Summary
Plasmodium species, the parasitic agents of malaria, invade erythrocytes to reproduce resulting in erythrocyte loss. However, a greater loss is caused by the elimination of uninfected erythrocytes, sometimes long after infection has been cleared. Using a mouse model, we found that Plasmodium infection induces the generation of anti-self antibodies that bind to the surface of uninfected erythrocytes from infected, but not uninfected, mice. These antibodies recognize phosphatidylserine, which is exposed on the surface of a fraction of uninfected erythrocytes during malaria. We find that phosphatidylserine-exposing erythrocytes are reticulocytes expressing high levels of CD47, a ‘do-not-eat-me’ signal, but the binding of anti-phosphatidylserine antibodies mediates their phagocytosis, contributing to anemia. In human patients with late post-malarial anemia, we found a strong inverse correlation between the levels of anti-phosphatidylserine antibodies and plasma hemoglobin, suggesting a similar role in humans. Inhibition of this pathway may be exploited for treating malarial anemia.
Introduction
Malaria-induced anemia involves both decreased erythropoiesis and increased removal of red blood cells (RBCs) (Haldar and Mohandas, 2009). For each RBC lysed directly due to Plasmodium infection, about 8 uninfected RBCs are killed in P. falciparum (Jakeman et al., 1999; Price et al., 2001) and 34 in P. vivax (Collins et al., 2003) infections. The removal of uninfected RBCs during infection may be a result of direct oxidative damage and/or transfer of oxidized lipids from infected to uninfected RBCs (Uyoga et al., 2012), which are recognized by macrophages and removed from the circulation (Arese et al., 2005). Additionally, loss of complement regulatory proteins coupled with increased levels of immune-complexes in malaria would render RBCs more susceptible to complement-mediated lysis (Stoute et al., 2003). However, a puzzling prolonged RBC loss is frequently observed in patients after successful anti-parasite treatment (Biemba et al., 1998; Price et al., 2001; Ritter et al., 1993; Woodruff et al., 1979), despite the levels of oxidative stress (Das and Nanda, 1999; Kulkarni et al., 2003) and complement regulatory proteins (Stoute et al., 2003) returning to normal levels after parasite clearance.
Malaria, like other infectious diseases that can lead to anemia (Toplak and Avcin, 2009; Vergani and Mieli-Vergani, 2013; von Landenberg et al., 2007), induces the generation of anti-self antibodies against a variety of antigens, such as RBC cytoskeletal (Berzins et al., 1983; Ternynck et al., 1991) and membrane (Arese et al., 2005; Zouali et al., 1986) proteins, enzymes (Ritter et al., 1993), sugar moieties (Ravindran et al., 1988; Satapathy et al., 1993), DNA (Adu et al., 1982; Daniel Ribeiro et al., 1984; Zouali et al., 1986) and phospholipids (Consigny et al., 2002; Facer and Agiostratidou, 1994; Jakobsen et al., 1993).
Using a mouse model of malaria we show here that anti-self antibodies generated during malaria recognize phosphatidylserine (PS), that is exposed in both infected and uninfected RBCs. Uninfected RBCs exposing PS are young RBCs expressing high levels of CD47, a molecule that inhibits phagocytosis (Sosale et al., 2015), yet in the presence of anti-PS antibodies macrophages efficiently phagocytize these cells. Transfer of affinity-purified anti-PS antibodies from Plasmodium-infected mice into other infected mice prolonged anemia after parasites had been cleared. Conversely, blocking of PS in infected mice resulted in faster recovery from anemia. These findings indicate that autoimmune anti-PS antibodies induced by malaria specifically bind to the surface of uninfected RBCs and mediate their clearance, contributing to anemia in mice. The analysis of the sera of human P. falciparum patients show that late post-malarial anemia correlates with high levels of anti-PS antibodies during the days of most pronounced anemia. Taken together, these results implicate anti-PS antibodies as mediators of post-malarial anemia.
Results
Anti-self antibodies recognize uninfected RBCs during malaria
As a model to study malarial anemia, we have used mice infected with Plasmodium yoelii 17XNL, a well-characterize non-lethal infection that induces strong anemia in mice with relatively low parasitemia (Figure 1A). In the sera of P. yoelii-infected mice we found anti-self antibodies that recognize lysates of RBCs from uninfected control mice. In contrast to antibodies against a Plasmodium antigen, merozoite surface protein 1 (MSP 1), whose levels are maintained after infection is cleared, anti-self antibodies decrease around the time of parasite clearance (Figure 1B). Anti-self anti-RBCs antibodies, first IgM (Figure S1) followed by IgG (Figure 1B), rise early in infection. Lysates of other cell types, such as lymphocytes or fibroblasts, are also recognized by antibodies that appear during infection (Figure S1 and (Ternynck et al., 1991)).
Figure 1.
Anti-self antibodies recognize uninfected RBCs during malaria. (A) Average of total (black line) and infected (gray line) RBCs per volume of blood in P. yoelii infected mice (n=5). (B) Antibodies (IgG) against RBC lysates (black line) and MSP 1 (gray line) were detected in the sera of P. yoelii infected mice (n=10) by ELISA. (C-E) RBCs from control uninfected mice (C) or from P. yoelii-infected mice (day 14 post infection) separated into uninfected (D) or infected (E) were incubated with affinity purified anti-RBC antibodies (red line), isotype matched irrelevant antibodies (blue line) or buffer alone (gray line), followed by secondary fluorescent antibodies. As control, we used RBCs from uninfected mice (filled gray plot) incubated with secondary antibodies. Insets show the average of mean fluorescence intensity for each condition from three independent mice. One representative experiment of three is shown. Average ± standard deviation is shown in each panel. *P < 0.05. See also Figure S1.
Next, we examined whether these anti-self antibodies that specifically recognize lysates of RBCs from uninfected control mice could also bind to these RBCs when they are intact. Anti-self antibodies were affinity-purified from sera of infected mice using RBC lysates as capture antigen. There was no detectable binding of purified anti-self antibodies to intact RBCs from uninfected mice (Figure 1C), but we observed that uninfected RBCs from P. yoelii-infected mice are recognized by anti-RBCs antibodies (Figure 1D). Infected RBCs unspecifically bind to antibodies (Figure 1E), as previously described (Facer, 1980; Mendis et al., 1983).
Anti-RBCs antibodies mediate phagocytosis of uninfected RBC and contribute to anemia in malaria
We next determined whether the anti-RBCs antibodies could contribute to the clearance of RBCs in malaria. We observed that anti-RBCs antibodies significantly increase macrophage phagocytosis of uninfected RBCs from P. yoelii-infected mice, but not of RBCs from control uninfected mice (Figure 2A). Infected RBCs were internalized at high proportions that were not increased by addition of anti-RBCs antibodies, which is in agreement with the unspecific binding of antibodies observed before and with the presence of numerous membrane modifications in these cells that are recognized directly by phagocytes (Giribaldi et al., 2001).
Figure 2.
Anti-self antibodies anti RBCs mediate phagocytosis of uninfected RBC and contribute to anemia in malaria. (A) RBCs from control uninfected mice (control), uninfected RBCs (uRBC) and infected RBCs (iRBC) from P. yoelii-infected mice (day 14 post infection) were labeled with DDAO and pre-incubated with buffer alone (control), isotype matched irrelevant antibodies (IgG) or affinity purified anti-RBC antibodies (anti-RBC). Phagocytosis was measured after incubation with splenocytes from uninfected control mice. DDAO fluorescence of F4/80+CD11b+ cells is shown. Lower panels show the average of mean fluorescence intensity for each condition from three independent mice. One representative experiment of two is shown. (B) P. yoelii-infected or control groups of mice (n=5) were injected with one dose of purified anti-RBC antibodies or isotype matched irrelevant immunoglobulins one day after infection. Number of RBCs per volume (cells × 106/µl; gray bars), hemoglobin concentration (g/dl; black bars) and hematocrit (percentage; white bars) was measured at day 7 post infection. All three parameters were found to be significantly lower in P. yoelii-infected mice injected with anti-RBC antibodies when compared to infected mice injected with isotype control antibodies (P < 0.01). Average of parasitemia of mice groups at day 7 post infection is shown in the right panel. One representative experiment of three is shown. (C) P. yoelii-infected groups of mice (n=5) were injected with one dose of purified anti-RBC antibodies (black) or isotype matched irrelevant antibodies (gray) on day 11 post infection. Number of RBCs per volume (cells × 106/µl) was measured at the indicated times and expressed as the ratio to the value at day 11. One representative experiment of two is shown. (D) Parasitemia of mice groups described in (c). Average ± standard deviation is shown in each panel. *P < 0.05; **P < 0.01. See also Figure S2.
Since anti-RBCs antibodies recognize intact uninfected RBCs from infected mice and facilitate their phagocytosis by macrophages, we then evaluated the role of these antibodies in malaria-induced anemia in vivo. Affinity-purified anti-RBCs or irrelevant isotype matched antibodies (Figure S2) were transferred into control or P. yoelii-infected mice one day after infection (Figure 2B). We found that anti-RBCs antibodies significantly increase anemia in infected animals at day 7 post-infection, but do not affect RBC levels in uninfected control mice (Figure 2B). This is in agreement with the observed binding profile (Figure 1C–E) and phagocytosis (Figure 2A) mediated by these antibodies, where only RBCs of infected mice, but not of uninfected, are recognized by anti-RBCs antibodies and phagocytized specifically. We also confirmed that the levels of parasitemia were similar in the infected groups, excluding the possibility of a direct effect of the parasite in the increased anemia (Figure 2B). RBC density, hematocrit and hemoglobin levels followed a similar pattern, confirming that the anemia is caused by a decrease in circulating RBCs.
We then analyzed the role of anti-RBCs antibodies in the late stage of P. yoelii infection (after day 11), where parasite levels are low, but anemia remains still at maximal levels (Figure 1A). This phase of infection provides a closer model to the anemia observed in human patients after treatment. Transfer of anti-RBCs antibodies to P. yoelii-infected mice during this late phase results in prolonged anemia compared to mice transferred with control antibodies (Figure 2C). Parasitemias are again similar in both groups of mice (Figure 2D).
Anti-RBCs antibodies recognize phosphatidylserine (PS) on uninfected RBCs from P. yoelii -infected mice and mediate their phagocytosis
Taken together, these results indicate that anti-RBCs antibodies recognize antigens that are present in lysates of RBC from control mice, but that normally are not exposed on the surface of these cells. However, anti-RBCs antibodies are able to recognize uninfected RBC in infected mice, suggesting that infection renders these self-antigens accessible on the surface of these RBCs. Since PS, a phospholipid that is flipped from the internal to the external side of apoptotic cells, is exposed in infected and uninfected RBCs during rodent malaria (Totino et al., 2010) and anti-PS antibodies are generated in human Plasmodium infections (Facer and Agiostratidou, 1994; Owens et al., 2005), we hypothesized that anti-self antibodies from infected mice recognize PS on RBCs facilitating clearance and contributing to anemia.
We observed that exposure of PS on RBCs increases with parasitemia during early infection and persists for several days after most parasites have been cleared (Figure 3A). At day 14 after infection when parasitemia is approximately 3%, there is 25% of total RBC presenting PS on their surface. We also found early rising IgM (Figure 3B) followed by IgG (Figure 3C) antibodies in the sera of infected mice that recognize PS. The sera of infected mice did not recognize p-2-glycoprotein I at different times after infection (data not shown), similarly to other infectious diseases (Sene et al., 2008). A high percentage (about 4%) of all IgG antibody-secreting spleen cells in infected mice were found to secrete anti-RBCs antibodies (Figure 3D). The percentage is similar for anti-PS antibodies, suggesting that a high proportion of anti-RBC antibodies recognize PS.
Figure 3.
Anti-self antibodies recognize PS on uninfected RBCs from P. yoelii-infected mice and mediate their phagocytosis. (A) Surface expression of PS in RBCs in P. yoelii-infected (black line) or uninfected (gray line) groups of mice (n=5). Data are expressed as percentage of RBCs positive for annexin V staining. One representative experiment of three is shown. Insets show uninfected (DAPI negative, top) and infected (DAPI positive, bottom) annexin V stained RBCs from infected mice. (B,C) Antibodies against PS, IgM (B) and IgG (C) were detected in the sera of P. yoelii infected mice (n=3 for each time point) by ELISA. (D) The percentage of antibody secreting cells (ASCs) for each antigen from the total ASCs in spleen was calculated by ELISPOT in plates coated with: control (BSA), RBC lysates, iRBC lysates or PS, in control uninfected (gray bars) and infected (black bars) mice. One representative experiment of three is shown. (E) Uninfected RBCs from groups of mice (n=6) control (gray bars) or P. yoelii-infected (day 14 post infection; black bars) were incubated independently with affinity purified anti-PS antibodies from other infected mice (anti-PS) or isotype matched irrelevant antibodies (IgG) followed by secondary fluorescent antibodies. RBCs were also pre-incubated with annexin V followed or not by anti-PS antibodies incubation. One representative experiment of two is shown. (F) Uninfected RBCs from control (gray bars) or P. yoelii-infected mice (n=3 mice; day 14 post infection; black bars) were labeled with DDAO and pre-incubated with isotype matched irrelevant antibodies (IgG), anti-RBC (anti-RBC) or anti PS (anti-PS) antibodies. RBCs were also pre-incubated with annexin V followed or not by anti-PS or anti-RBC antibodies as indicated. Phagocytosis was measured after incubation with splenocytes from control uninfected mice, as percentage of DDAO positive F4/80+CD11b+ cells. Average ± standard deviation is shown in each panel. *P < 0.05, **P < 0.01. One representative experiment of two is shown. See also Figures S3 and S4.
Anti-PS antibodies, affinity purified from sera of P. yoelii-infected mice using PS as capture antigen, recognize uninfected intact RBCs from infected, but not from uninfected control mice (Figure 3E). Pre-incubation with annexin V, which specifically binds to PS (van Engeland et al., 1998), decreases binding of anti-PS antibodies (Figure 3E). As control, it was confirmed that both affinity purified anti-PS and anti-self RBC antibodies recognize PS and RBC lysates by ELISA (Figure S3).
We next assessed whether anti-PS antibodies from infected mice facilitate RBC phagocytosis by macrophages. As expected, affinity-purified anti-PS antibodies have no effect on phagocytosis of RBCs from uninfected control mice (Figure 3F), since they do not expose PS on their surface. As observed before for total anti-self antibodies, there was no effect of anti-PS antibodies on phagocytosis of infected RBCs (Figure S4). In contrast, affinity-purified anti-PS antibodies enhance uptake of uninfected RBCs from infected mice (Figure 3F), suggesting that anti-PS antibodies can contribute to clearance of RBCs and anemia during malaria. This effect is inhibited by pre-incubation with annexin V, demonstrating its specificity. Annexin V also inhibited phagocytosis mediated by total anti-self antibodies, suggesting that the enhancing effect of these antibodies on phagocytosis is mostly due to binding to PS.
PS-exposing uninfected RBCs are reticulocytes that express high levels of CD47
We have observed that phagocytosis of uninfected RBCs from infected mice is low and is not inhibited by annexin V in the absence of anti-self antibodies (Figure 3F), indicating that direct binding of uninfected RBCs PS to the PS receptors in macrophages is not a major pathway for uptake in this assay. CD47, a marker of young RBCs (Oldenborg, 2004) and a major ‘do-not-eat me’ signal (Sosale et al., 2015), protects P. yoelii-infected RBCs from clearance (Banerjee et al., 2015). We therefore analyzed the expression levels of this marker in uninfected RBCs, which could explain the low uptake of RBCs in the absence of anti-PS antibodies.
A significant increase in CD47 expression in RBCs is observed during late infection, coinciding with an increase in reticulocytes and with PS exposure on RBCs (Figure 4A). We aimed to determine specifically whether RBCs exposing PS were also expressing high CD47 during infection. As described before (Banerjee et al., 2015), we found that infected RBCs co-express PS and CD47 in their surface (Figure S5). Notably, we also observed that virtually all uninfected RBCs exposing PS also express high levels of CD47 (Figure 4B,C). This is unusual, since newly generated RBCs that express high levels of CD47 do not normally expose PS (de Jong et al., 2001), which is usually found in aged or stressed RBCs (Buttari et al., 2015). During late malaria, the high level of PS exposure in uninfected RBCs would be counterbalanced by high CD47, keeping phagocytosis of uninfected RBCs at low levels. The presence of anti-PS antibodies shifts the balance towards high phagocytosis (Figure 3F) overcoming the protective effect of CD47, which could lead to anemia in vivo.
Figure 4.
Uninfected RBCs exposing PS during infection are reticulocytes that express high levels of the ‘do-not-eat-me signal’ CD47. Expression of different markers were analyzed in RBCs of P. yoelii infected mice (n=3). (A) Average of percentage RBCs showing high levels of: surface PS (white squares), surface CD47 (white circles), thiazole orange labeling (reticulocytes, black circles). Parasitemia (dashed line) and RBC density as percentage of day 0 (dash-dot line). (B,D) Analysis of surface expression of PS (annexin V), CD47 and thiazole orange (RNA) labeling (at day 15 post infection) in RBCs from control uninfected mice (left panels) and uninfected RBCs from infected mice (right panels). Gating strategy in Figure S5. (C,E) Quantification of RBCs double positive for surface PS and CD47 (C) or surface PS and thiazole orange (E). Average ± standard deviation is shown in each panel. One representative experiment of two is shown. See also Figure S5.
We next studied the levels of cytosolic RNA of RBCs expressing high CD47 and exposing PS, to determine whether they are actually newly formed RBCs, also called reticulocytes. We found that the uninfected RBCs exposing PS are predominantly reticulocytes (Figure 4D), indicating that young RBCs generated during infection expose PS earlier than under normal conditions. In the absence of anti-PS antibodies, these newly generated RBCs are not phagocytized, and would contribute to recover the loss of RBCs during infection, however, the presence of anti-PS antibodies would induce the clearance of these uninfected RBCs dampening the host efforts to regain normal RBCs levels and leading to prolonged anemia.
Anti-self PS antibodies correlate with late post-malarial anemia in humans
To study whether anti-PS antibodies are present in human malaria, we first analyzed the sera of P. falciparum-infected patients from an endemic area (Rourkela, India) and of hyperparasitemic non-immune travelers (France) in the day they received treatment. We observed that a high proportion of patients present significant levels of anti-PS antibodies, however no correlation was found with the levels of blood hemoglobin (Figure 5A,B). In the French cohort, twenty patients presenting anemia at the time of treatment or after, were followed up for one month. When we analyzed the levels of anti-PS antibodies in patients with late post-malarial anemia, that is, with a hemoglobin nadir at 2 to 3 weeks after treatment and a greater than 10% drop in hemoglobin level during this period, we observed a significant increase of anti-PS antibodies at this time (Figure 5C). This was not observed in patients with early post-malarial anemia (nadir at 3 to 7 days after treatment) (Figure 5D). The progression of anemia in all patients with available data at three weeks post treatment (n=11) strongly correlated (R2= 0.444) with the levels of anti-PS antibodies at this time (Figure 5E), indicating that high levels of anti-PS antibodies are associated with late post-malarial anemia.
Figure 5.
Anti-self PS antibodies correlate with late post-malarial anemia in humans. (A,B) Hemoglobin (Hb) and anti-PS antibody levels in the day of treatment (day 0) for 23 falciparum patients in Rourkela, India (A) and 35 patients in France (B). Lines show no correlation between variables (R2 > 0.03). (C,D) Hemoglobin (black) and anti-PS antibody (white) levels in patients with late (n=8) (C) or early (n=12) (D) or post-malarial anemia. Significant differences for each point with its day 0 value are indicated (*P < 0.05, **P < 0.01). (E) Anemia progression (hemoglobin on day 21/hemoglobin on day 3) and anti-PS antibody levels three weeks after treatment for falciparum patients in France (n=11). Line shows strong inverse correlation between variables (R2 = 0.44). Average ± standard deviation is shown in each panel. *P < 0.05; **P < 0.01.
Anti-self PS antibodies contribute to anemia in mouse models
To determine whether anti-PS antibodies play a role in malarial anemia, we transferred affinity-purified anti-PS antibodies or isotype control irrelevant antibodies into groups of P. yoelii-infected mice during late infection, when the levels of RBCs exposing PS are maximal. The amount of antibody transferred (400µg) is equivalent to the total amount of circulating anti-PS antibody in each mouse at day 14 after infection. We found that anti-PS antibodies delayed the recovery from anemia, keeping RBCs at very low levels for several days after parasite clearance (Figure 6A). The parasitemia of both groups of mice was similar (Figure 6B), allowing a direct comparison, but also confirming our in vitro observations that anti-PS antibodies do not play an important role in the phagocytosis of infected RBCs (Figure S5). More importantly, these results indicate that anti-PS antibodies play a role in the clearance of uninfected RBCs during malaria and contribute to anemia.
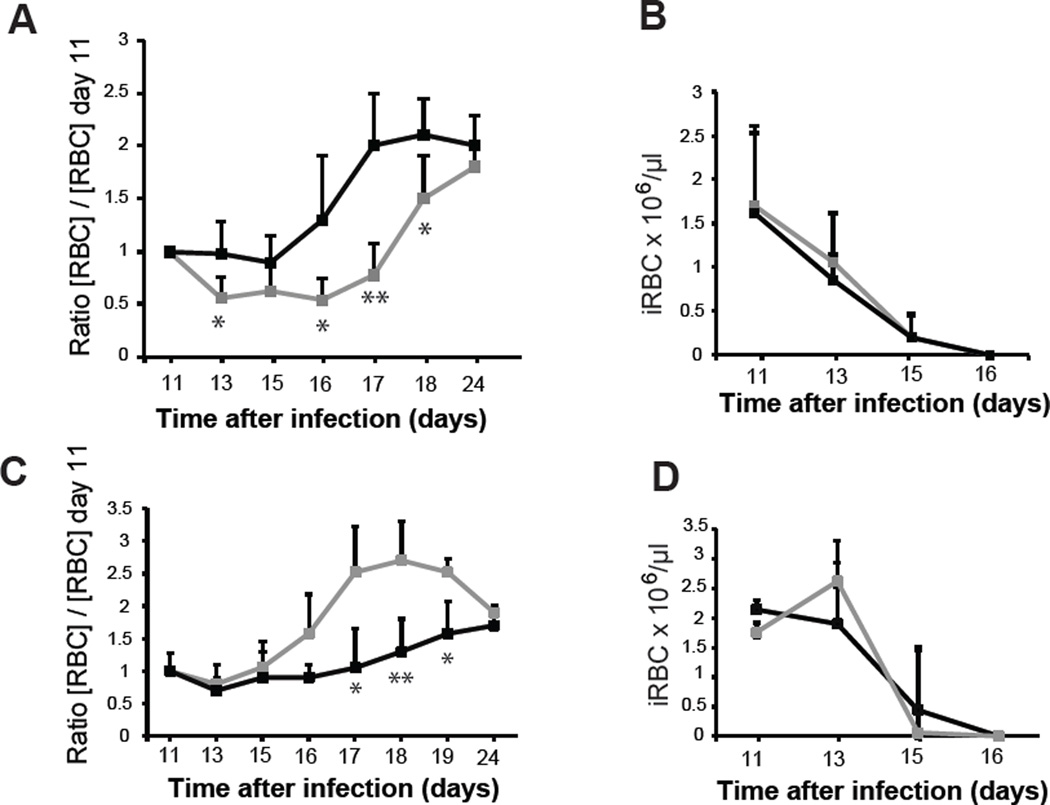
Figure 6.
Anti-self PS antibodies contribute to anemia in mouse models. (A) P. yoelii-infected groups of mice (n=5) were injected with one dose of purified anti-PS antibodies (gray line) or isotype matched irrelevant antibodies (black line) on day 11 post infection. Number of RBCs per volume was measured over time and expressed as the average of the ratio of variation for each mice (value on each day versus value at the day of injection (day 11). (B) Average of parasitemia of mice groups described in (A). (C) P. yoelii-infected groups of mice (n=5) were injected with annexin V (gray line) or vehicle alone (black line) at days 6 and 10 post infection. Number of RBCs per volume was measured in the indicated days and expressed as the average of the ratio for each mice of the value on the day of injection (day 11) versus the value on each day. (D) Average of parasitemia of mice groups described in (C). Average ± standard deviation is shown in each panel. *P < 0.05; **P < 0.01. One representative experiment of two is shown.
To further analyze the role of PS in late malarial anemia, we used annexin V as an inhibitor, since it prevents phagocytosis of uninfected RBCs by macrophages, as shown above. We observed a faster recovery from anemia in annexin V-treated mice (Figure 6C,D), suggesting that blocking of PS by annexin V binding would inhibit anti-PS antibody recognition of RBCs and anemia during late infection.
Discussion
Although a role for anti-self antibodies in malarial anemia has been repeatedly proposed (Ghosh, 2007; Helegbe et al., 2009; Ritter et al., 1993; Zuckerman, 1964), our results provide mechanistic evidence that anti-self antibodies mediate anemia in malaria. In particular, we found that anti-PS antibodies play a central role in the recognition of uninfected RBCs, targeting them for clearance.
Anti-PS antibodies are not found in healthy sera and are considered pathogenic, appearing during autoimmune diseases or infections (Elkon and Silverman, 2012). Diverse pathogens of viral, bacterial or parasitic origin such as Hepatitis C virus, HIV, Mycobacterium leprae or Leishmania induce anti-phospholipid antibodies, including anti-PS, in a high proportion of patients (Sene et al., 2008; Skouri et al., 2008). These antibodies may contribute to the clearance of intracellular pathogens by targeting infected cells, suggesting that anti-PS antibodies could act as a defense against intracellular pathogens.
We have observed that anti-PS antibodies generated during Plasmodium infection bind to both infected and uninfected RBC exposing PS. In the case of infected RBC, we did not observe increased phagocytosis after incubation with anti-PS or decreased parasitemia after transfer of anti-PS antibodies to infected mice. This is probably explained because the highly efficient recognition of infected RBCs damaged membranes by phagocytes cannot be further increased and suggests that the anti-parasitic role of these antibodies is not important in malaria.
Conversely, binding of anti-PS antibodies to uninfected RBCs resulted in increased phagocytosis in vitro and clearance in vivo. The accelerated clearance by phagocytes of uninfected RBCs, rather than hemolysis, appears to be the main contributor to RBC loss in malaria rodent models (Evans et al., 2006) and human disease (Lamikanra et al., 2007). Our results are in agreement with these observations and propose anti-PS antibodies as mediators for this mechanism.
In our mice experiments, we found that transfer of additional anti-PS antibodies to infected mice on day 11 results in prolonged anemia, presumably by facilitating the clearance of PS-exposing RBCs and delaying the recovery. This effect is reversed when mice receive annexin V, which would bind to PS and inhibit the binding of anti-PS antibodies, presumably resulting in higher levels of circulating RBCs.
PS represents an essential ‘eat-me’ signal for clearance of apoptotic cells, but exposure of PS alone is not sufficient to induce clearance by phagocytes (Ravichandran, 2011). We found that PS-exposing uninfected RBCs during infection also express high levels of the ‘don’t eat-me’ signal CD47, which prevents phagocytosis. It is not known whether the PS-exposing uninfected RBCs are fully functional, but exposure of PS on their membrane -induced by infection- could be improperly marking them for clearance. Three different lines of evidence support the idea that these cells would persist longer in the circulation but the appearance of anti-PS antibodies accelerates their clearance: (1) Phagocytosis of uninfected RBCs by macrophages in vitro is low, but it is greatly increased in the presence of anti-PS antibodies (Figure 3F); (2) High levels of CD47 in uninfected RBCs that expose PS (Figure 4B,C) indicates that even if the RBCs are expressing PS, the high levels of CD47 would protect them from phagocytosis in the absence of anti-PS antibodies; (3) Transfer of anti-PS antibodies to infected mice (Figure 6A) decreases the number of uninfected RBCs in blood.
Anti-PS antibodies would overcome the protective CD47 signal and would contribute to the removal of uninfected PS-exposing RBCs, generating anemia. Surprisingly, a high number of uninfected RBCs exposing PS are reticulocytes, indicating that malaria is inducing the exposure of PS in new RBCs generated during infection. Anti-PS antibodies would mediate the clearance of these RBCs during recovery from Plasmodium infection. In human malaria, it is characteristic that different patients develop post-malarial anemia at different times after treatment, after parasites have been cleared (Biemba et al., 1998; Price et al., 2001; Ritter et al., 1993; Woodruff et al., 1979), with nadir values of hemoglobin observed from 3 to 21 days post-treatment (Jaureguiberry et al., 2014; Rolling et al., 2014; Rolling et al., 2013). Interestingly, an increase in clearance of IgG-coated RBCs is observed in malaria patients after the parasite has been eliminated from the circulation (Ho et al., 1990).
Early anemia (at day 3 after treatment) is most likely a direct consequence of infection and caused, as described before, by dyserythropoiesis, oxidative damage and/or loss of complement regulatory proteins (Arese et al., 2005; Stoute et al., 2003; Uyoga et al., 2012). However, late anemia (at days 14 to 21) occurs at a time when oxidative stress, and complement regulatory proteins have already achieved normal levels in patients (Das and Nanda, 1999; Kulkarni et al., 2003; Stoute et al., 2003). In our analysis of human sera we observed that patients with late post-malarial anemia presented the highest anti-PS antibody levels two to three weeks after elimination of the parasite, coinciding with the time of most pronounced anemia. It is possible that the late increase in anti-PS antibodies may be triggering the clearance of uninfected RBCs that were still exposing PS on their surface as a consequence of the previous infection. Indeed, the finding that mouse reticulocytes generated during infection expose high levels of PS in their membranes suggests that these young, CD47 high, uninfected RBCs could persist in the blood, but the rise of anti-PS antibodies after two to three weeks would induce their clearance. Additionally, RBC clearance through anti-PS antibodies may be intensified in artesunate-treated patients, since the oxidative properties of this drug increase exposure of PS in RBC membranes (Alzoubi et al., 2014).
This study provides insights into the pathogenesis of malarial anemia and may lead to new therapeutic approaches through the inhibition of anti-PS mediated clearance of uninfected RBCs.
Experimental Procedures
Mice, parasites, and infections
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Institutional Animal Care and Use Committee of New York University School of Medicine, which is fully accredited by the Association For Assessment and Accreditation Of Laboratory Animal Care International (AAALAC). Female Swiss Webster mice 6 to 8 weeks old were purchased from the National Institutes of Health (Bethesda, MD) and The Jackson Laboratory (Bar Harbor, ME). To start blood-stage infections, mice were injected i.p. with 106 infected RBCs with nonlethal strain P. yoelii 17XNL per mouse resuspended in PBS, final volume 250 µl. To evaluate parasitemia, thin blood smears were made by bleeding mice from a nick in the tail. Smears were stained with KaryoMAX Giemsa (Life Technologies, Norwalk, CT), and a minimum of 500 RBCs per smear were counted.
P. yoelii 17XNL-infected RBCs were harvested by cardiac puncture of infected, anesthetized mice before the peak of parasitemia. RBCs were washed twice with PBS and separated from white blood cells by centrifugation at 2000 × g for 3 min. RBCs were then spun on an Accudenz (Accurate Chemical and Scientific, Westbury, NY) gradient to isolate schizonts and late trophozoite stage–infected RBCs, washed and resuspended in PBS.
To separate uninfected RBC from infected RBC from P. yoelii-mice, we first used LD MACS magnetic separation columns (Miltenyi Biotec, Auburn, CA) to remove infected RBC in the late phase of infection (schizonts). The RBCs not retained by the column (uninfected and early stage infected RBCs) were cultured at 37 °C, 5% CO2, 5% O2, 90% N2 to allow early stage iRBC progress into the schizont stage. In parallel, infected RBC retained in the column were eluted and cultured in the same conditions. After 20 h, cultures of uninfected RBCs were passed again through MACS magnetic separation columns to remove schizonts from remaining uninfected RBC. The purity of the uninfected RBC was 100%. As control, RBCs from uninfected control mice were cultured and passed through the columns in parallel and in the same conditions.
Flow cytometry and immunofluorescence
All flow cytometry was performed on a FACSCalibur or LSRII (Becton Dickinson, Franklin Lakes, NJ) and analyzed with FlowJo (Tree Star, Ashland, OR). All Abs for FACS were purchased from BioLegend, San Diego, CA.
For RBC surface expression analysis, RBCs from different days post infection were incubated with anti-RBC, anti-PS or control antibodies (all at 1 µg/µl; 4°C; 30 min). Control antibodies were isotype matched with anti-RBC and anti-PS purified antibodies. The relative concentration of each isotype control antibody was determined following the % of total reactivity for anti-RBC and anti-PS antibodies, as shown in Figure S2. Low endotoxin azide-free purified mouse isotype control IgG2b, IgG3, IgG2a and IgG1 (clones MPC-11, MG3–35, MG2a-53 and MG1–45, respectively) were used. As secondary antibody, PE-anti-IgG (Poly4053) was used.
To analyze the expression of PS and CD47 on the surface of RBC we used FITC-annexin V or PE-anti-CD47 (Biolegend, San Diego, CA) diluted in annexin V binding buffer (BD Biosciences, San Jose, CA) or standard FACs buffer, respectively. DAPI and Thiazole Orange (Sigma, St. Louis, MO) were added for DNA and RNA staining, respectively.
To analyze macrophages in the phagocytosis assay, splenocytes were labeled with FITC-anti-F4/80 (BM8) and PerCP-anti-CD11b (M1/70).
Phagocytosis
Mouse splenocyte suspensions were obtained by mechanical disruption of spleen through a cell strainer followed by osmotic lysis of RBCs with ammonium chloride/potassium hydrogen carbonate buffer. RBCs were labeled with DDAO (Invitrogen, Grand Island, NY) for 1 h at 37 °C followed by incubation with affinity purified anti-RBC, anti-PS or isotype matched irrelevant antibodies (as detailed above in flow cytometry section). All antibodies were added at 1 µg/µl for 30 min at 4 °C. RBCs were preincubated or not with annexin V (0.5 µM) (Sigma, St. Louis, MO) for 30 min at 4 °C. Splenocytes were then incubated for 30 min at 37 °C with the RBCs at 1:1 ratio splenocytes:RBCs. This ratio ensures non-saturating conditions for phagocytosis by splenocytes, that in most cases uptake only one or two RBCs, as can be monitored by the high fluorescence peaks. Cells were transferred to ice before staining for macrophages and FACs analysis.
Purification and detection of antibodies
The antibodies anti-RBC and anti-PS were purified from sera of mice infected with P. yoelii at day 14 post infection. Anti-RBC antibodies were purified using lysates of RBCs from control uninfected mice bound to High-Affinity Iodoacetyl Resin columns (Genscript, Piscataway, NJ). Anti-PS antibodies were purified with NeutroAvidin Agarose Resin column (Thermo scientific, Logan, UT), pre-incubated with biotin-PS 300 µg/ml (Echelon Biosciences, Salt Lake City, UT) overnight at 4 °C. Both columns were equilibrated with PBS buffer (pH 7.2). Column-bound antibody was eluted with 0.1 M glycine-HCl buffer (pH 2.5), neutralized with buffer 1M Tris HCl, pH 8.5, and concentrated with Vivaspin 20 (Sartorius stedim Biotech, Bohemia, NY).
For detection of anti-RBC and anti-merozoite surface protein 1 antibodies, ELISA plates were coated overnight at 4 °C with control uninfected RBCs lysate (20 µg/ml RBCs lysed with 10mM Tris HCl; pH 7.5 for 30 min at 4 °C) or recombinant P. yoelii merozoite surface protein 1 (obtained through MR4 [MRA-48] deposited by D.C. Kaslow) (5 µg/ml). An initial test was performed with sera from three different mice at different days after infection at different concentrations with serial dilutions. The antibody response (O.D.) was plotted against the dilutions to find the concentration that provided the maximal response but was still in the linear part of the curve to avoid saturation of the assay. Mice sera at 1:100 dilution in PBS was added to the wells and incubated for 2 h at 37 °C. For detection of antibodies recognizing splenocytes or NIH-3T3 lysates, plates were coated with splenocytes or 3T3 lysate (5 µg/ml) overnight at 4 °C. After washing, ELISAs were developed by incubation with anti-mouse IgG1, IgG2a, IgG2b, IgG3 subtypes or with total anti-mouse IgG or IgM, as indicated, labeled with HRP (GE Healthcare, Port Washington, NY) and developed with SigmaFast OPD (Sigma, St. Louis, MO). For detection of anti-PS antibodies a modification of the ELISA anti-phosphatidylserine IgG/IgM kit from Orgentec (Mainz, Germany) was used, replacing the secondary human antibody for a HRP-conjugated goat anti-mouse IgG (GE Healthcare, Port Washington, NY). Plates were analyzed with a Perkin Elmer Victor X3 2030 multilabel reader at 492 nm.
ELISPOT assay
Mouse splenocyte suspensions from uninfected control and P. yoelii– infected mice (day 10 post infection) were obtained as described for the phagocytosis assay. White blood cells were additionally purified in 45% Percoll at 25°C for 20 min at 1,500g to remove remaining RBCs. 5 ×104 cells were added per well and incubated in RPMI 1640 supplemented with 10% FBS in 96-well Costar 3590 ELISA plates (Corning Life Sciences, Tewksbury, MA) pre-coated with either capture anti-IgG (15µg/ml), infected RBC lysate, uninfected RBC lysate, PS (100µg/ml in ethanol) (Sigma, St. Louis, MO) or PBS 10% BSA as control, for 20h at 37°C with 5% CO2. Following extensive washings, anti-mouse IgG biotinylated detection antibody (Sigma, St. Louis, MO) was added at 1 µg/ml diluted in PBS 0.5% FBS for 2h at RT. Streptavidin-Horseradish Peroxidase (Mabtech AB, Nacka Strand, Sweden) was added diluted in PBS 0.5% FBS for 1h at RT. Plates were developed with TMB substrate (Mabtech AB, Nacka Strand, Sweden) for 15–20 min and then washed extensively with H2O. Spots were quantified by microscopy.
In vivo model of anemia
Mice were infected or not with P. yoelii one (Figure 2B) or 11 (Figure 2C,D; Figure 5) days before i.v. injection of 400 µg/mouse of affinity purified anti-RBC, anti-PS antibodies or isotype matched irrelevant antibodies. A mixture of purified commercial irrelevant IgG1 (clone MOPC 21), IgG2a (clone eBR2a), IgG2b (clone MPC-11) and IgG3 (clone MG3–35) (Biolegend) was injected into control mice in each experiment with the same proportions of each Ig subtype as determined for anti-RBC or anti-PS anti-sera, shown in Figure S2). After 7 days of infection (Figure 2B) or at the indicated times, RBC count (expressed as RBCs × 106/µl) was determined with AccuCheck counting beads (Invitrogen, Grand Island, NY) analyzed by FACs. The concentration of hemoglobin (g/dl) and hematocrit (%) was determined by NYU Hospitals Center clinical laboratories.
To determine the specificity of anti-PS antibodies effect on anemia, mice were infected with P. yoelii followed by i.v. injection of annexin V (200 µg/mouse, Sigma, St. Louis, MO) or vehicle (PBS) on days 6 and 10 post infection.
Statistical analysis
Statistical Analyses were performed with IBM SPSS v.20 software.
For mice experiments: Error bars represent the standard deviation from at least three replicates unless otherwise stated. We quantified P values using t-student or ANOVA when applicable. P < 0.05 was considered statistically significant.
For human samples: Linear regression was carried out to check the correlation between the dependent variable Hb day 21/Hb day 3 and anti-PS antibodies day 21 (PS21), showing a R=0.666 (R2=0.444) and p=0.025 and resulting in the next equation:
Hb day 3 was chosen for normalization because both groups of patients have similar levels of anemia on this day and it is only at later time points (day 21) when the two groups diverge in their levels of anemia.
Paired samples t-tests were carried out to compare significant variation in the mean of anti-PS antibodies and hemoglobin values after admission (time = 0).
Patients
Adult patients meeting at least one criterion for severe P. falciparum malaria were admitted to Ispat General Hospital in Rourkela, India, from January 2012 to March 2014 and treated intravenously with 2.4 mg/kg of artesunate (vial of 60 mg powder and solvent provided by manufacturers approved by the Government of India) at 0, 12, 24, and 48 h. Anemia was defined as blood hemoglobin below 12g/dL and 13g/dL for females and males, respectively. Blood samples from artesunate-treated patients were collected upon admission and analyzed for hemoglobin. Transfused patients were excluded from the analysis.
Adult patients meeting at least one criterion for severe P. falciparum malaria (2000) were admitted to public hospitals in France from May 2011 to December 2013 or to Ispat General Hospital in Rourkela, India, from January 2012 to March 2014. All French patients with imported severe malaria provided consent according to a procedure common to all National Reference Centers (CNR; available at: http://www.invs.sante.fr/Espace-professionnels/Centres-nationaux-de-reference/Textesreglementaires) in France on the behalf of the National Agency of Medicine and Health Product Safety. The Ile de France II Institutional Review Board has approved this approach as a non-research process (Article L1121-1of the French Code for Public Health) embedded in the surveillance missions of the CNR, officially empowered to collect information and biological samples (Article L1413-5 of the French Law No.2004–8069). Forms and data from all patients were collected as part of an observational program implemented by the CNR for Malaria.
Patients were treated intravenously with 2.4 mg/kg of artesunate at 0, 12, 24, and 48 h. Anemia was defined as blood hemoglobin below 12g/dL and 13g/dL for females and males, respectively. Intravenous artesunate (Guilin Pharmaceuticals, Shanghai, China) has been available in France since May 2011 through a named-patient program for imported severe malaria cases. All French patients were treated intravenously with 2.4 mg/kg of AS at 0, 12, 24, and 48 hours. Blood samples were obtained at the time of admission and on days 3, 7, 14, 21, and 28 for most patients, as recommended by the French High Committee for Public Health.
The study in India has approval for the use of human subjects from the Institutional Review Boards from the New York University School of Medicine and from the ISPAT General Hospital, Rourkela, India. All studies involving human subjects were conducted in accordance with the guidelines of the World Medical Association’s Declaration of Helsinki. All individuals and/or their legal guardians gave written informed consent. The clinical data and samples were de-identified using a unique study number, in accordance with the Health Insurance Portability and Accountability Act.
Blood samples from artesunate-treated patients were collected upon admission (in India) or were retrieved following collection in the setting of medical care at day 0, day 2 (+/−1), day 7 (+/−2), day 14 (+/−3), day 21 (+/−3) and day 28 (+/−3) (in France) and analyzed for hemoglobin. Transfused patients were excluded from the analysis. The plasma were then processed for anti-PS antibodies detection by ELISA (anti-phosphatidylserine IgG/IgM kit from Orgentec (Mainz, Germany).
Supplementary Material
Acknowledgments
This work was supported in part by a postdoctoral fellowship from the Ministerio de Ciencia e Innovacion, Spain, to CFA, by the National Institutes of Health (NIH) institutional training grant 5T32AI100853-03 to JRC, by a Burroughs Wellcome Fund Award for Investigators in the Pathogenesis of Infectious Disease to AR, by a Dana Foundation award for Neuroimmunology to AR and by the NIH National Institute of Allergy and Infectious Diseases grant U19AI089676-01S1 to SW. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Anti-MSP-1 antibody was obtained through BEI Resources, NIAID, NIH. We would like to thank the director of Ispat General Hospital in Rourkela, as well as the director of the Institute of Life Sciences in Bubhaneshwar for allowing us to use the institute’s facilities at Anusandhan Laboratory in Rourkela. We are thankful to Andre Ballesteros-Tato for helpful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
CFA designed and performed experiments, interpreted data and wrote manuscript, RR, PAN, JRC, SW, AM, ASM and SG performed experiments, JRC, JGD, PAN, PB, SW, AG and CFA interpreted data, AR interpreted data, designed experiments and wrote manuscript.
References
- Severe falciparum malaria. World Health Organization, Communicable Diseases Cluster. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2000;94(Suppl 1):S1–S90. [PubMed] [Google Scholar]
- Adu D, Williams DG, Quakyi IA, Voller A, Anim-Addo Y, Bruce-Tagoe AA, Johnson GD, Holborow EJ. Anti-ssDNA and antinuclear antibodies in human malaria. Clinical and experimental immunology. 1982;49:310–316. [PMC free article] [PubMed] [Google Scholar]
- Alzoubi K, Calabro S, Bissinger R, Abed M, Faggio C, Lang F. Stimulation of suicidal erythrocyte death by artesunate. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology. 2014;34:2232–2244. doi: 10.1159/000369666. [DOI] [PubMed] [Google Scholar]
- Arese P, Turrini F, Schwarzer E. Band 3/complement-mediated recognition and removal of normally senescent and pathological human erythrocytes. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology. 2005;16:133–146. doi: 10.1159/000089839. [DOI] [PubMed] [Google Scholar]
- Banerjee R, Khandelwal S, Kozakai Y, Sahu B, Kumar S. CD47 regulates the phagocytic clearance and replication of the Plasmodium yoelii malaria parasite. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:3062–3067. doi: 10.1073/pnas.1418144112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berzins K, Wahlgren M, Perlmann P. Studies on the specificity of anti-erythrocyte antibodies in the serum of patients with malaria. Clinical and experimental immunology. 1983;54:313–318. [PMC free article] [PubMed] [Google Scholar]
- Biemba G, Gordeuk VR, Thuma PE, Mabeza GF, Weiss G. Prolonged macrophage activation and persistent anaemia in children with complicated malaria. Tropical medicine & international health : TM & IH. 1998;3:60–65. doi: 10.1046/j.1365-3156.1998.00168.x. [DOI] [PubMed] [Google Scholar]
- Buttari B, Profumo E, Rigano R. Crosstalk between red blood cells and the immune system and its impact on atherosclerosis. BioMed research international. 2015;2015:616834. doi: 10.1155/2015/616834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins WE, Jeffery GM, Roberts JM. A retrospective examination of anemia during infection of humans with Plasmodium vivax. Am J Trop Med Hyg. 2003;68:410–412. [PubMed] [Google Scholar]
- Consigny PH, Cauquelin B, Agnamey P, Comby E, Brasseur P, Ballet JJ, Roussilhon C. High prevalence of co-factor independent anticardiolipin antibodies in malaria exposed individuals. Clinical and experimental immunology. 2002;127:158–164. doi: 10.1046/j.1365-2249.2002.01722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel Ribeiro CT, de Roquefeuil S, Druilhe P, Monjour L, Homberg JC, Gentilini M. Abnormal anti-single stranded (ss) DNA activity in sera from Plasmodium falciparum infected individuals. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1984;78:742–746. doi: 10.1016/0035-9203(84)90005-1. [DOI] [PubMed] [Google Scholar]
- Das BS, Nanda NK. Evidence for erythrocyte lipid peroxidation in acute falciparum malaria. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1999;93:58–62. doi: 10.1016/s0035-9203(99)90180-3. [DOI] [PubMed] [Google Scholar]
- de Jong K, Emerson RK, Butler J, Bastacky J, Mohandas N, Kuypers FA. Short survival of phosphatidylserine-exposing red blood cells in murine sickle cell anemia. Blood. 2001;98:1577–1584. doi: 10.1182/blood.v98.5.1577. [DOI] [PubMed] [Google Scholar]
- Elkon KB, Silverman GJ. Naturally occurring autoantibodies to apoptotic cells. Advances in experimental medicine and biology. 2012;750:14–26. doi: 10.1007/978-1-4614-3461-0_2. [DOI] [PubMed] [Google Scholar]
- Evans KJ, Hansen DS, van Rooijen N, Buckingham LA, Schofield L. Severe malarial anemia of low parasite burden in rodent models results from accelerated clearance of uninfected erythrocytes. Blood. 2006;107:1192–1199. doi: 10.1182/blood-2005-08-3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facer CA. Direct Coombs antiglobulin reactions in Gambian children with Plasmodium falciparum malaria. II. Specificity of erythrocyte-bound IgG. Clinical and experimental immunology. 1980;39:279–288. [PMC free article] [PubMed] [Google Scholar]
- Facer CA, Agiostratidou G. High levels of anti-phospholipid antibodies in uncomplicated and severe Plasmodium falciparum and in P. vivax malaria. Clinical and experimental immunology. 1994;95:304–309. doi: 10.1111/j.1365-2249.1994.tb06528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh K. Pathogenesis of anemia in malaria: a concise review. Parasitol Res. 2007;101:1463–1469. doi: 10.1007/s00436-007-0742-1. [DOI] [PubMed] [Google Scholar]
- Giribaldi G, Ulliers D, Mannu F, Arese P, Turrini F. Growth of Plasmodium falciparum induces stage-dependent haemichrome formation, oxidative aggregation of band 3, membrane deposition of complement and antibodies, and phagocytosis of parasitized erythrocytes. British journal of haematology. 2001;113:492–499. doi: 10.1046/j.1365-2141.2001.02707.x. [DOI] [PubMed] [Google Scholar]
- Haldar K, Mohandas N. Malaria, erythrocytic infection, and anemia. Hematology Am Soc Hematol Educ Program. 2009:87–93. doi: 10.1182/asheducation-2009.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helegbe GK, Huy NT, Yanagi T, Shuaibu MN, Yamazaki A, Kikuchi M, Yasunami M, Hirayama K. Rate of red blood cell destruction varies in different strains of mice infected with Plasmodium berghei-ANKA after chronic exposure. Malaria journal. 2009;8:91. doi: 10.1186/1475-2875-8-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho M, White NJ, Looareesuwan S, Wattanagoon Y, Lee SH, Walport MJ, Bunnag D, Harinasuta T. Splenic Fc receptor function in host defense and anemia in acute Plasmodium falciparum malaria. The Journal of infectious diseases. 1990;161:555–561. doi: 10.1093/infdis/161.3.555. [DOI] [PubMed] [Google Scholar]
- Jakeman GN, Saul A, Hogarth WL, Collins WE. Anaemia of acute malaria infections in non-immune patients primarily results from destruction of uninfected erythrocytes. Parasitology. 1999;119(Pt 2):127–133. doi: 10.1017/s0031182099004564. [DOI] [PubMed] [Google Scholar]
- Jakobsen PH, Morris-Jones SD, Hviid L, Theander TG, Hoier-Madsen M, Bayoumi RA, Greenwood BM. Anti-phospholipid antibodies in patients with Plasmodium falciparum malaria. Immunology. 1993;79:653–657. [PMC free article] [PubMed] [Google Scholar]
- Jaureguiberry S, Ndour PA, Roussel C, Ader F, Safeukui I, Nguyen M, Biligui S, Ciceron L, Mouri O, Kendjo E, et al. Postartesunate delayed hemolysis is a predictable event related to the lifesaving effect of artemisinins. Blood. 2014;124:167–175. doi: 10.1182/blood-2014-02-555953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni AG, Suryakar AN, Sardeshmukh AS, Rathi DB. Studies on biochemical changes with special reference to oxidant and antioxidants in malaria patients. Indian journal of clinical biochemistry : IJCB. 2003;18:136–149. doi: 10.1007/BF02867380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamikanra AA, Brown D, Potocnik A, Casals-Pascual C, Langhorne J, Roberts DJ. Malarial anemia: of mice and men. Blood. 2007;110:18–28. doi: 10.1182/blood-2006-09-018069. [DOI] [PubMed] [Google Scholar]
- Mendis KN, David PH, Hommel M, Carter R, Miller LH. Immunity to malarial antigens on the surface of Plasmodium falciparum-infected erythrocytes. Am J Trop Med Hyg. 1983;32:926–930. doi: 10.4269/ajtmh.1983.32.926. [DOI] [PubMed] [Google Scholar]
- Oldenborg PA. Role of CD47 in erythroid cells and in autoimmunity. Leukemia & lymphoma. 2004;45:1319–1327. doi: 10.1080/1042819042000201989. [DOI] [PubMed] [Google Scholar]
- Owens S, Chamley LW, Ordi J, Brabin BJ, Johnson PM. The association of anti-phospholipid antibodies with parity in placental malaria. Clinical and experimental immunology. 2005;142:512–518. doi: 10.1111/j.1365-2249.2005.02936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RN, Simpson JA, Nosten F, Luxemburger C, Hkirjaroen L, ter Kuile F, Chongsuphajaisiddhi T, White NJ. Factors contributing to anemia after uncomplicated falciparum malaria. Am J Trop Med Hyg. 2001;65:614–622. doi: 10.4269/ajtmh.2001.65.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravichandran KS. Beginnings of a good apoptotic meal: the find-me and eat-me signaling pathways. Immunity. 2011;35:445–455. doi: 10.1016/j.immuni.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravindran B, Satapathy AK, Das MK. Naturally-occurring anti-alpha-galactosyl antibodies in human Plasmodium falciparum infections--a possible role for autoantibodies in malaria. Immunol Lett. 1988;19:137–141. doi: 10.1016/0165-2478(88)90133-2. [DOI] [PubMed] [Google Scholar]
- Ritter K, Kuhlencord A, Thomssen R, Bommer W. Prolonged haemolytic anaemia in malaria and autoantibodies against triosephosphate isomerase. Lancet. 1993;342:1333–1334. doi: 10.1016/0140-6736(93)92248-r. [DOI] [PubMed] [Google Scholar]
- Rolling T, Agbenyega T, Issifou S, Adegnika AA, Sylverken J, Spahlinger D, Ansong D, Lohr SJ, Burchard GD, May J, et al. Delayed hemolysis after treatment with parenteral artesunate in African children with severe malaria--a double-center prospective study. The Journal of infectious diseases. 2014;209:1921–1928. doi: 10.1093/infdis/jit841. [DOI] [PubMed] [Google Scholar]
- Rolling T, Wichmann D, Schmiedel S, Burchard GD, Kluge S, Cramer JP. Artesunate versus quinine in the treatment of severe imported malaria: comparative analysis of adverse events focussing on delayed haemolysis. Malaria journal. 2013;12:241. doi: 10.1186/1475-2875-12-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satapathy AK, Das MK, Ravindran B. Murine malaria: anti-erythrocytic antibodies recognize N-acetyl neuraminic acid residues. Immunology. 1993;80:546–552. [PMC free article] [PubMed] [Google Scholar]
- Sene D, Piette JC, Cacoub P. Antiphospholipid antibodies, antiphospholipid syndrome and infections. Autoimmunity reviews. 2008;7:272–277. doi: 10.1016/j.autrev.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Skouri H, Gandouz R, Kraiem I, Harrabi I, Ben Said M Antibodies to anionic phospholipids and cofactors in kala-azar. Comparative study with malaria, toxoplasmosis and “autoimmune diseases”. Clinical and experimental rheumatology. 2008;26:894–902. [PubMed] [Google Scholar]
- Sosale NG, Spinler KR, Alvey C, Discher DE. Macrophage engulfment of a cell or nanoparticle is regulated by unavoidable opsonization, a species-specific ‘Marker of Self’ CD47, and target physical properties. Current opinion in immunology. 2015;35:107–112. doi: 10.1016/j.coi.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoute JA, Odindo AO, Owuor BO, Mibei EK, Opollo MO, Waitumbi JN. Loss of red blood cell-complement regulatory proteins and increased levels of circulating immune complexes are associated with severe malarial anemia. The Journal of infectious diseases. 2003;187:522–525. doi: 10.1086/367712. [DOI] [PubMed] [Google Scholar]
- Ternynck T, Falanga PB, Unterkirscher C, Gregoire J, da Silva LP, Avrameas S. Induction of high levels of IgG autoantibodies in mice infected with Plasmodium chabaudi. Int Immunol. 1991;3:29–37. doi: 10.1093/intimm/3.1.29. [DOI] [PubMed] [Google Scholar]
- Toplak N, Avcin T. Influenza and autoimmunity. Ann N Y Acad Sci. 2009;1173:619–626. doi: 10.1111/j.1749-6632.2009.04759.x. [DOI] [PubMed] [Google Scholar]
- Totino PR, Magalhaes AD, Silva LA, Banic DM, Daniel-Ribeiro CT, Ferreira-da-Cruz Mde F. Apoptosis of non-parasitized red blood cells in malaria: a putative mechanism involved in the pathogenesis of anaemia. Malaria journal. 2010;9:350. doi: 10.1186/1475-2875-9-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyoga S, Skorokhod OA, Opiyo M, Orori EN, Williams TN, Arese P, Schwarzer E. Transfer of 4-hydroxynonenal from parasitized to non-parasitized erythrocytes in rosettes. Proposed role in severe malaria anemia. British journal of haematology. 2012;157:116–124. doi: 10.1111/j.1365-2141.2011.09015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Engeland M, Nieland LJ, Ramaekers FC, Schutte B, Reutelingsperger CP. Annexin V-affinity assay: a review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry. 1998;31:1–9. doi: 10.1002/(sici)1097-0320(19980101)31:1<1::aid-cyto1>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Vergani D, Mieli-Vergani G. Autoimmune manifestations in viral hepatitis. Semin Immunopathol. 2013;35:73–85. doi: 10.1007/s00281-012-0328-6. [DOI] [PubMed] [Google Scholar]
- von Landenberg P, Lehmann HW, Modrow S. Human parvovirus B19 infection and antiphospholipid antibodies. Autoimmunity reviews. 2007;6:278–285. doi: 10.1016/j.autrev.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Woodruff AW, Ansdell VE, Pettitt LE. Cause of anaemia in malaria. Lancet. 1979;1:1055–1057. doi: 10.1016/s0140-6736(79)92952-0. [DOI] [PubMed] [Google Scholar]
- Zouali M, Druilhe P, Eyquem A. IgG-subclass expression of anti-DNA and anti-ribonucleoprotein autoantibodies in human malaria. Clinical and experimental immunology. 1986;66:273–278. [PMC free article] [PubMed] [Google Scholar]
- Zuckerman A. Autoimmunization and Other Types of Indirect Damage to Host Cells as Factors in Certain Protozoan Diseases. Exp Parasitol. 1964;15:138–183. doi: 10.1016/0014-4894(64)90014-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.