Abstract
Background
EBUS-guided transbronchial needle aspiration (TBNA) is important in the evaluation of thoracic lymphadenopathy. Reliably providing excellent diagnostic yield for malignancy, its diagnosis of sarcoidosis is inconsistent. Furthermore, when larger “core” biopsy samples of malignant tissue are required, TBNA may not suffice. The primary objective of this study was to determine if the sequential use of TBNA and a novel technique called cautery-assisted transbronchial forceps biopsies (ca-TBFB) was safe. Secondary outcomes included sensitivity and successful acquisition of tissue.
Methods
Fifty unselected patients undergoing convex probe EBUS were prospectively enrolled. Under EBUS guidance, all lymph nodes ≥ 1 cm were sequentially biopsied using TBNA and ca-TBFB. Safety and sensitivity were assessed at the nodal level for 111 nodes. Results of each technique were also reported on a per-patient basis.
Results
There were no significant adverse events. In nodes determined to be malignant, TBNA provided higher sensitivity (100%) than ca-TBFB (78%). However, among nodes with granulomatous inflammation, ca-TBFB exhibited higher sensitivity (90%) than TBNA (33%). For analysis based on patients rather than nodes, 6 of the 31 patients with malignancy would have been missed or understaged if the diagnosis was based on samples obtained by ca-TBFB. On the other hand, 3 of 8 patients with sarcoidosis would have been missed if analysis was based only on TBNA samples. In some cases only ca-TBFB acquired sufficient tissue for the core samples needed in clinical trials of malignancy.
Conclusions
The sequential use of TBNA and ca-TBFB appears to be safe. The larger samples obtained from ca-TBFB increased its sensitivity to detect granulomatous disease and provided specimens for clinical trials of malignancy when needle biopsies were insufficient. For thoracic surgeons and advanced bronchoscopists, we advocate ca-TBFB as an alternative to TBNA in select clinical scenarios.
MeSH key words: Bronchoscopy surgical procedures, lymphadenopathy
Introduction
Convex probe endobronchial ultrasound (EBUS), a safe minimally invasive technique with high diagnostic yield, has advanced the diagnosis of mediastinal and hilar lymphadenopathy. Guided by EBUS, real-time visualization of transbronchial needle aspiration (TBNA) is widely practiced. Per recent American College of Chest Physicians (ACCP) guidelines for invasive staging of non-small cell lung cancer (NSCLC), TBNA and other needle-based techniques are preferred [1]. The sensitivity using EBUS-TBNA for NSCLC is 89% [1] and up to 96% [2] for small cell lung cancer. A recent meta-analysis showed diagnostic sensitivity of TBNA for sarcoidosis to be significantly lower at 62%. Adjunct techniques, such as the use of concomitant transbronchial parenchymal biopsies, increased the yield to 81% [3]. A meta-analysis of EBUS-TBNA showed a pooled diagnostic accuracy of 79% for sarcoidosis [4]. TBNA is also used for clinical trials in patients with malignancy, however many such trials often specifically exclude needle-based samples.
The feasibility of passing small biopsy forceps (0.8–1.2 mm) through airways into adjacent lymph nodes has been reported previously. Samples obtained via forceps have demonstrated improved diagnostic yields for sarcoidosis and lymphoma [5–9]. However, this technique is unsuccessful in up to 28% of cases, usually due to an inability to penetrate the airway wall or lymph node capsule [6]. In this report we describe an innovative EBUS-guided technique using larger forceps and electrocautery to safely acquire tissue from mediastinal and hilar lymph nodes.
Materials and Methods
We prospectively enrolled 50 unselected patients older than 18 years referred for EBUS. In accordance with the published guidelines [1], patients with lymph nodes meeting size criteria (> 1 cm) or that are PET-avid undergo EBUS as the preferred initial invasive test in our institution. Patients were typically referred either to our outpatient multidisciplinary thoracic oncology program or hospitalized for evaluation. Referrals were predominantly from pulmonologists, thoracic surgeons and oncologists.
The sample size of 50 patients was chosen to be similar in size to prior studies that have evaluated the forceps technique [9]. In our prior analysis, we determined that we routinely evaluate more than 2 lymph node stations [10], making our anticipated nodal number greater than 100. The institutional review board at the Yale School of Medicine approved the study and all of its components (HIC number 1307012325).
Patients or surrogates were consented for both the procedure and the study and were interviewed for baseline data. EBUS was performed using conscious sedation without any artificial airway or ventilatory support. Following topical lidocaine and sedation with midazolam and fentanyl, the EBUS scope (Olympus BF-U180F with EU-ME1 processor) was used to perform a comprehensive mediastinal and hilar evaluation.
TBNA Protocol
The target lesions (lymph node or mass) were identified by EBUS and the jabbing technique advanced a 22-gauge needle (Olympus NA-201SX-4022) 20–30 times per pass with application of suction. On-site cytology was not typically used. TBNA was performed with 3 passes in each station for smaller nodes (5–9 mm) and with two passes for larger nodes (≥ 1 cm).
Cautery-Assisted Transbronchial Forceps Biopsy (ca-TBFB) Protocol
After identifying the target node or mass, an electrocautery knife (Olympus KD-31C-1; (Erbe VIO 300D – 40W)) was advanced through the EBUS scope. After visualizing the knife sheath come out the working channel, the knife was advanced to the airway wall. The EBUS image identified the target, cautery was applied, the knife was advanced, and penetration of the lymph node or adjacent mass was visualized with the EBUS scope. After this airway incision and nodal penetration, the knife was withdrawn and a 1.9 mm spiked forceps (Olympus FB-241K) was advanced into the target through the airway defect made by the cautery knife. The forceps were withdrawn to the proximal capsule, opened, passed through the diameter of the node, and closed. This was repeated 5 times per pass. Two passes were performed per station (Figure 1). Specimens were placed into formalin. Cases where forceps could not penetrate were recorded and an additional TBNA pass was performed so that each station had at least three passes, as recommended by guidelines [11].
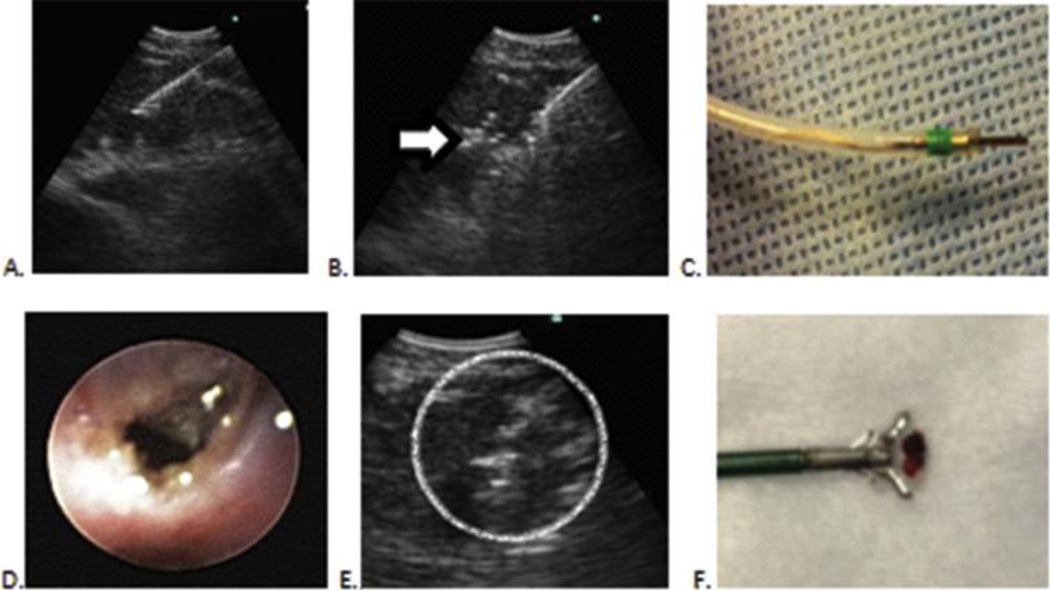
Figure 1. Technique employing EBUS-guided cautery-assisted forceps biopsies of lymph nodes.
A. EBUS-TBNA needle in lymph node
B. EBUS view with cautery knife inside same lymph node. Note mild artifact (Arrow)
C. Electrocautery knife that is inserted through working channel of EBUS scope
D. Mucosal incision seen after electrocautery incision.
E. Forceps seen inside lymph node (Circle)
F. Specimen obtained by forceps for surgical pathology
Using EBUS measurements, we assessed the distance between the airway wall and lymph node capsule on the last 81 samples. We determined the procedural time required for each technique in a subset of 10 subjects.
Assessment of Complications
We monitored all patients for bleeding, change in service level (outpatient to inpatient, inpatient to intensive care unit), and other clinically obvious post-procedural complications. We did not routinely obtain radiographs or lab work after the procedures.
To assess complications not obvious in the immediate post-procedure period, all patients were contacted by telephone 3–4 weeks post-procedure. Standard questions reviewed post-procedural fevers, chest pain, emergency room or physician visits, and other symptoms. Patient charts were also reviewed after 2 months to assess clinically documented procedure-related complications.
Pathology Processing
Specimens were sent to cytology (TBNA) and histopathology (ca-TBFB) after acquisition. TBNA samples were placed directly into Cytorich solution and ca-TBFB samples into formalin. The TBNA samples were spun down into pellets and subsequently intermixed with histogel. These specimens underwent the same process as pathologic tissue (ca-TBFB) where they were fixed, dehydrated, embedded in paraffin and cut for slides. For samples in which tissue quantity was minimal, we used the Cellient technique described by Andrew Fischer. In this, the cellular material/tissue was filtered for creating cellblocks. A cellblock was made of all specimens attained by TBNA and by forceps, and individual pathologists frequently obtained an intra-departmental diagnostic consensus. Both the TBNA and ca-TBFB samples were evaluated similarly using the cellblock specimens.
In order for granulomatous inflammation to be identified, the pathologists used well-defined criteria. Both architectural structure (cell clusters) and cell morphology were required for the diagnosis of granulomatous inflammation, not simply the presence of histiocytes. The cells of interest were epithelioid histiocytes intermixed with lymphocytes, with or without multi-nucleated giant cells.
Review of Pathologic Diagnoses
Cytology (TBNA) and histopathology (ca-TBFB) reports were reviewed and compared on a per-patient and per-nodal analysis. Using widely accepted criteria, biopsy was deemed successful if it identified lymphocytes or a specific disease entity. When any discrepancy was noted, we accepted the adjudication of a separate, independent pathologist. As such, all specimens were similarly prepared and analyzed, with direct comparison of diagnostic yield based on the cell blocks. Because the gold standard of mediastinoscopy was not universally appropriate, this process of pathological adjudication served as the de facto gold standard in this study. A final clinical diagnosis for each patient was determined by pathology and clinical information obtained during the follow-up period.
Statistical Analysis
Diagnostic yield on a nodal and patient basis was calculated for each procedure. Because mediastinoscopy was not routinely performed, sensitivity was calculated by dividing the number of nodes successfully diagnosed by one procedure (TBNA or ca-TBFB) over the total number of similar nodes positively identified by either technique (TBNA and ca-TBFB). Sensitivity was further examined in relation to lymph node location, size, and depth from the airway wall.
Results
Table 1 demonstrates baseline patient characteristics. Forty-nine had procedures using conscious sedation and one critically ill patient was already intubated for respiratory failure. In these patients, 140 nodes ≥ 5 mm were evaluated by TBNA and 100 met size criteria (≥1 cm) for both techniques. Seven nodes less than 1 cm and 4 central masses were also deemed clinically relevant, completing the 111.
Table 1.
Participant Characteristics (N=50)
| N [%] | |
|---|---|
| Age, mean (SD) | 62.2 (16.8) |
| Male gender | 30 [60.0] |
| Non-white race | 6 [12.0] |
| Patients with associated lung mass | 23 [46.0] |
| Patients with a history of malignancy | 25 [50.0] |
| Outpatient | 40 [80.0] |
Safety
Subsequent to ca-TBFB, two patients experienced temporary oozing of blood from bronchial mucosa. One required topical iced saline and lidocaine with epinephrine and the other resolved spontaneously. There were no cases of massive hemoptysis. One patient developed clinically obvious pneumomediastinum with subcutaneous emphysema and was admitted for observation. Another was diagnosed with an unrelated urinary tract infection and discharged home the following day. No other complications were clinically evident. Given that TBNA was immediately and consistently followed by ca-TBFB, neither the pneumomediastinum nor any other specific complication could be reliably attributed to either technique.
All patients were evaluated for complications at 3–4 weeks post-procedure. Of the four patients admitted during this follow-up period, charts were reviewed and no procedurally related complications were identified. One patient was admitted with a small bowel obstruction three days after the procedure, deemed unrelated to bronchoscopy. Another patient with brain metastases who underwent gamma knife surgery 6 days post-procedure was admitted for a seizure. Another patient was admitted with an isolated fever after receiving chemotherapy and improved without intervention or clear source despite evaluation. The fourth patient was admitted for induction chemotherapy.
During this initial follow-up period, there were two patient deaths. The first had been previously hospitalized for extensive thromboembolic disease and our procedure diagnosed advanced malignancy. The patient declined chemotherapy and died seven days post-procedure. The other patient had advanced malignancy and central airway obstruction, developed respiratory failure, and died 3 days post-procedure.
In addition to patient interviews within one month, a chart review was conducted two months post-procedure. We corroborated the chart review with patient interviews for complications and determined which patients underwent further diagnostic evaluation. Forty of the 50 patients had complete medical records and eight had partial records. Two patients received subsequent care outside of our institution. In our institutional practice, patients undergo mediastinoscopy in order to exclude false negative results when EBUS demonstrates only lymphocytes and there is substantive clinical suspicion of a pathologic process. During that time, 5 (10%) patients underwent mediastinoscopy or other surgical biopsy with no change in diagnosis compared to EBUS. While there were no cases of mediastinitis or abscess seen at mediastinoscopy, one necrotic node had a positive culture for Peptostreptococcus. During this follow-up, 21 patients had a total of 24 chest CT scans not ordered by study physicians for various clinical indications. No cases of mediastinitis or mediastinal abscesses were identified on CT scan although two patients had incidentally noted pneumomediastinum without clinical symptoms (scans obtained on days 1 and 6 post-procedure).
Successful biopsy by ca-TBFB
An average of 2.2 lymph nodes were biopsied per patient. Ca-TBFB acquired target tissue in parabronchial stations (89%) more consistently than paratracheal stations (65%) (Table 2). Biopsied nodes ranged from 6.9 mm to 36.5 mm and for analysis were grouped in sizes of <10 mm, 10–14.9 mm, 15–20 mm and greater than 20 mm. Within these groups, ca-TBFB yielded successful biopsies in 86%, 76%, 79% and 93% of the cases attempted, respectively. The median nodal depth from the airway lumen was 3.0 mm (range=1.5 to 6.7, interquartile range 1.6 mm), with no difference in acquisition with ca-TBFB for depths less than or greater than 3 mm (83% vs 82% respectively).
Table 2.
ca-TBFB1 Rate of Nodal Penetration by Lymph Node Characteristics
| ABILITY TO SUCCESSFULLY BIOPSY NODE BY LYMPH NODE LOCATION AND SIZE (N=111) | |||
| Location |
Specific Diagnosis/Lymphocytes (n=90) |
Total (n=111) |
Nodal Penetration |
| Paratracheal2 | 24 | 37 | 64.8% |
| Parabronchial3 | 66 | 74 | 89.2% |
| Lymph node size (mm) | |||
| 5–9.9 | 6 | 7 | 85.7% |
| 10–14.9 | 40 | 53 | 75.5% |
| 15–20 | 19 | 24 | 79.2% |
| >20 | 25 | 27 | 92.6% |
| ABILITY TO SUCCESSFULLY BIOPSY NODE BY LYMPH NODE DEPTH (n=81)** | |||
| Lymph node depth (mm) |
Specific Diagnosis/Lymphocytes (n=67) |
Total (n=81) |
Nodal Penetration |
| 1–1.9 | 5 | 5 | 100% |
| 2–3 | 30 | 37 | 81.1% |
| > 3 | 32 | 39 | 82.1% |
Ca-TBFB: Cautery-assisted transbronchial forceps biopsy, always performed using endobronchial ultrasound guidance
Paratracheal includes 4L, 4R, Pre-tracheal whereas parabronchial includes 7, 10R, 10L, 11R, 11L, 12R, 12L, Mass.
Depth collected on subset of cases.
The average times for two passes using TBNA and ca-TBFB were, respectively, 4 minutes 33 seconds and 4 minutes 12 seconds. For TBNA, most time was spent processing the specimen whereas ca-TBFB spent most time making the incision.
Sensitivity
TBNA successfully diagnosed 94 samples (85%) and ca-TBFB successfully diagnosed 89 (80%). The larger samples obtained via ca-TBFB exhibited a condition-specific sensitivity. In nodes determined to be malignant, TBNA established the diagnosis 100% of the time compared to only 78% using ca-TBFB (p<0.001), as shown in Table 3. In contrast, ca-TBFB established the diagnosis of granulomatous inflammation in 90% of the lymph nodes sampled, compared to 33% with TBNA (p<0.001).
Table 3.
Nodal Level Sensitivity by Biopsy Technique and Etiology (N=111 nodes)
| Conditions Positively Identified by a Consensual Gold Standard* |
Number of Nodes (Sensitivity in %) Positively Diagnosed per Biopsy Technique |
||
|---|---|---|---|
| Malignant Conditions (Total Nodes per Condition) | TBNA | ca-TBFB | p-value** |
| NSCLC (33) | 33 (100%) | 24 (73%) | 0.002 |
| Small Cell Lung Cancer (14) | 14 (100%) | 13 (93%) | *** |
| Lymphoma (5) | 5 (100%) | 4 (80%) | *** |
| Metastatic Other**** (2) | 2 (100%) | 1 (50%) | *** |
| Total Malignant (54) | 54 (100%) | 42 (78%) | <0.001 |
| Nonmalignant Conditions (Total Nodes per Condition) |
TBNA | ca-TBFB | p-value** |
| Granulomatous Inflammation (19) | 6 (32%) | 17 (85%) | *** |
| Infection (2) | 1 (50%) | 2 (100%) | *** |
| Lymphocytes Only (36) | 33 (92%) | 28 (78%) | 0.072 |
| Total Non-malignant (57) | 40 (70%) | 47 (82%) | *** |
| Overall Total | 94 (85%) | 89 (80%) | *** |
Abbreviations: TBNA = Transbronchial needle aspiration, in this study, always performed with endobronchial ultrasound guidance
ca-TBFB - Cautery-assisted transbronchial forceps biopsy, always performed with endobronchial ultrasound guidance
NSCLC – Non-small cell lung cancer
Two independent, blinded pathologists reached consensus with ties resolved by a third
From Fisher’s Exact Test
Underpowered to detect the indicated differences with given sample size
Metastatic Melanoma, Metastatic Renal Cell Carcinoma (1 node each)
We subsequently assessed the diagnostic yield based on a per-patient, rather than per-nodal, analysis. In our cohort, 31 patients had a final clinical diagnosis of malignancy, 10 patients had a diagnosis of benign nodes with only lymphocytes identified on biopsies, 8 patients had a diagnosis of sarcoidosis based on nodal granulomatous inflammation and 1 patient had Cryptococcus identified in the lymph node.
There were 24 clinically relevant diagnostic discrepancies identified between TBNA and ca-TBFB when analyzing results on a per-patient, rather than per-node, basis. Fifteen did not change the clinical stage of malignancy or the overall diagnosis (eg. sarcoidosis) and would therefore have had no clinical impact. Thus, in patients with malignancy, the N stage was identified similarly in both TBNA and ca-TBFB. However, ca-TBFB would have either missed or understaged 6 of the 31 patients with malignancy. To classify sarcoidosis in this analysis, the presence of granulomatous inflammation made in any node without malignancy or infection was sufficient. Of the eight patients with this diagnosis, TBNA alone would have failed to make a diagnosis in 3 of 8 patients with sarcoidosis.
Because the referring oncologists required non-needle specimens and no other sites of disease were available, nine patients underwent ca-TBFB solely to obtain core biopsies for clinic trials. Two patients received an alternate diagnosis of their lymphadenopathy (small cell carcinoma or granulomatous inflammation rather than the expected NSCLC), one patient died prior to specimen analysis by the trial, and five of the six remaining biopsies were successfully evaluated for clinical trials based on material obtained.
Discussion
To our knowledge, this is the first study demonstrating the potential benefit of using EBUS-guided electrocautery-induced airway incisions with standard forceps to acquire tissue from mediastinal or hilar lymph nodes. In cases where needle-acquired specimens do not suffice, the quantity of target tissue obtained by this innovative technique was adequate and enabled enrollment in clinical trials. Although overall sample size was small, results suggest ca-TBFB has a higher sensitivity for benign diseases such as sarcoidosis.
In this study, we have demonstrated that ca-TBFB appears to be safe. We had no significant bleeding, mediastinitis, pneumothorax or intraprocedural death. We feel that our research protocol that included both one- and two-month follow-up was sufficient for detecting major complications not identified during or immediately after the procedure. A recent review of more than 10,000 patients who had undergone EBUS described a 0.05% rate of significant adverse effects following the procedure [12]. Among the 5 EBUS complications documented during follow-up in that review, mediastinal infection and pneumothorax were noted. We did not have this experience. Although we did not, per our protocol, obtain post-procedural imaging, we observed no symptoms suggestive of pneumothorax. Although we may have underestimated clinically insignificant complications by not routinely performing post-procedural CT scans, any such complications did not require intervention.
Two patients died during the follow-up period. We do not believe these were related to the procedure. The first patient had advanced malignancy and, as a consequence of recurrent ongoing thrombotic episodes attributed to malignancy, care was changed to palliative. Because the second patient had progressive clinical deterioration due to airway obstruction prior to bronchoscopy, the procedure was performed to obtain trial specimens and palliation of symptoms via airway dilation. It is possible that the procedure contributed to his subsequent demise, although his death was attributed to heart disease. In a separate study with unpublished data presented at the 2013 International Conference of the American College of Chest Physicians, our group noted that 39% of 20 patients required electrocautery incisions to pass biopsy forceps into lymph nodes. Over a 10-day follow-up period in that cohort, there were no deaths. Finally, since finishing this study, we have adopted the ca-TBFB technique as a routine assessment tool for sarcoidosis, lymphoma and malignancy and for obtaining malignant core specimens for clinical trials. We have since performed more than 100 additional procedures with no periprocedural mortality.
Direct diagnostic comparison with mediastinoscopy was not feasible for two reasons: 1) mediastinoscopy was not routinely required in our patients and 2) patients with more advanced disease, as indicated by metastatic foci and multiple comorbidities, were often physically unfit to undergo mediastinoscopy because of their baseline medical status. Because mediastinoscopy was not employed here as a separate, independent gold standard, our data did not provide specificity. Our de facto gold standard was a clinical decision based on consensus of multiple pathologists, and in the case of TBNA, yields 100% sensitivity for malignancy because in every case, TBNA’s diagnosis was confounded with the gold standard. Because our sample was restricted to nodes that had been positively identified with either specific malignant conditions or specific non-malignant conditions, our aim was to compare the sensitivity of TBNA to ca-TBFB among nodes diagnosed apriori by our de facto gold standard. For this reason we have the information to calculate and compare sensitivities, i.e., the proportion of those correctly identified as having a condition among all those who do have the condition according to the stated gold standard. Conversely, because there are no true negatives within any of these subgroups (e.g., there are no persons in our sample free of cancer per our gold standard), there are none of the false negatives required for calculating specificity. The latter is the proportion of those who don’t have a condition per the gold standard who are correctly identified by the test technique as not having the index condition. Other statistics such as positive predictive value and negative predictive value require both sensitivity and specificity and population prevalence, and are therefore also precluded by the design of this experiment. Our inferential goal was very modest (i.e., testing for a simple difference) and we do not claim to be conducting tests of equivalence, superiority, or non-inferiority, each being more sophisticated than the simple test of differences presented here.
Given that we are testing for a simple difference of proportions, the power calculations generally support our modest aims and our findings. For instance, for the first row in Table 3 corresponding to NSCLC, for a sample of 33, and a goal of detecting a difference between proportions of 73% and 100% at an alpha of 5%, relative to the lower proportion of 73%, we have 80% power to detect a proportion of 94% with our sample of 33 in each group. The sample sizes for small cell lung cancer, lymphoma, and other cancers were prohibitively small, as indicated in a footnote. In every case in Table 3 where p-values are reported, the differences indicated were large enough to be detected with our modest samples.
Prior studies have analyzed the use of mini-forceps in select lymph node stations [4–9]. In this study, we biopsied unselected mediastinal and hilar stations and obtained averages comparable to practice patterns observed in the AQuIRE Registry [4]. Relative to paratracheal stations, we found higher sensitivity in subcarinal and hilar stations, likely because the less-acute angle of penetration.
In benign diseases such as sarcoidosis, the sensitivity of EBUS-TBNA was lower than for NSCLC. Because of this, alternative techniques such as transbronchial biopsy are often advocated, consequently exposing patients to iatrogenic risks like radiation and pneumothorax [3]. The use of ca-TBFB exposes patients to none of the risks inherent in these alternative approaches. We believe the larger pieces of intact tissue obtained by ca-TBFB enabled pathologists to more readily identify granulomas in this study.
We cannot readily explain why the sensitivity of TBNA for sarcoidosis was lower in our study than previously reported [3–4, 11]. One possible factor is that we limited the procedure to two passes per lymph node station with TBNA so that we could attain ca-TBFB without excessively prolonging the procedure. A recent study suggested that 3–5 passes in the largest accessible lymph nodes is necessary for evaluating sarcoidosis with EBUS-TBNA [13]. In other studies using mini-forceps, the sensitivity varied from 24% to 61% using 3–4 TBNA passes [5–7].
When discrepancies were re-reviewed by an independent pathologist, small granulomas were newly identified in 4 lymph nodes with TBNA. This improved TBNA’s diagnostic yield to 53% (10 out of 19 nodes), and may reflect inter-rater discrepancy on the smaller specimens. Notably, these granulomas were only detected after observation in the larger forceps-based samples.
In our cohort, TBNA was superior to ca-TBFB for diagnosis of malignancy. We can only speculate regarding these discrepancies. The angle of forceps penetration into the paratracheal nodes is acute, occasionally prohibiting the forceps from entering the node. Additional conjecture includes a fibrotic mediastinum from prior radiation or mediastinoscopy or calcification of nodes that made forceps penetration more difficult.
Because its false negative rate for diagnosis of malignancy is higher than that of TBNA, we do not advocate ca-TBFB for initial staging of malignancy. However, for patients with a previous history of malignancy, or high likelihood of malignancy suggested by imaging, we recommend the use of ca-TBFB for the acquisition of repeat biopsies needed for clinical trials. Because of our findings, it has become standard practice at our institution to use ca-TBFB for repeat biopsies, particularly when nodes or masses are subcarinal or parabronchial.
There are limitations to our study that merit comment. First of all, this study is a single operator experience in one institution. Although fellows participated, the electrocautery incision was made by a skilled bronchoscopist trained in interventional pulmonary medicine. Clinicians not familiar with EBUS or electrocautery may not achieve the level of procedural success reported here. Our study design did not evaluate accuracy against a true gold standard (mediastinoscopy). Detection of false-negatives was not routinely assessed in our study patients because most treating oncologists did not feel mediastinoscopy was warranted when EBUS was negative.
Our study did not enroll enough patients with lymphoma to determine the efficacy of ca-TBFB in its evaluation. Although the larger forceps biopsies might be intuitively superior to TBNA for evaluating nodal architecture, we cannot conclude this from our study. We did not use the 21 g TBNA needle when performing EBUS-TBNA and cannot address whether different needle size would influence sensitivity.
Conclusions
EBUS-guided ca-TBFB appears to be safe. The larger samples obtained from ca-TBFB increased the sensitivity of granulomatous disease and were useful for providing specimens in clinical trials for malignancy when needle biopsies were excluded. We strongly advocate this technique as an alternative to TBNA for thoracic surgeons and advanced bronchoscopists in select clinical scenarios.
Acknowledgments
We thank Cynthia Bensley, RN, for assistance in enrolling patients.
Funding: None
Footnotes
Conflict of Interest: None
Conception and design: KB, MP, JP
Analysis and interpretation: KB, MP, TM, KA, JP
Drafting of manuscript and important intellectual content: KB, MP, TM, JP
Independent review of pathology: RH
Contributor Information
Kyle Bramley, Email: Kyle.bramley@yale.edu.
Margaret A. Pisani, Email: Margaret.pisani@yale.edu.
Terrence E. Murphy, Email: Terrence.murphy@yale.edu.
Katy Araujo, Email: Katy.araujo@yale.edu.
Robert Homer, Email: Robert.homer@yale.edu.
Jonathan Puchalski, Email: Jonathan.puchalski@yale.edu.
References
- 1.Silvestri G, et al. Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e211S–e250S. doi: 10.1378/chest.12-2355. [DOI] [PubMed] [Google Scholar]
- 2.Wada H, et al. Lymph node staging by endobronchial ultrasound-guided transbronchial needle aspiration in patients with small cell lung cancer. Ann Thorac Surg. 2010;90(1):229–234. doi: 10.1016/j.athoracsur.2010.03.106. [DOI] [PubMed] [Google Scholar]
- 3.Agarwal R, Aggarwal AN, Gupta D. Efficacy and safety of conventional transbronchial needle aspiration in sarcoidosis: a systematic review and meta-analysis. Respir Care. 2013;58(4):683–693. doi: 10.4187/respcare.02101. [DOI] [PubMed] [Google Scholar]
- 4.Agarwal R, Srinivasan A, Aggarwal AN, Gupta D. Efficacy and safety of convex probe EBUS-TBNA in sarcoidosis: A systematic review and meta-analysis. Respiratory Medicine. 2012;106:883–892. doi: 10.1016/j.rmed.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 5.Eapen GA, et al. Complications, consequences, and practice patterns of endobronchial ultrasound-guided transbronchial needle aspiration: Results of the AQuIRE registry. Chest. 2013;143(4):1044–1053. doi: 10.1378/chest.12-0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herth FJ, et al. Endobronchial ultrasound-guided miniforceps biopsy in the biopsy of subcarinal masses in patients with low likelihood of non-small cell lung cancer. Ann Thorac Surg. 2008;85(6):1874–1878. doi: 10.1016/j.athoracsur.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 7.Franke KJ, et al. The contribution of endobronchial ultrasound-guided forceps biopsy in the diagnostic workup of unexplained mediastinal and hilar lymphadenopathy. Lung. 2012;190(2):227–232. doi: 10.1007/s00408-011-9341-0. [DOI] [PubMed] [Google Scholar]
- 8.Darwiche K, et al. Evaluation of a novel endobronchial ultrasound-guided lymph node forceps in enlarged mediastinal lymph nodes. Respiration. 2013;86(3):229–236. doi: 10.1159/000350867. [DOI] [PubMed] [Google Scholar]
- 9.Chrissian A, Misselhorn D, Chen A. Endobronchial-ultrasound guided miniforceps biopsy of mediastinal and hilar lesions. Ann Thorac Surg. 2011;92(1):284–288. doi: 10.1016/j.athoracsur.2011.03.069. [DOI] [PubMed] [Google Scholar]
- 10.Goyal G, Pisani M, Murphy T, et al. Advanced diagnostic bronchoscopy using conscious sedation and the laryngeal nerve block: tolerability, thoroughness, and diagnostic yield. Lung. 2014;192(6):905–913. doi: 10.1007/s00408-014-9607-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van derHeijden EH, Casal RF, Trisolini R, et al. Guideline for the acquisition and preparation of conventional and endobronchial ultrasound-guided transbronchial needle aspiration specimens for the diagnosis and molecular testing of patients with known or suspected lung cancer. Respiration. 2014;88(6):500–517. doi: 10.1159/000368857. [DOI] [PubMed] [Google Scholar]
- 12.von Bartheld MB, van Breda A, Annema JT. Complication Rate of Endosonography (Endobronchial and Endoscopic Ultrasound): A Systematic Review. Respiration. 2014;87:343–351. doi: 10.1159/000357066. [DOI] [PubMed] [Google Scholar]
- 13.Sun J, Yang H, Teng J, et al. Determining factors in diagnosing pulmonary sarcoidosis by endobronchial ultrasound-guided transbronchial needle aspiration. Ann Thorac Surg. 2014 doi: 10.1016/j.athoracsur.2014.09.029. [DOI] [PubMed] [Google Scholar]