Abstract
Purpose of review
Lungs are extremely susceptible to injury, and despite advances in surgical management and immunosuppression, outcomes for lung transplantation are the worst of any solid organ transplant. The success of lung transplantation is limited by high rates of primary graft dysfunction (PGD) due to ischemia-reperfusion (IR) injury characterized by robust inflammation, alveolar damage and vascular permeability. This review will summarize major mechanisms of lung IR injury with a focus on the most recent findings in this area.
Recent findings
Over the past 18 months numerous studies have described strategies to limit lung IR injury in experimental settings, which often reveal mechanistic insight. Many of these strategies involved the use of various anti-oxidants, anti-inflammatory agents, mesenchymal stem cells, and ventilation with gaseous molecules. Further advancements have been achieved in understanding mechanisms of innate immune cell activation, neutrophil infiltration, endothelial barrier dysfunction, and oxidative stress responses.
Summary
Methods for prevention of PGD after lung transplant are urgently needed, and understanding mechanisms of IR injury is critical for the development of novel and effective therapeutic approaches. In doing so, both acute and chronic outcomes of lung transplant recipients will be significantly improved.
Keywords: lung transplantation, ischemia-reperfusion injury, inflammation, primary graft dysfunction, innate immunity
INTRODUCTION
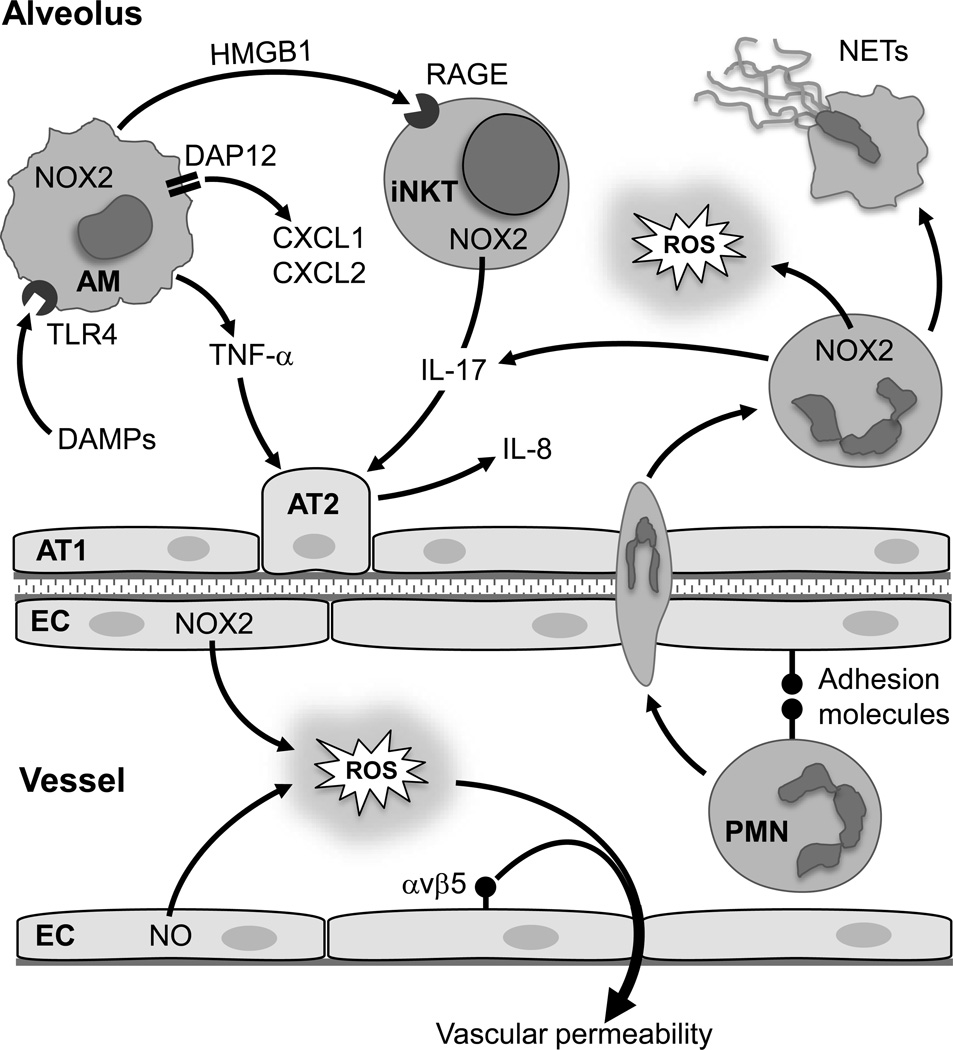
Inflammation typically occurs in response to infection, which is critical for pathogen eradication and tissue repair. However, ‘sterile inflammation’ can occur during non-infectious conditions including trauma, chemically induced injury, or ischemia [1]. In these cases, activation of the innate immune system promotes inflammation. In lung transplantation, organ ischemia and subsequent reperfusion is unavoidable and commonly leads to acute, sterile inflammation after transplant called ischemia-reperfusion (IR) injury. This is a major clinical issue because severe IR injury leads to primary graft dysfunction (PGD), which is the major source of both short- and long-term morbidity and mortality after lung transplantation [2,3]. Currently there are no therapeutic agents clinically utilized to specifically prevent IR injury, and treatment strategies are limited to supportive care. The annual number of lung transplantations keeps growing, and major research efforts have been aimed toward: 1) better preserving donor lungs, 2) expanding the donor lung pool, 3) reconditioning of marginal donor lungs via ex vivo lung perfusion, and 4) developing methods to prevent or treat IR injury. Thus understanding the cellular and molecular mechanisms of lung IR injury is paramount for the translation of these research efforts into clinical application. This article will provide a review of general mechanisms of lung IR injury with a focus on the most recent findings in this area. The complex nature of lung IR injury is illustrated in Figure 1, which highlights many of the inflammatory pathways discussed in this review that contribute to lung IR injury.
Figure 1. Inflammatory pathways contributing to lung IR injury.
After IR, generation of reactive oxygen species (ROS) by endothelial cells (EC) via NADPH oxidase (NOX2) and nitric oxide (NO), and activation of integrin αvβ5, promotes vascular permeability. Alveolar macrophages (AM) are activated, in part via binding of damage-associated molecular patterns (DAMPs) to toll-like receptor 4 (TLR4), to secrete neutrophil chemokines (CXCL1 and CXCL2) via DAP12, pro-inflammatory cytokines (e.g. TNF-α), and high mobility group box 1 (HMGB1). HMGB1 binds to receptor for advanced glycation end products (RAGE) to induce IL-17 secretion by invariant natural killer T cells (iNKT). TNF-α induces the production of IL-8, another potent chemokine, by alveolar type 2 epithelial cells (AT2); a process augmented by IL-17. Elevated chemokine levels and adhesion molecule expression on ECs and neutrophils (PMN) leads to binding and infiltration of neutrophils, which can release cytokines, ROS and form neutrophil extracellular traps (NETs). AT1, alveolar type 1 epithelial cell.
IR INJURY AND CLINICAL IMPACT
Ischemia (cessation of blood flow) and severe hypoxia of the explanted, donor lung is unavoidable before transplantation, and the use of cold preservation solutions are used to minimize ischemic injury. Because PGD is a complication that leads to severe early and long term consequences for lung transplant recipients, transplant teams tend to be very conservative in the selection of donor lungs. PGD, largely due to IR injury, is associated with significant early and late mortality after transplant, and severe PGD (PGD3) has an incidence of approximately 30% within 72 hours of transplant [3**]. PGD is also a risk factor for late graft rejection (bronchiolitis obliterans), the major cause of mortality in recipients beyond 1 year of transplant [4,5]. Clearly, methods for prevention of IR injury are deeply needed to improve both short- and long-term outcomes after lung transplantation.
IR INJURY ENTAILS ROBUST OXIDATIVE STRESS RESPONSES
Although reperfusion of the ischemic lung is mandatory to prevent irreversible ischemic damage, it also paradoxically promotes further damage and dysfunction; so-called IR injury. IR injury is a rapid and complex inflammatory response that entails endothelial and epithelial injury/dysfunction, release of cytokines and damage-associated molecular patterns (DAMPs), and vigorous innate immune responses including activation of alveolar macrophages, invariant natural killer T (iNKT) cells and neutrophils. Most of these responses are initiated by rapid and robust generation of reactive oxygen species (ROS) that leads to cell/tissue injury, activation of multiple cell types, lipid membrane peroxidation and secretion of inflammatory cytokines and DAMPs [6].
A recent study by Gielis et al. used electron spin resonance to quantify free radical formation after lung IR in mice and revealed a major radical ‘burst’ peaking one hour after reperfusion followed by a swift stabilization of the oxidative environment [7*]. This study also demonstrated acute oxidative stress in peripheral blood during ischemia and reperfusion. A study by Chatterjee et al. provided evidence that the cessation of blood flow during lung ischemia is “sensed” by the endothelium via a mechanosignaling cascade that depolarizes endothelial cell membranes and activates the phagocytic isoform of NADPH oxidase (NOX2) to generate ROS and nitric oxide, ultimately leading to oxidative injury and activation of signaling pathways that drive inflammation and cell death [8**]. This likely contributes to the development of life-threatening pulmonary edema in recipients with PGD. A recent analysis of patients from the Lung Transplant Outcomes Group (LTOG) cohort aimed to identify genetic variation in oxidative stress genes associated with PGD [9**]. This study identified several genes associated with PGD and concluded that donor-recipient interactions between functional single nucleotide polymorphisms in the NOX3 and NFE2L2 genes may be highly relevant. The effects of these interactions highlight the importance of oxidative stress detoxifiers (encoded by NFE2L2) in lung recipients and ROS generators (encoded by NOX3) in lung donors. Oxidative stress after IR causes DNA breaks, which in turn activates DNA nick sensor enzyme poly(ADP-ribose) polymerase (PARP) that plays an important role in DNA repair. Using a rat lung IR model, Hatachi et al. demonstrated a role for PARP in IR injury by showing that PARP inhibition attenuates inflammation and tissue damage caused by IR [10**].
Our laboratory has described a pivotal role for iNKT cells in the initiation of lung IR injury via an IL-17A-dependent mechanism [11], and we have shown that NOX2 in bone marrow-derived cells mediates lung IR injury [12]. In a recent follow-up study we demonstrated that activation of NOX2 in iNKT cells provides the mechanism for IL-17A production after IR leading to inflammation, neutrophil infiltration and pulmonary injury [13*]. We have previously demonstrated the potent ability of adenosine A2A receptor (A2AR) agonists to prevent IR injury [14], and our recent study also revealed that a primary mechanism for A2AR agonist-mediated protection entails inhibition of NOX2 in iNKT cells [13*]. Thus agonism of A2ARs on iNKT cells may be a novel therapeutic strategy to prevent primary graft dysfunction after lung transplantation.
INNATE IMMUNE RESPONSES
IR after lung transplant rapidly activates the innate immune system to promote inflammation and injury (see review in [15]). Receptors that mediate the response to microbial infection, notably toll-like receptors (TLRs), have also been implicated in lung IR injury. TLR4, the mammalian LPS receptor, has been shown to respond to endogenous molecules during sterile inflammation such as after IR [1]. Such endogenous molecules, termed DAMPs, include high mobility group box 1 (HMGB1), fibronectin, oxidized phospholipids and heat shock proteins among others. TLR4 has been implicated as a key modulator of lung IR injury [16], and Merry et al. recently utilized siRNA knockdown of TLR4 in rats to demonstrate that TLR4 activation, largely in alveolar macrophages, is important for the initiation of lung IR injury [17]. Phelan et al. used an in vitro model to confirm that TLR4 modulates cytokine responses of alveolar macrophages after acute hypoxia-reoxygenation [18]. A third study by this group demonstrated that TNF-α and IL-1β, secreted by alveolar macrophages, prime and amplify the response of endothelial and epithelial cells through parallel pathways after exposure to hypoxia-reoxygenation [19]. Finally, although IR injury is considered a sterile inflammatory condition, an interesting study by Prakash et al. suggests that the inflammatory response induced by lung IR is transient and strongly influenced by intestinal microbiota [20]. Here, treatment of mice with intestinally localized antibiotics was associated with attenuation of lung inflammatory markers, cell infiltration and edema in the lung after IR. These authors speculate that the commensal microbiota may provide an initial ‘priming’ signal that signals alveolar macrophages to generate IL-1β via inflammasome activation.
The activation and infiltration of innate immune cells, especially neutrophils as end-effectors of tissue injury, has been well documented after lung IR [11,21–23]. Infiltration of circulating host neutrophils into the graft is a key aspect of IR injury largely driven by potent chemokines, (e.g. IL-8 and CXCL2) produced by donor lung cells such as epithelium, endothelium or macrophages [24]. Several recent studies provide new insight into mechanisms of neutrophil-mediated IR injury. DAP12 is a membrane-associated protein expressed in myeloid cells that can augment or dampen innate inflammatory responses. Using DAP12−/− mice as donors or recipients, Spahn and colleagues showed that donor deficiency in DAP12 attenuates IR injury in a murine lung transplantation model [25**]. Further analysis demonstrated that DAP12 mediates the production of neutrophil chemoattractants, specifically CXCL2, and also revealed a trans-endothelial migration defect in DAP12−/− lungs that could be rescued by local administration of CXCL2. Thus DAP12 expression in macrophages mediates lung IR injury by promoting neutrophil trafficking. Another study by Sayah et al. showed that neutrophil extracellular traps (NETs) are induced after lung IR in two murine models and in human lung transplants [26**]. Considered by many to be an effector function of neutrophils, NETs are comprised of extracellular elaborations of DNA complexed with histones and neutrophil granular proteins and generated by a regulated cell death program called ‘NETosis’. In this study, NET formation was attenuated by DNase I treatment that that also correlated with reduced lung IR injury, and NETs were found to be more abundant in lungs of patients with PGD. Thus this study provided the first evidence of a pathogenic role for NETs in solid organ transplantation.
Early growth response-1 (Egr-1) is a zinc finger transcription factor that regulates the expression of multiple target genes including those involved in inflammatory cytokine and coagulant pathways. Using wild-type or Egr-1−/− mice, Yamamoto et al. demonstrated that lung function after transplantation was most improved when donor and recipient animals were Egr-1 deficient, and graft function was intermittently improved when either the donor or recipient animal was Egr-1 deficient [27]. Graft function correlated inversely with neutrophil infiltration, and Egr-1 expression was detected in neutrophil cytoplasm. These results suggest that graft Egr-1 has a greater contribution to lung IR injury through the regulation of neutrophil infiltration.
Mast cells can be important for initiation of immune responses via rapid generation of pro- or anti-inflammatory mediators, but their role in lung IR injury has remained unknown. Although mast cells can act as key regulators of tolerance depending on the nature of the mast cell subsets, their phenotype and environment, few studies have addressed a potential role for mast cells in lung IR injury. Greenland and colleagues recently reported that lung IR injury (extravascular lung water and endothelial permeability) in mast cell-deficient animals was similar to wild-type mice and concluded that mast cells are not necessary for the development of PGD [28].
ENDOTHELIAL BARRIER FUNCTION
Endothelial cell dysfunction and disruption of the endothelial barrier are hallmarks of lung IR injury and contribute greatly to PGD. As discussed above, depolarization of endothelial cell membranes induces ROS production and subsequent inflammation and leukocyte extravasation [8]. Integrins are surface receptors important in maintenance of the cell barrier, and integrin αvβ5 has been shown to mediate vascular leak [29]. Mallavia et al. recently used a murine lung transplant model to show that treatment of donor lungs with αvβ5 blocking antibody provided robust protection from IR injury, decreased vascular leak and neutrophil infiltration, and improved oxygenation following reperfusion [30*]. Although the mechanisms for αvβ5-mediated endothelial permeability remain unclear, these results have translational potential and may support the application of αvβ5 blocking strategies during ex vivo lung perfusion to prevent edema.
Endothelial barrier integrity as well as immune cell trafficking and function are several aspects attributed to the actions of sphingosine 1-phospate (S1P), a biologically active lipid mediator that signals via a family of five G protein-coupled receptors (S1PR1–5) [31]. Our group recently measured lung IR injury in mice treated with either FTY720 (a nonselective S1P receptor agonist) or VPC01091 (a selective S1PR1 agonist and S1PR3 antagonist) [32*]. We demonstrated that VPC01091 was equally protective as FTY720 against lung injury and vascular permeability after IR, suggesting that these protective mechanisms are primarily dependent on S1PR1 agonism. Use of selective S1PR1 agonists like VPC01091 may be a more effective approach to S1P receptor-targeted therapy because it avoids potential deleterious effects of S1P3-mediated profibrotic processes after lung injury.
Mammalian cells have an oxygen-sensing capacity as a protective response to hypoxic or ischemic injury, and hypoxia-inducible factors (HIFs) are well-described ‘master regulators’ of these cellular responses by regulating a multitude of genes affecting metabolism and inflammation after transplantation [33]. Zhao et al. used a rat model to demonstrate that HIF-1α contributes to pulmonary vascular dysfunction after lung IR and that a HIF-1α stabilizer, DMOG, provides significant protection [34]. A more recent study demonstrated the importance of aquaporin 1 (AQP1), a water channel protein expressed widely in vascular endothelium, in the resolution of lung IR injury through the stabilization of HIF-2α protein [35*]. Here, AQP1−/− mice had enhanced leukocyte infiltration and microvascular permeability as well as repressed HIF-2α stability, and HIF-2α overexpression restored the expression of angiogenic factors in pulmonary microvascular endothelial cells exposed to hypoxia-reoxygenation. These results suggest that AQP1 may participate in the resolution of lung IR injury via mediating HIF-2α stability and promoting angiogenesis.
STRATEGIES TO LIMIT IR INJURY
Many strategies have been shown to limit IR injury in experimental settings, which often reveal mechanistic insight. Most of these studies have been performed in animal models in which treatment was given either to the recipient or to the donor before lung reperfusion. Recent studies have identified a variety of strategies including: 1) anti-oxidant strategies using free radical scavengers or inhibitors of oxidant-producing enzymes (e.g. methylene blue [36] or N-acetylcysteine [37]); 2) anti-inflammatory strategies using inhibitors of pro-inflammatory transcription factors (e.g. inhibitor of Egr-1 [27]) or inflammatory mediators (e.g. α1-antitrypsin [38*], anti-cytokine [11], or anti-HMGB1 [39] agents); 3) ventilation with gaseous molecules (e.g. carbon monoxide [40]) or inhaled anesthetic sevoflurane [41] that display anti-inflammatory properties; 4) growth factors (e.g. keratinocyte growth factor-2 [42]); 5) dietary supplements such as creatine [43]; and 6) cell-based therapies such as application of mesenchymal stem cells [44,45]. In addition, a recent study by Hashimoto et al. showed that treatment with diannexin (a homodimer of annexin V, an anionic phospholipid-binding protein with potent anticoagulant activity) significantly reduced IR injury in a lung transplant model by reducing cell death and tissue inflammation [46].
Extracellular ATP (eATP), a DAMP molecule, has been shown to accumulate in rat lung isografts injured by prolonged cold preservation, both of which were attenuated by apyrase treatment [47]. This same group more recently confirmed the role of eATP in lung IR injury by demonstrating that apyrase treatment provided protection in a canine model of lung IR injury [48**]. Of particular interest, this study also demonstrated that human lung recipients with moderate to severe PGD had significantly higher eATP levels, and thus strategies aimed toward reducing eATP levels may be an area worth exploring to prevent PGD in transplant patients.
Pulmonary epithelial cells were the focus in a recent study by Kim et al. that utilized an in vitro model that simulates lung preservation and transplantation [49*]. They found that protein kinase C (PKCδ) was activated in epithelial cells during cold ischemia and that treatment with δV1-1, a peptide PKCδ inhibitor, reduced translocation of PKCδ and p53 to the mitochondria and also attenuated IR injury after lung transplantation in rats. Thus inhibition of PKCδ may be an effective strategy to prevent injury and necrosis after IR.
Several studies have explored the role of autophagy in lung IR injury. Autophagy is a homeostatic cellular process that degrades cytoplasmic components within the lysosome and is an important cytoprotective response to pathologic stresses such as after ischemia [50]. A study by Zhang et al. suggests that autophagy plays a protective role in lung IR injury via ERK1/2 signaling and that this effect may be enhanced by facilitating autophagy via rapamycin treatment [51]. This same group attributed enhanced autophagy levels as a mechanism for the attenuation of lung IR injury by administration of mesenchymal stem cells [52*]. However, some controversy exists as to the precise role of autophagy in lung IR injury as an earlier study concluded that inhibition of autophagy by 3-methyladenine ameliorates lung IR injury [53].
CONCLUSIONS
Lung IR injury is a complex, ‘sterile’ inflammatory condition involving rapid oxidative stress and subsequent responses by all cells globally within the lung, ultimately leading to breakdown of the endothelial and epithelial barriers resulting in life-threatening edema and defective gas exchange. Some of the major players are innate immune cells that are rapidly activated upon reperfusion and that induce direct tissue injury or augment inflammation via production of pro-inflammatory cytokines and DAMPs. Although many experimental studies have described various methods to prevent or dampen IR injury, these have not yet been translated into clinical use. Thus the thrust of future research should aim to better understand mechanisms of lung IR injury in order to identify more effective therapeutic targets and also aim to push the most promising therapies toward clinical trials. Because IR injury involves complex intra- and inter-cellular communications in the lung, therapies that simultaneously target multiple pathways and cell types will likely be required to most effectively treat or prevent PGD.
KEY POINTS.
Ischemia-reperfusion (IR) injury after lung transplantation is a real clinical issue because it often leads to primary graft dysfunction, the major cause of morbidity and mortality after transplant.
IR injury entails complex inflammatory communication between donor lung cells and infiltrating immune cells from the recipient, ultimately leading to breakdown of endothelial and epithelial barriers to result in life-threatening edema and organ dysfunction.
The robust oxidative stress that rapidly occurs upon reperfusion is a major initiating factor of IR injury, and recent studies have provided further understanding of the cells and pathways involved in order to help develop more effective targeted therapies.
Future studies should focus more on translating experimental results to clinical trials with the idea that the most effective therapies will likely target multiple, intracellular communications and intercellular pathways that lead to tissue injury and organ dysfunction.
Acknowledgments
None
FINANCIAL SUPPORT
This work was supported by NIH grants R01HL077301 (VEL) and R01HL119218 (VEL).
Abbreviations
- IR
ischemia-reperfusion
- PGD
primary graft dysfunction
- iNKT cell
invariant natural killer T cell
- ROS
reactive oxygen species
- DAMP
damage-associated molecular pattern
- TLR
toll-like receptor
- NET
neutrophil extracellular trap
- S1P
sphingosine 1-phospate
- HIF
hypoxia-inducible factor
- HMGB1
high mobility group box 1
- eATP
extracellular ATP
- PARP
poly(ADP-ribose) polymerase
Footnotes
CONFLICTS OF INTEREST
None
REFERENCES
- 1.Chen GY, Nunez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10:826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.den Hengst WA, Gielis JF, Lin JY, et al. Lung ischemia-reperfusion injury: a molecular and clinical view on a complex pathophysiological process. Am J Physiol Heart Circ Physiol. 2010;299:H1283–H1299. doi: 10.1152/ajpheart.00251.2010. [DOI] [PubMed] [Google Scholar]
- 3. Porteous MK, Diamond JM, Christie JD. Primary graft dysfunction: lessons learned about the first 72 h after lung transplantation. Curr Opin Organ Transplant. 2015;20:506–514. doi: 10.1097/MOT.0000000000000232. This review highlights the current state of the science regarding PGD and provides further insight into future development of preventative and treatment strategies.
- 4.Fiser SM, Tribble CG, Long SM, et al. Ischemia-reperfusion injury after lung transplantation increases risk of late bronchiolitis obliterans syndrome. Ann Thorac Surg. 2002;73:1041–1047. doi: 10.1016/s0003-4975(01)03606-2. [DOI] [PubMed] [Google Scholar]
- 5.Kreisel D, Krupnick AS, Puri V, et al. Short- and long-term outcomes of 1000 adult lung transplant recipients at a single center. J Thorac Cardiovasc Surg. 2011;141:215–222. doi: 10.1016/j.jtcvs.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 6.Ferrari RS, Andrade CF. Oxidative Stress and Lung Ischemia-Reperfusion Injury. Oxid Med Cell Longev. 2015;2015:590987. doi: 10.1155/2015/590987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gielis JF, Boulet GA, Briede JJ, et al. Longitudinal quantification of radical bursts during pulmonary ischaemia and reperfusion. Eur J Cardiothorac Surg. 2015;48:622–629. doi: 10.1093/ejcts/ezu518. In addition to revealing a major radical ‘burst’ peaking one hour after reperfusion in murine lungs, this study also demonstrated acute oxidative stress in peripheral blood during ischemia and reperfusion.
- 8. Chatterjee S, Nieman GF, Christie JD, Fisher AB. Shear stress-related mechanosignaling with lung ischemia: lessons from basic research can inform lung transplantation. Am J Physiol Lung Cell Mol Physiol. 2014;307:L668–L680. doi: 10.1152/ajplung.00198.2014. This study advances our understanding of the key events in the mechanosignaling cascade in the vasculature during lung ischemia. Endothelial mechanosignaling in response to ischemia leads to production of ROS, increased intracellular calcium and activation of eNOS.
- 9. Cantu E, Shah RJ, Lin W, et al. Oxidant stress regulatory genetic variation in recipients and donors contributes to risk of primary graft dysfunction after lung transplantation. J Thorac Cardiovasc Surg. 2015;149:596–602. doi: 10.1016/j.jtcvs.2014.09.077. This large, multicenter cohort study demonstrated an association between genes regulating oxidative stress and primary graft dysfunction. These results highlight the importance of oxidant stress detoxifiers in transplant recipients and ROS generators in lung donors.
- 10. Hatachi G, Tsuchiya T, Miyazaki T, et al. The poly(adenosine diphosphate-ribose) polymerase inhibitor PJ34 reduces pulmonary ischemia-reperfusion injury in rats. Transplantation. 2014;98:618–624. doi: 10.1097/TP.0000000000000305. Results from this study using a rat lung IR model suggest that a PARP inhibitor, PJ34, is protective after IR and thus PARP inhibitors may be a useful therapeutic strategy to help prevent or treat lung IR injury.
- 11.Sharma AK, LaPar DJ, Zhao Y, et al. Natural killer T cell-derived IL-17 mediates lung ischemia-reperfusion injury. Am J Respir Crit Care Med. 2011;183:1539–1549. doi: 10.1164/rccm.201007-1173OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang Z, Sharma AK, Marshall M, et al. NADPH oxidase in bone marrow-derived cells mediates pulmonary ischemia-reperfusion injury. Am J Respir Cell Mol Biol. 2009;40:375–381. doi: 10.1165/rcmb.2008-0300OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sharma AK, LaPar DJ, Stone ML, et al. NOX2 Activation of NKT Cells is Blocked by Adenosine A2A Receptor to Inhibit Lung Reperfusion Injury. Am J Respir Crit Care Med. 2016 Jan 12; doi: 10.1164/rccm.201506-1253OC. [Epub ahead of print] This is the first study to reveal that NOX2 activation is a mechanism for IL-17 production by iNKT cells after IR and also demonstrates that prevention of lung IR injury by adenosine A2A receptor agonism is via inhibition of NOX2 in iNKT cells.
- 14.Sharma AK, Laubach VE, Ramos SI, et al. Adenosine A2A receptor activation on CD4+ T lymphocytes and neutrophils attenuates lung ischemia-reperfusion injury. J Thorac Cardiovasc Surg. 2010;139:474–482. doi: 10.1016/j.jtcvs.2009.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kreisel D, Goldstein DR. Innate immunity and organ transplantation: focus on lung transplantation. Transpl Int. 2013;26:2–10. doi: 10.1111/j.1432-2277.2012.01549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zanotti G, Casiraghi M, Abano JB, et al. Novel critical role of Toll-like receptor 4 in lung ischemia-reperfusion injury and edema. Am J Physiol Lung Cell Mol Physiol. 2009;297:L52–L63. doi: 10.1152/ajplung.90406.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Merry HE, Phelan P, Doak MR, et al. Role of toll-like receptor-4 in lung ischemia-reperfusion injury. Ann Thorac Surg. 2015;99:1193–1199. doi: 10.1016/j.athoracsur.2014.12.062. [DOI] [PubMed] [Google Scholar]
- 18.Phelan P, Merry HE, Hwang B, Mulligan MS. Differential toll-like receptor activation in lung ischemia reperfusion injury. J Thorac Cardiovasc Surg. 2015;149:1653–1661. doi: 10.1016/j.jtcvs.2015.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Merry HE, Phelan P, Doaks M, et al. Functional roles of tumor necrosis factor-alpha and interleukin 1-Beta in hypoxia and reoxygenation. Ann Thorac Surg. 2015;99:1200–1205. doi: 10.1016/j.athoracsur.2014.11.042. [DOI] [PubMed] [Google Scholar]
- 20.Prakash A, Sundar SV, Zhu YG, et al. Lung ischemia-reperfusion is a sterile inflammatory process influenced by commensal microbiota in mice. Shock. 2015;44:272–279. doi: 10.1097/SHK.0000000000000415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fiser SM, Tribble CG, Long SM, et al. Lung transplant reperfusion injury involves pulmonary macrophages and circulating leukocytes in a biphasic response. J Thorac Cardiovasc Surg. 2001;121:1069–1075. doi: 10.1067/mtc.2001.113603. [DOI] [PubMed] [Google Scholar]
- 22.Welbourn CR, Goldman G, Paterson IS, et al. Pathophysiology of ischaemia reperfusion injury: central role of the neutrophil. Br J Surg. 1991;78:651–655. doi: 10.1002/bjs.1800780607. [DOI] [PubMed] [Google Scholar]
- 23.Zhao M, Fernandez LG, Doctor A, et al. Alveolar macrophage activation is a key initiation signal for acute lung ischemia-reperfusion injury. Am J Physiol Lung Cell Mol Physiol. 2006;291:L1018–L1026. doi: 10.1152/ajplung.00086.2006. [DOI] [PubMed] [Google Scholar]
- 24.Kobayashi Y. The role of chemokines in neutrophil biology. Front Biosci. 2008;13:2400–2407. doi: 10.2741/2853. [DOI] [PubMed] [Google Scholar]
- 25. Spahn JH, Li W, Bribriesco AC, et al. DAP12 expression in lung macrophages mediates ischemia/reperfusion injury by promoting neutrophil extravasation. J Immunol. 2015;194:4039–4048. doi: 10.4049/jimmunol.1401415. By using a murine lung transplant model, this study uncovers a role for DAP12 in exacerbating lung IR injury. DAP12 signaling in donor lung alveolar macrophages promotes their survival and mediates local chemokine production, leading to neutrophil infiltration and lung injury after transplantation.
- 26. Sayah DM, Mallavia B, Liu F, et al. Neutrophil extracellular traps are pathogenic in primary graft dysfunction after lung transplantation. Am J Respir Crit Care Med. 2015;191:455–463. doi: 10.1164/rccm.201406-1086OC. This is the first study that describes a pathogenic role for NETs in solid organ transplantation, suggesting that NETs may be an effective therapeutic target to prevent PGD. In addition to describing NETs in two experimental murine PGD models, which was attenuated by DNase I treatment, NETs were also found to be more abundant in patients with PDG.
- 27.Yamamoto S, Yamane M, Yoshida O, et al. Early Growth Response-1 Plays an Important Role in Ischemia-Reperfusion Injury in Lung Transplants by Regulating Polymorphonuclear Neutrophil Infiltration. Transplantation. 2015;99:2285–2293. doi: 10.1097/TP.0000000000000783. [DOI] [PubMed] [Google Scholar]
- 28.Greenland JR, Xu X, Sayah DM, et al. Mast cells in a murine lung ischemia-reperfusion model of primary graft dysfunction. Respir Res. 2014;15:95. doi: 10.1186/s12931-014-0095-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Su G, Hodnett M, Wu N, et al. Integrin alphavbeta5 regulates lung vascular permeability and pulmonary endothelial barrier function. Am J Respir Cell Mol Biol. 2007;36:377–386. doi: 10.1165/rcmb.2006-0238OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mallavia B, Liu F, Sheppard D, Looney MR. Inhibiting integrin alphavbeta5 reduces ischemia-reperfusion injury in an orthotopic lung transplant model in mice. Am J Transplant. 2015 doi: 10.1111/ajt.13605. These authors showed that antibody-based blockade of αvβ5 integrin is broadly protective in a clinically relevant murine lung transplant model by reducing vascular permeability and inflammation as well as improving function
- 31.Proia RL, Hla T. Emerging biology of sphingosine-1-phosphate: its role in pathogenesis and therapy. J Clin Invest. 2015;125:1379–1387. doi: 10.1172/JCI76369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stone ML, Sharma AK, Zhao Y, et al. Sphingosine-1-phosphate receptor 1 agonism attenuates lung ischemia-reperfusion injury. Am J Physiol Lung Cell Mol Physiol. 2015;308:L1245–L1252. doi: 10.1152/ajplung.00302.2014. Although S1P and FTY720 have been shown to be protective following experimental lung transplantation, the role of the S1P receptor(s) have remained unclear. This study suggests that S1PR1 is the receptor responsible for S1P-mediated protection from lung IR injury.
- 33.Akhtar MZ, Sutherland AI, Huang H, et al. The role of hypoxia-inducible factors in organ donation and transplantation: the current perspective and future opportunities. Am J Transplant. 2014;14:1481–1487. doi: 10.1111/ajt.12737. [DOI] [PubMed] [Google Scholar]
- 34.Zhao X, Jin Y, Li H, et al. Hypoxia-inducible factor 1 alpha contributes to pulmonary vascular dysfunction in lung ischemia-reperfusion injury. Int J Clin Exp Pathol. 2014;7:3081–3088. [PMC free article] [PubMed] [Google Scholar]
- 35. Ge H, Zhu H, Xu N, et al. Increased Lung Ischemia-reperfusion Injury in Aquaporin 1 Null Mice is Mediated via Decreased HIF-2alpha Stability. Am J Respir Cell Mol Biol. 2015 Dec 9; doi: 10.1165/rcmb.2014-0363OC. [Epub ahead of print] This study clarifies the role of AQP1 in the resolution of lung injury after IR by demonstrating that AQP1 promotes resolution by promoting angiogenesis and HIF-2α stability.
- 36.Abreu Mda M, Pazetti R, Almeida FM, et al. Methylene blue attenuates ischemia--reperfusion injury in lung transplantation. J Surg Res. 2014;192:635–641. doi: 10.1016/j.jss.2014.07.043. [DOI] [PubMed] [Google Scholar]
- 37.Forgiarini LF, Forgiarini LA, Jr, da Rosa DP, et al. N-acetylcysteine administration confers lung protection in different phases of lung ischaemia-reperfusion injury. Interact Cardiovasc Thorac Surg. 2014;19:894–899. doi: 10.1093/icvts/ivu258. [DOI] [PubMed] [Google Scholar]
- 38. Gao W, Zhao J, Kim H, et al. alpha1-Antitrypsin inhibits ischemia reperfusion-induced lung injury by reducing inflammatory response and cell death. J Heart Lung Transplant. 2014;33:309–315. doi: 10.1016/j.healun.2013.10.031. α1-antitrypsin has been shown to have anti-inflammatory effects, and this study is the first to demonstrate that administration of α1-antitrypsin may be a safe and effective therapy for the treatment of lung IR injury after transplantation.
- 39.Sharma AK, LaPar DJ, Stone ML, et al. Receptor for advanced glycation end products (RAGE) on iNKT cells mediates lung ischemia-reperfusion injury. Am J Transplant. 2013;13:2255–2267. doi: 10.1111/ajt.12368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meng C, Ma L, Liu J, et al. Inflation with carbon monoxide in rat donor lung during cold ischemia phase ameliorates graft injury. Exp Biol Med (Maywood) 2015 Aug 19; doi: 10.1177/1535370215600550. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chai J, Long B, Liu X, et al. Effects of sevoflurane on tight junction protein expression and PKC-alpha translocation after pulmonary ischemia-reperfusion injury. Exp Mol Med. 2015;47:e167. doi: 10.1038/emm.2015.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fang X, Wang L, Shi L, et al. Protective effects of keratinocyte growth factor-2 on ischemia-reperfusion-induced lung injury in rats. Am J Respir Cell Mol Biol. 2014;50:1156–1165. doi: 10.1165/rcmb.2013-0268OC. [DOI] [PubMed] [Google Scholar]
- 43.Almeida FM, Oliveira-Junior MC, Souza RA, et al. Creatine supplementation attenuates pulmonary and systemic effects of lung ischemia and reperfusion injury. J Heart Lung Transplant. 2015 Jul 4; doi: 10.1016/j.healun.2015.06.012. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 44.Lu W, Si YI, Ding J, et al. Mesenchymal stem cells attenuate acute ischemia-reperfusion injury in a rat model. Exp Ther Med. 2015;10:2131–2137. doi: 10.3892/etm.2015.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tian W, Liu Y, Zhang B, et al. Infusion of mesenchymal stem cells protects lung transplants from cold ischemia-reperfusion injury in mice. Lung. 2015;193:85–95. doi: 10.1007/s00408-014-9654-x. [DOI] [PubMed] [Google Scholar]
- 46.Hashimoto K, Kim H, Oishi H, et al. Annexin V homodimer protects against ischemia reperfusion-induced acute lung injury in lung transplantation. J Thorac Cardiovasc Surg. 2015 Nov 11; doi: 10.1016/j.jtcvs.2015.10.112. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 47.Sugimoto S, Lin X, Lai J, et al. Apyrase treatment prevents ischemia-reperfusion injury in rat lung isografts. J Thorac Cardiovasc Surg. 2009;138:752–759. doi: 10.1016/j.jtcvs.2009.04.049. [DOI] [PubMed] [Google Scholar]
- 48. Ibrahim M, Wang X, Puyo CA, et al. Human recombinant apyrase therapy protects against canine pulmonary ischemia-reperfusion injury. J Heart Lung Transplant. 2015;34:247–253. doi: 10.1016/j.healun.2014.09.034. Extracellular ATP (eATP) is a DAMP that can promote acute lung injury. This study documented elevated eATP levels in dog lungs after IR as well as human lung transplant recipients with PGD. Apyrase treatment provided significant protection from lung IR injury. Thus strategies that target eATP may prove effective in preventing PGD after transplantation.
- 49. Kim H, Zhao J, Zhang Q, et al. deltaV1-1 reduces pulmonary ischemia reperfusion-induced lung injury by inhibiting necrosis and mitochondrial localization of PKCdelta and p53. Am J Transplant. 2015 doi: 10.1111/ajt.13445. [Epub ahead of print] This study utilized a novel in vitro model of simulated IR to elucidate cellular changes in epithelial cells during lung IR injury. PKCδ was shown to mediate IR-induced inflammatory responses, and use of δV1-1, a PKCδ inhibitor, was effective in attenuating lung IR injury.
- 50.Murrow L, Debnath J. Autophagy as a stress-response and quality-control mechanism: implications for cell injury and human disease. Annu Rev Pathol. 2013;8:105–137. doi: 10.1146/annurev-pathol-020712-163918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhang D, Li C, Zhou J, et al. Autophagy protects against ischemia/reperfusion-induced lung injury through alleviating blood-air barrier damage. J Heart Lung Transplant. 2015;34:746–755. doi: 10.1016/j.healun.2014.12.008. The role of autophagy in lung IR injury is not understood. This study provides evidence that promotion of autophagy with rapamycin protects lungs from IR injury partly through maintaining tight junctions in endothelial cells. These data have important implications to therapies directed at reducing pulmonary edema after transplantation.
- 52. Li J, Zhou J, Zhang D, et al. Bone marrow-derived mesenchymal stem cells enhance autophagy via PI3K/AKT signalling to reduce the severity of ischaemia/reperfusion-induced lung injury. J Cell Mol Med. 2015;19:2341–2351. doi: 10.1111/jcmm.12638. This study explored the protective roles of mesenchymal stem cells and autophagy in lung IR injury by demonstrating that mesenchymal stem cells attenuate lung IR injury by enhancing autophagy via the PI3K/Akt signaling pathway.
- 53.Zhang J, Wang JS, Zheng ZK, et al. Participation of autophagy in lung ischemia-reperfusion injury in vivo. J Surg Res. 2013;182:e79–e87. doi: 10.1016/j.jss.2012.11.014. [DOI] [PubMed] [Google Scholar]