] | Acute | Appetite, satiety, food-related brain activity, and rewards circuits [111] | Healthy men and women |
| Weight | Chronic | Weight loss [111, 112] | Healthy men |
| Insulin sensitivity [113] | Acute | Improved peripheral insulin sensitivity, blood-brain barrier transport correlates with peripheral glucose |
Healthy women |
MCI mild cognitive impairment, AD Alzheimer’s disease, DM diabetes mellitus, CNS central nervous system, PET-FGD positron-emission tomographic imaging, fMRI functional magnetic resonance imaging, fALFF fractional amplitude of low frequency fluctuation
IN administration of insulin plays an important role in improving mood [109] and cognitive task performance in healthy [71, 83, 86] and in memory-impaired individuals [51, 71]. IN administration of insulin increased the feeling of well-being and self-confidence and decreased anger in a cohort of healthy participants acutely and after 8 weeks of daily treatment with 40 IU [83], which supports the results of previous studies evaluating the effects of IN insulin on mood [110]. In healthy adults, prolonged intranasal insulin intake improved long-term declarative memory [83, 86], as well as verbal working memory performance [106]. Similarly, important improvements have been found in patients with mild cognitive impairment (MCI) and AD after intranasal insulin administration: a study measuring cognitive tasks after administering 20 IU of daily IN insulin in patients with early AD demonstrated improved attention, functional status, and verbal information retention in the treated versus the placebo group after 21 days of therapy [84]. Treatment with 20 IU of intranasal insulin led to a significant improvement in delayed memory, enhanced the performance in general cognition measures in patients with AD, and improved functional status in both AD and MCI patients over a 4-month period [71]. In the same study, subjects treated with 40 IU of INI also performed better on functional metrics, as well as general cognition measures. Additional effects on regulation of body weight and body composition were found after 8 weeks of treatment with 40 IU of insulin, with a great impact in reduction of weight and body fat [114], possibly linked to an increase in cerebral blood flow in the insular cortex [115], which could represent a potential treatment for obesity, another important stroke risk factor.
Following a distinctly different logic, the Stroke Hyperglycemia Insulin Network Effort (SHINE) trial (NCT01369069) is investigating the safety and efficacy of intensive glucose-lowering randomizing acute stroke patients to either targeted glucose concentration with the use of intravenous glucose or standard of care subcutaneous insulin. The main benefit of insulin in this trial is expected to be exerted through its hypoglycemic action, which is distinctly different from the mode of action of IN insulin as described above; among other properties, IN insulin does not yield a hypoglycemic response which would make its concurrent use with intravenous insulin possible and safe.
Safety and Risk of Hypoglycemia
A very significant advantage and common denominator to all the studies using IN insulin as a therapeutic intervention to improve cognitive function is that none of them have demonstrated significant adverse effects [51, 70, 71, 83, 84, 86]. It was safe and well tolerated by the vast majority of subjects. The minimal risk of hypoglycemia is reinforced by its lack of efficacy as glucose-lowering treatment in type 1 [116, 117] and type 2 diabetes [113, 118]. A very small (4 %) acute lowering of serum glucose in healthy young subjects was attributed to systemic absorption of small amounts of insulin mainly through inhalation and normalized by 60 min [104]. The single dose of 40 IU of IN insulin used in clinical studies did not have significant effects on serum or subcutaneous glucose levels, heart rate, or blood pressure [93, 106], acutely or chronically [105] .
IGF-1, Animal, and Human Data
Physiology and Mechanisms of Action
IGF-I constitutes a single-chain polypeptide with structural homology to the proinsulin and plays an essential role in metabolic functions such as glucose metabolism. It is produced mainly by the liver in response to the endocrine growth hormone stimulus; its bioavailability is regulated by IGF-binding proteins [107, 108], and its actions are mediated by specific membrane receptors, abundantly expressed in the brain [108]. During the normal aging process, IGF-1 levels decline and low IGF-1 levels have been correlated with frailty and decrease in cognitive abilities [111]. It plays an important role in the development, cell differentiation, plasticity, and survival of the nervous system. It promotes proliferation, differentiation, and maturation of neuronal and glial cells across all stages of cellular development [108, 112, 119] and mediates neurite formation, axogenesis, synaptogenesis [112], and myelin formation [119, 120]. It is also critically implicated in key synaptic processes such as long-term potentiation and long-term depression, which underlie memory, learning, and neuroplasticity by regulating the synthesis and trafficking of glutamate and γ-aminobutyric acid receptor subunits, alteration of ion channel activity and neuronal excitability, and structural changes in the synapse [112].
Its neuroprotective effects are exerted largely through the same mechanisms detailed above for insulin: in vitro studies have shown it to protect against excitotoxicity and oxidative stress [121–123] and to exert a strong antiapoptotic effect [112]. In vivo studies confirmed protection against hypoxic–ischemic insults [124], and it is generally viewed as a neuronal survival factor [125]. Besides limiting the acute phase damage, IGF-I could play a major role in the long-term recovery process from ischemic stroke, enhancing regeneration: it stimulates in vitro proliferation and differentiation of neural and oligodendrocyte progenitors [120] and myelin expression [119], which are probably the underlying mechanisms of its stimulating effect on remyelination [119] and explain the beneficial effect on white matter in addition to neurons. Its pivotal role in neuroplasticity [112] can be particularly important for the recovering brain after a stroke [126, 127].
Human Epidemiologic Observations
Epidemiologic studies in humans have reported an inverse relation between plasma IGF-1 levels and risk of ischemic stroke [128] Serum IGF-1 levels decline with age, lack of exercise, and in metabolic syndrome, and a low IGF-1 level is independently associated with increased risk of stroke [107]. Low circulating IGF-1 levels are associated with worse outcome after stroke [129, 130] although this correlation should be interpreted cautiously and not taken to necessarily imply a causative link.
Studies in Animal Stroke Models
Although no human studies in stroke have been reported, several studies have evaluated the effect of IGF-1 administration in experimental middle cerebral artery occlusion (MCAO) AIS models, using several different administration methods:
Subcutaneous administration of 200 μg/day of IGF-1 for 7 days starting 30 min after the insult resulted in reduced final infarct volume and a significant improvement of functional outcome [131]. In another study, acute administration of IGF-1 30 min before or 2 h after MCAO followed by 24 h reperfusion in diabetic rats decreased the lesion volume measured by MRI as well as the number of apoptotic cells in the cortical penumbra area [132]. A different study tested the IGF-1 gene transfer using adenovirus-associated IGF-1 constructs in mice using a Sendai virus [133]. Gene transfer proved to be neuroprotective and increased survival rates when applied 30 min after bilateral artery occlusion [133]. IGF-1 administered directly into the cerebral ventricles 30 min after stroke induction resulted in reduced infarct size and improved functional outcome [131], and topical IGF-1 application on the cerebral cortex had a similar effect of reduced infarct size and improved neuronal survival [134].
Three studies investigated the efficacy of IN administered IGF-1 in MCAO model in male rats: IGF-1 administered 2, 4, or 6 h following MCAO revealed a time-dependent beneficial effect, with 54 % (vs. 39 and 29 %, respectively) reduction in stroke volume in the animals treated at the 2 h time-point [135]. Reduced apoptosis and significant improvement in motor and sensory function were seen in the same study [135]. A similarly designed study utilizing the same IGF-1 dose (150 μg) in which animals where treated 10 min and 24 and 48 h following MCAO corroborated the potential time-sensitive effect of IGF-1, as the functional improvement was already evident at day 1 [76]. A pattern of dose-dependent effect also emerged, with the dose of 37.5 μg providing no imaging or functional benefit [76], as opposed to 75 [136] and 150 μg [76, 135].
Despite the universally positive outcomes of the reported animal studies, several shortcomings should be acknowledged: potentially neuroprotective anesthetics such as halothane, isoflurane, and pentobarbital were used, potentially augmenting the effect of IGF-1. Most of the studies were conducted using normal adult rats, diabetes- and hypertension-free, both conditions highly prevalent in human subjects with stroke and known to influence the short-term effect and recovery in MCAO animals [137, 138]. Many of the studies were characterized by lack of power analysis, randomization of animals and blind induction of treatment [139] and especially regarding the intranasal route, lack of replication from independent groups [135, 136, 139]. In short, several of the criteria set forth by the Stroke Therapy Academy Industry Round Table (STAIR) for optimal conduct of preclinical research in stroke [140] were not met. However, these studies have shed light on some important insights regarding the usefulness of IGF-1 in animal models and its potential application as neuroprotective agent for human beings.
Discussion
Insulin presents an appealing candidate neuroprotectant agent for AIS. Its complex physiology and pleiotropic CNS effects allow it to intervene in several key pathologic processes that unfold in the immediate aftermath of an ischemic insult (Fig. 1). This offers a relative advantage compared to previously tested neuroprotectants that were mostly focused on singular mechanisms, which was a possibly significant reason for failure, given the complexity and interconnection of neuronal death mechanisms in AIS. Other compounds with putative neuroprotective properties considered for intranasal delivery in stroke patients tend have a more narrow focus on a specific step of the ischemic cascade: deferoxamine, an iron chelator, which has been shown to improve outcomes in rat stroke models [141] mainly exerts its effect by limiting the effects of iron toxicity and ensuing inflammation. IN caspase-9 inhibitor targets exclusively a specific step of the apoptotic pathway [142]. Erythropoietin, which has been found to increase the efficacy of IN IGF-1 in animal stroke models, primarily acts through the PIK-3 apoptotic pathway [143]. Vascular endothelial growth factor, also shown to improve outcomes in rat stroke models, exerts its effect mainly through angiogenesis, although it seems to have additional neuroprotective properties, rendering its effect to be more diverse [144]. The neuroprotectant potential of hypothermia is being currently evaluated in several trials (Cooling Plus Best Medical Treatment Versus Best Medical Treatment Alone for Acute Ischaemic Stroke (EuroHYP-1; NCT01833312), Hypothermia in Acute Ischemic Stroke—Surface Versus Endovascular Cooling, (HAIS-SE; NCT01665885), The Intravascular Cooling in the Treatment of Stroke 2/3 Trial (ICTuS2/3; NCT01123161), Hypothermia in Acute Stroke With Thrombolysis Imaging Evaluation of Revascularization (HASTIER; NCT01778855) utilizing a similar rationale of pleiotropic effect on several deleterious mechanisms. It should be noted that the similarity between the two approaches is limited to the conceptual level, as their modes and duration of delivery, potential side effects, and tolerability differ significantly.
Additionally, insulin is naturally occurring in the CNS, and therefore, its administration could be viewed as restoration or augmentation of physiologic processes as opposed to introducing novel molecules that irreversibly block or modify singular molecular pathways or receptors. The latter can lead to untoward consequences either in the form of side effects, as occurred with the NMDA competitive antagonist dextrorphan and its phenylcyclidine-like properties causing seizures and hallucinations [22], or more unpredictable disruption of potentially beneficial recovery mechanisms. As an example, it is well established that excitotoxicity plays a central role in neuronal death following AIS [17]. However, the same glutamate receptor-mediated mechanisms are also implicated in normal brain functions, including restorative mechanisms such as long-term potentiation [14, 145], which might be crucial for stroke recovery. It is likely that some of the previously tried neuroprotectants failed, although potentially beneficial in the acute phase, because of their interference with the neuroplasticity process. Data from healthy and cognitively impaired human subjects have not raised any concerns over the long-term effects of intranasal insulin administration on cognition and mood; on the contrary, all relevant studies indicate a beneficial effect [71, 83, 84].
The intranasal route presents several considerable advantages: it is feasible, painless, and well tolerated by patients. It does not require special equipment, intravenous administration, or sterilization techniques. It has been tested extensively in human subjects without any safety concerns. It dramatically improves penetration to the CNS, bypassing the BBB, which has been a significant limitation of practically every study with systemic administration of medications targeting the CNS. Given that the systemic circulation is bypassed, there are no first-pass hepatic metabolism and no concerns of systemic side effects. In the case of insulin, this is particularly significant, as systemic administration would inevitably increase the risk of hypoglycemia, which could have detrimental effect on the injured brain [146, 147].
Despite the significant potential advantages of IN insulin in AIS, current limitations and gaps of knowledge should be acknowledged: although there is robust clinical data supporting the efficacy and safety of IN insulin in healthy adults and subjects with MCI or AD, there is a paucity of data from human subjects and animal models of AIS. Extrapolating the positive effect on cognition in otherwise healthy adults to AIS patients without prior careful in vivo experiments confers risks. A common reason for failure of prior neuroprotectant studies was escalation to phase II or phase III trials without robust preliminary and animal data. Therefore, carefully planned experiments fulfilling the Stroke Treatment Academic Industry Roundtable (STAIR) criteria [140] should ideally be performed before introducing the treatment to human AIS subjects. Given its good safety, efficacy, and tolerability profile in healthy adults and MCI and AD patients, a case for careful phase I trial of IN insulin in AIS patients could be made. It should be noted IN insulin fulfills several of the consensus recommendations set forth by the VII Stroke Treatment Academic Industry Roundtable (STAIR), regarding priorities for research on neuroprotective and/or adjunctive therapies for AIS [148]: as stated above, its mechanism of action is pleiotropic, conceptually akin to hypothermia (which is often cited as prototypical example of pleiotropic intervention in the STAIR recommendations). Its intranasal mode of delivery is selective and maximizes delivery to the CNS, while minimizing systemic exposure.
Several other issues remain currently unaddressed: when is the optimal time for treatment initiation? Following the dogma “time is brain” and adopting an emergent approach similar to IV tissue plasminogen activator administration protocol are probably reasonable, and this is further supported from preliminary animal studies that indicate a time-dependent benefit from IN administration of IGF-1 [76]. Rapid administration as close to the stroke onset as possible is recommended by the STAIR committee as a measure to maximize the neuroprotective benefit [144] and should be incorporated in every future animal or human study in the acute phase of stroke. This adherence to a strict time window is considered a sine qua non in acute stroke neuroprotection, to maximize the possibility that the study will yield a benefit; non-adherence to a strict time window might solely undermine the potential success an otherwise promising treatment.
Duration of treatment is another area of uncertainty: the postulated physiologic effects of insulin suggest that it might be beneficial beyond the hyperacute phase of AIS, as it is implicated in neuronal regeneration. This is further supported by more prolonged use in AD patients [71, 84], with a clear benefit and no safety concerns. The optimal dose remains unknown; the 20–40 IU doses utilized in AD subjects might be efficacious in AIS, but given the significant differences in the pathophysiology of the two processes, a dose escalation study is necessary.
Lastly, an important issue that remains unclear is whether insulin or IGF-1 would yield more favorable results. Though closely related, they are not necessarily identical and might have differing or complementary effects in AIS. A detailed discussion of the differences between the two molecules’ structural and functional properties and CNS actions would exceed the scope of this review. Interestingly, IN insulin has been tested extensively in human subjects but with non-AIS pathology, whereas IGF-1 has been almost exclusively tested in animal stroke models, both with beneficial effects. Given their high structural and functional homologies, it would be reasonable to apply a similar research approach and logic to both molecules. However, significant scientific rigor and caution are necessary in evaluating theses strategies to avoid extrapolating assumptions from one molecule to the other and misattributing properties that are not robustly justified by the experimental data. Direct comparison studies, first in animal stroke models, appear to be a necessary future step in our effort to accurately characterize the efficacy of each molecule and guide the decision on which of the two might be more suitable for further development. In summary, intranasally administered insulin presents an attractive candidate therapeutic agent in AIS with pleiotropic neuroprotective actions, encouraging data from human and animal studies and a simple, safe, and well-tolerated route of administration. Further, carefully designed animal and human studies are required to establish its efficacy as a neuroprotectant in AIS. Lessons learned from prior failures of initially promising neuroprotective therapies should be taken into account in order to avoid methodological and study design flaws that could limit the performance of a promising therapeutic agent.
Acknowledgments
The authors wish to thank Katharina Dormanns, PhD candidate Brains Trust Research Group, BlueFern Supercomputing Unit (University of Canterbury), for her contribution in the design of the figures presented in the paper.
Dr V.N. has received grants from the NIH–National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (5R21-DK-084463-02 and 1R01 DK103902-01A1) and National Institute on Aging (NIA) (1R01-AG-0287601-A2) and National Instittute of Diabetes and Digestive and Kidney Diseases (1R01DK103902-01A1) related to this study, and V.N. received salaries from these grants.
Abbreviations
- IN
Intranasal
- AIS
Acute ischemic stroke
- ICH
Intracerebral hemorrhage
- BBB
Blood–brain barrier
- CNS
Central nervous system
- IGF-1
Insulin-like growth factor 1
Footnotes
Conflict of Interest Drs Lioutas, Alfaro-Martinez, Bedoya, Chung, Pimentel, and Novak declare that they have no competing interests.
References
- 1.Kernan WN, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(7):2160–236. doi: 10.1161/STR.0000000000000024. [DOI] [PubMed] [Google Scholar]
- 2.Lozano R, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murray CJ, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2197–223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 4.Jauch EC, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(3):870–947. doi: 10.1161/STR.0b013e318284056a. [DOI] [PubMed] [Google Scholar]
- 5.Ciccone A, Valvassori L. Endovascular treatment for acute ischemic stroke. N Engl J Med. 2013;368(25):2433–4. doi: 10.1056/NEJMc1304759. [DOI] [PubMed] [Google Scholar]
- 6.Broderick JP, et al. Endovascular therapy after intravenous t-PA versus t-PA alone for stroke. N Engl J Med. 2013;368(10):893–903. doi: 10.1056/NEJMoa1214300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kidwell CS, et al. A trial of imaging selection and endovascular treatment for ischemic stroke. N Engl J Med. 2013;368(10):914–23. doi: 10.1056/NEJMoa1212793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goyal M, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372(11):1019–30. doi: 10.1056/NEJMoa1414905. [DOI] [PubMed] [Google Scholar]
- 9.Campbell BC, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372(11):1009–18. doi: 10.1056/NEJMoa1414792. [DOI] [PubMed] [Google Scholar]
- 10.Berkhemer OA, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372(1):11–20. doi: 10.1056/NEJMoa1411587. [DOI] [PubMed] [Google Scholar]
- 11.Donnan GA, et al. Penumbral selection of patients for trials of acute stroke therapy. Lancet Neurol. 2009;8(3):261–9. doi: 10.1016/S1474-4422(09)70041-9. [DOI] [PubMed] [Google Scholar]
- 12.Ebinger M, et al. Imaging the penumbra—strategies to detect tissue at risk after ischemic stroke. J Clin Neurosci. 2009;16(2):178–87. doi: 10.1016/j.jocn.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 13.Zheng Z, Yenari MA. Post-ischemic inflammation: molecular mechanisms and therapeutic implications. Neurol Res. 2004;26(8):884–92. doi: 10.1179/016164104X2357. [DOI] [PubMed] [Google Scholar]
- 14.Ward NS, Cohen LG. Mechanisms underlying recovery of motor function after stroke. Arch Neurol. 2004;61(12):1844–8. doi: 10.1001/archneur.61.12.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vexler ZS, Tang XN, Yenari MA. Inflammation in adult and neonatal stroke. Clin Neurosci Res. 2006;6(5):293–313. doi: 10.1016/j.cnr.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Q, Tang XN, Yenari MA. The inflammatory response in stroke. J Neuroimmunol. 2007;184(1–2):53–68. doi: 10.1016/j.jneuroim.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hazell AS. Excitotoxic mechanisms in stroke: an update of concepts and treatment strategies. Neurochem Int. 2007;50(7–8):941–53. doi: 10.1016/j.neuint.2007.04.026. [DOI] [PubMed] [Google Scholar]
- 18.Moskowitz MA, Lo EH. Neurogenesis and apoptotic cell death. Stroke. 2003;34(2):324–6. doi: 10.1161/01.str.0000054047.14853.ad. [DOI] [PubMed] [Google Scholar]
- 19.Sairanen T, et al. Apoptosis dominant in the periinfarct area of human ischaemic stroke–a possible target of antiapoptotic treatments. Brain. 2006;129:189–99. doi: 10.1093/brain/awh645. Pt 1. [DOI] [PubMed] [Google Scholar]
- 20.Broughton BR, Reutens DC, Sobey CG. Apoptotic mechanisms after cerebral ischemia. Stroke. 2009;40(5):e331–9. doi: 10.1161/STROKEAHA.108.531632. [DOI] [PubMed] [Google Scholar]
- 21.Yuan J. Neuroprotective strategies targeting apoptotic and necrotic cell death for stroke. Apoptosis. 2009;14(4):469–77. doi: 10.1007/s10495-008-0304-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Albers GW, et al. Safety, tolerability, and pharmacokinetics of the N-methyl-D-aspartate antagonist dextrorphan in patients with acute stroke. Dextrorphan Study Group. Stroke. 1995;26(2):254–8. doi: 10.1161/01.str.26.2.254. [DOI] [PubMed] [Google Scholar]
- 23.Davis SM, et al. Termination of acute stroke studies involving selfotel treatment. ASSIST Steering Committed. Lancet. 1997;349(9044):32. doi: 10.1016/s0140-6736(05)62166-6. [DOI] [PubMed] [Google Scholar]
- 24.Lees KR. Cerestat and other NMDA antagonists in ischemic stroke. Neurology. 1997;49(5 Suppl 4):S66–9. doi: 10.1212/wnl.49.5_suppl_4.s66. [DOI] [PubMed] [Google Scholar]
- 25.Muir KW, et al. Magnesium for acute stroke (intravenous magnesium efficacy in stroke trial): randomised controlled trial. Lancet. 2004;363(9407):439–45. doi: 10.1016/S0140-6736(04)15490-1. [DOI] [PubMed] [Google Scholar]
- 26.Sacco RL, et al. Glycine antagonist in neuroprotection for patients with acute stroke: GAIN Americas: a randomized controlled trial. JAMA. 2001;285(13):1719–28. doi: 10.1001/jama.285.13.1719. [DOI] [PubMed] [Google Scholar]
- 27.Horn J, et al. Very Early Nimodipine Use in Stroke (VENUS): a randomized, double-blind, placebo-controlled trial. Stroke. 2001;32(2):461–5. doi: 10.1161/01.str.32.2.461. [DOI] [PubMed] [Google Scholar]
- 28.Bath PM, et al. Tirilazad for acute ischaemic stroke. Cochrane Database Syst Rev. 2001;4:CD002087. doi: 10.1002/14651858.CD002087. [DOI] [PubMed] [Google Scholar]
- 29.Shuaib A, et al. NXY-059 for the treatment of acute ischemic stroke. N Engl J Med. 2007;357(6):562–71. doi: 10.1056/NEJMoa070240. [DOI] [PubMed] [Google Scholar]
- 30.Lees KR, et al. NXY-059 for acute ischemic stroke. N Engl J Med. 2006;354(6):588–600. doi: 10.1056/NEJMoa052980. [DOI] [PubMed] [Google Scholar]
- 31.Lees KR, et al. Additional outcomes and subgroup analyses of NXY-059 for acute ischemic stroke in the SAINT I trial. Stroke. 2006;37(12):2970–8. doi: 10.1161/01.STR.0000249410.91473.44. [DOI] [PubMed] [Google Scholar]
- 32.Davalos A, et al. Oral citicoline in acute ischemic stroke: an individual patient data pooling analysis of clinical trials. Stroke. 2002;33(12):2850–7. doi: 10.1161/01.str.0000038691.03334.71. [DOI] [PubMed] [Google Scholar]
- 33.Davalos A, et al. Citicoline in the treatment of acute ischaemic stroke: an international, randomised, multicentre, placebo-controlled study (ICTUS trial) Lancet. 2012;380(9839):349–57. doi: 10.1016/S0140-6736(12)60813-7. [DOI] [PubMed] [Google Scholar]
- 34.Use of anti-ICAM-1 therapy in ischemic stroke: results of the enlimomab acute stroke trial. Neurology. 2001;57(8):1428–34. doi: 10.1212/wnl.57.8.1428. [DOI] [PubMed] [Google Scholar]
- 35.Taoufik E, Probert L. Ischemic neuronal damage. Curr Pharm Des. 2008;14(33):3565–73. doi: 10.2174/138161208786848748. [DOI] [PubMed] [Google Scholar]
- 36.Arundine M, Tymianski M. Molecular mechanisms of glutamate-dependent neurodegeneration in ischemia and traumatic brain injury. Cell Mol Life Sci. 2004;61(6):657–68. doi: 10.1007/s00018-003-3319-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chapman KZ, et al. A rapid and transient peripheral inflammatory response precedes brain inflammation after experimental stroke. J Cereb Blood Flow Metab. 2009;29(11):1764–8. doi: 10.1038/jcbfm.2009.113. [DOI] [PubMed] [Google Scholar]
- 38.Danton GH, Dietrich WD. Inflammatory mechanisms after ischemia and stroke. J Neuropathol Exp Neurol. 2003;62(2):127–36. doi: 10.1093/jnen/62.2.127. [DOI] [PubMed] [Google Scholar]
- 39.Vila N, et al. Levels of anti-inflammatory cytokines and neurological worsening in acute ischemic stroke. Stroke. 2003;34(3):671–5. doi: 10.1161/01.STR.0000057976.53301.69. [DOI] [PubMed] [Google Scholar]
- 40.Mattson MP, Culmsee C, Yu ZF. Apoptotic and antiapoptotic mechanisms in stroke. Cell Tissue Res. 2000;301(1):173–87. doi: 10.1007/s004419900154. [DOI] [PubMed] [Google Scholar]
- 41.Schulingkamp RJ, et al. Insulin receptors and insulin action in the brain: review and clinical implications. Neurosci Biobehav Rev. 2000;24(8):855–72. doi: 10.1016/s0149-7634(00)00040-3. [DOI] [PubMed] [Google Scholar]
- 42.Margolis RU, Altszuler N. Insulin in the cerebrospinal fluid. Nature. 1967;215(5108):1375–6. doi: 10.1038/2151375a0. [DOI] [PubMed] [Google Scholar]
- 43.King GL, Johnson SM. Receptor-mediated transport of insulin across endothelial cells. Science. 1985;227(4694):1583–6. doi: 10.1126/science.3883490. [DOI] [PubMed] [Google Scholar]
- 44.Banks WA. The source of cerebral insulin. Eur J Pharmacol. 2004;490(1–3):5–12. doi: 10.1016/j.ejphar.2004.02.040. [DOI] [PubMed] [Google Scholar]
- 45.Duarte AI, Moreira PI, Oliveira CR. Insulin in central nervous system: more than just a peripheral hormone. J Aging Res. 2012;2012:384017. doi: 10.1155/2012/384017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Adamo M, Raizada MK, LeRoith D. Insulin and insulin-like growth factor receptors in the nervous system. Mol Neurobiol. 1989;3(1–2):71–100. doi: 10.1007/BF02935589. [DOI] [PubMed] [Google Scholar]
- 47.Hopkins DF, Williams G. Insulin receptors are widely distributed in human brain and bind human and porcine insulin with equal affinity. Diabet Med. 1997;14(12):1044–50. doi: 10.1002/(SICI)1096-9136(199712)14:12<1044::AID-DIA508>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 48.Albrecht J, Wroblewska B, Mossakowski MJ. The binding of insulin to cerebral capillaries and astrocytes of the rat. Neurochem Res. 1982;7(4):489–94. doi: 10.1007/BF00965500. [DOI] [PubMed] [Google Scholar]
- 49.Horsch D, Kahn CR. Region-specific mRNA expression of phosphatidylinositol 3-kinase regulatory isoforms in the central nervous system of C57BL/6J mice. J Comp Neurol. 1999;415(1):105–20. [PubMed] [Google Scholar]
- 50.Recio-Pinto E, Rechler MM, Ishii DN. Effects of insulin, insulin-like growth factor-II, and nerve growth factor on neurite formation and survival in cultured sympathetic and sensory neurons. J Neurosci. 1986;6(5):1211–9. doi: 10.1523/JNEUROSCI.06-05-01211.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reger MA, et al. Effects of intranasal insulin on cognition in memory-impaired older adults: modulation by APOE genotype. Neurobiol Aging. 2006;27(3):451–8. doi: 10.1016/j.neurobiolaging.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 52.Plum L, Belgardt BF, Bruning JC. Central insulin action in energy and glucose homeostasis. J Clin Invest. 2006;116(7):1761–6. doi: 10.1172/JCI29063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bingham EM, et al. The role of insulin in human brain glucose metabolism: an 18fluoro-deoxyglucose positron emission tomography study. Diabetes. 2002;51(12):3384–90. doi: 10.2337/diabetes.51.12.3384. [DOI] [PubMed] [Google Scholar]
- 54.Figlewicz DP, Benoit SC. Insulin, leptin, and food reward: update 2008. Am J Physiol Regul Integr Comp Physiol. 2009;296(1):R9–19. doi: 10.1152/ajpregu.90725.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Plitzko D, Rumpel S, Gottmann K. Insulin promotes functional induction of silent synapses in differentiating rat neocortical neurons. Eur J Neurosci. 2001;14(8):1412–5. doi: 10.1046/j.0953-816x.2001.01740.x. [DOI] [PubMed] [Google Scholar]
- 56.Plum L, Schubert M, Bruning JC. The role of insulin receptor signaling in the brain. Trends Endocrinol Metab. 2005;16(2):59–65. doi: 10.1016/j.tem.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 57.Dandona P, et al. Insulin inhibits intranuclear nuclear factor kappaB and stimulates IkappaB in mononuclear cells in obese subjects: evidence for an anti-inflammatory effect? J Clin Endocrinol Metab. 2001;86(7):3257–65. doi: 10.1210/jcem.86.7.7623. [DOI] [PubMed] [Google Scholar]
- 58.Aljada A, et al. Insulin inhibits the pro-inflammatory transcription factor early growth response gene-1 (Egr)-1 expression in mononuclear cells (MNC) and reduces plasma tissue factor (TF) and plasminogen activator inhibitor-1 (PAI-1) concentrations. J Clin Endocrinol Metab. 2002;87(3):1419–22. doi: 10.1210/jcem.87.3.8462. [DOI] [PubMed] [Google Scholar]
- 59.Dandona P, et al. Insulin suppresses plasma concentration of vascular endothelial growth factor and matrix metalloproteinase-9. Diabetes Care. 2003;26(12):3310–4. doi: 10.2337/diacare.26.12.3310. [DOI] [PubMed] [Google Scholar]
- 60.Garg R, et al. Hyperglycemia, insulin, and acute ischemic stroke: a mechanistic justification for a trial of insulin infusion therapy. Stroke. 2006;37(1):267–73. doi: 10.1161/01.STR.0000195175.29487.30. [DOI] [PubMed] [Google Scholar]
- 61.Aljada A, et al. Insulin inhibits the expression of intercellular adhesion molecule-1 by human aortic endothelial cells through stimulation of nitric oxide. J Clin Endocrinol Metab. 2000;85(7):2572–5. doi: 10.1210/jcem.85.7.6677. [DOI] [PubMed] [Google Scholar]
- 62.Huang SS, et al. The essential role of endothelial nitric oxide synthase activation in insulin-mediated neuroprotection against ischemic stroke in diabetes. J Vasc Surg. 2014;59(2):483–91. doi: 10.1016/j.jvs.2013.03.023. [DOI] [PubMed] [Google Scholar]
- 63.Philpott KL, et al. Activated phosphatidylinositol 3-kinase and Akt kinase promote survival of superior cervical neurons. J Cell Biol. 1997;139(3):809–15. doi: 10.1083/jcb.139.3.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dimmeler S, et al. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399(6736):601–5. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 65.McKay MK, Hester RL. Role of nitric oxide, adenosine, and ATP-sensitive potassium channels in insulin-induced vasodilation. Hypertension. 1996;28(2):202–8. doi: 10.1161/01.hyp.28.2.202. [DOI] [PubMed] [Google Scholar]
- 66.Duarte AI, et al. Insulin neuroprotection against oxidative stress is mediated by Akt and GSK-3beta signaling pathways and changes in protein expression. Biochim Biophys Acta. 2008;1783(6):994–1002. doi: 10.1016/j.bbamcr.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 67.Ueda H. Prothymosin alpha plays a key role in cell death mode-switch, a new concept for neuroprotective mechanisms in stroke. Naunyn Schmiedebergs Arch Pharmacol. 2008;377(4–6):315–23. doi: 10.1007/s00210-007-0254-7. [DOI] [PubMed] [Google Scholar]
- 68.Song J, et al. Axons guided by insulin receptor in Drosophila visual system. Science. 2003;300(5618):502–5. doi: 10.1126/science.1081203. [DOI] [PubMed] [Google Scholar]
- 69.Guyot LL, et al. The effect of topical insulin on the release of excitotoxic and other amino acids from the rat cerebral cortex during streptozotocin-induced hyperglycemic ischemia. Brain Res. 2000;872(1–2):29–36. doi: 10.1016/s0006-8993(00)02426-4. [DOI] [PubMed] [Google Scholar]
- 70.Novak V, et al. Enhancement of vasoreactivity and cognition by intranasal insulin in type 2 diabetes. Diabetes Care. 2014;37(3):751–9. doi: 10.2337/dc13-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Craft S, et al. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial. Arch Neurol. 2012;69(1):29–38. doi: 10.1001/archneurol.2011.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang H, et al. Intranasal insulin enhanced resting-state functional connectivity of hippocampal regions in type 2 diabetes. Diabetes. 2014 doi: 10.2337/db14-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lochhead JJ, Thorne RG. Intranasal delivery of biologics to the central nervous system. Adv Drug Deliv Rev. 2012;64(7):614–28. doi: 10.1016/j.addr.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 74.Costantino HR, et al. Intranasal delivery: physicochemical and therapeutic aspects. Int J Pharm. 2007;337(1–2):1–24. doi: 10.1016/j.ijpharm.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 75.Migliore MM, et al. Brain delivery of proteins by the intranasal route of administration: a comparison of cationic liposomes versus aqueous solution formulations. J Pharm Sci. 2010;99(4):1745–61. doi: 10.1002/jps.21939. [DOI] [PubMed] [Google Scholar]
- 76.Liu XF, et al. Intranasal administration of insulin-like growth factor-I bypasses the blood–brain barrier and protects against focal cerebral ischemic damage. J Neurol Sci. 2001;187(1–2):91–7. doi: 10.1016/s0022-510x(01)00532-9. [DOI] [PubMed] [Google Scholar]
- 77.Hanson LR, Frey WH., 2nd Intranasal delivery bypasses the blood–brain barrier to target therapeutic agents to the central nervous system and treat neurodegenerative disease. BMC Neurosci. 2008;9(Suppl 3):S5. doi: 10.1186/1471-2202-9-S3-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Merkus FW, van den Berg MP. Can nasal drug delivery bypass the blood–brain barrier?: questioning the direct transport theory. Drugs R&D. 2007;8(3):133–44. doi: 10.2165/00126839-200708030-00001. [DOI] [PubMed] [Google Scholar]
- 79.Merkus P, et al. Direct access of drugs to the human brain after intranasal drug administration? Neurology. 2003;60(10):1669–71. doi: 10.1212/01.wnl.0000067993.60735.77. [DOI] [PubMed] [Google Scholar]
- 80.Thorne RG, et al. Delivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neuroscience. 2004;127(2):481–96. doi: 10.1016/j.neuroscience.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 81.Ross TM, et al. Intranasal administration of interferon beta bypasses the blood–brain barrier to target the central nervous system and cervical lymph nodes: a non-invasive treatment strategy for multiple sclerosis. J Neuroimmunol. 2004;151(1–2):66–77. doi: 10.1016/j.jneuroim.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 82.Hilsted J, et al. Intranasal insulin therapy: the clinical realities. Diabetologia. 1995;38(6):680–4. doi: 10.1007/BF00401839. [DOI] [PubMed] [Google Scholar]
- 83.Benedict C, et al. Intranasal insulin improves memory in humans. Psychoneuroendocrinology. 2004;29(10):1326–34. doi: 10.1016/j.psyneuen.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 84.Reger MA, et al. Intranasal insulin improves cognition and modulates beta-amyloid in early AD. Neurology. 2008;70(6):440–8. doi: 10.1212/01.WNL.0000265401.62434.36. [DOI] [PubMed] [Google Scholar]
- 85.Park CR, et al. Intracerebroventricular insulin enhances memory in a passive-avoidance task. Physiol Behav. 2000;68(4):509–14. doi: 10.1016/s0031-9384(99)00220-6. [DOI] [PubMed] [Google Scholar]
- 86.Benedict C, et al. Intranasal insulin improves memory in humans: superiority of insulin aspart. Neuropsychopharmacology. 2007;32(1):239–43. doi: 10.1038/sj.npp.1301193. [DOI] [PubMed] [Google Scholar]
- 87.Reger MA, et al. Intranasal insulin administration dose-dependently modulates verbal memory and plasma amyloid-beta in memory-impaired older adults. J Alzheimers Dis. 2008;13(3):323–31. doi: 10.3233/jad-2008-13309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Freiherr J, et al. Intranasal insulin as a treatment for Alzheimer’s disease: a review of basic research and clinical evidence. CNS Drugs. 2013;27(7):505–14. doi: 10.1007/s40263-013-0076-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Banks WA, Owen JB, Erickson MA. Insulin in the brain: there and back again. Pharmacol Ther. 2012;136(1):82–93. doi: 10.1016/j.pharmthera.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cholerton B, Baker LD, Craft S. Insulin, cognition, and dementia. Eur J Pharmacol. 2013;719(1–3):170–9. doi: 10.1016/j.ejphar.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cholerton B, et al. Insulin and sex interactions in older adults with mild cognitive impairment. J Alzheimers Dis. 2012;31(2):401–10. doi: 10.3233/JAD-2012-120202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schioth HB, et al. Brain insulin signaling and Alzheimer’s disease: current evidence and future directions. Mol Neurobiol. 2012;46(1):4–10. doi: 10.1007/s12035-011-8229-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Benedict C, et al. Immediate but not long-term intranasal administration of insulin raises blood pressure in human beings. Metabolism. 2005;54(10):1356–61. doi: 10.1016/j.metabol.2005.04.026. [DOI] [PubMed] [Google Scholar]
- 94.Figlewicz DP. Insulin, food intake, and reward. Semin Clin Neuropsychiatry. 2003;8(2):82–93. doi: 10.1053/scnp.2003.50012. [DOI] [PubMed] [Google Scholar]
- 95.Farris W, et al. Insulin-degrading enzyme regulates the levels of insulin, amyloid beta-protein, and the beta-amyloid precursor protein intracellular domain in vivo. Proc Natl Acad Sci U S A. 2003;100(7):4162–7. doi: 10.1073/pnas.0230450100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Humpel C. Chronic mild cerebrovascular dysfunction as a cause for Alzheimer’s disease? Exp Gerontol. 2011;46(4):225–32. doi: 10.1016/j.exger.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Exalto LG, et al. An update on type 2 diabetes, vascular dementia and Alzheimer’s disease. Exp Gerontol. 2012;47(11):858–64. doi: 10.1016/j.exger.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 98.Dede DS, et al. Assessment of endothelial function in Alzheimer’s disease: is Alzheimer’s disease a vascular disease? J Am Geriatr Soc. 2007;55(10):1613–7. doi: 10.1111/j.1532-5415.2007.01378.x. [DOI] [PubMed] [Google Scholar]
- 99.Yang Y, et al. I ntranas a l i nsulin a m eliorates t au hyperphosphorylation in a rat model of type 2 diabetes. J Alzheimers Dis. 2013;33(2):329–38. doi: 10.3233/JAD-2012-121294. [DOI] [PubMed] [Google Scholar]
- 100.Wang X, et al. Insulin deficiency exacerbates cerebral amyloidosis and behavioral deficits in an Alzheimer transgenic mouse model. Mol Neurodegener. 2010;5:46. doi: 10.1186/1750-1326-5-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu Y, et al. Deficient brain insulin signalling pathway in Alzheimer’s disease and diabetes. J Pathol. 2011;225(1):54–62. doi: 10.1002/path.2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hoyer S. The aging brain. Changes in the neuronal insulin/insulin receptor signal transduction cascade trigger late-onset sporadic Alzheimer disease (SAD). A mini-review. J Neural Transm. 2002;109(7–8):991–1002. doi: 10.1007/s007020200082. [DOI] [PubMed] [Google Scholar]
- 103.Jauch-Chara K, et al. Intranasal insulin suppresses food intake via enhancement of brain energy levels in humans. Diabetes. 2012;61(9):2261–8. doi: 10.2337/db12-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Heni M, et al. Nasal insulin changes peripheral insulin sensitivity simultaneously with altered activity in homeostatic and reward-related human brain regions. Diabetologia. 2012;55(6):1773–82. doi: 10.1007/s00125-012-2528-y. [DOI] [PubMed] [Google Scholar]
- 105.McInnes GT. The expanding role of angiotensin receptor blockers in the management of the elderly hypertensive. Curr Med Res Opin. 2003;19(5):452–5. doi: 10.1185/030079903125001992. [DOI] [PubMed] [Google Scholar]
- 106.Benedict C, et al. Differential sensitivity of men and women to anorexigenic and memory-improving effects of intranasal insulin. J Clin Endocrinol Metab. 2008;93(4):1339–44. doi: 10.1210/jc.2007-2606. [DOI] [PubMed] [Google Scholar]
- 107.Dong X, et al. The relationship between serum insulin-like growth factor I levels and ischemic stroke risk. PLoS One. 2014;9(4):e94845. doi: 10.1371/journal.pone.0094845. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 108.Benarroch EE. Insulin-like growth factors in the brain and their potential clinical implications. Neurology. 2012;79(21):2148–53. doi: 10.1212/WNL.0b013e3182752eef. [DOI] [PubMed] [Google Scholar]
- 109.Kern W, et al. Improving influence of insulin on cognitive functions in humans. Neuroendocrinology. 2001;74(4):270–80. doi: 10.1159/000054694. [DOI] [PubMed] [Google Scholar]
- 110.Kern W, et al. Central nervous system effects of intranasally administered insulin during euglycemia in men. Diabetes. 1999;48(3):557–63. doi: 10.2337/diabetes.48.3.557. [DOI] [PubMed] [Google Scholar]
- 111.Kalmijn S, et al. A prospective study on circulating insulin-like growth factor I (IGF-I), IGF-binding proteins, and cognitive function in the elderly. J Clin Endocrinol Metab. 2000;85(12):4551–5. doi: 10.1210/jcem.85.12.7033. [DOI] [PubMed] [Google Scholar]
- 112.Fernandez AM, Torres-Aleman I. The many faces of insulin-like peptide signalling in the brain. Nat Rev Neurosci. 2012;13(4):225–39. doi: 10.1038/nrn3209. [DOI] [PubMed] [Google Scholar]
- 113.Frauman AG, Jerums G, Louis WJ. Effects of intranasal insulin in non-obese type II diabetics. Diabetes Res Clin Pract. 1987;3(4):197–202. doi: 10.1016/s0168-8227(87)80039-6. [DOI] [PubMed] [Google Scholar]
- 114.Hallschmid M, et al. Towards the therapeutic use of intranasal neuropeptide administration in metabolic and cognitive disorders. Regul Pept. 2008;149(1–3):79–83. doi: 10.1016/j.regpep.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 115.Schilling TM, et al. Intranasal insulin increases regional cerebral blood flow in the insular cortex in men independently of cortisol manipulation. Hum Brain Mapp. 2014;35(5):1944–56. doi: 10.1002/hbm.22304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lalej-Bennis D, et al. Six month administration of gelified intranasal insulin in 16 type 1 diabetic patients under multiple injections: efficacy vs subcutaneous injections and local tolerance. Diabetes Metab. 2001;27(3):372–7. [PubMed] [Google Scholar]
- 117.Frauman AG, et al. Long-term use of intranasal insulin in insulin-dependent diabetic patients. Diabetes Care. 1987;10(5):573–8. doi: 10.2337/diacare.10.5.573. [DOI] [PubMed] [Google Scholar]
- 118.Lalej-Bennis D, et al. Efficacy and tolerance of intranasal insulin administered during 4 months in severely hyperglycaemic Type 2 diabetic patients with oral drug failure: a cross-over study. Diabet Med. 2001;18(8):614–8. doi: 10.1046/j.1464-5491.2001.00528.x. [DOI] [PubMed] [Google Scholar]
- 119.Chesik D, De Keyser J, Wilczak N. Insulin-like growth factor system regulates oligodendroglial cell behavior: therapeutic potential in CNS. J Mol Neurosci. 2008;35(1):81–90. doi: 10.1007/s12031-008-9041-2. [DOI] [PubMed] [Google Scholar]
- 120.Espinosa-Jeffrey A, et al. Transferrin regulates transcription of the MBP gene and its action synergizes with IGF-1 to enhance myelinogenesis in the md rat. Dev Neurosci. 2002;24(2–3):227–41. doi: 10.1159/000065698. [DOI] [PubMed] [Google Scholar]
- 121.Heck S, et al. Insulin-like growth factor-1-mediated neuroprotection against oxidative stress is associated with activation of nuclear factor kappaB. J Biol Chem. 1999;274(14):9828–35. doi: 10.1074/jbc.274.14.9828. [DOI] [PubMed] [Google Scholar]
- 122.Matsuzaki H, et al. Activation of Akt kinase inhibits apoptosis and changes in Bcl-2 and Bax expression induced by nitric oxide in primary hippocampal neurons. J Neurochem. 1999;73(5):2037–46. [PubMed] [Google Scholar]
- 123.Vincent AM, et al. IGF-I prevents glutamate-induced motor neuron programmed cell death. Neurobiol Dis. 2004;16(2):407–16. doi: 10.1016/j.nbd.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 124.Johnston BM, et al. Insulin-like growth factor-1 is a potent neuronal rescue agent after hypoxic-ischemic injury in fetal lambs. J Clin Invest. 1996;97(2):300–8. doi: 10.1172/JCI118416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Russo VC, et al. The insulin-like growth factor system and its pleiotropic functions in brain. Endocr Rev. 2005;26(7):916–43. doi: 10.1210/er.2004-0024. [DOI] [PubMed] [Google Scholar]
- 126.Torres-Aleman I. Insulin-like growth factors as mediators of functional plasticity in the adult brain. Horm Metab Res. 1999;31(2–3):114–9. doi: 10.1055/s-2007-978707. [DOI] [PubMed] [Google Scholar]
- 127.Torres Aleman I. Role of insulin-like growth factors in neuronal plasticity and neuroprotection. Adv Exp Med Biol. 2005;567:243–58. doi: 10.1007/0-387-26274-1_10. [DOI] [PubMed] [Google Scholar]
- 128.Johnsen SP, et al. Insulin-like growth factor (IGF) I, −II, and IGF binding protein-3 and risk of ischemic stroke. J Clin Endocrinol Metab. 2005;90(11):5937–41. doi: 10.1210/jc.2004-2088. [DOI] [PubMed] [Google Scholar]
- 129.Aberg D, et al. Serum IGF-I levels correlate to improvement of functional outcome after ischemic stroke. J Clin Endocrinol Metab. 2011;96(7):E1055–64. doi: 10.1210/jc.2010-2802. [DOI] [PubMed] [Google Scholar]
- 130.Denti L, et al. Insulin-like growth factor 1 as a predictor of ischemic stroke outcome in the elderly. Am J Med. 2004;117(5):312–7. doi: 10.1016/j.amjmed.2004.02.049. [DOI] [PubMed] [Google Scholar]
- 131.Schabitz WR, et al. Delayed neuroprotective effect of insulin-like growth factor-i after experimental transient focal cerebral ischemia monitored with MRI. Stroke. 2001;32(5):1226–33. doi: 10.1161/01.str.32.5.1226. [DOI] [PubMed] [Google Scholar]
- 132.Rizk NN, et al. Insulin like growth factor-1 (IGF-1) decreases ischemia-reperfusion induced apoptosis and necrosis in diabetic rats. Endocrine. 2007;31(1):66–71. doi: 10.1007/s12020-007-0012-0. [DOI] [PubMed] [Google Scholar]
- 133.Shirakura M, et al. Postischemic administration of Sendai virus vector carrying neurotrophic factor genes prevents delayed neuronal death in gerbils. Gene Ther. 2004;11(9):784–90. doi: 10.1038/sj.gt.3302224. [DOI] [PubMed] [Google Scholar]
- 134.Wang JM, et al. Reduction of ischemic brain injury by topical application of insulin-like growth factor-I after transient middle cerebral artery occlusion in rats. Brain Res. 2000;859(2):381–5. doi: 10.1016/s0006-8993(00)02008-4. [DOI] [PubMed] [Google Scholar]
- 135.Liu XF, et al. The window of opportunity for treatment of focal cerebral ischemic damage with noninvasive intranasal insulin-like growth factor-I in rats. J Stroke Cerebrovasc Dis. 2004;13(1):16–23. doi: 10.1016/j.jstrokecerebrovasdis.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 136.Liu XF, et al. Non-invasive intranasal insulin-like growth factor-I reduces infarct volume and improves neurologic function in rats following middle cerebral artery occlusion. Neurosci Lett. 2001;308(2):91–4. doi: 10.1016/s0304-3940(01)01982-6. [DOI] [PubMed] [Google Scholar]
- 137.Rizk NN, Rafols J, Dunbar JC. Cerebral ischemia induced apoptosis and necrosis in normal and diabetic rats. Brain Res. 2005;1053(1–2):1–9. doi: 10.1016/j.brainres.2005.05.036. [DOI] [PubMed] [Google Scholar]
- 138.Rizk NN, Rafols JA, Dunbar JC. Cerebral ischemia-induced apoptosis and necrosis in normal and diabetic rats: effects of insulin and C-peptide. Brain Res. 2006;1096(1):204–12. doi: 10.1016/j.brainres.2006.04.060. [DOI] [PubMed] [Google Scholar]
- 139.Kooijman R, et al. Insulin-like growth factor I: a potential neuro-protective compound for the treatment of acute ischemic stroke? Stroke. 2009;40(4):e83–8. doi: 10.1161/STROKEAHA.108.528356. [DOI] [PubMed] [Google Scholar]
- 140.Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke. 1999;30(12):2752–8. doi: 10.1161/01.str.30.12.2752. [DOI] [PubMed] [Google Scholar]
- 141.Hanson LR, et al. Intranasal deferoxamine provides increased brain exposure and significant protection in rat ischemic stroke. J Pharmacol Exp Ther. 2009;330(3):679–86. doi: 10.1124/jpet.108.149807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Akpan N, et al. Intranasal delivery of caspase-9 inhibitor reduces caspase-6-dependent axon/neuron loss and improves neurological function after stroke. J Neurosci. 2011;31(24):8894–904. doi: 10.1523/JNEUROSCI.0698-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Fletcher L, et al. Intranasal delivery of erythropoietin plus insulin-like growth factor-I for acute neuroprotection in stroke. Laboratory investigation. J Neurosurg. 2009;111(1):164–70. doi: 10.3171/2009.2.JNS081199. [DOI] [PubMed] [Google Scholar]
- 144.Yang JP, et al. The dose-effectiveness of intranasal VEGF in treatment of experimental stroke. Neurosci Lett. 2009;461(3):212–6. doi: 10.1016/j.neulet.2009.06.060. [DOI] [PubMed] [Google Scholar]
- 145.Di Lazzaro V, et al. Motor cortex plasticity predicts recovery in acute stroke. Cereb Cortex. 2010;20(7):1523–8. doi: 10.1093/cercor/bhp216. [DOI] [PubMed] [Google Scholar]
- 146.Witsch J, et al. Hypoglycemic encephalopathy: a case series and literature review on outcome determination. J Neurol. 2012;259(10):2172–81. doi: 10.1007/s00415-012-6480-z. [DOI] [PubMed] [Google Scholar]
- 147.Fujioka M, et al. Specific changes in human brain after hypoglycemic injury. Stroke. 1997;28(3):584–7. doi: 10.1161/01.str.28.3.584. [DOI] [PubMed] [Google Scholar]
- 148.Albers GW, et al. Stroke Treatment Academic Industry Roundtable (STAIR) recommendations for maximizing the use of intravenous thrombolytics and expanding treatment options with intra-arterial and neuroprotective therapies. Stroke. 2011;42(9):2645–50. doi: 10.1161/STROKEAHA.111.618850. [DOI] [PubMed] [Google Scholar]