Abstract
Objective
A retrospective chart review was performed to determine patient outcomes before and after partnership by gynecologic oncologists (GYN/ONC) with a sarcoma center (SC) for patients with recurrent unresectable/metastatic (RM) uterine leiomyosarcoma (uLMS).
Methods
58 RM patients, identified from medical records of uLMS patients cared for by either GYN/ONC service and/or the SC between 1/1/2000–4/1/2014, were audited for patient and tumor characteristics, outcomes, and clinical trials enrollments.
Results
Of the 58 patients, 26 patients (48%) were treated by GYN/ONC alone and 32 were treated by a combination of GYN/ONC and SC (52%). Age, race, tumor size, grade, presence of lymphovascular invasion, cervical involvement, and FIGO stage at diagnosis were not statistically different between the two groups. There was a significant difference between the number of clinical trial enrollments (0.07 vs 0.84 trials/patient, p<0.001) and the number of chemotherapy regimens prescribed (2.67 vs 4.29/patient, p=0.03) by GYN/ONC vs SC; the latter was driven by the number of clinical trial enrollments by the SC. Sixty-nine percent of patients referred to the SC were enrolled in at least one clinical trial, while just 8% of patients in the GYN/ONC group were enrolled in at least one clinical trial, a difference that is significant (p<0.0001).
Conclusions
Referral of RM uLMS patients by GYN/ONC to a dedicated clinical trials-based SC resulted in an increase in the number of chemotherapy regimens prescribed and clinical trial enrollments. Partnership between GYN/ONC and a dedicated SC with access to clinical trials should be encouraged for all RM uLMS patients.
Keywords: Uterine Leiomyosarcoma, Clinical Trials, Overall Survival, Gynecologic Oncology, Medical Oncology, Sarcoma Center
Introduction
Sarcomas represent approximately 8% of all uterine malignancies, and uterine leiomyosarcoma (uLMS) is the most common form (1). uLMS is typically diagnosed in peri-and post-menopausal women between the ages of 51–56 years of age, though it can occur at any adult age (2). Although most women with suspected uterine sarcomas are asymptomatic at presentation, many report irregular bleeding patterns or a rapidly enlarging pelvic mass(3). Sixty percent of patients present with disease limited to the uterus, and remission rates vary from 20–60% based upon the extent of disease at the time of primary resection(3–5). Generally, uLMS is associated with a poor prognosis and five-year overall survival (OS) rates of 25%(2, 6). Stage at presentation is the most important prognostic indicator for uLMS (6, 7). Even for patients with stage I and II disease, there is a 70% recurrence rate(3, 5). Relapse often occurs as metastases to the lungs or the liver due to its hematogenous spread(3, 5)
Due to the rarity of uLMS, there have been few clinical trials that test new agents dedicated solely to RM disease. Chemotherapy for patients with RM uLMS is considered to be palliative. Though the National Comprehensive Cancer Center (NCCN) guidelines state that the standard of care is participation in a clinical trial, the actual enrollment of uLMS patients in clinical trials is only 3%, well below the target participation rates of 10–15%(8). As systemic therapy for advanced uLMS generally follows the recommendations for adult soft tissue sarcoma (STS)(9), referral of uLMS patients to sarcoma centers (SC) for participation in clinical trials for adult STS has the potential to increase both the proportion of gynecology oncology patients with uLMS enrolled and the exposure of uLMS patients to potential sequential therapeutic agents. However, at this time, access to a wide range of adult STS clinical trials, with a few exceptions, is limited to cancer centers with a dedicated sarcoma group. No studies to date have compared clinical trial enrollments and OS of gynecology oncology patients with RM uLMS treated at a cancer center with an emphasis on clinical trials-based sarcoma treatment to those managed by gynecology oncologists (GYN/ONC) alone. We hypothesized that active referral to a specialized center for the treatment of soft tissue sarcomas would increase clinical trial enrollment and improve outcomes of patients with RM uLMS. The Siteman Cancer Center Sarcoma Program at Barnes Jewish Hospital / Washington University School of Medicine (SC) was formalized in 2010 and consists of a multidisciplinary team of adult and pediatric medical oncologists who specialize in the treatment of sarcoma, gynecologic oncologists, surgical pathologists, surgical and orthopedic oncologists, interventional radiologists and radiation oncologists who work together to improve patient survival for patients with these rare tumors.
Methods
Patient Population
From January 1, 2000 to April 1, 2014, 67 patients diagnosed with RM uLMS were managed at Barnes Jewish Hospital / Siteman Cancer Center at Washington University in St. Louis. Patients were managed by either the gynecology oncology department, a medical oncology center specializing in sarcoma, or by both departments concurrently or independently. Patient databases for both the Siteman Cancer Center and the gynecologic oncology department identified 58 patients with pathological diagnosis of uLMS and recurrent unresectable and/or metastatic disease. Nine additional patients with uLMS experienced NED after surgical debulking and adjuvant therapy and thus were excluded from the analysis because their disease was resectable and had not recurred.
Staging was defined according to the 2009 International Federation of Obstetricians and Gynecologists (FIGO) surgical staging system. For patients operated on prior to 2009, stage was determined retrospectively from post-surgical pathological assessments. Histological classification was performed according to the World Health Organization classification for uLMS. In each case, the pathology records were reviewed to confirm diagnosis and characteristics of tumors were abstracted from original pathology reports. Once pathological diagnosis was confirmed, the medical records were reviewed for demographics, chemotherapy treatments, and outcome parameters.
The diagnosis of RM disease was made by either pathological confirmation or radiographic diagnosis. At diagnosis of RM disease, patients subsequently received chemotherapy and/or palliative radiation. Subsequent therapy was at the discretion of the treating gynecologic oncologist, or if the patient had been referred to the SC, by the medical oncologist specializing in the treatment of sarcoma. Clinical trial enrollment was undertaken by either gynecology oncology or medical oncology. Follow up of patients was performed using information reported in the clinical history. When information about survival or death was not sufficiently detailed in the clinical history or electronic medical record, verification and date of death was sought from state death registries and/or obituaries.
Statistical analysis
OS was used as the primary endpoint. OS was defined as the time from the time of initial relapse or diagnosis of metastatic disease to death from any cause or last date of contact. OS was also determined from the date of the diagnosis of RM disease to death from any cause or last date of contact. Continuous and categorical variables were compared by a Kruskal-Wallis test and the Fisher Exact (or chi-square) test, respectively. Kaplan-Meier (KM) curves were generated that provide unadjusted survival estimates for all patients and across group. Differences between groups were determined by log-rank tests. Univariate analyses through Cox proportional-hazards models were considered to evaluate the interested variables for OS. All statistical tests were two-sided using an α = 0.05 level of significance. SAS Version 9.3 (Cary, NC) was used to perform all statistical analyses.
Results
Patient Characteristics
During the study period of 2000–2013, fifty-eight patients with RM uLMS were diagnosed and treated at Siteman Cancer Center / Barnes Jewish Hospital and Washington University School of Medicine. The average age of all patients was 54 years. Twenty-six patients were treated exclusively by GYN/ONC, while 32 patients were treated by a medical oncologist specializing in sarcoma treatment (SC) or treated in conjunction with both services. Twenty-two of those patients in the SC group were initially treated by GYN/ONC, with cross over to the SC group after referral. Forty-two patients (72.4%) were Caucasian, while 16 patients (27.6%) were African American (TABLE 1).
Table 1. Clinical Features of Patients with Leiomyosarcoma.
Demographic characteristics of RM uLMS patients, including age and race; clinical tumor characteristics including presence of cervical involvement, grade, tumor size, average number of mitotic figures, presence of LVSI (lymphovascular space invasion), and FIGO stage.
| Variable | Gyn Oncology (n=26) | Medical Oncology (n=32) | P[1] |
|---|---|---|---|
| Age at diagnosis (yr), mean (range) | 57 (38–88) | 52 (31–67) | 0.25 |
| Race | 0.92 | ||
| Caucasian | 19 | 23 | |
| African American | 7 | 9 | |
| Cervical Involvement[2] | 0.47 | ||
| No | 13 | 18 | |
| Yes | 6 | 4 | |
| Grade[2] | 0.64 | ||
| Low | 3 | 2 | |
| High | 19 | 26 | |
| Tumor Size (cm), mean (range)[3] | 13.9 (4–35) | 12.5 (3.5–27) | 0.6 |
| Avg # Mitotic Figures, mean (range)[3] | 21.9 (3–70) | 17.1 (5–66) | 0.14 |
| LVSI[2] | 0.97 | ||
| No | 6 | 7 | |
| Yes | 10 | 12 | |
| FIGO Stage (2009) | 0.28 | ||
| Unstaged/Unk | 4 | 4 | |
| I | 2 | 9 | |
| II | 1 | 3 | |
| III | 3 | 2 | |
| IV | 16 | 14 |
Chi-square or Fisher Exact test for categorical variable; Kruskal-Wallis Test for continuous variable.
The denominator for the percentages is the sum of patients across all categories in each group, respectively, excluding missing values.
Missing values
Stage of Disease and Tumor Characteristics
In the gynecology oncology treatment group, 16 patients (61.5%) were diagnosed with stage IV disease at the time of treatment, 3 with stage III disease (11.5%), 1 with stage II disease (3.8%), and 2 with stage I disease (7.7%). In the treatment group cared for by the SC or GYN/ONC and the SC, 14 patients (43.8%) were diagnosed with stage IV disease, 2 with stage III (6.3%) disease, 3 with stage II disease (9.4%), and 9 with stage I (28.1%) disease. There was no statistically significant difference in the stage distribution between the two treatment groups (p=0.28). Regardless of the stage of disease prior to initial treatment, all patients included for analysis were diagnosed with RM uLMS (TABLE 1).
A majority of uLMS were diagnosed as high grade (86.4% in the GYN/ONC group vs 92.9% in the combined referral group, p=0.64). The average number of mitotic figures per 10/hpf was 21.9 in the gynecology oncology group and 17.1 in the combined referral group, a difference that was not significant. The average tumor size in the largest dimension per pathology report was 13.9 centimeters (cm) in the GYN/ONC treatment group, and 12.5 cm in the SC group, with no difference between the two groups. There was no significant difference between the frequency of lympho-vascular invasion (LVI) or uterine cervical involvement between the two treatment groups (TABLE 1).
Treatment Regimens
Neoadjuvant chemotherapy was not given. The number of patients who were prescribed adjuvant chemotherapy was not different between the GYN/ONC treatment group and the SC treatment group (11 vs. 14 patients, p=0.99). Fifteen patients in the GYN/ONC treatment group did not receive adjuvant chemotherapy. Eighteen patients in the SC did not receive adjuvant chemotherapy. In regards to palliative therapy, all thirty-two patients referred to the SC treatment group were prescribed palliative chemotherapy, while 16 patients (61.5%) in the GYN/ONC treatment group were prescribed palliative chemotherapy (TABLE 2). Of the remaining 10 patients in the GYN/ONC treatment group who did not receive palliative therapy, four died within 30 days of staging surgery, three elected for hospice treatment at the time of the diagnosis of RM disease, two patients had yet to progress after having resection of metastatic disease, and data regarding specific types of palliative treatment was incomplete for one patient.
Table 2. Clinical Trial Enrollment by Service.
Description of clinical trial enrollment by the GYN/ONC service compared to the SC service.
| Chemotherapy | GYN/ONC (n=26) | SC (n=32) | P[1] |
|---|---|---|---|
| Palliative (# Pts prescribed), (%) | 16 (61.5) | 32 (100) | <0.001 |
| No Palliative (# Pts prescribed), (%) | 10 (38.5) | 0 (0) | |
| Total Regimens (average), mean (range)[2] | 2.67 (1–8) | 4.29 (1–11) | 0.03 |
| Off-trial Regimens (average), mean (range)[2] | 2.67 (1–8) | 3.06 (0–8) | 0.25 |
| Clinical Trial Enrollment | < 0.0001 | ||
| No | 24 (92%) | 10 (31%) | |
| Yes | 2 (8%) | 22 (69%) |
Chi-square or Fisher Exact test for categorical variable; Kruskal-Wallis Test for continuous variable.
Missing values
Forty-six of 58 patients (79%) received adjuvant or palliative chemotherapy as prescribed by either GYN/ONC or SC treatment groups. Of those patients that received adjuvant or palliative chemotherapy, 39 out of 49 (80%) first-line regimens consisted of a gemcitabine and docetaxel combination. One patient was enrolled in a clinical trial that included gemcitabine and docetaxel as first-line therapy, and the additional first-line regimens prescribed by both treatment groups are listed in Table 3. Thirty-seven of 58 patients (64%) received a second line regimen, which included doxorubicin single agent (22% of second-line regimens), gemcitabine and docetaxel combination (14% of second-line regimens), or hormonal treatment (letrozole, anastrozole) (14% of second-line regimens), with additional prescribed regimens listed in Table 3. Four patients were enrolled in a clinical trial as second-line treatment. Additionally, a spectrum of single and combination agent chemotherapy regimens were employed beyond second line, including pazopanib, megestrol acetate, ifosfamide, docetaxel, carboplatin, and paclitaxel.
Table 3. Chemotherapy Regimens.
Quantitative description of chemotherapy regimens, including clinical trial regimens, that RM uLMS patients were enrolled as first-line, second-line, third-line, and fourth-line and greater.
| Regimen | Number | Percent |
|---|---|---|
| First-line (n=49) | ||
| Gemcitabine / docetaxel | 39 | 80 |
| Liposomal doxorubicin | 2 | 4 |
| Megestrol acetate | 2 | 4 |
| Gemcitabine / docetaxel / doxorubicin | 1 | 2 |
| Clinical Trials: Gemcitabine / docetaxel / MORAb-4 (1pt) | 1 | 2 |
| Aromatase inhibitor (anastrozole) | 1 | 2 |
| Gemcitabine | 1 | 2 |
| Doxorubicin | 1 | 2 |
| Pazopanib | 1 | 2 |
| Second-line (n=37) | ||
| Doxorubicin | 8 | 22 |
| Gemcitabine / docetaxel | 5 | 14 |
| Aromatase inhibitor (letrozole, anastrozole) | 5 | 14 |
| Clinical Trials: tivosanib (2 pts), doxorubicin / TH302 (1pt), doxorubicin / PDSF-α ab (1pt) | 4 | 11 |
| Liposomal doxorubicin | 4 | 11 |
| Dacarbazine | 2 | 5 |
| Gemcitabine | 2 | 5 |
| Additional agents: Pazopanib megestrol acetate, doxorubicin / ifosfamide, doxorubicin / dacarbazine, paclitaxel, carboplatin / liposomal doxorubicin, carboplatin / paclitaxel | <5% each | |
| Third-line (n=27) | ||
| Clinical Trial: Dacarbazine vs. eribulin (2 pts), dacarbazine (2 pts), tivozanib (2pts), doxorubicin / TH302 (1pt) | 7 | 26 |
| Gemcitabine / docetaxel | 7 | 26 |
| Pazopanib | 5 | 19 |
| Aromatase inhibitor (letrozole, anastrozole) | 3 | 12 |
| Ifosfamide | 2 | 8 |
| Additional agents: Docetaxel, paclitaxel, Doxorubicin / ifosfamide | <5% each | |
| Fourth-line + | ||
| All agents: IMC-A12, paclitaxel, ifosfamide, temozolomide, pazopanib, bevacizumab, cisplatin, GM2/GD2/GD3 vaccine, navelbine, PI3 kinase inhibitor, gemcitabine, everolimus, sunitinib, 5-FU, carboplatin, docetaxel, dacarbazine, PDGF-α antibody, megestrol acetate, doxorubicin, iritecan, tivozanib, eribulin, anastrozole, | ||
Clinical Trial Enrollment and Survival
When comparing the total number of chemotherapy regimens (adjuvant, palliative, and clinical trials) prescribed by either GYN/ONC or SC, there was a significant difference between the total number of chemotherapy regimens prescribed by the two groups (2.67 by GYN/ONC vs 4.29 by SC, p=0.03), while the number of non-trial regimens (adjuvant and palliative) prescribed did not differ (2.67 by GYN/ONC vs 3.06 by SC, p=0.25) (Table 2). Sixty-nine percent of patients referred to the SC were enrolled in at least one clinical trial, while just 8% of patients in the GYN/ONC group were enrolled in at least one clinical trial, a difference that is significant (p<0.0001).
There was a significant difference between the number of clinical trial enrollments in the gynecology oncology group and the combined treatment group (2 vs 22, p<0.001) (Table 2). Of those 22 patients who were treated initially by GYN/ONC and later referred to the SC, they were enrolled in a median of 7 cycles prior to referral (range 2–18 cycles), none of which were delivered as part of a clinical trial. Among all 24 patients enrolled in a clinical trial, 16% were enrolled on a trial as first line chemotherapy, 25% as second line chemotherapy, 29% as third line chemotherapy, and 29% were enrolled as in a clinical trial at fourth-line or greater chemotherapy (Table 3). The number of non-trial based chemotherapy regimens and the number of patients who received adjuvant chemotherapy was not significantly different between the two groups, confirming that both the GYN/ONC and SC treatment groups utilized adjuvant chemotherapy and a similar number of non-trial chemotherapy regimens (Table 2). Ultimately, the difference in the total number of chemotherapy regimens was driven by the clinical trial enrollment between the two treatment groups (Table 2). Therefore, while the number of adjuvant and palliative chemotherapy regimens prescribed by gynecology and medical oncology are not statistically different, referral to an SC resulted in greater clinical trial enrollment for patients with RM uLMS.
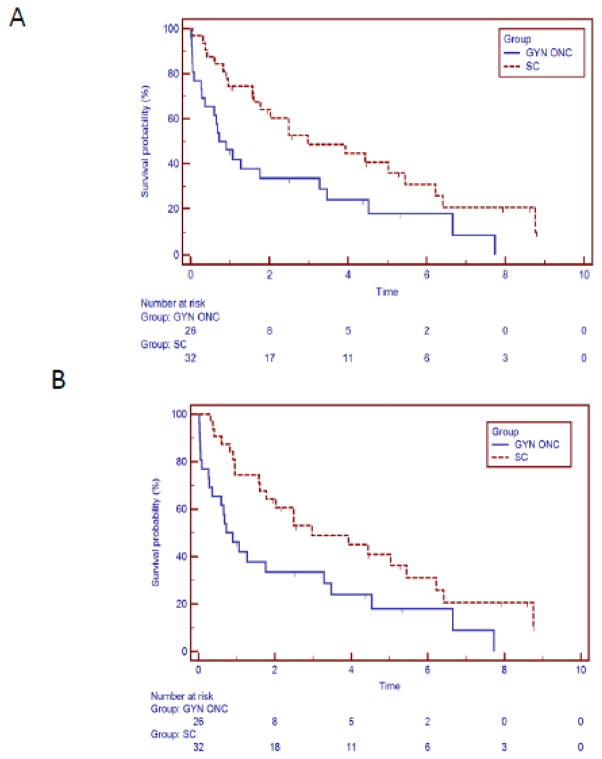
Additionally, the median OS of patients in the SC treatment group from the time of diagnosis is 2.97 years while the median OS in GYN/ONC treatment group is 0.9 years. At 1, 3, and 5 years, OS from diagnosis was lower in GYN/ONC group (48%, 35%, and 19%, respectively) than SC (77%, 51%, and 42%, respectively) (log rank p=0.0142, Figure 1A). OS was significantly higher in the SC group compared to GYN/ONC group [HR 0.47, 95% confidence interval (CI) 0.25–0.87, p=0.0167]. Additionally, median OS from time of diagnosis of RM disease was better in the SC treatment group when compared to the GYN/ONC treatment group (2.97 years vs. 0.9 years, HR 0.49, 95% CI 0.26–0.92, p=0.014) (FIGURE 1B). In a subset analysis excluding those patients in the GYN/ONC treatment group who did not receive palliative chemotherapy secondary to postoperative death, there remained a trend toward OS from the time of diagnosis (p=0.08) and the time of diagnosis of RM disease (p=0.09).
FIGURE 1.
A. 26 Patients treated by GYN/ONC and 32 patients treated by the partnership of GYN/ONC and SC demonstrates an overall survival benefit from the time of diagnosis when treated by a multidisciplinary program (median survival 0.9 vs. 2.97 years, p=0.018). B. Overall Survival from the Diagnosis of RM Disease: 26 Patients treated by GYN/ONC and 32 patients treated by the partnership of GYN/ONC and SC demonstrates an overall survival benefit from the time of diagnosis of RM disease when treated by a multidisciplinary program (median survival 0.9 vs. 2.97 years, p=0.014).
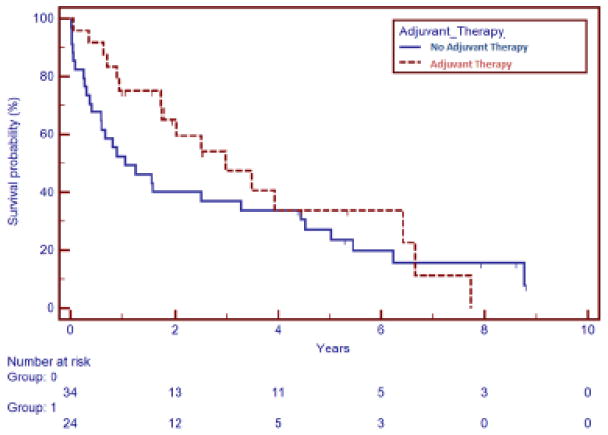
We analyzed our small data set to determine if there was an effect of adjuvant chemotherapy on OS for uLMS; currently, for patients with early stage disease (stage I or II), it is not clear from the literature if any intervention improves the survival outcomes when compared to post-surgical surveillance(10–12). In this retrospective cohort, there was no difference in OS from the date of diagnosis between patients who received adjuvant chemotherapy and those that did not (HR 0.75, 95% CI 0.41–1.35, p=0.33), which is consistent with previous publications (FIGURE 2). Those patients that did not receive adjuvant chemotherapy included those observed after a diagnosis of stage I disease, those that declined therapy, and those that presented with metastatic disease.
FIGURE 2.
Adjuvant Therapy and Overall Survival: No overall survival benefit from the time of diagnosis was noted by the use of adjuvant chemotherapy in a cohort of 58 patients. Thirty-four patients did not receive adjuvant chemotherapy and 24 patients did receive adjuvant chemotherapy (p=0.33).
Discussion
The treatment of patients with uLMS is challenging and has long been the primary responsibility of gynecologic oncologists. Unlike other adult STS, there are separate NCCN guidelines for uLMS written by the Uterine Neoplasm panel(13), as it is believed that LMS that originate in the uterus may be a distinct subgroup of tumors based on gene expression patterns(14). We explored the effects of the newly formed SC at Washington University at St. Louis on outcomes for uLMS patients. We found that, within the limitations of this retrospective study, those patients that were referred to a dedicated SC were enrolled in significantly more clinical trials and had a median OS advantage of 2.07 years over those patients who were exclusively cared for by gynecology oncologists alone. This data supports a multidisciplinary approach that includes the participation of a sarcoma center for the treatment of RM uLMS.
Currently, the cornerstone of treatment for uLMS involves surgical resection via hysterectomy with bilateral salpingo-oophorectomy and additional surgery as necessary to achieve optimal debulking of tumor(3, 4, 15, 16). Although no randomized clinical trial has shown an OS benefit of adjuvant chemotherapy in the treatment of uLMS, Hensley et al reported in a prospective phase II trial of 46 patients with completely resected stage I,II,III, or IV uLMS that the combination of four cycles of fixed-dose rate gemcitabine and docetaxel in patients with uterus-limited disease resulted in a 2-year PFS of 78% with a 57% 3-year PFS (12). A randomized, phase III study is currently enrolling patients with high-risk resected stage I uLMS to receive either gemcitabine and docetaxel followed by doxorubicin or observation, a study that may clarify if combination chemotherapy after surgery is an effective treatment(17). However, at this time, for complete resection of uterine-limited LMS, the standard approach to management with adjuvant chemotherapy remains controversial(18).
Unfortunately, likely due to the rarity of the tumor, few clinical trials of RM uLMS have been performed that did not include other STS histologies. The combination of gemcitabine and docetaxel was shown to have response rates of 36% in the first line and 27% in the second line treatment of uLMS when used for those with metastatic disease(19, 20). For those patients with recurrent uLMS, NCCN guidelines state that enrollment in clinical trial is preferred and should be considered if an appropriate trial is available(13). However, in the United States, only 2–4% of eligible cancer patients are actually enrolled in National Cancer Institute (NCI)-sponsored treatment trials(21), and a recent Institute of Medicine report found that 40% of NCI sponsored trials close without meeting accrual goals and nearly one third of phase III trials close because of poor accrual(22). Such low accruals for cancer treatment trials may delay identification of optimal regimens, include therapies that are of limited benefit, or lead to the rejection of potentially beneficial regimens(23).
There have been many potential barriers identified for cancer clinical trial enrollment, including those attributed to the physician, the patient, and the system. We demonstrated that partnership with a trials-based SC dramatically increases participation in clinical trials. This may be due to the expectation, as instructed by a gynecologic oncologist to a patient at referral, that a patient will likely be recommended to enroll on a clinical trial when referred to the SC. One study of 235 patients referred to a NCI-designated comprehensive cancer center found that only 20% of patients potentially eligible or phase II/III trials were offered enrollment, but 75% of those patients enrolled on clinical trials(24). The presence of oncology specialists and hospital cancer programs affect accrual to cancer treatment trials, and noted that the presence of oncology specialists (per ASCO membership status) is significantly associated with the state’s clinical trials accrual, with a greater number of ASCO physicians per 1000 cancer patients, the greater number of clinical trial accruals. Similarly, the presence of ACOS (American College of Surgeons)-approved cancer programs was significantly associated with clinical trial accruals(25). Specifically, a population-based assessment of specialty physician involvement in cancer clinical trials reported that 87.8% of medical oncologists, 66.1% of radiation oncologists, and 35% of surgeons (general, thoracic, colorectal, surgical oncology, and other subspecialists) reported referring or enrolling one or more patients in clinical trials during the previous 12 months. Physicians affiliated with a CCOP (Community Clinical Oncology Program) or NCI-designated cancer center vs. those with no such affiliations were more likely to participate in clinical trials(26). There is a paucity of data in regards to the utility of a gynecology oncology-medical oncology multidisciplinary approach for the treatment of rare gynecologic tumors; however, with our small single-institution study, we found that active referral of patients with RM uLMS to a medical oncologist with a specialization in sarcoma treatment resulted in increased clinical trial enrollment. Given our findings after the recent establishment of our dedicated sarcoma center, it appears additional investigation into this query is feasible on a larger scale, perhaps with a multi-institutional retrospective or even as part of a prospective study involving uLMS.
There are several limitations to our study including the general rarity of uLMS and the small number of patients available for analysis from this single institution. However, as the results from our single-institution retrospective study demonstrate the potential for increased clinical trial enrollments for uLMS, we believe these findings warrant reporting at this time, with the potential for a larger cohort from this institution or a multi-institutional cohort reported at a later time. Additionally, there is the inherent selection bias found in all retrospective studies; specifically, as data regarding performance status was not available for all patients, we cannot account for potential differences in performance status and its effect on OS between the two groups. Additionally, although we did not detect a significant difference in age between the two groups, our analysis is limited by the small number of patients in each group. We did not address the possibility that patients were offered a clinical trial prior to the offering of more traditional adjuvant therapy, patients that were eligible for clinical trials that declined enrollment, or reasons that patients elected for hospice treatment. In addition, we were not able to delineate clinical decision making regarding referral to the SC; it is possible that GYN/ONC patients who were not referred to the SC for clinical trial enrollment were poor candidates for additional therapy and thus more likely to have poorer outcomes. We also could not account for what or how many clinical trials were accruing during the period of study in order to report on the number of patients that were eligible for enrollment and not accrued, nor account for the fact that the SC group represents a more modern series of patients and the difference in OS between the two groups may be related to improvements in adjuvant treatment or supportive care. Finally, we did not take into consideration the role that patient demographic or socioeconomic characteristics may have played into the patient’s choice to enroll in a clinical trial.
Our findings confirm that the use of adjuvant chemotherapy and palliative chemotherapy outside of clinical trials for patients with RM uLMS is consistent between the two treatment groups at this institution. The difference in delivery of care lies in the increase in total number of chemotherapy regimens and clinical trial enrollments with the referral of RM uLMS patients to a dedicated trials-based sarcoma center. The trend in OS that remained after those patients in the GYN/ONC treatment group who died postoperatively prior to palliative chemotherapy would suggest that, in the era prior to referral of RM uLMS patients to the SC, there was an increased number of patients with RM disease that elected for hospice care in lieu of palliative chemotherapy. This is likely secondary to the expectation that most, if not all patients with RM uLMS who are referred to the SC have an expectation to discuss clinical trial enrollment. However, it is impossible to surmise patient reasons for electing to undergo palliative chemotherapy and/or clinical trials from this retrospective review. It is also possible that the additional lines of therapy provided through clinical trial enrollment may explain the OS benefit seen in this retrospective analysis; however, many of the clinical trials the patients were involved with have yet to be reported and are likely to show a wide range of efficacy in the treatment of RM uLMS. Referral to a medical oncologist at a specialized treatment center may also expose the patient to palliative chemotherapy regimens that have shown activity in STS, and to which uLMS may also respond. Additionally, the successive application of several palliative chemotherapy regimens may provide incremental delays in recurrence or progression and/or more extensive spread of disease, thus ultimately delaying the functional decline of the patient.
The rarity of uLMS and paucity of active clinical trials for this disease make evidence-based treatment of recurrent and/or metastatic uLMS particularly challenging. Although many factors have been shown to affect clinical trial enrollment, the involvement of a dedicated sarcoma center on accrual numbers for gynecologic cancers has not yet been investigated. Our data suggests that given the increased number of clinical trial enrollments and increased number of chemotherapy regimens that resulted from a partnership between gynecology oncology and a dedicated sarcoma center, a multidisciplinary approach to the care of all uLMS patients should be encouraged. Moreover, our findings of improved survival in those patients referred to a dedicated sarcoma center, albeit from a small single-institution cohort, demonstrates the potential of future similar investigations to improve the outcomes of women with this rare and aggressive disease.
Highlights.
Identified patients with RM uLMS from two services (gynecologic oncology & sarcoma center) in one academic referral center
Clinical characteristics, outcomes, & trial enrollments compared for patients treated by GYN/ONC service, the SC, or both
Referrals to the SC were enrolled in a greater number of clinical trials than those by the GYN/ONC service.
Partnership between GYN/ONC and a SC with access to clinical trials should be encouraged for all RM uLMS patients.
Footnotes
Conflict of Interest Statement: All conflicts of interest have been reported as noted on the individual conflict of interest forms.
Financial Disclosures: None
Funding Sources: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Amant F, Moerman P, Neven P, Timmerman D, Van Limbergen E, Vergote I. Endometrial cancer. Lancet. 2005;366(9484):491–505. doi: 10.1016/S0140-6736(05)67063-8. [DOI] [PubMed] [Google Scholar]
- 2.Giuntoli RL, 2nd, Metzinger DS, DiMarco CS, Cha SS, Sloan JA, Keeney GL, et al. Retrospective review of 208 patients with leiomyosarcoma of the uterus: prognostic indicators, surgical management, and adjuvant therapy. Gynecologic oncology. 2003;89(3):460–9. doi: 10.1016/s0090-8258(03)00137-9. [DOI] [PubMed] [Google Scholar]
- 3.Ramondetta LMBT, Broaddus R, Jhingran A. In: Gynecologic cancer. Buzdar AUFR, editor. xix. New York: Springer; 2006. p. 311. [Google Scholar]
- 4.Gadducci A, Cosio S, Romanini A, Genazzani AR. The management of patients with uterine sarcoma: a debated clinical challenge. Critical reviews in oncology/hematology. 2008;65(2):129–42. doi: 10.1016/j.critrevonc.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 5.Major FJ, Blessing JA, Silverberg SG, Morrow CP, Creasman WT, Currie JL, et al. Prognostic factors in early-stage uterine sarcoma. A Gynecologic Oncology Group study. Cancer. 1993;71(4 Suppl):1702–9. doi: 10.1002/cncr.2820710440. [DOI] [PubMed] [Google Scholar]
- 6.Gadducci A, Landoni F, Sartori E, Zola P, Maggino T, Lissoni A, et al. Uterine leiomyosarcoma: analysis of treatment failures and survival. Gynecologic oncology. 1996;62(1):25–32. doi: 10.1006/gyno.1996.0185. [DOI] [PubMed] [Google Scholar]
- 7.Farid M, Ong WS, Tan MH, Foo LS, Lim YK, Chia WK, et al. The influence of primary site on outcomes in leiomyosarcoma: a review of clinicopathologic differences between uterine and extrauterine disease. American journal of clinical oncology. 2013;36(4):368–74. doi: 10.1097/COC.0b013e318248dbf4. [DOI] [PubMed] [Google Scholar]
- 8.Harrison JD, Carter J, Young JM, Solomon MJ. Difficult clinical decisions in gynecological oncology: identifying priorities for future clinical research. International Journal of Gynecological Cancer. 2006;16(1):1–7. doi: 10.1111/j.1525-1438.2006.00424.x. [DOI] [PubMed] [Google Scholar]
- 9.Reichardt P. The treatment of uterine sarcomas. Ann Oncol. 2012;23(Suppl 10):x151–7. doi: 10.1093/annonc/mds359. [DOI] [PubMed] [Google Scholar]
- 10.Omura GA, Blessing JA, Major F, Lifshitz S, Ehrlich CE, Mangan C, et al. A randomized clinical trial of adjuvant adriamycin in uterine sarcomas: a Gynecologic Oncology Group Study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1985;3(9):1240–5. doi: 10.1200/JCO.1985.3.9.1240. [DOI] [PubMed] [Google Scholar]
- 11.Hensley ML, Ishill N, Soslow R, Larkin J, Abu-Rustum N, Sabbatini P, et al. Adjuvant gemcitabine plus docetaxel for completely resected stages I–IV high grade uterine leiomyosarcoma: Results of a prospective study. Gynecologic oncology. 2009;112(3):563–7. doi: 10.1016/j.ygyno.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 12.Hensley ML, Wathen JK, Maki RG, Araujo DM, Sutton G, Priebat DA, et al. Adjuvant therapy for high-grade, uterus-limited leiomyosarcoma: results of a phase 2 trial (SARC 005) Cancer. 2013;119(8):1555–61. doi: 10.1002/cncr.27942. [DOI] [PubMed] [Google Scholar]
- 13.(NCCN). NCCN. NCCN Clinical Practice Guidelines in Oncology. Uterine Neoplasms Version 2.2015. 2015 [cited 2015 December 15]. Available from: http://www.nccn.org/professionals/physician_gls/pdf/uterine.pdf.
- 14.Rao UN, Finkelstein SD, Jones MW. Comparative immunohistochemical and molecular analysis of uterine and extrauterine leiomyosarcomas. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 1999;12(11):1001–9. [PubMed] [Google Scholar]
- 15.Zivanovic O, Leitao MM, Iasonos A, Jacks LM, Zhou Q, Abu-Rustum NR, et al. Stage-specific outcomes of patients with uterine leiomyosarcoma: a comparison of the international Federation of gynecology and obstetrics and american joint committee on cancer staging systems. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27(12):2066–72. doi: 10.1200/JCO.2008.19.8366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leitao MM, Jr, Zivanovic O, Chi DS, Hensley ML, O’Cearbhaill R, Soslow RA, et al. Surgical cytoreduction in patients with metastatic uterine leiomyosarcoma at the time of initial diagnosis. Gynecologic oncology. 2012;125(2):409–13. doi: 10.1016/j.ygyno.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 17.Institute. GOGNC. ClinicalTrialsgov [Internet] Bethesda (MD): National Library of Medicine (US); 2000. Gemcitabine hydrochloride and docetaxel followed by doxorubicin hydrochloride or observation in treating patients with high-risk uterine leiomyosarcoma previously removed by surgery. [cited 2015 Feb 13] Available from: https://clinicaltrialsgov/ct2/show/NCT01533207 NLM Identifier: NCT01533207. [Google Scholar]
- 18.Hensley ML. Role of chemotherapy and biomolecular therapy in the treatment of uterine sarcomas. Best practice & research Clinical obstetrics & gynaecology. 2011;25(6):773–82. doi: 10.1016/j.bpobgyn.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 19.Hensley ML, Blessing JA, Mannel R, Rose PG. Fixed-dose rate gemcitabine plus docetaxel as first-line therapy for metastatic uterine leiomyosarcoma: a Gynecologic Oncology Group phase II trial. Gynecologic oncology. 2008;109(3):329–34. doi: 10.1016/j.ygyno.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hensley ML, Blessing JA, Degeest K, Abulafia O, Rose PG, Homesley HD. Fixed-dose rate gemcitabine plus docetaxel as second-line therapy for metastatic uterine leiomyosarcoma: a Gynecologic Oncology Group phase II study. Gynecologic oncology. 2008;109(3):323–8. doi: 10.1016/j.ygyno.2008.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dellson P, Nilbert M, Bendahl PO, Malmstrom P, Carlsson C. Towards optimised information about clinical trials; identification and validation of key issues in collaboration with cancer patient advocates. European journal of cancer care. 2011;20(4):445–54. doi: 10.1111/j.1365-2354.2010.01207.x. [DOI] [PubMed] [Google Scholar]
- 22.Nass SJMH, Mendelsohn J. Committee on Cancer Clinical Trials and the NCI Cooperative Group Program. Institute of Medicine, National Academies Press; 2010. A national cancer clinical trials system for the 21st century: reinvigorating the NCI Cooperative Group Program. [PubMed] [Google Scholar]
- 23.Manders DB, Paulsen A, Richardson DL, Kehoe SM, Miller DS, Lea JS. Factors associated with clinical trial screening failures in gynecologic oncology. Gynecologic oncology. 2014;134(3):450–4. doi: 10.1016/j.ygyno.2014.06.023. [DOI] [PubMed] [Google Scholar]
- 24.Albrecht TL, Eggly SS, Gleason ME, Harper FW, Foster TS, Peterson AM, et al. Influence of clinical communication on patients’ decision making on participation in clinical trials. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26(16):2666–73. doi: 10.1200/JCO.2007.14.8114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sateren WB, Trimble EL, Abrams J, Brawley O, Breen N, Ford L, et al. How sociodemographics, presence of oncology specialists, and hospital cancer programs affect accrual to cancer treatment trials. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2002;20(8):2109–17. doi: 10.1200/JCO.2002.08.056. [DOI] [PubMed] [Google Scholar]
- 26.Klabunde CN, Keating NL, Potosky AL, Ambs A, He Y, Hornbrook MC, et al. A population-based assessment of specialty physician involvement in cancer clinical trials. Journal of the National Cancer Institute. 2011;103(5):384–97. doi: 10.1093/jnci/djq549. [DOI] [PMC free article] [PubMed] [Google Scholar]