Abstract
Evidence suggests that individuals who report fewer total hours of sleep are more likely to be overweight or obese. Few studies have prospectively evaluated weight-loss success in relation to reported sleep quality and quantity. This analysis sought to determine the association between sleep characteristics and weight loss in overweight or obese women enrolled in a randomized clinical trial of a weight-loss program. We hypothesized that in overweight/obese women, significant weight loss would be demonstrated more frequently in women who report a better Pittsburgh Sleep Quality Index (PSQI) Global Score or sleep >7 h/night as compared to women who report a worse PSQI score or sleep ≤7 h/night. Women of ages 45.5 ± 10.4 (mean ± SD) years and BMI of 33.9 ± 3.3 (n = 245) were randomized and completed PSQI at baseline and 6 months; 198 had weight change assessed through 24 months. At baseline, 52.7% reported PSQI scores above the clinical cutoff of 5. Better subjective sleep quality increased the likelihood of weight-loss success by 33% (relative risk (RR), 0.67; 95% confidence interval (CI), 0.52–0.86), as did sleeping >7 h/night. A worse Global Score at 6 months was associated with a 28% lower likelihood of continued successful weight loss at 18 months, but unassociated by 24 months. These results suggest that sleep quality and quantity may contribute to weight loss in intervention-based studies designed to promote weight control in overweight/obese adult women.
INTRODUCTION
Obesity is a major public health problem. Over the past several decades, obesity rates have risen to the degree that currently one billion adults are overweight and one-third of US adults meet the criteria for obesity (1). The National Center for Health Statistics reported that in 2003–2004 the prevalence of overweight and obesity among adults in the United States approached 65% (2). A subgroup of the population at high risk for overweight is adult women (≥18 years of age), particularly postmenopausal women, for whom prevalence approaches 70% (3). According to the National Health and Nutrition Examination Survey (NHANES) 2007–2008, 35.5% of women were obese and 72.3% were overweight or obese (4). The proposed reasons for the surge in overweight and obesity among adult women in the United States are widely debated (3) and include excess energy intake, physical inactivity, genetic predisposition (5,6), weight gain retention after pregnancy (7) and the obesogenic environment (8).
More recently, there has been heightened interest in the role of sleep in weight control and obesity, particularly among women (9). Sleep duration among adults and children is declining worldwide. In the 1960’s, US adults reported sleeping an average of 8–8.9 h/night (10). By 2008, the National Sleep Foundation survey estimated that nightly sleep duration had decreased to an average of 6.6 h/night, and one study reported that mean sleep duration was only 5–6 h/night with rates lowest in employed individuals (11). The recommended sleep duration suggested by the National Sleep Foundation is between 7 and 9 h/night, based on epidemiological evidence indicating that individuals who sleep <6 or >9 h/night have higher mortality rates than those who sleep 7–8 h/night (12–14).
Chronic sleep duration of ≤6 h/night has also been associated with higher BMI (15,16). Even 7 h/night is insufficient according to a longitudinal analyses of NHANES that demonstrated significantly higher rates of obesity in adults who experienced less than 7 h average sleep duration per night (17). What is not well described is the relationship between sleep and outcomes of weight-loss interventions. Using a well-characterized dataset of overweight/obese women participating in a randomized clinical trial of a weight-loss program, we sought to determine whether Pittsburgh Sleep Quality Index (PSQI) score or sleep duration at the time of study enrollment was associated with greater success with weight loss. Additionally, we wanted to explore if additional items on the PSQI were associated with weight-loss success and if sleep score evaluated after 6 months of intervention (weight loss) was associated with longer-term weight loss (weight at 12, 18, and 24 months). We hypothesized that in overweight/obese women significant weight loss (≥10% of baseline body weight) would be demonstrated more frequently in women who sleep ≥7 h/night or who report a better PSQI Global Score as compared to women who sleep <7 h/night or report worse PSQI Score.
METHODS AND PROCEDURES
Study design
This study is an ancillary study within a randomized clinical trial of a commercial weight-loss program (Jenny Craig, Carlsbad, CA) over a period of 24 months and has been previously described (18). Participants were enrolled at four sites: University of California, San Diego, La Jolla, CA; University of Arizona, Tucson, AZ; University of Minnesota, Minneapolis, MN; and Kaiser Permanente Center for Health Research, Portland, OR. This ancillary study involved a subgroup of women who provided self-report information on sleep characteristics at baseline and 6 months. Weight change (responsiveness to intervention in relation to sleep) included weight measurements in this same subgroup through the 24-month time point. This study was approved by the institutional review board of all participating institutions.
Recruitment and intervention
Participants were recruited using targeted recruitment flyers disseminated via electronic mail and onsite, word-of-mouth, radio advertisement, and electronic medical record screening systems. Responders completed a telephone-based eligibility screening. All participants completed the informed consent process during the initial clinic visit. Women assigned to two of the study groups participated in the commercial multifaceted weight-loss program, which included an energy-reduced diet prescription, recommendations to increase physical activity and behavioral counseling. The diet component of the program included prepackaged prepared food items that were incorporated and were accompanied by increased vegetables and fruit. The approach was tailored so that participants could choose regular foods when preferred. Women assigned to the remaining arm served as a usual care comparison group, in which they were provided general weight-loss counseling with a dietetics professional at baseline and 6 months. Women were enrolled in the trial for a 24-month period.
Study participants
Eligibility criteria for this ancillary study included those for the parent study, which were: Aged ≥18 years; BMI 25–40 kg/m2 and a minimum of 15 kg over ideal weight as defined by actuarial tables; not pregnant or breastfeeding or planning to become pregnant in the next 2 years; willing to participate in any of the three study groups over a 2-year period; no eating disorders, food allergies or intolerances; and willing and able to perform a simple step test for assessing cardiopulmonary fitness. Current active involvement in another diet intervention study or organized weight-loss program, or having a history or presence of a significant psychiatric disorder or any other condition that, in the investigator’s judgment, would interfere with participation in the trial, also disqualified women. For this ancillary study, women were recruited after the initiation of recruitment for the parent study, rendering 159 women unavailable for baseline measures (see Figure 1; CONSORT diagram); 24 did not complete the baseline questionnaire and 12 the 6-month questionnaire; 2 were excluded for reasons related to previous diagnosis of obstructive sleep disorder which was self-reported via recruitment health history questionnaire. The final analytical cohort included 245 women. Analysis of longer-term maintenance of weight loss included a subgroup of 198 of the 245 women who demonstrated any weight loss at 6 months and provided repeated measurement of body weight through 24 months.
Figure 1.
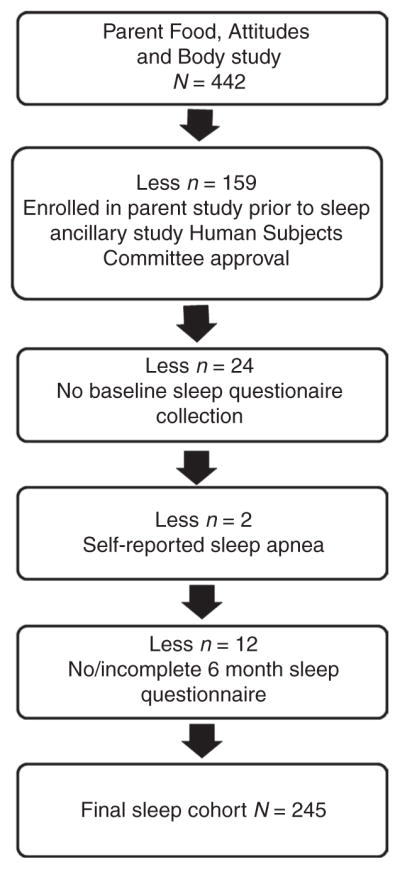
CONSORT of study sample for Sleep Evaluation within the Food, Attitudes, and Body Weight Loss intervention Study (n = 442 reduced to n = 245).
PSQI
The Pittsburg Sleep Quality Index (PSQI) Questionnaire was administered at baseline and 6 months in order to assess sleep duration and quality in the ancillary study participants. The PSQI is a clinical sleep behavior questionnaire validated for use in patients with insomnia (19), cancer (20), and Parkinson’s disease (21); however, it is widely used in general populations as well (22). The PSQI assesses a 1-month interval and contains 19 questions regarding sleep quality, duration, and behavior using Likert scales from “0” to “3”, and an additional five questions rated by the bed partner, if applicable. In the event that a bed partner was not present, the participant was counseled to self-report a response, but only if a bed partner had reported any observations of sleep behaviors to them within the past month. The questionnaire was graded into seven component scores: Sleep quality, sleep latency, habitual sleep efficiency, sleep disturbance, sleeping medication use, and daytime dysfunction; participants also recorded the time they went to bed and the time they woke up and these values were used to compute the component score for sleep duration. In each case, a score of “0” indicated no difficulty, while “3” indicated extreme difficulty. These seven component scores were then summed to determine the participant’s Global Sleep Score, with a range of 0–21 points, “0” indicating no difficulty, and “21” indicating severe difficulty. Global Scores >5 indicated significant sleep complaints (22).
Anthropometric measurements
Height was measured without shoes to the nearest half-inch using standardized procedures and a wall-mounted stadiometer, and weight was measured without shoes or overgarments on a calibrated upright balance scale to the nearest half-pound. Waist and hip circumferences were measured to the nearest half-inch according to study protocol. Women removed outer clothing and stood upright in front of a full-length mirror while a Gulick II measuring tape was extended around the waist and then hip. Waist measures referenced the iliac crest as a referent anatomical site, and hip was measured at the widest point of the hip area. The site study coordinator performed all measures at baseline and at 6, 12, 18, and 24 months, although the focus of this ancillary study was primarily on weight change at 6 months. Furthermore, for this evaluation of sleep characteristics and weight loss, clinically relevant weight loss was defined as change in body weight to 6-month time point of ≥10% baseline weight.
Statistical analyses
Because the focus of this study was on weight loss and not study group assignment, the three study arms were collapsed for evaluation in order to assure adequate statistical power to test the study hypothesis; this ancillary study had no a priori hypothesis to evaluate sleep as a modifier of weight loss in relation to specific interventional approaches used to promote weight loss (i.e., study group assignment). Participant characteristics at baseline were compared between women reporting sleeping >7 or ≤7 h/night (17) using t-tests (age, BMI, waist and hip circumference, waist-to-hip ratio), Wilcoxon rank-sum tests (education and income) or χ2-tests (menopausal status) (ethnicity). Component (0, 1, 2, or 3) and Global (0–21) Sleep Scores at baseline and 6 months were compared using paired signed-rank tests. Dichotomized Global Sleep Scores (≤5 vs. > 5) at baseline and 6 months were compared using McNemar’s χ2-test. Relative risks (RRs) and 95% confidence intervals (CIs) were calculated for associations between each sleep score and successful weight loss. Component sleep scores were dichotomized, thereby comparing “good” sleepers (component score = 0) vs. all others (component score > 0). There was one exception: the sleep disturbances variable was dichotomized differently (score ≤1 vs. >1) as 25% of the subjects reported a score of zero for this component. Potential confounding was assessed for baseline weight, BMI, waist circumference, hip circumference, waist-to-hip ratio, intervention arm, age, menopausal status, ethnicity, and income. None of the aforementioned variables changed the association by at least 10%, however, adjustments were made for age, BMI, race/ethnicity, and intervention arm given likelihood based on the general literature of some confounding in association with these characteristics. Potential associations between sleep scores at 6 months and successful weight loss over time (12, 18, and 24 months) were also tested, restricting to women who demonstrated any weight loss at 6 months (i.e., at least 1 kg) and defining successful weight loss as ≥ 10% baseline weight (NHLBI, 1998). Furthermore, we investigated the relationship between sleep and waist circumference (instead of weight loss) but found no significant associations (data not shown). All statistical analyses were performed using Stata 11.2 (StataCorp, College Station, TX), and all statistical tests were two-sided.
RESULTS
This ancillary study included 245 pre- and postmenopausal women. The study sample was predominantly non-Hispanic white, with a mean ± SD age of 45.5 ± 10.4 years. The majority (87.8%) had at least some college education, and 41.2% were postmenopausal. Participant characteristics at baseline were compared between women reporting >7 vs. ≤7 h of sleep per night (Table 1). As might be expected, age was marginally associated (P = 0.051) and menopausal status significantly associated with sleep quantity. Women reporting more sleep (>7 h/night) were younger and more likely to be premenopausal than women reporting less sleep. By 6 months, mean weight loss was 7.8 kg, with 44.1% of women showing a weight loss of ≥10% of their baseline weight (Table 2). Of note, 198 of the women in this ancillary study completed the 24-month intervention and provided data for analysis of sleep and longer-term weight control. In this subsample, 87.4% demonstrated any weight loss (i.e., ≥1 kg) at 6 months, and 73.1% demonstrated any weight loss at 24 months.
Table 1.
Baseline characteristics of study participants (n = 245), stratified by sleep duration at baseline: mean ± SD (median) or n (%)
| Characteristic | >7 h (n = 89) | ≤7 h (n = 156) | P valuea |
|---|---|---|---|
| Age (years) | 43.7 ± 11.3 (44) | 46.4 ± 9.8 (48) | 0.051 |
| Education | 0.540 | ||
| Some high school | 0 (0.00) | 1 (0.64) | |
| High school graduate | 10 (11.2) | 19 (12.2) | |
| Some college | 36 (40.5) | 66 (42.3) | |
| College graduate | 18 (20.2) | 31 (19.9) | |
| Some post-college education | 25 (28.1) | 39 (25.0) | |
| Race/ethnicity | 0.309 | ||
| Non-Hispanic white | 67 (75.3) | 109 (69.9) | |
| Non-Hispanic black | 5 (5.62) | 18 (11.5) | |
| Hispanic | 14 (15.7) | 24 (15.4) | |
| Other | 3 (3.37) | 5 (3.21) | |
| Household income | 0.887 | ||
| <$25,000 | 4 (4.49) | 5 (3.21) | |
| $25,000–$49,000 | 21 (23.6) | 44 (28.2) | |
| $50,000–$74,000 | 30 (33.7) | 44 (28.2) | |
| $75,000–$99,000 | 19 (21.4) | 31 (19.9) | |
| ≥$100,000 | 15 (16.9) | 32 (20.5) | |
| BMI (kg/m2) | 34.2 ± 3.5 (34) | 33.7 ± 3.2 (34) | 0.316 |
| Waist circumference (cm) | 109 ± 9.8 (109) | 108 ± 9.1 (108) | 0.852 |
| Hip circumference (cm) | 120 ± 7.4 (119) | 119 ± 8.0 (118) | 0.421 |
| Waist-to-hip ratio | 0.91 ± 0.1 (0.9) | 0.91 ± 0.1 (0.9) | 0.539 |
| Menopausal status | 0.019 | ||
| Premenopausal | 61 (68.5) | 83 (53.2) | |
| Postmenopausal | 28 (31.5) | 73 (46.8) |
P calculated from nonparametric rank-sum test (education and income), χ2-test (ethnicity and menopause), and t-test (age, BMI, waist circumference, hip circumference, and waist-to-hip ratio).
Table 2.
Body weight and sleep component and Global Sleep Scores as measured by the Pittsburgh Sleep Quality Index (PSQI) at baseline and 6 months (n = 245)
| Body weight or sleep score | Baseline n (%) | 6 months n (%) | P valuea |
|---|---|---|---|
| Body weight (kg), mean ± SD | 91.8 ± 10.0 | 83.9 ± 10.5 | <0.001 |
| Subjective sleep quality | 0.216 | ||
| 0: Very good | 74 (30.2) | 71 (29.0) | |
| 1: Fairly good | 143 (58.4) | 136 (55.5) | |
| 2: Fairly bad | 23 (9.39) | 33 (13.5) | |
| 3: Very bad | 5 (2.04) | 5 (2.04) | |
| Sleep latency score | 0.101 | ||
| 0: 0 | 105 (42.9) | 92 (37.6) | |
| 1: 1–2 | 92 (37.6) | 100 (40.8) | |
| 2: 3–4 | 38 (15.5) | 39 (15.9) | |
| 3: 5–6 | 10 (4.08) | 14 (5.71) | |
| Sleep duration | 0.092 | ||
| 0: >7 h | 89 (36.3) | 79 (32.2) | |
| 1: 6–7 h | 95 (38.8) | 97 (39.6) | |
| 2: 5–6 h | 57 (23.3) | 61 (24.9) | |
| 3: <5 h | 4 (1.63) | 8 (3.27) | |
| Habitual sleep efficiency | 0.821 | ||
| 0: >85% | 173 (70.6) | 174 (71.0) | |
| 1: 75–84% | 49 (20.0) | 44 (18.0) | |
| 2: 65–74% | 15 (6.12) | 18 (7.35) | |
| 3: <65% | 8 (3.27) | 9 (3.67) | |
| Sleep disturbances score | 0.039 | ||
| 0: 0 | 10 (4.08) | 6 (2.45) | |
| 1: 1–9 | 179 (73.1) | 170 (69.4) | |
| 2: 10–18 | 55 (22.5) | 67 (27.4) | |
| 3: 19–27 | 1 (0.41) | 2 (0.82) | |
| Sleeping medication use | 0.018 | ||
| 0: None during past month | 193 (78.8) | 182 (74.3) | |
| 1: <1 time/week | 22 (8.98) | 25 (10.2) | |
| 2: 1–2 times/week | 13 (5.31) | 13 (5.31) | |
| 3: ≥3 times/week | 17 (6.94) | 25 (10.2) | |
| Daytime dysfunction score | 0.383 | ||
| 0: 0 | 97 (39.6) | 88 (35.9) | |
| 1: 1–2 | 132 (53.9) | 139 (56.7) | |
| 2: 3–4 | 15 (6.12) | 17 (6.94) | |
| 3: 5–6 | 1 (0.41) | 1 (0.41) | |
| Global Sleep Score | 0.013 | ||
| 0–4: Good sleep | 116 (47.4) | 104 (42.5) | |
| 5–21: Poor sleep | 129 (52.7) | 141 (57.6) |
P calculated from nonparametric, paired sign-rank test.
PSQI scores were calculated at baseline and 6 months for 245 participants. At baseline, 116 (47.4%) women reported a PSQI Global Sleep Score <5 (Table 2). At 6 months, the proportion of women with a PSQI Global Score below 5 was 42.5%, a difference that was statistically significant (P = 0.01) and appeared to be related to a change in sleep disturbance score and result in greater use of sleep medications (Table 2).
Potential associations between baseline sleep score and successful weight loss at 6 months also were evaluated, controlling for age, BMI, race/ethnicity and weight-loss study intervention arm. Weight-loss “success” was defined as losing ≥10% body weight from baseline measure (23). Women with not very good (fair/poor) subjective sleep quality (i.e., component score >0) were significantly less likely to achieve successful weight loss than women with a subjective sleep quality score of zero (RR, 0.67; 95% CI, 0.52–0.86; Table 3). Likewise, women who reported sleeping ≤7 h/night were significantly less likely to achieve successful weight loss than women who reported sleeping >7 h/night (RR, 0.70; 95% CI, 0.54–0.91). These two component sleep scores (subjective sleep quality and sleep duration) were strongly correlated (r = 0.39, P < 0.0001); however, calculating RRs stratified on the alternate measure suggested that these factors are independently associated with successful weight loss; similar results were also demonstrated using a model including both terms (data not shown). Use of sleep medications was significantly associated unsuccessful weight loss (RR, 0.61; 95% CI, 0.40–0.93). Global Sleep Score was marginally associated with weight-loss success (RR, 0.79; 95% CI, 0.60–1.02).
Table 3.
Association between Pittsburgh Sleep Quality Index (PSQI) sleep scores at baseline and successful weight loss at 6 months (n = 245)
| Sleep score at baseline | Unsuccessfula weight loss | Successfula weight loss | Crude | Adjustedb |
|---|---|---|---|---|
|
| ||||
| n (%) | RR (95% CI) | |||
| Subjective sleep quality | 0.65 (0.50–0.86) | 0.67 (0.52–0.86) | ||
| 0: Very good | 31 (22.6) | 43 (39.8) | ||
| 1–3: Not very good | 106 (77.4) | 65 (60.2) | ||
| Sleep latency score | 0.87 (0.66–1.15) | 0.86 (0.67–1.12) | ||
| 0: No difficulty | 55 (40.2) | 50 (46.3) | ||
| 1–3: Some difficulty | 82 (59.9) | 58 (53.7) | ||
| Sleep duration | 0.71 (0.54–0.94) | 0.70 (0.54–0.91) | ||
| 0: >7 h | 41 (29.9) | 48 (44.4) | ||
| 1–3:≤7 h | 96 (70.1) | 60 (55.6) | ||
| Habitual sleep efficiency | 0.80 (0.57–1.12) | 0.78 (0.55–1.10) | ||
| 0: >85% | 92 (67.2) | 81 (75.0) | ||
| 1–3: ≤85% | 45 (32.9) | 27 (25.0) | ||
| Sleep disturbances | 1.02 (0.73–1.42) | 0.98 (0.71–1.36) | ||
| 0–1: None/few | 106 (77.4) | 83 (76.9) | ||
| 2–3: Some | 31 (22.6) | 25 (23.2) | ||
| Sleeping medication use | 0.60 (0.38–0.94) | 0.61 (0.40–0.93) | ||
| 0: None | 100 (73.0) | 93 (86.1) | ||
| 1–3: Some | 37 (27.0) | 15 (13.9) | ||
| Daytime dysfunction | 1.07 (0.80–1.43) | 1.03 (0.79–1.36) | ||
| 0: None | 56 (40.9) | 41 (38.0) | ||
| 1–3: Some | 81 (59.1) | 67 (62.0) | ||
| Global Sleep Score | 0.80 (0.61–1.07) | 0.79 (0.60–1.02) | ||
| 0–4: Good sleep | 59 (43.1) | 57 (52.8) | ||
| 5–21: Poor sleep | 78 (56.9) | 51 (47.2) | ||
CI, confidence interval; RR, relative risk.
Unsuccessful weight loss defined as losing <10% baseline body weight between baseline and 6 months; successful weight loss defined as losing ≥10% baseline body weight between baseline and 6 months.
Adjusted for age, BMI, race/ethnicity, and intervention arm.
To explore the relationship between sleep score after weight loss (PSQI measured at 6 months) and demonstrated maintenance of weight-loss over time, RRs for maintaining successful weight loss were calculated for each time point after 6 months among women demonstrating any weight loss from baseline through 24 months (n = 208 at 12 months, 205 at 18 months, and 198 at 24 months). Consistent with our other findings, women who reported “very good” sleep quality score or an average nightly sleep duration of >7 h showed increased likelihood of successfully maintaining weight loss at 12 and 18 months, although these associations were attenuated and nonsignificant at 24 months (Table 4). In addition, a worse habitual sleep efficiency score (≤85%) was associated with a 38% lower likelihood of successful weight-loss maintenance at 18 months (RR, 0.62; 95% CI, 0.42–0.92). Overall PSQI Global Sleep Scores of ≥5 were associated with a lower likelihood of longer-term successful weight loss (RR, 0.80; 95% CI, 0.62–1.02 and RR, 0.72; 95% CI, 0.54–0.95 at 12 and 18 months, respectively). This association was attenuated by 24 months and no longer significant.
Table 4.
Association between Pittsburgh Sleep Quality Index (PSQI) sleep scores at 6 months and successful weight loss over 24 months, restricted to women with any weight loss at 6 months (≥ 1 kg): RR (95% CI)
| Sleep score at 6 months | 12 months (n = 208) | 18 months (n = 205) | 24 months (n = 198) |
|---|---|---|---|
| Subjective sleep quality | 0.83 (0.64–1.06) | 0.74 (0.55–0.98) | 0.88 (0.63–1.22) |
| Sleep latency | 1.01 (0.77–1.32) | 0.85 (0.63–1.14) | 1.16 (0.82–1.64) |
| Sleep duration | 0.64 (0.50–0.81) | 0.76 (0.57–1.00) | 0.85 (0.61–1.19) |
| Habitual sleep efficiency | 0.85 (0.63–1.14) | 0.62 (0.42–0.92) | 0.79 (0.52–1.21) |
| Sleep disturbances | 0.99 (0.75–1.31) | 0.83 (0.60–1.15) | 1.03 (0.73–1.45) |
| Sleeping medication use | 1.02 (0.76–1.35) | 0.82 (0.57–1.16) | 1.00 (0.67–1.50) |
| Daytime dysfunction | 0.84 (0.66–1.09) | 0.87 (0.65–1.17) | 0.97 (0.69–1.35) |
| Global Sleep Score | 0.80 (0.62–1.02) | 0.72 (0.54–0.95) | 1.03 (0.74–1.43) |
CI, confidence interval; RR, relative risk.
Successful weight loss defined as losing ≥ 10% body weight between baseline and each follow-up time point. Adjusted for age, BMI, race/ethnicity, and intervention arm.
DISCUSSION
This study is among the first to report the relation between sleep and weight-loss success in a large sample of overweight or obese women participating in a study of a weight-loss intervention. The findings support the a priori hypothesis that higher sleep quality score and longer sleep duration, as measured by PSQI, are associated with greater likelihood of successful weight loss. The findings support available descriptive literature that has documented the association between shorter sleep duration and risk for overweight and obesity (10,15–16,24), but are among the first to suggest sleep characteristics may influence weight-loss success. Importantly, we dichotomized sleep duration at < or ≥7 h despite some evidence that the relationship between sleep duration and obesity may be U- or J-shaped. This was necessary because only three women reported sleeping ≥9 h/night. It seems that our clinical trial sample therefore differed from epidemiological population samples in this regard. This difference likely reflects the “healthy” volunteer effect commonly demonstrated in recruitment samples participating in diet intervention trials.
There are several possible explanations for our observed associations between PSQI scores and weight loss. A crossover study by Nedeltcheva et al., suggested that individuals consumed more snacks as well as snacks higher carbohydrate content (particularly between 7 PM and 7 AM) when subjected to restricted sleep (5.5 h) vs. normal sleep (8.5 h) duration for 3-week periods (25). These results support the hypothesis that reduced sleep duration may not only provide increased time available for snacking, but also greater carbohydrate consumption, thereby increasing total energy intake and subsequently impairing weight loss. A cross-sectional study among Chinese adults also found a significant association between sleep duration and greater energy consumption, but in this population the excess intake was contributed by dietary fat (26). Specifically, adults sleeping <7 h/night had a significantly higher intake of energy from fat than did those sleeping 7–9 h/night.
Furthermore, inadequate sleep is associated with altered neuroendocrine appetite control characterized by reduced leptin and increased ghrelin concentrations, which are hormones that promote satiety and hunger, respectively (15). This phenomenon was demonstrated by individuals participating in the Wisconsin Sleep Cohort Study (n = 1,024), suggesting that reductions in sleep duration may be associated with increased physiological hunger cues (5). In one of the few prospective crossover trials evaluating the role of sleep on appetite, a study by Spiegel et al., recruited twelve healthy male participants, who were within 10% of ideal body weight, and randomized them first to either restricted sleep duration (4 h) or an extended sleep duration (10 h) (11). The results showed that sleep-restriction led to a 24% increase in hunger ratings, and a 23% increase in appetite, compared with extended sleep. Another crossover-design trial (n = 11, two 14-day observation periods at least 3 months apart) tested the hypothesis that sleep curtailment would promote increased energy intake. These studies support our findings suggesting that sleep duration is a contributing factor in weight control. Of note one limitation to our study is the fact that neither dietary intake nor snacking patterns were assessed in this study, preventing further exploration of effect modification by diet.
Our results did not find a significant association between any of the sleep domains evaluated and waist circumference or waist-to-hip ratio, indicators of central adiposity (data not shown). Yet evidence from a descriptive study by Rontoyanni et al., showed an inverse association between body fatness and nocturnal sleep duration in 30 women (27). In their report, each 1-h decrease in self-reported sleep duration was associated with a 2.8% increase in body fat, after controlling for energy intake and age. Furthermore, in a sample of 1,024 adults participating in the population-based longitudinal Wisconsin Sleep Cohort Study, mean BMI was shown to differ in participants who reported mean sleep durations of 8 or 5 h, respectively (28). In our sample of overweight and obese women enrolling in a weight-loss trial, the mean BMIs were not different in women sleeping <7 vs. >7 h/night, likely related to the limited BMI range required for study eligibility.
In one of the few studies that evaluated weight loss during two distinct 14-day sleep duration periods (5.5 vs. 8.5 h/night), weight loss was not significantly different between sleep periods (n = 10). However, the body composition lost differed, with fat mass comprising the majority of weight lost in the group sleeping 8.5 h/night and lean body mass comprising the majority of weight lost in the group sleeping 5.5 h/night. These results suggest that sleep may play a role in preserving lean body mass during weight loss (29) that could promote higher resting energy expenditure and greater weight-loss over time.
Independent of sleep duration, subjective sleep quality has been evaluated as a predictor of BMI, but not in relation to successful weight loss. Poor subjective Sleep Quality score, as evaluated using the PSQI, was shown to be associated with increased BMI in African American women participating in the Cardiovascular Health Epidemiology Study (30). In that analysis, sleep duration, sleep disturbance, and daytime dysfunction also were significant predictors of obesity. Although our study did not find daytime dysfunction or sleep disturbance to be predictors of weight-loss success, sleep duration was significantly associated with successful weight loss (RR, 0.70; 95% CI, 0.51–0.96).
Habitual sleep efficiency, while not associated with successful weight loss at 6 months, became a significant factor at 18 months, suggesting that continual (habitual) unhealthy sleep may reduce the potential for successful long-term weight loss and maintenance. No other studies have specifically evaluated this relationship. However, physical activity is associated with improved sleep efficiency (31) and weight-loss success and maintenance (32,33). Engaging in at least 200 minutes of weekly, moderate-intensity physical activity, such as brisk walking, is recommended by the 2008 Physical Activity Guidelines for Americans (34) and has been identified as an important predictor of long-term weight loss success in the National Weight Loss Cohort (35). The intervention used here did not target increased physical activity beyond basic guidance on the topic and also did not specifically measure physical activity. It is unknown to what extent activity may or may not have changed and/or contributed to weight loss in these women.
This study is the first prospective study to evaluate sleep as a contributor to weight-loss success, and one of a few to evaluate sleep and weight associations with a relatively large sample size. Several limitations should be considered. First, our study was not able to quantify sleep duration and sleep quality or assess sleep disordered breathing in a sleep laboratory using polysomnography, which is the gold standard in sleep research. However, we did assess history of sleep apnea by self-report and applied this as an exclusionary criteria for sampling. Furthermore, the PSQI is a well-established questionnaire that has been validated among many study populations. Future research may also want to include actigraphy, which is an accelerometer that has the capability to record continuous sleep-wake activity over multiple days; some devices can also quantify energy expenditure. Additionally, data on dietary intake and quantifiable physical activity were not available, which would help to more fully elucidate the association between dietary intakes and sleep duration or quality. Given the large measurement error demonstrated in relation to self-reported energy intake among overweight women, it is not clear that these additional data would provide meaningful results for interpretation (36). Finally, central or total adiposity was not directly measured to more fully characterize the relationship between sleep and body composition; rather, indirect measures of central adiposity, including circumferences and BMI, were used instead.
The results of this study suggest that overweight/obese women who enter a weight-loss program would have a higher chance of continued weight-loss success (at 6 months as well as later time points) if sleep quality is high and/or sleep duration averages >7 h/night at the time of study enrollment. These findings may be important in the overall approach to obesity management among women in that sleep may be a modifiable component of weight-loss therapy. Further research to characterize sleep in the context of weight-loss interventions should be pursued as targeted efforts to improve sleep quality and duration could be implemented, thereby promoting greater weight-loss success.
Acknowledgments
This study was funded by Jenny Craig, Carlsbad, CA. The study is registered in http://clinicaltrials.gov/, registration number NCT00640900. An additional source of support included the graduate assistantship provided to Kelly Morrow as a graduate student in Nutritional Sciences at the University of Arizona in Tucson, AZ. C.A.T. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The sponsor, Jenny Craig, Inc., had a minimal role in the design and protocol development. By contractual agreement, scientists at the University of California, San Diego, and the other participating institutions had responsibility and independence regarding data management, analysis, and publication. The funding sponsor had no role in the collection, analysis, or interpretation of the data; or in the preparation, review, or approval of the manuscript.
Footnotes
DISCLOSURE
The authors declared no conflict of interest.
See the online ICMJE Conflict of Interest Forms for this article.
References
- 1.World Health Organization. [Accessed 10 June 2011];Information Sheet on Obesity and Overweight. 2011 < http://www.who.int/dietphysicalactivity/publications/facts/obesity/en/>.
- 2.Kumanyika SK, Obarzanek E, Stettler N, et al. American Heart Association Council on Epidemiology and Prevention, Interdisciplinary Committee for Prevention. Population-based prevention of obesity: the need for comprehensive promotion of healthful eating, physical activity, and energy balance: a scientific statement from American Heart Association Council on Epidemiology and Prevention, Interdisciplinary Committee for Prevention (formerly the expert panel on population and prevention science) Circulation. 2008;118:428–464. doi: 10.1161/CIRCULATIONAHA.108.189702. [DOI] [PubMed] [Google Scholar]
- 3.Daniels J. Obesity: America’s epidemic. Am J Nurs. 2006;106:40–9. doi: 10.1097/00000446-200601000-00028. quiz 49. [DOI] [PubMed] [Google Scholar]
- 4.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 5.Trenell MI, Marshall NS, Rogers NL. Sleep and metabolic control: waking to a problem? Clin Exp Pharmacol Physiol. 2007;34:1–9. doi: 10.1111/j.1440-1681.2007.04541.x. [DOI] [PubMed] [Google Scholar]
- 6.Hebebrand J, Hinney A. Environmental and genetic risk factors in obesity. Child Adolesc Psychiatr Clin N Am. 2009;18:83–94. doi: 10.1016/j.chc.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 7.Baker JL, Gamborg M, Heitmann BL, et al. Breastfeeding reduces postpartum weight retention. Am J Clin Nutr. 2008;88:1543–1551. doi: 10.3945/ajcn.2008.26379. [DOI] [PubMed] [Google Scholar]
- 8.Devine CM, Nelson JA, Chin N, Dozier A, Fernandez ID. “Pizza is cheaper than salad”: assessing workers’ views for an environmental food intervention. Obesity (Silver Spring) 2007;15(Suppl 1):57S–68S. doi: 10.1038/oby.2007.388. [DOI] [PubMed] [Google Scholar]
- 9.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 10.Van Cauter E, Knutson KL. Sleep and the epidemic of obesity in children and adults. Eur J Endocrinol. 2008;159(Suppl 1):S59–S66. doi: 10.1530/EJE-08-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141:846–850. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- 12.Wingard DL, Berkman LF, Brand RJ. A multivariate analysis of health-related practices: a nine-year mortality follow-up of the Alameda County Study. Am J Epidemiol. 1982;116:765–775. doi: 10.1093/oxfordjournals.aje.a113466. [DOI] [PubMed] [Google Scholar]
- 13.Miller MA, Cappuccio FP. Inflammation, sleep, obesity and cardiovascular disease. Curr Vasc Pharmacol. 2007;5:93–102. doi: 10.2174/157016107780368280. [DOI] [PubMed] [Google Scholar]
- 14.Chen R, Takahashi T, Kanda T. Bariatric surgery for morbid obesity. N Engl J Med. 2007;356:2176–2183. [PubMed] [Google Scholar]
- 15.Knutson KL, Van Cauter E. Associations between sleep loss and increased risk of obesity and diabetes. Ann N Y Acad Sci. 2008;1129:287–304. doi: 10.1196/annals.1417.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Cauter E, Knutson KL. Sleep and the epidemic of obesity in children and adults. Eur J Endocrinol. 2008;159:S59–S66. doi: 10.1530/EJE-08-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gangwisch JE, Malaspina D, Boden-Albala B, Heymsfield SB. Inadequate sleep as a risk factor for obesity: analyses of the NHANES I. Sleep. 2005;28:1289–1296. doi: 10.1093/sleep/28.10.1289. [DOI] [PubMed] [Google Scholar]
- 18.Rock CL, Flatt SW, Sherwood NE, et al. Effect of a free prepared meal and incentivized weight loss program on weight loss and weight loss maintenance in obese and overweight women: a randomized controlled trial. JAMA. 2010;304:1803–1810. doi: 10.1001/jama.2010.1503. [DOI] [PubMed] [Google Scholar]
- 19.Morgan K, Thompson J, Dixon S, Tomeny M, Mathers N. Predicting longer-term outcomes following psychological treatment for hypnotic-dependent chronic insomnia. J Psychosom Res. 2003;54:21–29. doi: 10.1016/s0022-3999(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 20.Beck SL, Schwartz AL, Towsley G, Dudley W, Barsevick A. Psychometric evaluation of the Pittsburgh Sleep Quality Index in cancer patients. J Pain Symptom Manage. 2004;27:140–148. doi: 10.1016/j.jpainsymman.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 21.Wang G, Cheng Q, Zeng J, et al. Sleep disorders in Chinese patients with Parkinson’s disease: validation study of a Chinese version of Parkinson’s disease sleep scale. J Neurol Sci. 2008;271:153–157. doi: 10.1016/j.jns.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 22.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 23.National Heart Lung and Blood Institute. [Accessed 10 June 2011];Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults: The Evidence Report. 1998 < http://www.nhlbi.nih.gov/guidelines/obesity/ob_gdlns.htm>.
- 24.Chaput JP, Klingenberg L, Sjödin A. Do all sedentary activities lead to weight gain: sleep does not. Curr Opin Clin Nutr Metab Care. 2010;13:601–607. doi: 10.1097/MCO.0b013e32833ef30e. [DOI] [PubMed] [Google Scholar]
- 25.Nedeltcheva AV, Kilkus JM, Imperial J, et al. Sleep curtailment is accompanied by increased intake of calories from snacks. Am J Clin Nutr. 2009;89:126–133. doi: 10.3945/ajcn.2008.26574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shi Z, McEvoy M, Luu J, Attia J. Dietary fat and sleep duration in Chinese men and women. Int J Obes (Lond) 2008;32:1835–1840. doi: 10.1038/ijo.2008.191. [DOI] [PubMed] [Google Scholar]
- 27.Rontoyanni VG, Baic S, Cooper AR. Association between nocturnal sleep duration, body fatness, and dietary intake in Greek women. Nutrition. 2007;23:773–777. doi: 10.1016/j.nut.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 28.Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1:e62. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nedeltcheva AV, Kilkus JM, Imperial J, Schoeller DA, Penev PD. Insufficient sleep undermines dietary efforts to reduce adiposity. Ann Intern Med. 2010;153:435. doi: 10.1059/0003-4819-153-7-201010050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bidulescu A, Din-Dzietham R, Coverson DL, et al. Interaction of sleep quality and psychosocial stress on obesity in African Americans: the Cardiovascular Health Epidemiology Study (CHES) BMC Public Health. 2010;10:581. doi: 10.1186/1471-2458-10-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Watenpaugh DE. The role of sleep dysfunction in physical inactivity and its relationship to obesity. Curr Sports Med Rep. 2009;8:331–338. doi: 10.1249/JSR.0b013e3181c27834. [DOI] [PubMed] [Google Scholar]
- 32.Hill JO, Thompson H, Wyatt H. Weight maintenance: what’s missing? J Am Diet Assoc. 2005;105:S63–S66. doi: 10.1016/j.jada.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 33.Donnelly JE, Blair SN, Jakicic JM, et al. American College of Sports Medicine. American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41:459–471. doi: 10.1249/MSS.0b013e3181949333. [DOI] [PubMed] [Google Scholar]
- 34.Physical Activity Guidelines Advisory Committee Report to the Secretary of Health and Human Services. [Accessed 23 April 2011];US Department of Health and Human Services web site. 2008 < http://www.health.gov/PAGuidelines/committeereport.aspx>.
- 35.Catenacci VA, Ogden LG, Stuht J, et al. Physical activity patterns in the National Weight Control Registry. Obesity (Silver Spring) 2008;16:153–161. doi: 10.1038/oby.2007.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abbot JM, Thomson CA, Ranger-Moore J, et al. Psychosocial and behavioral profile and predictors of self-reported energy underreporting in obese middle-aged women. J Am Diet Assoc. 2008;108:114–119. doi: 10.1016/j.jada.2007.10.007. [DOI] [PubMed] [Google Scholar]


