Abstract
We describe a versatile and quick route to cationic gold(I) complexes containing N-heterocyclic carbenes and a second ancillary ligand (such as phosphanes, phosphites, arsines and amines) of interest for the synthesis of compounds with potential catalytic and medicinal applications. The general synthetic strategy has been applied to the preparation of novel cationic heterobimetallic ruthenium(II)-gold(I) complexes that are highly cytotoxic on renal cancer Caki-1 and colon cancer HCT 116 cell lines while showing a synergistic effect and being more selective than their monometallic counterparts.
Graphical Abstract
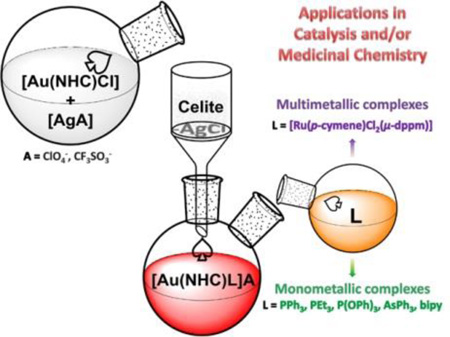
Novel synthetic strategy to incorporate a second neutral ligand in [gold(I)-NHC]+ fragments for the preparation of mono and multimetallic compounds.
The chemistry of gold(I) complexes containing N-heterocyclic carbenes1 has developed rapidly in the past decade with the findings of their relevant applications as homogeneous catalysts1–7 and their potential as anticancer and antimicrobial agents7–14 (including some heterometallic complexes15,16). The beneficial effects of a second ligand in processes catalyzed by gold(I)-N-heterocyclic compounds have been described.17 Cationic gold(I) N-heterocyclic carbene complexes containing phosphanes of the type [(NHC)AuPR3]I (NHC = 1,3-diethylbenzimidazol-2-ylidene) have been described recently by Ott et al. as very efficient enzyme inhibitors with promising potential as anticancer chemotherapeutics.18,19
The preparation of these complexes involves the addition of NaCO3 to the N-heterocyclic imidazolium iodide at 50 °C followed by addition the corresponding chlorido(phosphane) gold(I) derivatives with reactions times ranging from 8 to 60 hours, and subsequent purification by extraction in CH2Cl2/H2O.18,19 This method limits the nature of the anion to that present in the imidazolium salt precursor. A second method developed by Nolan et al. for the preparation of [(IPr)Au(PR3)]+ compounds involves protonolysis of [(IPr)Au(OH)] derivatives with appropriate PR3.HBF4 salts (RT and reaction times of 14 hours).20
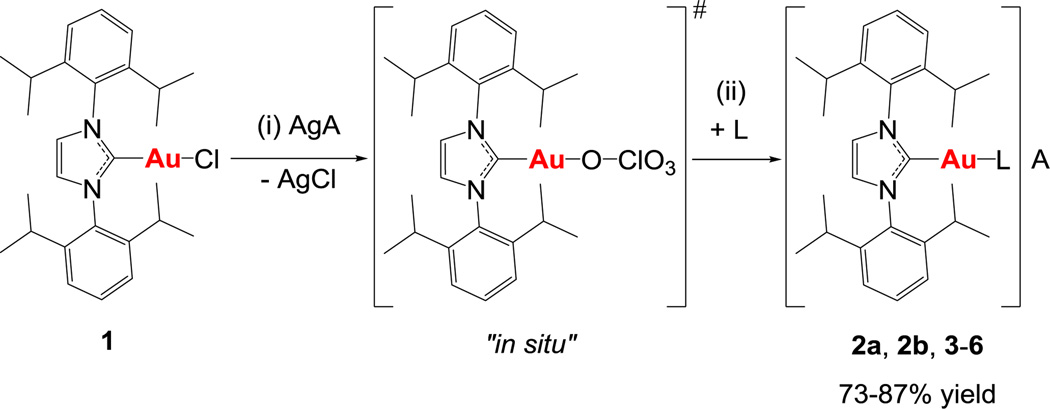
We describe here a simple and efficient synthetic method to obtain gold(I)-N-heterocyclic carbenes with a second ancillary ligand by abstraction of the chloride with silver perchlorate in compounds [(NHC)AuCl]21 and subsequent addition of the ancillary ligand (Scheme 1, total reaction time 30 min). The reaction scheme is quite general and different phosphanes such as PPh3 and PEt3 can be used. We also synthesized cationic complexes containing NHC and a phosphite P(OEt3)3, triphenylarsine AsPh3 and bipyridine (bipy) as second ligand.
Scheme 1.
Preparation of gold complexes [(IPr)Au(L)]A, L = PPh3, A = ClO4− (2a), A = CF3SO3− (2b), A = ClO4−, L = PEt3 (3), L = P(OPh)3 (4), L = AsPh3 (5), L = bipy (6). (i) AgClO4 or AgOSO2CF3 (1 eq.), CH2Cl2/Diethyl ether 1:1, from 0 °C to RT, 15’. (ii) L (1 eq.), CH2Cl2/Diethyl ether 2:1, RT, 15 min.
Gold(I) compounds with hydrogen-bond-supported heterocyclic carbene (HBHC) and nitrogen acyclic carbene (NAC) of the type [(carbene)Au(AsPh3)][SbF6] have been described by Espinet et al. by one pot reaction of gold precursors [(carbene)AuCl] and AgSBF6/AsPh3 (2–5 h reactions).17 Cationic gold(I) compounds of the type [(NHC)Au(α-diimine)]X (X = BF4−, SbF6−) have also been reported by Braunstein et al. by one pot reaction of [(NHC)AuCl]/α-diimine and TlPF6 or AgX (24 h reaction).21
For different synthetic/catalytic purposes it may be relevant to separate the AgCl formed in the abstraction reaction before addition of the second ancillary ligand. We have demonstrated that the use of a silver salt containing an anion that can be covalently coordinated to gold, allows for the separation of solutions (after filtration of AgCl) containing in situ generated [(NHC)AuOClO3] species that are stable for at least 72h at 5 °C, and during 2 hours at RT. The covalent nature of the OClO3− group in the intermediate has been confirmed by IR spectroscopy (spectra and explanations in the ESI). The addition of different ancillary ligands to these solutions leads to immediate formation of stable cationic species (2–6) that precipitate in the reaction media in high yields and that can be separated by filtration without further purification (Scheme 1). The reaction can also be performed with silver triflate (AgOSO2CF3) (2b). It should be noted that compound 4 is an stable precursor to the phosphane derivatives by displacement of the more labile AsPh3.
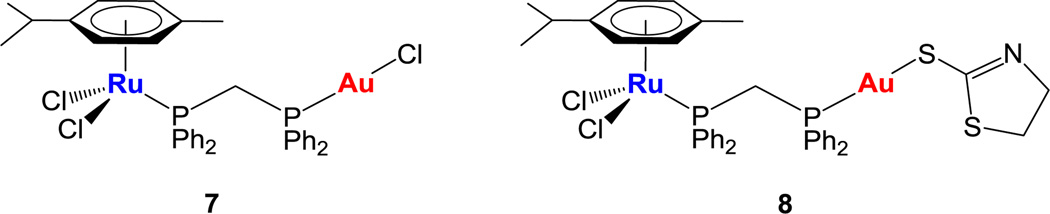
We have used this general synthetic method to incorporate a phosphane fragment containing a second metal center and generate new cationic heterobimetallic ruthenium-gold complexes. Our group has been involved in the preparation of heterometallic complexes containing gold(I) phosphane moeties as potential cancer chemotherapeutics.23–26 The hypothesis is that the incorporation of two active metals in the same molecule may improve their activity as anti-tumor agents due to interaction of the different metals with multiple biological targets (cooperative effect) or by the improved chemicophysical properties of the resulting heterometallic compound (synergism). We have prepared a number of titanocene-gold derivatives with high efficacy in ovarian and prostate cancer in vitro23 and in renal cancer both in vitro24 and in vivo25. We and others have also reported recently on the design of heterometallic ruthenium-gold complexes with relevant in vitro properties against HCT 116 colon cancer cell lines (7 and 8 in Chart 1).26 We found in most cases a synergistic effect of the heterometallic compound when compared to its monometallic counterparts (either alone or in combination).23–26
Chart 1.
Previous heterometallic Ru-Au complexes synthesized in our group.26
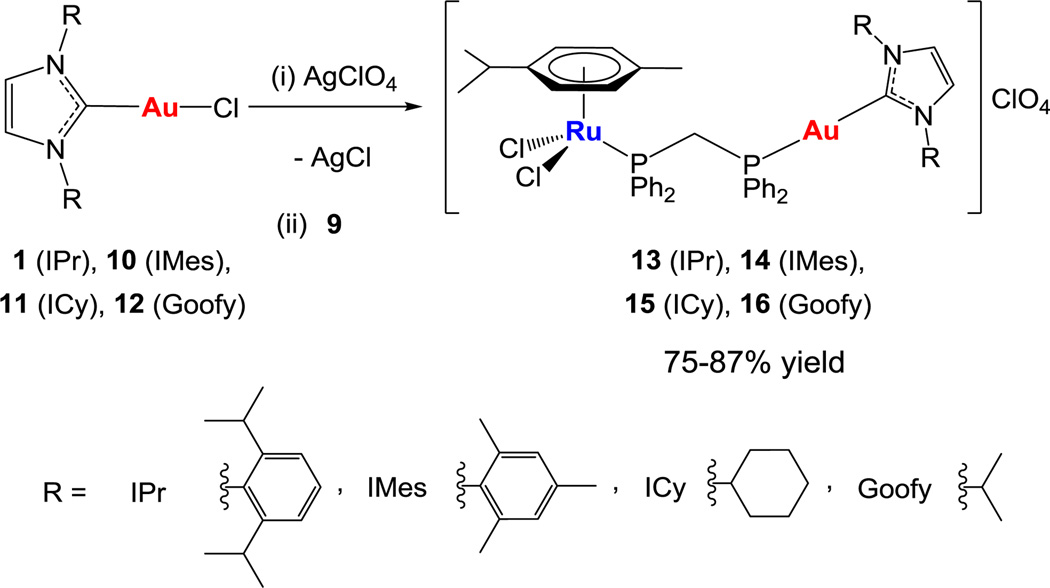
We aimed to incorporate gold(I)-N-heterocyclic fragments to [Ru(p-cymene)Cl2(η1-dppm)] 9 species to improve the pharmacological profile of previously reported heterometallic complexes 7 and 8. Gold NHC complexes display strong antimitochondrial effects, which can be attributed to both their cationic and lipophilic characters.27,28 Combining the potential antimetastatic/cytotoxic properties of [Ru(p-cymene)Cl2(η1-dppm)] 929 and the antimitochondrial/TrRx inhibition effects of [(NHC)Au]+ and [(NHC)Au(PR3)]+species10,18,19 may give rise to potent cancer chemotherapeutics.
Following the general reaction scheme reported here we obtained cationic heterobimetallic Ru-Au complexes (13–16) with four different N-heterocyclic carbenes in high yields (Scheme 2). These compounds could not have been prepared following any of the previously reported methods.17–20,22 The new heterometallic compounds (see ESI for detailed synthetic procedure and characterization) are soluble in DMSO/H2O, DMSO/PBS or DMSO/media (1:99) mixtures at micromolar concentrations, which is relevant for subsequent biological testing.
Scheme 2.
Preparation of heterometallic ruthenium−gold complexes [Ru(p-cymene)Cl2(μ-dppm)Au(NHC)]ClO4. (i) AgClO4 (1 eq.), CH2Cl2/Diethyl ether 1:1, from 0 °C to RT, 15 min. (ii) [Ru(p-cymene)Cl2(η1-dppm)] 9 (1 eq.), CH2Cl2/Diethyl ether 2:1, RT, 15 min.
Compounds 13–16 were characterized unambiguously by 31P{1H}, 1H and 13C{1H} NMR and IR spectroscopy, MS ESI spectrometry and by elemental analysis (see experimental section in ESI). In addition, the ionic nature of the compounds can be ascertained by conductivity measurements that confirm that they are 1:1 electrolytes. The anionic nature of the ClO4− is also confirmed by IR spectroscopy (ESI). The 31P{1H} NMR spectra in CDCl3 of the heterometallic complexes show two distinct doublets at 26.7/20.0 ppm (13), 25.3/21.1 ppm (14), 28.7/21.5 (15) and 28.1/21.4 ppm (16) due to coordination of the dppm ligand to the Au(I) and Ru(II) centers respectively. Moreover, the signals corresponding to the carbons of the imidazolium ring show a doublet in all cases due to the coupling between the carbenic (2JPC ~ 128Hz) and the ethylenic (4JPC ~ 3Hz) carbons and the phosphorous of the dppm ligand bound to the gold center.
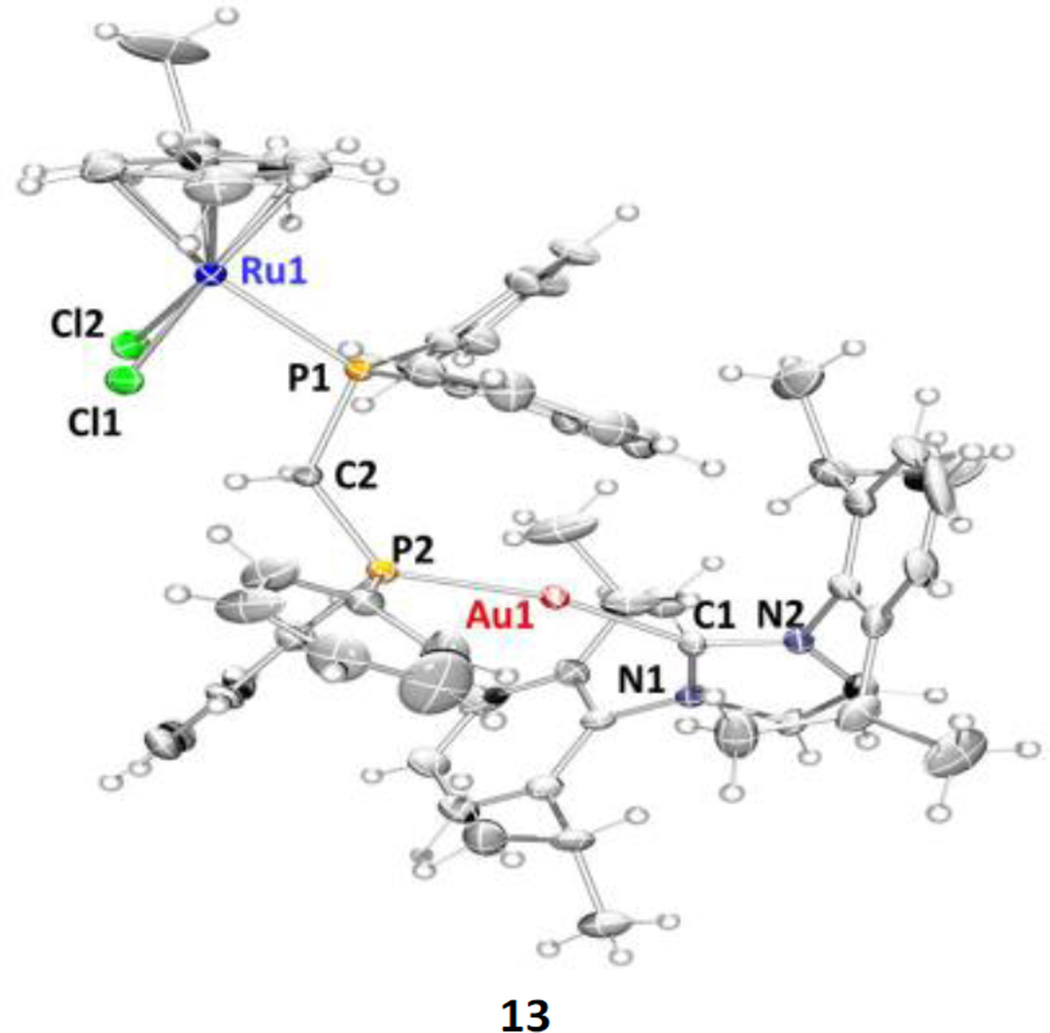
Single crystals of compounds 13 and 14 were isolated as bright orange needles in mixtures of dichloromethane/Et2O. The structure of 13 is depicted below along including selected angles and distances. The structure of 14 is collected in the ESI along with crystallographic data and tables of selected distances and angles for both 13 and 14 (quite similar).
Coordination bond lengths and angles of the two metal ions in 13 and 14 (ESI) are in agreement with those found for similar complexes retrieved in a search in the CSD (v. 1.16)26,30 and in the structure of previously described [Ru(p-cymene)Cl2(μ-dppm)AuCl] (7).26 The bond lengths Au(1)-P(2) and Au(1)-C(1) are respectively 2.228(7) and 2.052(12) Å while the angle P(2)-Au(1)-C(1) is 165.3(4)° showing a stronger deviation of the linearity preferred by gold(I) and being considerably smaller that the angle of 177.88(9)° found for heterobimetallic 7.26 The ruthenium(II) ion exhibits the expected pseudo octahedral three legged piano-stool arrangement common for half-sandwich Ru(II) phosphine complexes and as found in 7.
The stability of the new heterometallic compounds 13–16 was evaluated by 31P{1H} and 1H NMR spectroscopy in DMSO-d6, and by UV−vis spectroscopy in DMSO/PBS solution. NMR experiments were performed in neat DMSO-d6 due to the low solubility of the compounds in mixtures of DMSO-d6/D2O in concentrations larger than 100 micromolar. The compounds are stable in DMSO-d6 with half-life times ranging from 2 to 15 days. The UV−vis spectra of selected compound 15 (micromolar concentration) in 1:99 DMSO/PBS solution did not change in 24 h, with a half-life >72 h (see ESI) indicating the stability of these complexes in physiological media.
The cytotoxicity of the heterometallic complexes [Ru(p-cymene)Cl2(μ-dppm)Au(NHC)]ClO4 (13–16) and monometallic gold(I) complexes [(NHC)AuCl] (1, 10–12 in Scheme 2) was assayed by monitoring their ability to inhibit cell growth using the BluePresto™ assay (see details in the ESI). In this assay, human renal Caki-1 and human colon HCT 116 and DLD1 cancer cell lines and non-tumorigenic human embryonic kidney cell lines HEK-293T were incubated with the indicated compound for 72 hours and compared for sensitivity to cisplatin, and [Ru(p-cymene)Cl2(η1-dppm)] 9. The results are summarized in Table 1 (a complete Table including data on the DLD1 cell line and the combination of monometallic 9 and 1,10–12 can be found in the ESI). The heterometallic compounds are more toxic to the renal cancer cell line (Caki-1 cells) and colon HCT 116 than cisplatin, and monometallic [(NHC)AuCl] (1, 10–12) and [Ru(p-cymene)Cl2(η1-dppm)] 9.29 Importantly, all heterometallic RuAu complexes are considerably less toxic to the non-tumorigenic human embryonic kidney cell line (HEK-293T) than cisplatin and [Ru(p-cymene)Cl2(η1-dppm)] 9. Monometallic [(NHC)AuCl] have also IC50 values above 100 micromolar for the HEK cell line but are less toxic to the renal cancer cell line Caki-1. We studied the effect of the combination of monometallic gold 1, 10–12 and ruthenium 9 compounds (1:1 equivalents) in Caki-1 cell lines at 72 h which in all cases gave IC50 values larger than those of the bimetallic compounds (ESI). This fact supports the idea that there is indeed a synergistic effect for the heterometallic complexes in their in vitro activity on the renal cancer cell line.
Table 2.
IC50 values (µM) in human cell lines were determined with monometallic [(NHC)AuCl] 1, 10–12 monometallic [Ru(p-cymene)Cl2(η1-dppm)] 9, and new heterometallic Ru-Au compounds (13–16).a
| Caki-1 | HEK-293T | HCT 116 | |
|---|---|---|---|
| [(NHC)AuCl] | |||
| 1 | 27.1 ± 2.0 | 61.5 ± 5.1 | 39.7 ± 4.9 |
| 10 | 21.2 ± 1.6 | >100 | 31.2 ±3.2 |
| 11 | 58.8 ± 3.9 | >100 | 59.1 ± 5.8 |
| 12 | 17.5 ± 2.2 | >100 | 27.7 ± 4.9 |
| [Ru(p-cymene)Cl2(η1-dppm)] 9 | 35.6 ± 3.7 | 81.6 ± 3.0 | 18.2 ± 2.2 |
| New cationic Ru-Au | |||
| 13 | 5.2 ± 0.9 | 73.2± 2.5 | 8.1 ± 1.8 |
| 14 | 14.1 ± 1.9 | >100 | 5.22 ± 0.7 |
| 15 | 3.8 ± 0.6 | >100 | 6.4 ± 1.0 |
| 16 | 12.7 ± 2.7 | >100 | 9.6± 3.1 |
| cisplatin | 29 ± 4.11b | 3.27 ± 0.13b | --- |
All compounds were dissolved in 1% of DMSO and diluted with water before addition to cell culture medium for a 72 h incubation period. Cisplatin was dissolved in H2O.
Values from reference 24.
We analyzed the interactions of 13–16 with representative DNA molecules. Specifically, we carried out gel electrophoresis studies to disclose the effects of the new heterobimetallic compounds on plasmid (pBR322) DNA (ESI). Compound [Ru(p-cymene)Cl2(η1-dppm)] 9 weakly reduced supercoiling in plasmid DNA although it was able to bind DNA in a non-intercalative fashion.31 Most Gold(I)-N-heterocyclic carbene complexes (unless designed to target DNA quadruplexes) are known to not interact or interact only weakly with DNA.10 Treatment with increasing amounts of heterometallic RuAu derivatives 13–16 did not affect the mobility of the faster-running supercoiled form (Form I) even at the highest molar ratios (ESI). The lack of interaction between the heterobimetallic compounds and plasmid (pBR322) DNA (already observed for previously described [Ru(p-cymene)Cl2(μ-dppm)AuX] compounds, X = Cl 7, thiazoline 8) points out that other biomolecular targets are probably implicated in the cell death pathway.
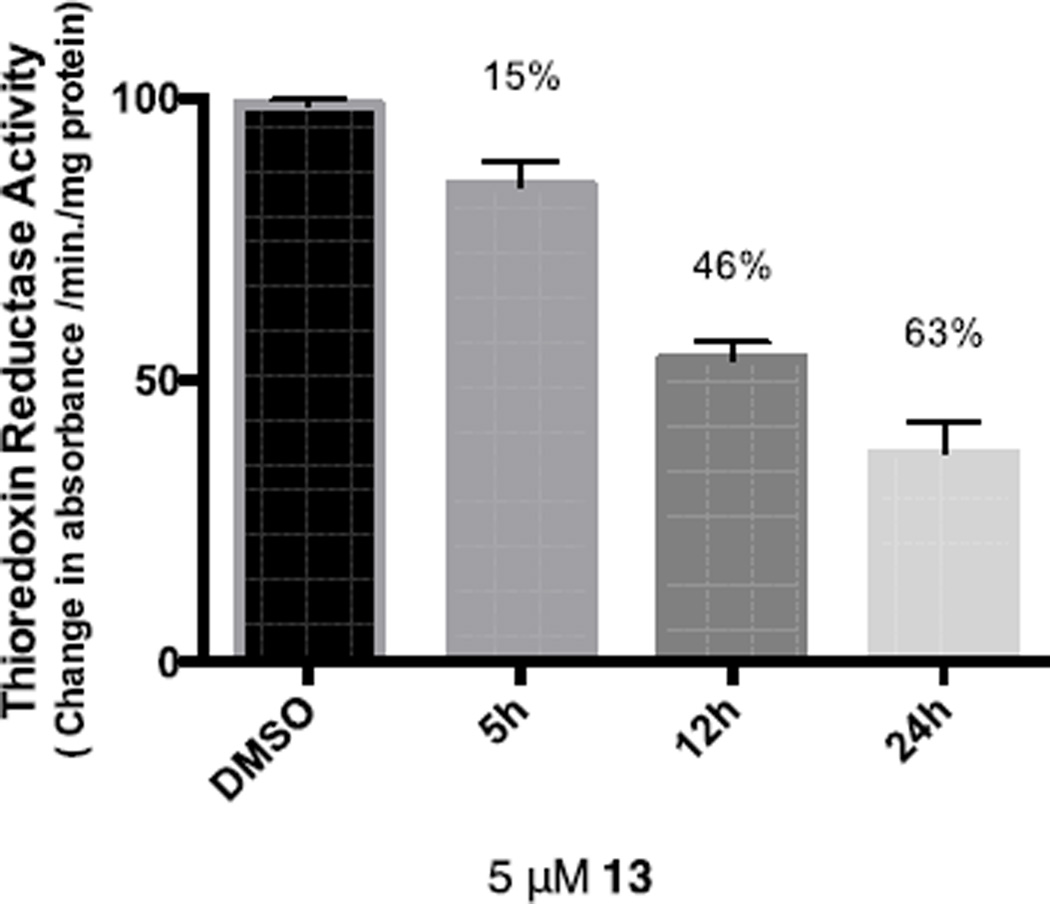
Changes in cell anti-oxidant capacity are a characteristic of many chemo-resistant cancers, and overexpression of thioredoxin reductase (TrRx) is among the key defense and survival mechanisms of cisplatin-resistant cells. We have reported on the inhibition of TrRx in Caki-1 cells by Auranofin and heterometallic titanocene-gold complexes.25 Since Au-NHC compounds are known to inhibit TrRx, we measured the activity of thioredoxin reductase in Caki-1 cells, following incubation with compounds 13, 1 and 9. We found thioredoxin reductase activity to be lower in cells treated with 5 µM of 1, 9, 13, with an observed inhibition of 17%, 44% and 63% respectively after a 24 hour incubation (Figure 2 and see figures for all compounds in the ESI). This experiment clearly showed that the inhibition of TrRx is more efficient for the heterometallic complex 13 [Ru(p-cymene)Cl2(μ-dppm)Au(IPr)]ClO4 when compared to monometallic [(IPr)AuCl] derivative 1 and [Ru(p-cymene)Cl2(η1-dppm)] 9.
Figure 2.
Thioredoxin reductase activity in 13 treated Caki-1 cells. Activity of endogenous Caki-1 thioredoxin reductase from soluble whole cell lysates following incubation with 5 µM of compound 13 or 0.1% DMSO for 5, 12 and 24 hours.
In conclusion, the general synthetic strategy described here may be used for the preparation of cationic gold(I)-N-heterocyclic carbene complexes containing a second neutral ligand. The mild reaction conditions and the complete separation of silver salts before the addition of the second ligand, has allowed for the preparation of novel heterometallic RuAu complexes that have resulted highly active in vitro against renal and colon cancer cell lines while being more selective and active than the monometallic counterparts (either alone or in combination). The biological activity of these new derivatives is currently being further explored.
We envision that the versatility of this method will enable the synthesis of a broad range of compounds (e.g. multimetallic systems, polymers, nanocarriers) for which the incorporation of [Au(NHC)] species may improve their biological or catalytic applications.
Supplementary Material
Figure 1.
ORTEP view of the molecular structures of 13 showing the labelling scheme. Labelling for hydrogen and carbon atoms is omitted for clarity. A drawing of the molecular structure containing relevant carbon atoms labelled is provided in the ESI. Selected distances (Å) and angles (°): Au(1)-P(2) = 2.228(7), Au(1)-C(1) = 2.052(12), Ru(1)-P(1) = 2.352(3), Ru(1)-Cl(1) = 2.409(3), Ru(1)-Cl(2) = 2.422(3), Ru(1)-Z* = 1.719; P(2)-Au(1)-C(1) = 165.3(4), Cl(1)-Ru(1)-Cl(2) = 87.65(11), Cl(1)-Ru(1)-P(1) =85.39(10), Cl(2)-Ru(1)-P(1) = 86.80(10), Ru(1)-P(1)-Z*= 132.32. Z* = centroid of p-cymene ring.
Acknowledgments
We thank the National Cancer Institute (NCI) for grant 1SC1CA182844 (M.C.) M.C. is grateful to Mr. Leonard Tow and the Tow Foundation for a Tow Professorship (2015–2017).
Footnotes
Electronic Supplementary Information (ESI) available: Experimental section (general Information, instrumentation and synthetic procedures), NMR, IR, UV-Vis and MS-ESI+HR spectra, crystallographic data, interaction with plasmid pBR322 DNA, cell culture and cell viability assays and inhibition of thioredoxin reductase.
Notes and references
- 1.Wurm T, Asiri AM, Hasmi ASK. In: N-Heterocyclic Carbenes. Nolan SP, editor. Vol. 9. Wiley-VHC; 2014. p. 243. [Google Scholar]
- 2.Wang Y-M, Lackner AD, Toste FD. Acc. Chem. Res. 2014;47:889. doi: 10.1021/ar400188g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gatineau D, Goddard JP, Mouries-Mansuy V, Fensterbank L. Israel J. Chem. 2013;53:892. [Google Scholar]
- 4.Loh CCJ, Enders D. Chem. A Eur. J. 2012;18:10212. doi: 10.1002/chem.201200287. [DOI] [PubMed] [Google Scholar]
- 5.Nicolas M. RSC Catalysis Series. 2011;6:317. [Google Scholar]
- 6.Alcazaro M, Stork T, Anoop A, Thiel W, Furstner A. Angew. Chem. Int. Ed. 2010;49:2542. doi: 10.1002/anie.200907194. [DOI] [PubMed] [Google Scholar]
- 7.Nicolas M, Nolan SP. Chem. Soc. Rev. 2008;37:1776. doi: 10.1039/b711132k. [DOI] [PubMed] [Google Scholar]
- 8.Tacke M. J. Organomet. Chem. 2015;782:17. [Google Scholar]
- 9.Lazreg F, Cazin CSJ. In: N-Heterocyclic Carbenes. Nolan SP, editor. Vol. 7. Wiley-VHC; 2014. p. 173. [Google Scholar]
- 10.Bertrand B, Casini A. Dalton Trans. 2014;43:4209. doi: 10.1039/c3dt52524d. [DOI] [PubMed] [Google Scholar]
- 11.Oehninger L, Ott I. Dalton Trans. 2013;42:3269. doi: 10.1039/c2dt32617e. [DOI] [PubMed] [Google Scholar]
- 12.Liu W, Gust R. Chem. Soc. Rev. 2013;42:755. doi: 10.1039/c2cs35314h. [DOI] [PubMed] [Google Scholar]
- 13.Hindi KM, Panzer MJ, Tessier CA, Cannon CL, Youngs WJ. Chem. Rev. 2009;109:3859. doi: 10.1021/cr800500u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernández GA, Vela Gurovic MS, Olivera NL, Chopa AB, Silbestri GF. J. Inorg. Biochem. 2014;135:54. doi: 10.1016/j.jinorgbio.2014.03.001. and refs. therein. [DOI] [PubMed] [Google Scholar]
- 15.Bertrand B, Citta A, Franken IL, Picquet M, Folda A, Scalcon V, Rigobello MP, Le Gendre P, Casini A, Bodio E. J. Biol. Inorg. Chem. 2015;20:1005. doi: 10.1007/s00775-015-1283-1. [DOI] [PubMed] [Google Scholar]
- 16.Boselli L, Carraz M, Mazeres S, Paloque L, Gonzalez G, Benoit-Vical F, Valentin A, Hemmert C, Gornitzka H. Organometallics. 2015;34:1046. [Google Scholar]
- 17.Ramiro Z, Bartolome C, Espinet P. Eur. J. Inorg. Chem. 2014:5499. doi: 10.1021/ic101059c. [DOI] [PubMed] [Google Scholar]
- 18.Rubianni R, Salassa L, de Almeida A, Casini A, Ott I. Chem Med Chem. 2014;9:1205. doi: 10.1002/cmdc.201400056. [DOI] [PubMed] [Google Scholar]
- 19.Rubianni R, Can S, Kitanovic I, Alborzinia H, Stefanopoulou M, Kokoschka M, Monchgesang S, Sheldrick WS, Wolf S, Ott I. J. Med. Chem. 2011;54:8646. doi: 10.1021/jm201220n. [DOI] [PubMed] [Google Scholar]
- 20.Gaillard S, Nun P, Slawin AMZ, Nolan SP. Organometallics. 2010;29:5402. [Google Scholar]
- 21.de Fremont P, Clavier H, Rosa V, Avilés T, Braunstein P. Organometallics. 2011;30:2241. [Google Scholar]
- 22.Visbal R, Laguna A, Gimeno MC. Chem. Commun. 2013;49:5642. doi: 10.1039/c3cc42919a. [DOI] [PubMed] [Google Scholar]
- 23.González-Pantoja JF, Stern M, Jarzecki AA, Royo E, Robles-Escajeda E, Varela-Ramirez A, Aguilera RJ, Contel M. Inorg. Chem. 2011;50:11099. doi: 10.1021/ic201647h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fernández-Gallardo J, Elie BT, Florian J, Sulzmaier J, Sanaú M, Ramos JW, Contel M. Organometallics. 2014;33:6669. doi: 10.1021/om500965k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fernández-Gallardo J, Elie BT, Sadhukha T, Prabha S, Sanaú M, Rotenberg SA, Ramos JW, Contel M. Chem. Sci. 2015;6:5269. doi: 10.1039/c5sc01753j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Massai L, Fernández-Gallardo J, Guerri A, Arcangelic A, Pillozzic S, Contel M, Messori L. Dalton Trans. 2015;44:11067. doi: 10.1039/c5dt01614b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baker MV, Barnard PJ, Berners-Price SJ, Brayshaw SK, Hickey JL, Skelton BW, White AH. Dalton Trans. 2006:3708. doi: 10.1039/b602560a. [DOI] [PubMed] [Google Scholar]
- 28.Hickey JL, Ruhayel RA, Barnard PJ, Baker MV, Berners-Price SJ, Filipovska A. J. Am. Chem. Soc. 2008;130:12570. doi: 10.1021/ja804027j. [DOI] [PubMed] [Google Scholar]
- 29.Das S, Sinha S, Britto R, Somasundaram K, Samuelsom AG. J. Inorg. Biochem. 2010;104:93. doi: 10.1016/j.jinorgbio.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 30.Allen FH. Acta Cryst B. 2002;B58:380. doi: 10.1107/s0108768102003890. [DOI] [PubMed] [Google Scholar]
- 31.Cutts SM, Casta A, Panousis C, Parsons PG, Sturm RA, Phillips DR. Methods Mol. Biol. 1997;90:95. doi: 10.1385/0-89603-447-X:95. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.