SUMMARY
The intestinal epithelium is a single cell layer that facilitates the absorption of nutrients but also provides a tight barrier to prevent pathogen invasion and dissemination of commensal microbes. Specialized epithelial cells of the gastrointestinal tract achieve this front-line defense by working in concert with lymphoid, myeloid, and stromal cells to secrete an array of factors that limit direct contact between the epithelium and infectious agents. The importance of these mechanisms is underscored by the ability of enteric pathogens to target these mechanisms to achieve invasion and dissemination. This review highlights recent advances in our understanding of these intricate molecular and cellular mechanisms adopted by these cells to promote spatial segregation and barrier maintenance.
INTRODUCTION
The gastrointestinal (GI) tract represents the largest surface area in the body, and requires protection from infectious and non-infectious threats continuously introduced during ingestion. Prevention or rapid resolution of an infection is tantamount to survival, as evidenced by the fact that diarrheal disease caused by enteric pathogens remains a leading cause of childhood mortality. Also, a sustained immune response directed towards intestinal microbes is implicated in chronic digestive disorders, such as inflammatory bowel diseases (IBD) Crohn’s disease and ulcerative colitis. Despite the need for an impregnable defense, the intestinal epithelium is only one cell thick to accommodate the exchange of nutrients and water. Additionally, the epithelium of the lower GI tract (small intestine and colon) exists in close apposition to trillions of microbes that aid digestion. Although the presence of these microbionts (members of the microbiota) is generally beneficial, dissemination to extra-intestinal organs or the overgrowth of toxigenic members can be catastrophic. Hence, the mucosal immune system of the gut is faced with the extraordinary challenge of coexisting with microbionts and simultaneously preventing a breach in a single layer of epithelial cells.
The solution to this problem is the incessant production of an array of secreted factors that limit direct contact between the epithelium and infectious agents, thereby avoiding unnecessary battles. Specialized intestinal epithelial cell (IEC) lineages play an active role in achieving this task, and work in concert with lymphoid, myeloid, and stromal cells of the lamina propria, the underlying connective tissue. Pathogens have evolved complex tactics to foil these defense mechanisms in an attempt to access the favorable environment beyond the epithelial barrier. Microbionts, in contrast, are often integrated into the host’s defensive plan and promote resistance to invasion by these pathogens. In this article, we review these defense mechanisms intrinsic to IECs of the lower GI tract. We will emphasize recent progress in understanding the molecular mechanisms by which spatial segregation is established.
ANATOMICAL FEATURES OF THE LOWER GI TRACT
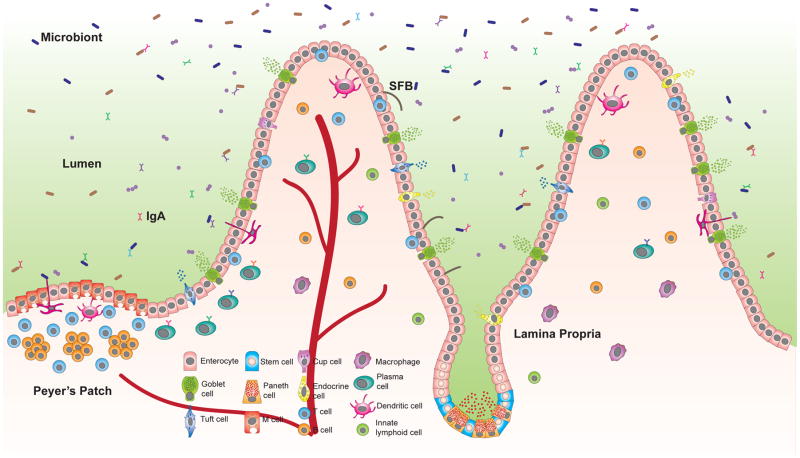
Defense mechanisms must accommodate anatomical structures that reflect the function of the organ. Most of the enzymatic breakdown of food occurs in the small intestine where the surface area available for nutrient absorption is maximized by finger-like protrusions called villi, which are predominantly covered by absorptive columnar epithelial cells known as enterocytes (Marchiando, 2016). Between villi are the crypts of Lieberkuhn, invaginations that shield stem cells, which give rise to all the IEC lineages and are identified based on expression of Lgr5 (Figure 1). These crypts include mucus-producing goblet cells found throughout the GI tract, and Paneth cells located in the base of the small intestinal crypts where they secrete antimicrobial molecules and Wnt ligands to protect and maintain the stem cell niche (Sato et al., 2011). The forest of villi is interrupted by occasional lymphoid nodules referred to as Peyer’s patches. Paneth cells and Peyer’s patches are enriched in the lowest part of the small intestine (ileum) where the density of bacteria is greater. The epithelium above Peyer’s patches include microfold (M) cells, which are IECs that allow luminal contents to pass through and encounter antigen presenting cells (APCs) below. Although critical for immuno-surveillance, M cells increase vulnerability to infection by serving as a point of entry for pathogens.
Figure 1. Specialized epithelial cell lineages promote barrier protection in the small intestine.
Stem cells located in the crypts give rise to intestinal epithelial cells (IECs) with specialized function. Enterocytes predominate the single layer epithelium and participate in the exchange of nutrients. Paneth cells reside in the base of the crypts and secrete antimicrobial peptides, while other types of IECs migrate up the crypt-villus axis. Goblet cells produce mucins that form a physical barrier against invading pathogens and microbionts. Tuft cells secrete cytokines that help initiate immune responses to parasites. M cells line the Peyer’s patches and mediate transport of luminal antigens and bacteria to dendritic cells that initiate B and T cell responses, including production of a diverse repertoire of IgA by plasma cells in the lamina propria, the underlying connective tissue. IECs then transport IgA to the lumen with the polymeric IgA receptor (pIgR). IECs also stimulate cytokine production by innate lymphoid cells (ILCs) in response to adherent microbionts such as segmented filamentous bacteria (SFB), and produce many inflammatory mediators. The function of enteroendocrine cells (which secrete hormones) and other IECs like cup cells in host defense require investigation.
The unabsorbed contents of the small intestine pass by the cecal pouch and the appendix before entering the colon where the density of bacteria is increased further. Antibiotic regiments that deplete these bacteria render the host vulnerable to excessive colonization by opportunistic pathogens such as Clostridium difficile. For this reason, restoring microbionts to the colon through fecal transplantation is receiving considerable attention as a therapeutic intervention for intestinal disease involving host-microbe interactions (Pamer, 2014). The colon does not have villi, and instead has extended crypts, which facilitate uptake of water and metabolites produced by microbionts. The colon also contains gut associated lymphoid tissue (GALT) resembling Peyer’s patches of the small intestine.
IECs migrate from the crypt base towards the tip of the villus (or crypt in the colon) as they undergo several rounds of cell division and are then shed into the lumen (Marchiando, 2016). This continuous but regulated turnover can serve as a defense strategy that prevents pathogens from establishing a foothold. For instance, during nematode infection, the rate of IEC turnover is accelerated by production of the type-2 cytokine interleukin-13 (IL-13), leading to an “epithelial escalator” that facilitates expulsion of the parasite (Cliffe et al., 2005). Also, tight junctional complexes consisting of claudins, occludins, zonula occludens and junctional adhesion molecules, create a seal between neighboring IECs and regulate epithelial permeability to ions, nutrients, and water, while guarding against the entry of microbes between cells. However, the dynamic regulation of unconventional claudin-2 ion channels and other components of the tight junctions allows paracellular transport, and when dysregulated during an immune response, can contribute to barrier breach by bacteria and the sustained inflammation associated with IBD (Marchiando, 2016; Weber et al., 2015). Therefore, the most effective way to prevent a barrier breach is to establish spatial segregation between IECs and luminal microbes.
MUCUS AND ITS GLYCOSYLATION
This separation is partially achieved by goblet cells that secrete heavily glycosylated mucins that oligomerize through disulfide bonds to form mucus. O-linked oligosaccharide modification of the conserved Proline-Threonine-Serine (PTS) repeats in the mucin domain maintains the integrity of the epithelial barrier (Fu et al., 2011). These glycan chains create sticky binding sites in mucus that trap microbes along with antibodies, antimicrobial molecules, and even bacteriophages that can kill the ensnared bacteria (Barr et al., 2013). Mice deficient in Muc2, the most abundant intestinal mucin, lose the ability to contain microbionts within the lumen and are highly susceptible to infection by the model Gram-negative pathogen Citrobacter rodentium (Bergstrom et al., 2010), underscoring this critical role of mucus in defense. The colon contains two layers of mucus, where the inner layer is composed of mucins attached to the epithelium that ensures the absence of bacteria in this region, and the outer loose layer serves as a habitat and nutritional source for microbionts (Johansson et al., 2008). In contrast, enterocytes in the small intestine release the metalloprotease meprin-β, which detaches Muc2 from the epithelium through cleavage, creating loose mucus that carries microbes down the GI tract (Schutte et al., 2014). Despite this lack of an inner dense layer of mucus in the small intestine, the zone surrounding the villi are kept bacteria-free by the high concentration of antimicrobial molecules and antibodies that will be discussed below.
The interactions between mucins and microbes are bidirectional. Muc2 remains attached to the small intestinal epithelium in germ-free mice because the presence of bacteria is necessary for meprin-β secretion (Schutte et al., 2014). Microbionts with mucolytic activity (the ability to degrade mucus) can modulate the diversity of the gut microbiota by altering their ecological niches. A balance in these mucus-degrading commenals is crucial for the host because enrichment of certain genera including Desulfovibrio, Bacteroides, Parabacteroides, and Prevotella are associated with increased permeability of the mucus layer (Jakobsson et al., 2014). Mice deficient in Nod2, a bacterial sensor associated with Crohn’s disease, display an expansion of Bacteroides vulgatus, which is responsible for a reduction in goblet cell mucin granules and Muc2 expression in the small intestine (Ramanan et al., 2014). Additionally, some enteric pathogens use their mucolytic activity to reach the underlying epithelium. The protozoan Entamoeba histolytica secretes cysteine proteases that dissolve mucus by cleaving the less glycosylated and unprotected C-terminal region of Muc2 (Lidell et al., 2006). In an example of subversion, Listeria monocytogenes exploits the process of mucus production to cross the epithelial barrier. E-cadherin, the binding partner for L. monocytogenes invasion protein internalin A, is transiently exposed on the luminal surface of villi by goblet cells that are reorgnizing their tight junctions following granule release, thus allowing the bacteria to engage this surface molecule and breach the epthelium (Nikitas et al., 2011).
Recent findings have increased our understanding of goblet cell regulation and revealed unappreciated ways in which these cells provide feedback to immune cells to further coordinate defense. IECs respond to helminth infections by releasing the cytokines thymic stromal lymphopoietin (TSLP), IL-33, and IL-25 to initiate production of type-2 cytokines IL-4 and IL-13 by Th2 cells and group 2 innate lymphoid cells (ILC2s), which then mediate goblet cell hyperplasia and mucus production. A previously obscure IEC called tuft cells orchestrate this type-2 immunity in response to protozoa and helminths by producing IL-25, in a manner dependent on the taste-chemosensor Trpm5 (Gerbe et al., 2016; Howitt et al., 2016; von Moltke et al., 2016). In turn, goblet cells regulate lymphocyte function by delivering luminal antigens to lamina propria CD103+ dendritic cells (DCs), APCs that promote lymphocyte migration, IgA production, and regulatory T cell (Treg) differentiation (McDole et al., 2012). Also, muc2 binds glycan receptors on DCs to induce anti-inflammatory β-catenin signaling and maintain gut homeostasis (Shan et al., 2013). Thus, like other host defense strategies, glycosylation is subject to exploitation by pathogens.
FUCOSYLATION
Fucosylation is another type of glycosylation that is receiving increasing attention as a mediator of communication between IECs and lymphoid cells. Microbionts that attach to the epithelium, such as segemented filamentous bacteria (SFB), induce group 3 innate lympohid cells (ILC3s) to produce IL-22, which signals back to the epithelium. IL-22 induces IEC production of serum amyloid alpha (SAA), which induces Th17 cell differentiation and IgA production (Atarashi et al., 2015; Sano et al., 2015). Additionally, IL-22 induces fucosyltransferase 2 (Fut2) expression by IECs, which attaches fucose groups to the epithelial surface and protects against enteric Salmonella enterica Typhimurium invasion (Goto et al., 2014). Similarly, epithelial IL-22 receptor (IL-22RA1) signaling promotes intestinal fucosylation by inducing Fut2, and prevents lethal Citrobacter rodentium infection (Pham et al., 2014). Fut2 can also be induced when ILC3s are stimulated by IL-23 from DCs exposed to lipopolysaccharide (LPS) (Pickard et al., 2014). Fucosylated molecules that are released into the small intestinal lumen are consumed by microbionts in the colon, providing a nutrient source for these commenals and thus additional defense against pathogens (Pickard et al., 2014). Consistent with these findings, FUT2 mutation in humans alters the composition of the gut microbiota and confers susceptibility to IBD (Rausch et al., 2011). However, because pathogens often use oligosaccharides for binding, individuals with FUT2 mutations are protected from certain infectious diseases such as gastroenteritis caused by the prominent GII.4 norovirus strains (Lindesmith et al., 2003). Thus, fucosylation plays an important role in regulating the epithelial barrier, microbiota content, and susceptibility to infection.
ANTIMICROBIAL MOLECULES AND IgA
The granules produced by Paneth cells are a major source of antimicrobial molecules, which complement the physical separation created by mucus produced by goblet cells. The presence of the gut microbiota becomes intolerable when Paneth cell function is disrupted (Adolph et al., 2013). In support of a central role of these IECs in barrier maintenance, the appearance of dysfunctional Paneth cells is a remarkably consistent and evolutionarily conserved consequence when Crohn’s disease susceptibility genes are mutated, including Atg16l1 (Cadwell et al., 2008; Lassen et al., 2014), Irgm1 (Liu et al., 2013), Nod2 (Ramanan et al., 2014; VanDussen et al., 2014), and Xbp1 (Kaser et al., 2008). Paneth cell antimicrobial gene expression, and consequently epithelial barrier integrity, is dependent on the histone deacetylase Hdac3 and toll like receptor (TLR) recognition of microbionts (Alenghat et al., 2013; Vaishnava et al., 2008). Like SAA, many antimicrobials produced by Paneth cells and other IECs are also induced by IL-22. Among these are the C-type lectin family of RegIII molecules, which catalyze the formation of hexameric membrane-permeabilizing pores upon binding bacterial surface phospholipids, thus inducing bacterial cell lysis (Mukherjee et al., 2014). RegIII has a particularly important role in establishing a 50μm sterile zone separating the small intestinal epithelium from bacteria (Vaishnava et al., 2011). In the colon, production of RegIII proteins is dependent on Lrrc19, an epithelial cell surface receptor that binds microbionts and controls their relative abundance (Cao et al., 2016).
Neutralization of a broader range of infectious agents, including eukaryotic pathogens and viruses, is achieved through the production of many additional microbicidal molecules including lysozyme, phospholipase A2, and cysteine-rich cationic peptides referred to as defensins. The α-defensins are the best characterized among IEC-derived antimicrobials, and shown to protect against Gram-negative pathogens and destabilization of the microbiota (Salzman et al., 2010). Biologically active a-defensins produced by small intestinal Paneth cells are detected in the colon (Mastroianni and Ouellette, 2009), indicating they are stable and capable of long-range action. While over 20 α-defensins (cryptdins) have been identified in mice, only 2 α-defensins, α-defensin 5 (HD5) and α-defensin 6 (HD6), have been characterized in humans. Although a-defensins generally function by disrupting membrane integrity, human α-defensin 6 (HD6) can assemble to form nanonets that entangle bacteria (Chu et al., 2012). Thus, the assortment of antimicrobial molecules produced by IECs, and their mechanisms of action, matches the diversity of infectious agents encountered in the intestinal environment.
IgA produced by lamina propria plasma cells is transported through IECs by the polymeric immunoglobulin receptor (pIgR) to reach the lumen where it is the most abundant antibody, and sequesters microbes and antigens in a process known as ‘immune exclusion’. The expression of pIgR in IECs is regulated by cytokines, such as IL-17, and both classical and alternative pathways of NFκB activation downstream of TLR signaling (Bruno et al., 2011; Cao et al., 2012). Pathogens and microbionts can proteolytically destruct IgA complexes to favor their own stable colonization over other bacteria in the intestine (Moon et al., 2015). Bacteria that reach the inner mucus layer induce the most potent IgA response, and hence, IgA coating is a defining feature of inflammatory microbionts that pose the greatest threat to barrier integrity (Palm et al., 2014). Thus, IECs play an active role in lymphocyte-mediated spatial segregation by sampling antigens from aggressive bacteria at Peyer’s patches, and transporting IgA specific to these bacteria back into the lumen.
CELLULAR MECHANISMS THAT SUPPORT EPITHELIAL DEFENSE
Immune signaling cascades and stress response pathways that are active in many cellular compartments have IEC-specific functions that are essential for maintaining the barrier (Table 1). The NFκB and RipK1 pathway counteracts signals downstream of cytokines and microbes to prevent epithelial cell death and ensuing inflammation (Dannappel et al., 2014; Nenci et al., 2007; Takahashi et al., 2014). Also, packaging and secretion of granule contents places a tremendous burden on Paneth and goblet cells, and requires pathways that offset the stress caused by an environment undergoing constant flux. Autophagy (macroautophagy) is a key homeostasis pathway in which cellular materials including organelles are sequestered in double-membrane vesicles (autophagosome) and targeted to the lysosome for degradation and recycling. A genetic variant of ATG16L1 encodes a destabilized protein that interferes with autophagosome formation and increases susceptibility to Crohn’s disease (Lassen et al., 2014; Murthy et al., 2014), consistent with a particularly important role of autophagy in host-microbe interactions in the gut. In the small intestine, inactivation of autophagy in Paneth cells causes a decrease in the number of structurally intact granules and inflammatory gene expression (Adolph et al., 2013; Cadwell et al., 2008; Conway et al., 2013). In the colon, deficiency in the Nlrp6 inflammasome or Atg16l1 mutation impairs autophagy and mucus secretion by goblet cells (Lassen et al., 2014; Wlodarska et al., 2014). Whereas Paneth cell autophagy is associated with endoplasmic reticulum (ER) function, goblet cell autophagy mediates the trafficking and function of NADPH oxidase, which produces reactive oxygen species (ROS) necessary to trigger calcium-dependent mucus secretion (Patel et al., 2013). Another function of autophagy in IECs is targeting internalized bacteria for degradation. TLR signaling induces autophagy to prevent S. Typhimurium accumulation within enterocytes (Benjamin et al., 2013). Therefore, autophagy supports Paneth cells, goblet cells, and bacterial killing within enterocytes, which can all contribute to defense against invasive pathogens (Conway et al., 2013; Lassen et al., 2014).
Table 1. In vivo evidence of intestinal epithelial-intrinsic gene function.
The table lists examples in which key factors are exclusively inhibited in the intestinal epithelium through breeding mice expressing the Cre recombinase from the villin promoter (villin-Cre) with mice harboring a “floxed” allele of the indicated genes. The established function of the gene and the main phenotype of the animal are indicated. These examples provide physiological evidence of epithelial-intrinsic functions of conserved cellular factors and pathways.
| Gene | Function | Phenotype | Reference |
|---|---|---|---|
| Atg16L1, Atg5 | Autophagy | Increased dissemination of Salmonella Typhimurium to extra-intestinal sites. | (Conway et al., 2013) (Benjamin et al., 2013) |
| RipK1 | Cell death signaling | Increased epithelial apoptosis, spontaneous enteritis. | (Dannappel et al., 2014; Takahashi et al., 2014) |
| E-cadherin | Cell adhesion | Susceptible to Yersinia enterocolitica infection and chemically induced colitis. | (Schneider et al., 2010) (Grill et al., 2015) |
| TLR5 | Recognition of bacterial flagellin | Spontaneous dissemination of microbionts to extra-intestinal sites. | (Chassaing et al., 2014) |
| Xbp1 | ER stress | Spontaneous enteritis and chemically induced colitis. | (Kaser et al., 2008) |
| Klf4 | Transcriptional regulator of proliferation and differentiation | Suppressed NFkB signaling and resistant to chemically induced colitis. | (Ghaleb et al., 2014) |
| Pparγ | Transcriptional regulator of fatty acid and glucose metabolism | Increased susceptibility to chemically induced colitis. | (Adachi et al., 2006; Mohapatra et al., 2010) |
| IL-18R | IL-18 cytokine receptor | Increased susceptibility to chemically induced colitis. | (Nowarski et al., 2015) |
| IL-25 | Inflammatory cytokine | Increased susceptibility to chemically induced colitis. | (Reynolds et al., 2015) |
| Pofut1 | Notch signaling | Spontaneous enterocolitis and translocation of Gram-negative bacteria to extra-intestinal sites. | (Guilmeau et al., 2008) |
| NEMO/Ikkγ, Ikkβ and Ikkα | NFκB signaling | Spontaneous chronic intestinal inflammation. | (Nenci et al., 2007) |
| Caspase-8, c-Flip | Apoptosis | Spontaneous enteritis and colitis. | (Wittkopf et al., 2013) (Gunther et al., 2011) |
Random mutagenesis experiments in mice provide unbiased evidence that the unfolded protein response (UPR) in IECs activated downstream of ER stress contributes to a balanced coexistence with intestinal bacteria. Point mutations in Muc2 that generate protein aggregates and disrupt ER homeostasis in goblet cells lead to spontaneous colitis (Heazlewood et al., 2008). Mutation of the membrane trafficking molecule Yipf6 causes inflammation throughout the intestine associated with dilation of the ER and Paneth and goblet cell granule defects (Brandl et al., 2012). Similarly, targeted deletion of the UPR activating transcription factor 6 (Atf6), or point mutation in an enzyme that processes Atf6, confers hypersensitivity to colitis (Brandl et al., 2009; Cao et al., 2013). Deletion of the ER-resident disulfide isomerase Anterior Gradient 2 (Agr2) also causes Paneth and goblet cell defects associated with unmitigated ER stress (Zhao et al., 2010). Although autophagy and ER stress are individually important, simultaneous deletion of Atg16l1 and the UPR transcription factor Xbp1 in Paneth cells act synergistically to elicit severe transmural inflammation in the small intestine resembling Crohn’s disease (Adolph et al., 2013), suggesting that these pathways work together to alleviate the secretory burden placed on Paneth cells.
ROS are directly antimicrobial, but as illustrated by their role in autophagy-mediated mucin granule secretion (Patel et al., 2013), they also possess essential signaling functions in the intestine. In the Drosophila midgut and mammalian intestine, colonization by microbiont Lactobacillus species induces epithelial proliferation by stimulating ROS production within enterocytes through NADPH oxidase 1 (Nox1) (Jones et al., 2013). Also, a soluble peptide derived from the plasma-membrane associated protein Annexin-1 is generated during wounding, which signals through a formyl peptide receptor (Fpr) to activate Nox1. ROS generated through this process facilitate wound repair by oxidizing and inactivating PTEN and PTP-Pest, phosphatases that negatively regulate kinases involved in cell migration (Leoni et al., 2013). In contrast, bacterially-induced ROS generated through the Duox pathway in the aging Drosophila intestine causes oxidative stress in intestinal stem cells, dysplasia, and a large increase in the amount of intestinal bacteria. Restoring expression of the peptidoglycan recognition protein SC2 (PGRP-SC2), an inhibitor of innate immune signaling in the epithelium, reduces bacterial burden in the intestine and increases lifespan (Guo et al., 2014). This counterintuitive observation demonstrates that a heightened state of immunity can decrease IEC defense by disrupting tissue architecture.
Many of these pathways and gene expression programs involved in IEC-intrinsic defense are co-regulated downstream of microbial sensing. As discussed above, TLR activation in IECs induces the expression of antimicrobial genes, autophagy, and ROS. Also, infection of IECs by adherent-invasive E. coli (AIEC) activates autophagy downstream of hypoxia-inducible transcription factor 1α (HIF1α) (Mimouna et al., 2014). HIF1α regulates the expression of many pro-barrier genes in IECs undergoing accelerated O2 consumption following exposure to butyrate produced by microbionts (Kelly et al., 2015). These examples illustrate how IECs can directly sense and respond to the environment. However, deployment of IEC-intrinsic defense strategies often requires microbial sensing by non-IECs. In the example of TLR signaling and intestinal homeostasis, the seminal finding that the gut microbiota is protective during chemically-induced colitis (Rakoff-Nahoum et al., 2004) is explained by a surprising B cell- intrinsic role of the TLR adaptor Myd88 (Kirkland et al., 2012). Also, Paneth and goblet cell degranulation is triggered by lymphoid cells that produce interferon (IFN)-γ (Farin et al., 2014). Similarly, the paucity of mucin granules in Nod2-deficient mice is dependent on excess IFN-γ production by lymphocytes (Ramanan et al., 2014).
A major challenge is to understand how cytokine levels are fine-tuned to avoid inopportune responses from IECs. IL-18 produced following microbiont activation of Aim2, a cytosolic DNA sensor, protects against colitis (Hu et al., 2015). However, removal of IL-18 binding protein (IL-18bp), an inhibitor of IL-18, leads to excessive IL-18 receptor signaling in the epithelium, which inhibits goblet cell maturation and increases susceptibility to colitis (Nowarski et al., 2015). The ability to distinguish different infectious threats is also central to mounting an appropriate response. Nlrp6 in IECs promotes a protective IL-18 response when sensing metabolites from the microbiota (Levy et al., 2015), but induces an antiviral interferon response in the presence of viral RNA (Wang et al., 2015). Although many questions remain regarding IL-18 signaling in the gut, we are beginning to understand how exact levels of IL-18 and other immune mediators are tightly controlled, and moving beyond defining a pathway as simply inflammatory versus anti-inflammatory.
CONCLUDING REMARKS
The multifaceted functions of IECs are essential for a stable relationship with microbionts and responding to pathogens. Many of these functions promote spatial segregation. However, once an infectious agent passes through the imposing physical and chemical barrier created by mucus, antibodies, and antimicrobials, IECs continue to promote barrier function through complex communication networks involving APCs and lymphoid cells beneath the barrier. Although not the focus of this review, IECs are an important source of cytokines and other immune mediators that control lymphocyte migration and differentiation, such as retinoic acid, TGFβ, and IL-33 (Peterson and Artis, 2014). The identification of tuft cells as the source of IL-25 illustrates that much more research is necessary to appreciate the origin and regulation of these IEC-derived factors. In addition, the precise functions and cellular pathways of IEC lineages such as cup cells and enteroendocrine cells, and their interactions with other epithelial, immune, and neuronal cell types require further characterization (Grun et al., 2015).
Advanced culturing techniques, including enteroid and organoid systems, are promising ways to investigate mechanisms of IEC-instrinsic defense. Also, co-cultures of IECs with immune cells and sophisticated gene-targeting approaches in animal models can help identify the obscure signals that mediate bidirectional communication. Despite the appreciation that an enormous number of T cells are embedded within the epithelial layer, how IECs and these heterogeneous intra-epithelial lymphocytes (IELs) coordinate barrier protection is poorly understood (Cheroutre et al., 2011). Finally, ongoing research is revealing how interactions between microbionts affect host physiology, and molecular techniques are allowing detailed examination of transkingdom interactions involving other occupants of the GI tract, such as beneficial and pathogenic viruses (Cadwell, 2015; Kernbauer et al., 2014). A major goal in infectious and inflammatory disease therapy is to identify strategies that enhance the host’s ability to tolerate the presence of offending microbes. Given that IEC-intrinsic defense has evolved to achieve this exact task in the remarkably demanding environment of the gut, we suggest that therapeutic breakthroughs may come from integrating the progress highlighted in this review with innovations in cell culture, gnotobiotic animal models, and microbiont manipulation.
Acknowledgments
We would like to thank Thaddeus Stappenbeck for input on the manuscript. KC is supported by US National Institute of Health (NIH) grants DK103788, DK093668, and HL123340, and is a Burroughs Wellcome Fund Investigator in the Pathogenesis of Infectious Diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adolph TE, Tomczak MF, Niederreiter L, Ko HJ, Bock J, Martinez-Naves E, Glickman JN, Tschurtschenthaler M, Hartwig J, Hosomi S, et al. Paneth cells as a site of origin for intestinal inflammation. Nature. 2013 doi: 10.1038/nature12599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi M, Kurotani R, Morimura K, Shah Y, Sanford M, Madison BB, Gumucio DL, Marin HE, Peters JM, Young HA, et al. Peroxisome proliferator activated receptor gamma in colonic epithelial cells protects against experimental inflammatory bowel disease. Gut. 2006;55:1104–1113. doi: 10.1136/gut.2005.081745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alenghat T, Osborne LC, Saenz SA, Kobuley D, Ziegler CG, Mullican SE, Choi I, Grunberg S, Sinha R, Wynosky-Dolfi M, et al. Histone deacetylase 3 coordinates commensal-bacteria-dependent intestinal homeostasis. Nature. 2013;504:153–157. doi: 10.1038/nature12687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atarashi K, Tanoue T, Ando M, Kamada N, Nagano Y, Narushima S, Suda W, Imaoka A, Setoyama H, Nagamori T, et al. Th17 Cell Induction by Adhesion of Microbes to Intestinal Epithelial Cells. Cell. 2015;163:367–380. doi: 10.1016/j.cell.2015.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr JJ, Auro R, Furlan M, Whiteson KL, Erb ML, Pogliano J, Stotland A, Wolkowicz R, Cutting AS, Doran KS, et al. Bacteriophage adhering to mucus provide a non-host-derived immunity. Proc Natl Acad Sci U S A. 2013;110:10771–10776. doi: 10.1073/pnas.1305923110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin JL, Sumpter R, Jr, Levine B, Hooper LV. Intestinal epithelial autophagy is essential for host defense against invasive bacteria. Cell Host Microbe. 2013;13:723–734. doi: 10.1016/j.chom.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergstrom KS, Kissoon-Singh V, Gibson DL, Ma C, Montero M, Sham HP, Ryz N, Huang T, Velcich A, Finlay BB, et al. Muc2 protects against lethal infectious colitis by disassociating pathogenic and commensal bacteria from the colonic mucosa. PLoS Pathog. 2010;6:e1000902. doi: 10.1371/journal.ppat.1000902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandl K, Rutschmann S, Li X, Du X, Xiao N, Schnabl B, Brenner DA, Beutler B. Enhanced sensitivity to DSS colitis caused by a hypomorphic Mbtps1 mutation disrupting the ATF6-driven unfolded protein response. Proc Natl Acad Sci U S A. 2009;106:3300–3305. doi: 10.1073/pnas.0813036106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandl K, Tomisato W, Li X, Neppl C, Pirie E, Falk W, Xia Y, Moresco EM, Baccala R, Theofilopoulos AN, et al. Yip1 domain family, member 6 (Yipf6) mutation induces spontaneous intestinal inflammation in mice. Proc Natl Acad Sci U S A. 2012;109:12650–12655. doi: 10.1073/pnas.1210366109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno ME, Frantz AL, Rogier EW, Johansen FE, Kaetzel CS. Regulation of the polymeric immunoglobulin receptor by the classical and alternative NF-kappaB pathways in intestinal epithelial cells. Mucosal Immunol. 2011;4:468–478. doi: 10.1038/mi.2011.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadwell K. The virome in host health and disease. Immunity. 2015;42:805–813. doi: 10.1016/j.immuni.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadwell K, Liu JY, Brown SL, Miyoshi H, Loh J, Lennerz JK, Kishi C, Kc W, Carrero JA, Hunt S, et al. A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature. 2008;456:259–263. doi: 10.1038/nature07416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao AT, Yao S, Gong B, Elson CO, Cong Y. Th17 cells upregulate polymeric Ig receptor and intestinal IgA and contribute to intestinal homeostasis. J Immunol. 2012;189:4666–4673. doi: 10.4049/jimmunol.1200955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao S, Su X, Zeng B, Yan H, Huang Y, Wang E, Yun H, Zhang Y, Liu F, Li W, et al. The Gut Epithelial Receptor LRRC19 Promotes the Recruitment of Immune Cells and Gut Inflammation. Cell Rep. 2016;14:695–707. doi: 10.1016/j.celrep.2015.12.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao SS, Zimmermann EM, Chuang BM, Song B, Nwokoye A, Wilkinson JE, Eaton KA, Kaufman RJ. The unfolded protein response and chemical chaperones reduce protein misfolding and colitis in mice. Gastroenterology. 2013;144:989–1000. e1006. doi: 10.1053/j.gastro.2013.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassaing B, Ley RE, Gewirtz AT. Intestinal epithelial cell toll-like receptor 5 regulates the intestinal microbiota to prevent low-grade inflammation and metabolic syndrome in mice. Gastroenterology. 2014;147:1363–1377. e1317. doi: 10.1053/j.gastro.2014.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheroutre H, Lambolez F, Mucida D. The light and dark sides of intestinal intraepithelial lymphocytes. Nat Rev Immunol. 2011;11:445–456. doi: 10.1038/nri3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu H, Pazgier M, Jung G, Nuccio SP, Castillo PA, de Jong MF, Winter MG, Winter SE, Wehkamp J, Shen B, et al. Human alpha-defensin 6 promotes mucosal innate immunity through self-assembled peptide nanonets. Science. 2012;337:477–481. doi: 10.1126/science.1218831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cliffe LJ, Humphreys NE, Lane TE, Potten CS, Booth C, Grencis RK. Accelerated intestinal epithelial cell turnover: a new mechanism of parasite expulsion. Science. 2005;308:1463–1465. doi: 10.1126/science.1108661. [DOI] [PubMed] [Google Scholar]
- Conway KL, Kuballa P, Song JH, Patel KK, Castoreno AB, Yilmaz OH, Jijon HB, Zhang M, Aldrich LN, Villablanca EJ, et al. Atg16l1 is required for autophagy in intestinal epithelial cells and protection of mice from Salmonella infection. Gastroenterology. 2013;145:1347–1357. doi: 10.1053/j.gastro.2013.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannappel M, Vlantis K, Kumari S, Polykratis A, Kim C, Wachsmuth L, Eftychi C, Lin J, Corona T, Hermance N, et al. RIPK1 maintains epithelial homeostasis by inhibiting apoptosis and necroptosis. Nature. 2014;513:90–94. doi: 10.1038/nature13608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farin HF, Karthaus WR, Kujala P, Rakhshandehroo M, Schwank G, Vries RG, Kalkhoven E, Nieuwenhuis EE, Clevers H. Paneth cell extrusion and release of antimicrobial products is directly controlled by immune cell-derived IFN-gamma. J Exp Med. 2014;211:1393–1405. doi: 10.1084/jem.20130753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Wei B, Wen T, Johansson ME, Liu X, Bradford E, Thomsson KA, McGee S, Mansour L, Tong M, et al. Loss of intestinal core 1-derived O-glycans causes spontaneous colitis in mice. J Clin Invest. 2011;121:1657–1666. doi: 10.1172/JCI45538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerbe F, Sidot E, Smyth DJ, Ohmoto M, Matsumoto I, Dardalhon V, Cesses P, Garnier L, Pouzolles M, Brulin B, et al. Intestinal epithelial tuft cells initiate type 2 mucosal immunity to helminth parasites. Nature. 2016;529:226–230. doi: 10.1038/nature16527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaleb AM, Laroui H, Merlin D, Yang VW. Genetic deletion of Klf4 in the mouse intestinal epithelium ameliorates dextran sodium sulfate-induced colitis by modulating the NF-kappaB pathway inflammatory response. Inflamm Bowel Dis. 2014;20:811–820. doi: 10.1097/MIB.0000000000000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y, Obata T, Kunisawa J, Sato S, Ivanov II, Lamichhane A, Takeyama N, Kamioka M, Sakamoto M, Matsuki T, et al. Innate lymphoid cells regulate intestinal epithelial cell glycosylation. Science. 2014;345:1254009. doi: 10.1126/science.1254009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill JI, Neumann J, Hiltwein F, Kolligs FT, Schneider MR. Intestinal E-cadherin Deficiency Aggravates Dextran Sodium Sulfate-Induced Colitis. Dig Dis Sci. 2015;60:895–902. doi: 10.1007/s10620-015-3551-x. [DOI] [PubMed] [Google Scholar]
- Grun D, Lyubimova A, Kester L, Wiebrands K, Basak O, Sasaki N, Clevers H, van Oudenaarden A. Single-cell messenger RNA sequencing reveals rare intestinal cell types. Nature. 2015;525:251–255. doi: 10.1038/nature14966. [DOI] [PubMed] [Google Scholar]
- Guilmeau S, Flandez M, Bancroft L, Sellers RS, Tear B, Stanley P, Augenlicht LH. Intestinal deletion of Pofut1 in the mouse inactivates notch signaling and causes enterocolitis. Gastroenterology. 2008;135:849–860. 860 e841–846. doi: 10.1053/j.gastro.2008.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunther C, Martini E, Wittkopf N, Amann K, Weigmann B, Neumann H, Waldner MJ, Hedrick SM, Tenzer S, Neurath MF, et al. Caspase-8 regulates TNF-alpha-induced epithelial necroptosis and terminal ileitis. Nature. 2011;477:335–339. doi: 10.1038/nature10400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Karpac J, Tran SL, Jasper H. PGRP-SC2 promotes gut immune homeostasis to limit commensal dysbiosis and extend lifespan. Cell. 2014;156:109–122. doi: 10.1016/j.cell.2013.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heazlewood CK, Cook MC, Eri R, Price GR, Tauro SB, Taupin D, Thornton DJ, Png CW, Crockford TL, Cornall RJ, et al. Aberrant mucin assembly in mice causes endoplasmic reticulum stress and spontaneous inflammation resembling ulcerative colitis. PLoS Med. 2008;5:e54. doi: 10.1371/journal.pmed.0050054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howitt MR, Lavoie S, Michaud M, Blum AM, Tran SV, Weinstock JV, Gallini CA, Redding K, Margolskee RF, Osborne LC, et al. Tuft cells, taste-chemosensory cells, orchestrate parasite type 2 immunity in the gut. Science. 2016 doi: 10.1126/science.aaf1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Peng L, Kwak YT, Tekippe EM, Pasare C, Malter JS, Hooper LV, Zaki MH. The DNA Sensor AIM2 Maintains Intestinal Homeostasis via Regulation of Epithelial Antimicrobial Host Defense. Cell Rep. 2015;13:1922–1936. doi: 10.1016/j.celrep.2015.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsson HE, Rodriguez-Pineiro AM, Schutte A, Ermund A, Boysen P, Bemark M, Sommer F, Backhed F, Hansson GC, Johansson ME. The composition of the gut microbiota shapes the colon mucus barrier. EMBO reports. 2014 doi: 10.15252/embr.201439263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson ME, Phillipson M, Petersson J, Velcich A, Holm L, Hansson GC. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci U S A. 2008;105:15064–15069. doi: 10.1073/pnas.0803124105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RM, Luo L, Ardita CS, Richardson AN, Kwon YM, Mercante JW, Alam A, Gates CL, Wu H, Swanson PA, et al. Symbiotic lactobacilli stimulate gut epithelial proliferation via Nox-mediated generation of reactive oxygen species. Embo J. 2013;32:3017–3028. doi: 10.1038/emboj.2013.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaser A, Lee AH, Franke A, Glickman JN, Zeissig S, Tilg H, Nieuwenhuis EE, Higgins DE, Schreiber S, Glimcher LH, et al. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell. 2008;134:743–756. doi: 10.1016/j.cell.2008.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly CJ, Zheng L, Campbell EL, Saeedi B, Scholz CC, Bayless AJ, Wilson KE, Glover LE, Kominsky DJ, Magnuson A, et al. Crosstalk between Microbiota-Derived Short-Chain Fatty Acids and Intestinal Epithelial HIF Augments Tissue Barrier Function. Cell Host Microbe. 2015;17:662–671. doi: 10.1016/j.chom.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernbauer E, Ding Y, Cadwell K. An enteric virus can replace the beneficial function of commensal bacteria. Nature. 2014;516:94–98. doi: 10.1038/nature13960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkland D, Benson A, Mirpuri J, Pifer R, Hou B, DeFranco AL, Yarovinsky F. B cell-intrinsic MyD88 signaling prevents the lethal dissemination of commensal bacteria during colonic damage. Immunity. 2012;36:228–238. doi: 10.1016/j.immuni.2011.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassen KG, Kuballa P, Conway KL, Patel KK, Becker CE, Peloquin JM, Villablanca EJ, Norman JM, Liu TC, Heath RJ, et al. Atg16L1 T300A variant decreases selective autophagy resulting in altered cytokine signaling and decreased antibacterial defense. Proc Natl Acad Sci U S A. 2014;111:7741–7746. doi: 10.1073/pnas.1407001111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leoni G, Alam A, Neumann PA, Lambeth JD, Cheng G, McCoy J, Hilgarth RS, Kundu K, Murthy N, Kusters D, et al. Annexin A1, formyl peptide receptor, and NOX1 orchestrate epithelial repair. J Clin Invest. 2013;123:443–454. doi: 10.1172/JCI65831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy M, Thaiss CA, Zeevi D, Dohnalova L, Zilberman-Schapira G, Mahdi JA, David E, Savidor A, Korem T, Herzig Y, et al. Microbiota-Modulated Metabolites Shape the Intestinal Microenvironment by Regulating NLRP6 Inflammasome Signaling. Cell. 2015;163:1428–1443. doi: 10.1016/j.cell.2015.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidell ME, Moncada DM, Chadee K, Hansson GC. Entamoeba histolytica cysteine proteases cleave the MUC2 mucin in its C-terminal domain and dissolve the protective colonic mucus gel. Proc Natl Acad Sci U S A. 2006;103:9298–9303. doi: 10.1073/pnas.0600623103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindesmith L, Moe C, Marionneau S, Ruvoen N, Jiang X, Lindblad L, Stewart P, LePendu J, Baric R. Human susceptibility and resistance to Norwalk virus infection. Nat Med. 2003;9:548–553. doi: 10.1038/nm860. [DOI] [PubMed] [Google Scholar]
- Liu B, Gulati AS, Cantillana V, Henry SC, Schmidt EA, Daniell X, Grossniklaus E, Schoenborn AA, Sartor RB, Taylor GA. Irgm1-deficient mice exhibit Paneth cell abnormalities and increased susceptibility to acute intestinal inflammation. Am J Physiol Gastrointest Liver Physiol. 2013;305:G573–584. doi: 10.1152/ajpgi.00071.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchiando KCaA. Function of Epithelial Barriers. In: Stahl RABaPD., editor. Encyclopedia of Cell Biology. Waltham, MA: Academic Press; 2016. pp. 687–694. [Google Scholar]
- Mastroianni JR, Ouellette AJ. Alpha-defensins in enteric innate immunity: functional Paneth cell alpha-defensins in mouse colonic lumen. J Biol Chem. 2009;284:27848–27856. doi: 10.1074/jbc.M109.050773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDole JR, Wheeler LW, McDonald KG, Wang B, Konjufca V, Knoop KA, Newberry RD, Miller MJ. Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. Nature. 2012;483:345–349. doi: 10.1038/nature10863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimouna S, Bazin M, Mograbi B, Darfeuille-Michaud A, Brest P, Hofman P, Vouret-Craviari V. HIF1A regulates xenophagic degradation of adherent and invasive Escherichia coli (AIEC) Autophagy. 2014;0 doi: 10.4161/15548627.2014.984275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohapatra SK, Guri AJ, Climent M, Vives C, Carbo A, Horne WT, Hontecillas R, Bassaganya-Riera J. Immunoregulatory actions of epithelial cell PPAR gamma at the colonic mucosa of mice with experimental inflammatory bowel disease. PLoS One. 2010;5:e10215. doi: 10.1371/journal.pone.0010215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon C, Baldridge MT, Wallace MA, Burnham CA, Virgin HW, Stappenbeck TS. Vertically transmitted faecal IgA levels determine extra-chromosomal phenotypic variation. Nature. 2015;521:90–93. doi: 10.1038/nature14139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S, Zheng H, Derebe MG, Callenberg KM, Partch CL, Rollins D, Propheter DC, Rizo J, Grabe M, Jiang QX, et al. Antibacterial membrane attack by a pore-forming intestinal C-type lectin. Nature. 2014;505:103–107. doi: 10.1038/nature12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy A, Li Y, Peng I, Reichelt M, Katakam AK, Noubade R, Roose-Girma M, Devoss J, Diehl L, Graham RR, et al. A Crohn’s disease variant in Atg16l1 enhances its degradation by caspase 3. Nature. 2014 doi: 10.1038/nature13044. [DOI] [PubMed] [Google Scholar]
- Nenci A, Becker C, Wullaert A, Gareus R, van Loo G, Danese S, Huth M, Nikolaev A, Neufert C, Madison B, et al. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature. 2007;446:557–561. doi: 10.1038/nature05698. [DOI] [PubMed] [Google Scholar]
- Nikitas G, Deschamps C, Disson O, Niault T, Cossart P, Lecuit M. Transcytosis of Listeria monocytogenes across the intestinal barrier upon specific targeting of goblet cell accessible E-cadherin. J Exp Med. 2011;208:2263–2277. doi: 10.1084/jem.20110560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowarski R, Jackson R, Gagliani N, de Zoete MR, Palm NW, Bailis W, Low JS, Harman CC, Graham M, Elinav E, et al. Epithelial IL-18 Equilibrium Controls Barrier Function in Colitis. Cell. 2015;163:1444–1456. doi: 10.1016/j.cell.2015.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palm NW, de Zoete MR, Cullen TW, Barry NA, Stefanowski J, Hao L, Degnan PH, Hu J, Peter I, Zhang W, et al. Immunoglobulin A coating identifies colitogenic bacteria in inflammatory bowel disease. Cell. 2014;158:1000–1010. doi: 10.1016/j.cell.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamer EG. Fecal microbiota transplantation: effectiveness, complexities, and lingering concerns. Mucosal Immunol. 2014;7:210–214. doi: 10.1038/mi.2013.117. [DOI] [PubMed] [Google Scholar]
- Patel KK, Miyoshi H, Beatty WL, Head RD, Malvin NP, Cadwell K, Guan JL, Saitoh T, Akira S, Seglen PO, et al. Autophagy proteins control goblet cell function by potentiating reactive oxygen species production. Embo J. 2013;32:3130–3144. doi: 10.1038/emboj.2013.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson LW, Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol. 2014;14:141–153. doi: 10.1038/nri3608. [DOI] [PubMed] [Google Scholar]
- Pham TA, Clare S, Goulding D, Arasteh JM, Stares MD, Browne HP, Keane JA, Page AJ, Kumasaka N, Kane L, et al. Epithelial IL-22RA1-mediated fucosylation promotes intestinal colonization resistance to an opportunistic pathogen. Cell Host Microbe. 2014;16:504–516. doi: 10.1016/j.chom.2014.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickard JM, Maurice CF, Kinnebrew MA, Abt MC, Schenten D, Golovkina TV, Bogatyrev SR, Ismagilov RF, Pamer EG, Turnbaugh PJ, et al. Rapid fucosylation of intestinal epithelium sustains host-commensal symbiosis in sickness. Nature. 2014;514:638–641. doi: 10.1038/nature13823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Ramanan D, Tang MS, Bowcutt R, Loke P, Cadwell K. Bacterial Sensor Nod2 Prevents Inflammation of the Small Intestine by Restricting the Expansion of the Commensal Bacteroides vulgatus. Immunity. 2014 doi: 10.1016/j.immuni.2014.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausch P, Rehman A, Kunzel S, Hasler R, Ott SJ, Schreiber S, Rosenstiel P, Franke A, Baines JF. Colonic mucosa-associated microbiota is influenced by an interaction of Crohn disease and FUT2 (Secretor) genotype. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:19030–19035. doi: 10.1073/pnas.1106408108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JM, Lee YH, Shi Y, Wang X, Angkasekwinai P, Nallaparaju KC, Flaherty S, Chang SH, Watarai H, Dong C. Interleukin-17B Antagonizes Interleukin-25-Mediated Mucosal Inflammation. Immunity. 2015;42:692–703. doi: 10.1016/j.immuni.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzman NH, Hung K, Haribhai D, Chu H, Karlsson-Sjoberg J, Amir E, Teggatz P, Barman M, Hayward M, Eastwood D, et al. Enteric defensins are essential regulators of intestinal microbial ecology. Nat Immunol. 2010;11:76–83. doi: 10.1038/ni.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano T, Huang W, Hall JA, Yang Y, Chen A, Gavzy SJ, Lee JY, Ziel JW, Miraldi ER, Domingos AI, et al. An IL-23R/IL-22 Circuit Regulates Epithelial Serum Amyloid A to Promote Local Effector Th17 Responses. Cell. 2015;163:381–393. doi: 10.1016/j.cell.2015.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M, Barker N, Shroyer NF, van de Wetering M, Clevers H. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469:415–418. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider MR, Dahlhoff M, Horst D, Hirschi B, Trulzsch K, Muller-Hocker J, Vogelmann R, Allgauer M, Gerhard M, Steininger S, et al. A key role for E-cadherin in intestinal homeostasis and Paneth cell maturation. PLoS One. 2010;5:e14325. doi: 10.1371/journal.pone.0014325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutte A, Ermund A, Becker-Pauly C, Johansson ME, Rodriguez-Pineiro AM, Backhed F, Muller S, Lottaz D, Bond JS, Hansson GC. Microbial-induced meprin beta cleavage in MUC2 mucin and a functional CFTR channel are required to release anchored small intestinal mucus. Proc Natl Acad Sci U S A. 2014;111:12396–12401. doi: 10.1073/pnas.1407597111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan M, Gentile M, Yeiser JR, Walland AC, Bornstein VU, Chen K, He B, Cassis L, Bigas A, Cols M, et al. Mucus enhances gut homeostasis and oral tolerance by delivering immunoregulatory signals. Science. 2013;342:447–453. doi: 10.1126/science.1237910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N, Vereecke L, Bertrand MJ, Duprez L, Berger SB, Divert T, Goncalves A, Sze M, Gilbert B, Kourula S, et al. RIPK1 ensures intestinal homeostasis by protecting the epithelium against apoptosis. Nature. 2014;513:95–99. doi: 10.1038/nature13706. [DOI] [PubMed] [Google Scholar]
- Vaishnava S, Behrendt CL, Ismail AS, Eckmann L, Hooper LV. Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface. Proc Natl Acad Sci U S A. 2008;105:20858–20863. doi: 10.1073/pnas.0808723105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaishnava S, Yamamoto M, Severson KM, Ruhn KA, Yu X, Koren O, Ley R, Wakeland EK, Hooper LV. The antibacterial lectin RegIIIgamma promotes the spatial segregation of microbiota and host in the intestine. Science. 2011;334:255–258. doi: 10.1126/science.1209791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanDussen KL, Liu TC, Li D, Towfic F, Modiano N, Winter R, Haritunians T, Taylor KD, Dhall D, Targan SR, et al. Genetic variants synthesize to produce paneth cell phenotypes that define subtypes of Crohn’s disease. Gastroenterology. 2014;146:200–209. doi: 10.1053/j.gastro.2013.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Moltke J, Ji M, Liang HE, Locksley RM. Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature. 2016;529:221–225. doi: 10.1038/nature16161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Zhu S, Yang L, Cui S, Pan W, Jackson R, Zheng Y, Rongvaux A, Sun Q, Yang G, et al. Nlrp6 regulates intestinal antiviral innate immunity. Science. 2015;350:826–830. doi: 10.1126/science.aab3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber CR, Liang GH, Wang Y, Das S, Shen L, Yu AS, Nelson DJ, Turner JR. Claudin-2-dependent paracellular channels are dynamically gated. Elife. 2015;4 doi: 10.7554/eLife.09906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittkopf N, Gunther C, Martini E, He G, Amann K, He YW, Schuchmann M, Neurath MF, Becker C. Cellular FLICE-like inhibitory protein secures intestinal epithelial cell survival and immune homeostasis by regulating caspase-8. Gastroenterology. 2013;145:1369–1379. doi: 10.1053/j.gastro.2013.08.059. [DOI] [PubMed] [Google Scholar]
- Wlodarska M, Thaiss CA, Nowarski R, Henao-Mejia J, Zhang JP, Brown EM, Frankel G, Levy M, Katz MN, Philbrick WM, et al. NLRP6 inflammasome orchestrates the colonic host-microbial interface by regulating goblet cell mucus secretion. Cell. 2014;156:1045–1059. doi: 10.1016/j.cell.2014.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F, Edwards R, Dizon D, Afrasiabi K, Mastroianni JR, Geyfman M, Ouellette AJ, Andersen B, Lipkin SM. Disruption of Paneth and goblet cell homeostasis and increased endoplasmic reticulum stress in Agr2−/− mice. Developmental biology. 2010;338:270–279. doi: 10.1016/j.ydbio.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]